Abstract
Treatment-resistant depression affects up to 20% of individuals suffering from major depressive disorder (MDD). The medications currently available to treat depression, including serotonin re-uptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs), fail to produce adequate remission of depressive symptoms for a large number of patients. The monoamine hypothesis upon which these medications are predicated should be expanded and revised as research elucidates alternative mechanisms of depression and effective methods to treat the underlying pathologic consequences. Research into the role of tryptophan degradation and the kynurenine pathway in the setting of inflammation has brought new insight into potential etiologies of MDD. Further investigation into the connection between inflammatory mediators, tryptophan degradation, and MDD can provide many targets for novel antidepressant therapies. Thus, this review will highlight the role of the kynurenine pathway in the pathophysiology of depression, as well as a novel therapeutic target to classic and new modulators to treat depression based on findings from preclinical and clinical studies.
Keywords: Kynurenine pathway; Indoleamine 2,3–dioxygenase; Tryptophan; Inflammation; Depression
1. INTRODUCTION
Mental illness is a pervasive category of disorders that account for a larger proportion of disability in developed countries than any other illness, including cancer and heart disease (Reeves et al., 2011). Depressive disorders are also a global issue and the leading cause of burden and disability worldwide, according to the 2010 Global Burden of Disease of the World Health Organization (Ferrari et al., 2013). Major Depressive Disorder (MDD) is a significant public health issue within the United States that affects roughly 3% of adults, or approximately 9 million people (Reeves et al., 2011). The economic burden of MDD in the United States was estimated to be $210 billion in 2010, an increase in over 20% since 2005 (Greenberg et al., 2015). The burden this places on the patients, health care system, and economy as a whole indicates the importance of efficacious treatment of mental illnesses and continued research into the biological etiologies of these complex conditions.
The underlying cause of depression has been difficult to elucidate due to the heterogeneous nature of the disease and is based on cluster of symptoms derived from different etiologies. The monoamine deficiency hypothesis has historically been used to explain how depressive symptoms arise from insufficient levels of monoamine neurotransmitters (Delgado, 2006; Schildkraut, 1965). The serotonergic hypothesis was later developed by Van Praag and Korf (1971), followed by the dopaminergic hypothesis of Willner et al. (1990). However, as evidenced by the latent response to antidepressant medications, specifically reuptake inhibitors, and the prevalence of treatment-resistant depression, it is necessary to continue researching alternative treatment methodologies (Trivedi et al., 2008).
Early generations of antidepressants were the monoamine oxidase inhibitors (MOAIs) and the tricyclic antidepressants (TCAs). The mechanism of action of MOAIs is irreversible inhibition of monoamine oxidase, the enzyme responsible for degradation of serotonin, norepinephrine, and dopamine, thereby increasing synaptic concentrations of these neurotransmitters. TCAs act by decreasing reuptake of serotonin and norepinephrine in the presynaptic neurons, effectively increasing their synaptic concentration (DeBattista, 2012). While MAOIs and TCAs do effectively decrease symptoms of depression in a subset of individuals, they are limited by their considerable side effects and are no longer prescribed as first line treatments for depression (Elhwuegi, 2004).
Current first line antidepressant drugs are selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs). The selectivity of these drugs has resulted in a treatment option that is better tolerated with fewer side effects, as compared to the MOAIs and TCAs (DeBattista, 2012). However, despite improvements in receptor targeting, SSRIs are no more efficacious than the older TCAs at relieving symptoms of depression (Millan, 2006). Improvements in the side effect profile may increase compliance with treatment programs, but increased efficacy is the ultimate goal for successful pharmacologic management of depression.
Adjuvant therapy with atypical antipsychotics, such as quetiapine, aripiprazole, and olazapine, has been shown to augment the therapeutic effects of SSRIs and has been approved for use in treatment-resistant depression (Bobo and Shelton, 2010; Connolly and Thase, 2011). The detrimental health effects of prolonged atypical antipsychotic use are considered a significant hindrance to the long-term use of this combination, despite the potential benefits. Side effects included significant weight gain, dyslipidemia, and altered glucose metabolism (Davey et al., 2012).
Another important consideration regarding currently available antidepressant treatments is the slow onset of action before clinical improvement is attained. Often, SSRIs and SNRIs require weeks to months of treatment before patients report a diminishment of symptoms (O’Leary et al., 2014). There is also a higher risk of suicide and other deliberate acts of self-harm during the first month of treatment with antidepressants. It has been suggested this is due to an improvement in physical energy that precedes improvements in depressive mood and negative thoughts (Conwell and Heisel, 2012). Identifying faster acting antidepressants is clearly crucial to increasing compliance and decreasing the harmful effects of delayed treatment.
Similarly, related to insufficient therapeutic response of the currently available antidepressants, at least 20% of depressed patients are treatment-resistant—defined as nonresponse to two different pharmacologic classes taken at optimal dose and for sufficient period of time (Berlim and Turecki, 2007). Additionally, approximately 50% of those who are diagnosed with MDD will experience a recurrent or chronic course of the illness (Crown, 2002). Major depression is a complex disorder in which gene-environment interactions affects many areas of the body; it stands to reason that manipulating one molecule or neurotransmitter may not effectively cure the disease (Myint, 2012a). The insufficient therapeutic effect of the currently available antidepressants has moved the field to focus on alternative mechanisms beyond the monoamine hypothesis. Indeed, over the past two decades there has been a shift from the monoamine hypothesis to pathways involving neuroplasticity impairment. Since patients with autoimmune and inflammatory disorders such as diabetes and fibromyalgia often present with depressive symptoms, it has been proposed that depression may be linked to inflammation (McInnis et al., 2014). Indeed, patients with depression had an increase in serum levels of proinflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) (O’Brien et al., 2007). Such pro-inflammatory state has been documented in depressed adolescents as well compared to healthy non-depressed adolescents, suggesting that it may play a role early in the course of illness, suggesting that increased inflammation in MDD is not due to chronicity effects (Gabbay et al 2009). The kynurenine pathway (KP) has been hypothesized to play a key role in processes linking peripheral inflammation and CNS alterations by i) reducing tryptophan availability, and ii) production of oxygen radicals and highly potent neurotoxins (Hochstrasser et al., 2011). Thus, tryptophan degradation and its role in the availability of serotonin have brought attention to the kynurenine pathway as a potential target for future research into alternative treatments for depression.
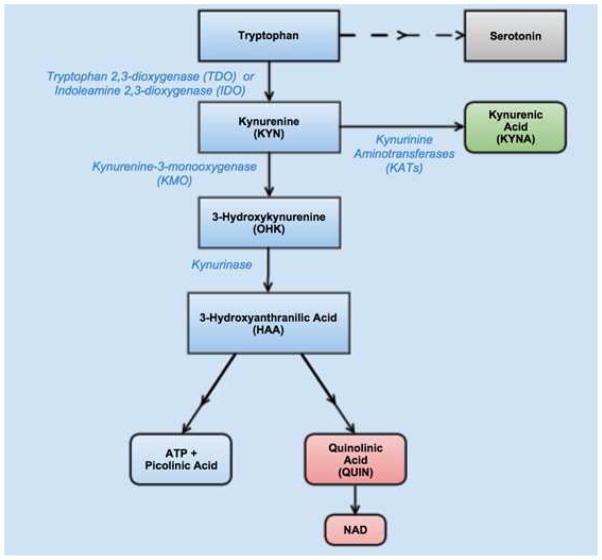
About 99% of tryptophan (TRP) is metabolized by tryptophan 2,3-dioxygenase (TDO) into kynurenine (KYN) in the liver. However, during active inflammation, indoleamine 2,3 –dioxygenase (IDO) is activated in extrahepatic tissues to convert TRP to KYN (Leklem, 1971). Kynurenine by itself in not neuroactive but instead is compartmentally hydroxylated by kynurenine-3-monooxygenase (KMO) into 3-hydroxykynurenine (OHK). Further cleavage of OHK by kynureninase yields 3-hydroxyanthranilic acid (3-HAA). After production of 3-HAA, there are two possible degradation arms: one proceeds with complete oxidation of 3-HAA to form adenosine triphosphate (ATP) and a small quantity of picolinic acid (PIC), while the other pathway produces quinolinic acid (QUIN) which is eventually degraded into nicotinamide adenine dinucleotide (NAD) (Leklem, 1971). Conversely, an alternative pathway is the conversion of KYN to kynurenic acid (KYNA)—a glutamate and 17-nicotinic acetylcholine receptor antagonist—by kynurenine aminotransferases (KATs) (Lopresti et al., 2014). Figure 1 portrays the tryptophan degradation pathway.
Figure 1.
The tryptophan degradation pathway. Tryptophan is degraded by either tryptophan 2,3-dioxygenase (TDO) or indoleamine 2,3-dioxygenase (IDO) into kynurenine (KYN). Kynurenine is further degraded into either 3-Hydroxykynurenine (OHK) by kynurenine-3-monooxygenase (KMO) or into kynurenic acid (KYNA) by kynurinine aminotransferases (KATs). OHK goes on to be degraded by kynurinase to produce 3-hydroxyanthranillic acid (HAA). Finally, HAA can be degraded into either ATP or small amounts of picolinic acid or into quinolinic acid (QUIN) and further into NAD. KYNA has been hypothesized to be neuroprotective while QUIN has been hypothesized to be neurodegradative.
The KYN pathway also plays a role in the metabolism of glucose. The ATP and HAA formed from this pathway activate glycolysis (Quagliariello and Papa, 1964), through which glycogen is stored in the cells to be utilized in case of stress or glucose need. QUIN has also been shown to inhibit gluconeogenesis (Lardy, 1971). It appears that under normal physiological conditions in the brain, the KYN pathway serves mainly for glycogen storage and the production of small amounts of NAD required for ATP synthesis in the central nervous system (Myint, 2012a, b).
TRP depletion is therefore the result of enhanced tryptophan catabolism by TDO in the liver (Takikawa, 2005) and IDO in the lungs, placenta, blood, and brain (Heyes et al., 1993; Mellor and Munn, 1999). Furthermore, serotonin is degraded by IDO into formyl-5-hydroxykynuramine, in addition to the degradation by monoamine oxidase (Pertz and Back, 1988). The enhanced degradation of tryptophan towards kynurenine and away from production of serotonin has been termed the “kynurenine shunt” (Lapin and Oxenkrug, 1969; Mangoni 1974).
The tryptophan degradation pathway and the kynurenine shunt have been connected to a number of psychiatric conditions, suggesting that this biochemical process may have far reaching implications. Patients with BD have shown decreased neuroprotective kynurenine metabolites in their hippocampus and amygdala when compared to control patients (Savitz et al., 2015a). Schizophrenic patients were also identified as having an imbalance between the neuroprotective and neurotoxic metabolites of tryptophan degradation (Kegel et al., 2014). MDD is often associated with a systemic pro-inflammatory state that can be tied to increasing levels of IDO activity in the peripheral tissue and the brain (Heyes et al., 1993). This review will present current research that identifies the kynurenine pathway as a prevalent component of the pathophysiology of depression from studies with animal models and human. In addition, this review will highlight studies that focus on treatment targets associated with the kynurenine pathway and antidepressant responses.
2. SEARCH STRATEGY
For this narrative review, the PubMed/MEDLINE database was searched with the following Boolean terms: “kynurenine”[Mesh] OR “kynureninase”[Supplementary Concept] OR “kynurenine pathway” OR “indolamine-2,3-dioxygenase” OR “tryptophan 2,3-dioxygenase” OR “kynurenic acid” OR “quinolinic acid” OR “anthranilic acid” OR “3 –Hydroxykynurenine” AND “Depression”[Mesh] OR “Depressive Disorder”[Mesh] OR “Depressive Disorder, Major”[Mesh] OR “Depression”; through March 3rd, 2015.
Observational and experimental studies in human and animal models investigating the role of components of the kynurenine pathway in the pathophysiology of MDD were included. Review articles, case reports, as well as studies that included participants with bipolar depression were excluded. The overall methodological quality of retrieved references was considered for final inclusion. No language restrictions were applied. The citation tracking of included reports was searched in Google Scholar for potentially eligible articles for this review.
3. RESULTS AND DISCUSSION
3.1 Kynurenine pathway in the pathophysiology of depression and its comorbidities
3.1.1 Evidence from human studies: central effects
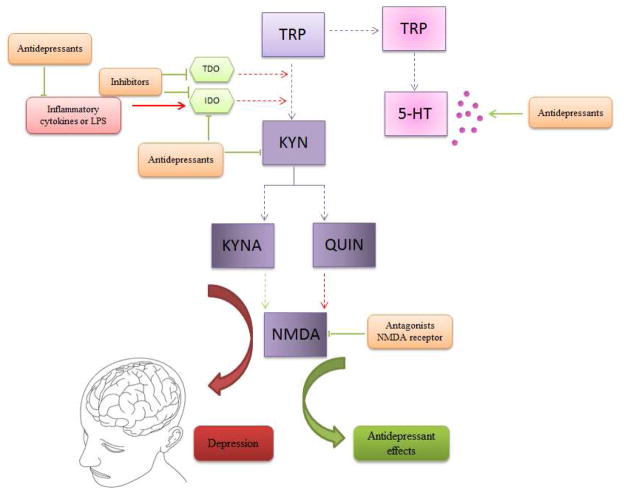
Recent studies have shown that the kynurenine pathway (KP) plays an important role in depression (Figure 2) (Myint et al., 2007, 2012a, b). Positive correlations were drawn between KYN, QUIN, KYNA and specific pro-inflammatory immunological variables in the cerebrospinal fluid (CSF), and depressive symptoms in patients with hepatitis on IFN-α treatment (Raison et al., 2009). The pro-inflammatory status of patients with major depression has been associated with increases in pro-inflammatory cytokines interleukin-2 (IL-2), IL-6, TNF-α and IFN-γ in addition to decreases in anti-inflammatory cytokines IL-4 and IL-10 (Myint, 2012a). A proton MR spectroscopy study revealed a positive correlation relating KYN, 3-HAA and tCho (cell membrane turnover biomarker) in the right caudate and left putamen with melancholia in adolescent MDD (Gabbay et al., 2010b).
Figure 2.
The role of kynurenine pathway (KP) in the pathophysiology of depression and as a therapeutic target. Proinflammatory cytokines as well LPS may induce indoleamine 2,3-dioxygenase (IDO) activation, which in turn increase KYN levels and its toxic metabolite quinolinic acid (QUIN). In addition, IDO activation is linked to serotonin (5-HT) depletion. Increased inflammation and toxic KP activation, including excitotoxicity by n-methyl-D-aspartate (NMDA) receptor, as well as decreased 5-HT levels are associated with pathophysiology of depression (red arrows). On the other hand, antidepressant drugs acting to decrease pro-inflammatory cytokines, kynurenine (KYN) levels and its metabolites. Antidepressants also increase 5-HT levels. Inhibitors of TDO and IDO enzymes decrease KYN levels and exert antidepressant effects. Antagonists of NMDA receptor, such as ketamine decrease excitotoxicity induced by NMDA receptor activation and exert antidepressant effects (green arrows).
The KYN pathway is seem to be involved with other psychiatric diseases, such as schizophrenia and bipolar disorder. However, the neurobiological effects found in KYN pathway it seems to be different in these diseases and MDD. A recent study measuring the levels of QUIN and KYNA in patients with schizophrenia found depressed QUIN/KYNA ratios in the CSF of schizophrenic patients as compared to controls (Kegel et al., 2014). In fact, elevated KYNA is one of the most consistently found deviations in patients with schizophrenia and BD with psychotic features (Schwarcz et al., 2001; Miller et al., 2006, Linderholm et al., 2012). On the other hand, Savitz et al. (2015a) found a reduction in the neuroprotective KYNA/QUIN ratio, but no difference in the individual KYN metabolites in BD patients, compared to health controls. Reduced KYNA/QUIN ratio in BD patients was linked to amygdala and hippocampus volume reduction (Savitz et al., 2015a). Furthermore, IDO downregulation and 5-HT upregulation has been associated with the antidepressant properties of valproate, an agent used as an antiepileptic and mood stabilizer (Qiu et al., 2014). This pathologic shift towards KYNA and away from QUIN production runs contrary to the major depression model in which KYNA is neuroprotective and QUIN is neurotoxic. Interestingly, interleukin-1 receptor antagonist, QUIN, and KYN were significantly elevated in MDD patients, compared with healthy controls, whereas KYNA, tryptophan, and KYN were positively correlated with hippocampal and amygdala volume in MDD patients (Savitz et al., 2015c), suggesting that an immune-related imbalance in the relative metabolism of KYNA and QUIN predisposes to depression-associated dendritic atrophy and anhedonia (Savitz et al., 2015c).
The final products of the tryptophan degradation pathway have also been shown to directly alter activity of certain receptors in the brain. QUIN is an N-methyl-D-aspartate receptor (NMDA-R) agonist (Schwarcz et al., 1983). Accumulation of QUIN in the brain results in excitotoxicity due to stimulation of NMDA receptors (Okuda et al., 1998). This neurodegenerative effect is counteracted by KYNA, which is an NMDA-R antagonist (Perkins and Stone, 1982). KYNA can thereby act to protect neurons against the excitotoxicity of QUIN (Kim and Choi, 1987). An imbalance between the neuroprotective effects of KYNA and the neurotoxic effects of QUIN was demonstrated in major depression as well as INFα-treatment depression (Myint et al., 2012a,b). When the catabolism of tryptophan is enhanced by increased IDO activity, the production of KYNA is outpaced by the production of OHK by KMO. Activated monocytes in the body can then continue the degradation pathway to produce excess QUIN (Chiarugi et al., 2001). Erhardt et al. (2013) demonstrated that elevated CSF levels of QUIN and IL-6, but not KYNA, were positivity correlated with suicide attempts in patients with MDD. On the other hand, Sublette et al. (2012) showed higher levels of KYN in MDD with history of suicide attempt as compared to MDD without history of suicide attempt; however, in attempters, there was elevated KYN levels and a positive correlation with cytokines activation (Sublette et al., 2011), showing an association between inflammation and KYN pathway stimulation. Also, Bay-Richter et al. (2015) revealed that levels of QUIN increased and KYNA decreased over time in suicidal patients versus healthy controls; high levels of IL-6 were also related to more severe suicidal symptoms. A recent study in adolescents documented similar findings of increased KYN/TRP in suicidal depressed adolescents (Gabbay et al., 2015). Increased levels of pro-inflammatory cytokines, such as IL-6, may be involve with microglia and astrocytes cells activation. Astrocytes and microglia have been identified as the primary site for tryptophan catabolism in the brain (Grant et al., 2000). While the astrocytes are shown to produce mainly KYNA, microglia and macrophages produce mainly QUIN that is then degraded to NAD by neighboring microglia (Guillemin et al., 2001). In studies of major depression, loss of astrocytes has been observed (Rajkowska et al., 1999). This loss might be partly due to the increased toxic KYN metabolites resulting from the pro-inflammatory state induced by the imbalance between the neurotoxic KYN metabolites and the neuroprotective KYNA (Myint, 2012a, b). This pathway, in the absence of inflammation or immune challenge, produces the small amount of NAD required by the central nervous system (Leklem, 1971). Complete depletion of NAD is fatal to the cells, especially if they are under stress, since ATP formation in these cells is dependent on NAD (Myint, 2012a, b).
Microglia is known to produce inflammatory mediators, so it is possible that these pro-inflammatory cytokines may induce the KP in the brain (Myint et al., 2012a, b). Elevated levels of microglial density have been found in the postmortem examination of suicidal patients with major depression and schizophrenia (Steiner et al., 2008). In addition, the activation of the KP in microglia leads to formation of potentially neurotoxic KYN metabolites, such as QUIN (Mayhew et al., 2015). In a postmortem study, an association was found between severe depression and increased density of QUIN immunopositive microglia in the anterior cingulate cortex (Stone et al., 2000). A similar study identified significantly increased QUIN immunoreactivity in the prefrontal cortex of patients with MDD and bipolar depression (Steiner et al., 2011). However, in the hippocampus from patients suffering uni- and bipolar depression a reduction in QUIN-immunoreactive microglia was observed (Busse et al., 2014). This indicates that microglia might exert a toxic or neuroprotective role, depending on brain area.
3.1.2 Evidence from human studies: peripheral effects
It is well known that the central nervous system (CNS) is in constant communication with the peripheral body systems. Many studies have used peripheral markers to better understand diffuse nature of mood disorder pathologies, such as depression. The IDO enzyme is activated in extrahepatic tissue, such as the lungs, kidneys, spleen, blood, and brain, during situations of inflammation, infection or oxidative stress (Heyes et al., 1993; Mellor and Munn, 1999). In addition, IDO activity is increased by the pro-inflammatory molecule interferon-γ (IFN-γ) (Carlin et al., 1987). IDO is most likely involved in the pathophysiology of depression (Kim et al., 2012) and may even be the link between inflammation and depression (Quak et al., 2014). IDO is activated by pro-inflammatory cytokines, including IL-6 and IL-1β, and IDO activity is associated with a decrease in serotonin content and an increase in KYN content, which in turn actives QUIN and glutamate receptor (Kim et al., 2012; Heyes and Lackner, 1990). Findings from Raison et al. (2009) suggest that peripheral activation of IDO leads to parallel activation of the KP in the brain. In a population-based Young Finns Studies demonstrated a positive correlation between peripheral activation of IDO and depressive symptoms at baseline and follow-up (Elovainio et al., 2012), and with depressive symptoms and carotid atherosclerosis in women (Elovainio et al., 2011). Recently, higher levels of serum IDO and inflammatory mediators (TNF-α, IFN-γ and C-reactive protein) and lower levels of 5-HT were found at baseline in MDD women compared to health controls (Zoga et al., 2014). On the other hand, undergoing effective treatment decreased IDO and TNF-α and these effects were positively linked to patient improvement (Zoga et al., 2014). Myint et al. (2013) found an association between serum IFN-γ gene CA repeat polymorphisms, higher KYN concentrations, and an increase in serum TRP breakdown in patients with depression. In addition, a study showed that 53.7% of patients in treatment with IFN-α develop depressive symptoms associated with TRP depletion and a high neurotoxic challenge (KYN to kynurenic acid quotient) (Baranyi et al., 2013). In patients with malignant melanoma on IFN-α therapy, an increase in plasma KYN and neopterin concentrations, as well as in the KYN/TRP ratio was observed (Capuron et al., 2003). Moreover, MDD patients not receiving antidepressants showed lower TRP concentrations, which were associated with depressive symptoms (Capuron et al., 2003).
In melancholic MDD adolescents KYN/TRP ratios in blood were elevated and TRP concentrations were reduced compared to non-melancholic MDD adolescents (Gabbay et al., 2010a). A study performed in adolescents with anhedonia and MDD found a significant correlation between IDO activity in blood and anhedonia. The correlation was even more significant when medicated patients were excluded, potentially due to the anti-inflammatory effects of antidepressants (Gabbay et al., 2012). These findings displayed an important role of KYN pathway in anhedonia in adolescents suffering MDD.
Somatization in depression was associated with higher serum levels of IDO activity (Anderson et al., 2012), KAT activity, and with enhanced neurotoxic potential (Maes et al., 2011). On the other hand, Hughes et al. (2012) found no differences in IDO expression, plasma KYN levels and metabolites levels in MDD patients, when compared to controls. A reduction in circulating TRP concentrations was noted (Hughes et al., 2012). Increases in IL-6, decreases in KYNA, or increases in the KYN/KYNA ratio showed a significant association with the development of depressive symptoms in patients with cytokine therapy induced depression (Wichers et al., 2005). Interestingly, IDO activation via IL-6 is a pathway involved with regulation of TRP availability (Anderson et al., 2013). The KYN/KYNA ratio is used to estimate how much of the KYN is degraded into KYNA. The amount of KYNA was found to be significantly lower in depressed patients than healthy controls, indicating an imbalance in the kynurenine pathway. This imbalance persisted, even after treatment with SSRIs for six weeks (Myint et al., 2012b). Several studies have indicated that the presence of depression with other comorbid conditions, such cancer, HIV and pain, may be related, at least in part, to dysregulation in the KYN pathway. In cancer patients, KYN levels pre- and post-treatment were measured; KYN levels predicted the trajectory of depression and were an important factor associated with depression and fatigue (Pertl et al., 2013). The severity of depressive symptoms in HIV-infected patients was associated with lower plasma levels of TRP and a higher plasma KYN/TRP ratio (Martinez et al., 2014).
In individuals with Type D personality, which is related to negative affectivity in social situations, increased symptoms of depression and anxiety were noted (Altmaier et al., 2013). In addition, in Type D individuals, lower levels of TRP and KYN were related, but not associated, with depression or anxiety (Altmaier et al., 2013). Positive correlation in TRP catabolism was also found in patients with depression and irritable bowel syndrome (IBS) (Fitzgerald et al., 2008). Individuals with severe IBS exhibited higher levels of KYN/TRP ratio, compared to less severe symptoms and controls, and were more than doubled when linked to depression and anxiety compared to less severe (Fitzgerald et al., 2008). Among patients with coronary artery disease, IDO peripheral activation was correlated with severity of depressive symptoms (Swardfager et al., 2009). Also, a meta-analysis of depressive patients showed significantly decreased TRP levels, which was associated with an increase in KYN/KYNA ratio (Ogawa et al., 2014). Plasma from patients with chronic pain and depression had an elevated KYN/TRP ratio (Kim et al. 2012). A recent study found a reduction of the KYNA/QUIN ratio in serum of depressed and remitted phases of MDD patients, compared to healthy controls (Savitz et al., 2015b). Moreover, a negative correlation between KYNA/QUIN and anhedonia in depressed patients was demonstrated, as well as a negative correlation between lifetime number of depressive episodes and KYNA/QUIN, and a positive correlation between the number of months in remission and KYNA/QUIN (Savitz et al., 2015b).
It has also been proposed that the imbalanced KYN pathway can induce glial-neuronal network impairment that might contribute to the recurrent and chronic nature of MDD (Myint et al., 2012a, b). Patients with MDD show lower tryptophan availability and higher tryptophan breakdown, in addition to lower mean plasma KYNA (Myint et al., 2007). However, a difference in plasma kynurenine or tryptophan concentrations was not found (Myint et al., 2007). The tryptophan-kynurenine pathway provides a link between the immune activation present in MDD and the neurochemical and cellular abnormalities that have been attributed to mood disorders (Myint, 2012a, b). This evidence suggests that the KYN pathway in periphery could be associated with KYN toxic pathway activation in the CNS, leading to neuroinflammatory processes.
3.1.3 Evidence from preclinical studies: central and peripheral effects
The role of the kynurenine pathway in central and peripheral processes is often studied concurrently with animal models of depression. Henry et al. (2009) found that immune system stimulation with lipopolysaccharide (LPS) has been shown to induce cytokine production in the mouse brain with concurrent production of IDO mRNA in microglia. In an animal model of depression, LPS administration produced depressive-like effects and was associated with a more pronounced induction of peripheral and brain IDO and a higher turnover rate of brain serotonin when compared to young adult mice at 24 hours post-LPS injection (Goubout et al., 2008). Mice injected with LPS also displayed depressive-like behavior in the forced swimming test (FST), paralleled by an increase in the KYN/TRP ratio in the serum and IDO in the brainstem (Dobos et al., 2012). A study using western blot analysis correlated an increase in aromatic L-amino acid decarboxylase and IDO in the hippocampus of stressed rats with progression of depressive behavior (Jia et al., 2013). The effects of the TRP-KYN pathway are different in periphery and brain areas. For example, Laugeray et al. (2010) found that rats subjected to chronic stress had an increase in TRP catabolism along the KP in the periphery, whereas in the amygdala and striatum TRP was preferentially metabolized to QUIN, and in the cingulate cortex QUIN was reduced. In addition, the KYN/TRP ratio in the periphery was linked to the magnitude of depressive and anxiety-like behaviors (Laugeray et al., 2011). The effects of LPS on the KP may vary depending on the strain of rodent. In fact, mRNA expression of IDO enzymes and immune activation was altered in BALB-c mice, but not in the C57BL/6J strain after LPS administration (Browne et al., 2012). On the other hand, intracerebroventricular administration of LPS in C57BL/6J mice induced depressive-like behavior and increased IDO expression, synthesis and secretion of TNF-α and IL-6, and activation of inducible isoform of nitric oxide synthase (iNOS) in the hippocampus (Fu et al., 2010). The effects of LPS was time dependent (Fu et al., 2010), suggesting that differences in C57BL/6J mice exerted by LPS could be influenced by time and route administration.
Depressive-like behavior and increased TRP catabolism in mice was extended after infection with Mycobacterium bovis, Bacillus Calmette-Guérin (BCG) (Kelley et al., 2013). KYN pathway was also strongly associated with depressive-like behavior in rodents treated with HIV transactivator of transcription protein (Lawson et al., 2011). BCG in rodents induced depressive and sickness behavior, however only depressive-like behavior was associated with increased levels of TNF-α and peripheral IDO activation (Moreau et al., 2005, 2008). A study using a mouse model of metabolic syndrome (MetS), which is linked to activation of cytokines in brain tissue, showed that LPS administration in MetS mice significantly increased brain KYN/TRP ratio, IL-1β and TNF-α (Dinel et al., 2014). LPS injection in MetS mice was associated with a decrease in hippocampal brain-derived neurotrophic factor (BDNF) (Dinel et al., 2014), an important protein involved in neuroplasticity and the pathophysiology of depression (Ignácio et al., 2014; Réus et al., 2013). Mice subjected to UCMS demonstrated a higher peripheral KYN pathway activity (Laugeray et al., 2010). Viral mimetic Poly I:C induced symptoms of depression and anxiety in rats accompanied by increased expression of IL-1β, IL-6, TNF-α and CD11b and by decreased expression of BDNF and it’s receptor TrkB in the hippocampus and frontal cortex (Gibney et al., 2013).
In conclusion, multiple animal models of depression, including chronic stress or depressive-like behavior induced by infection or LPS, have been associated with kynurenine pathway activation both centrally and peripherally.
3.2 Kynurenine pathway as a potential strategy for the treatment of depression: evidences from in vitro, in vivo and human studies
The fact that standard antidepressants usually require approximately one month or more to manifest their antidepressant effects suggest that regulation of pathways other than the monoaminergic system, such as neuroplasticity or immune system regulation, could be involved with symptom improvement in depressive patients (Berton and Nestler, 2006). Moreover, the higher risk of suicide and other deliberate acts of self-harm during the first month of treatment is not uncommon. It has been thought to be due to a mismatch in symptom improvement; that is, physical energy improves first, while resolution of depressive mood and negative thoughts is more gradual (Conwell and Heisel, 2012).
Previous studies have shown that monoaminergic-based antidepressants also act on the KYN pathway. Immune system or KYN pathway modulators have been shown to produce antidepressant effects (Figure 2). The antidepressant sertraline produced a reduction in the KYN/MEL (melatonin) ratio and 3-Hydroxykynurenine (3-OHKY)/MEL ratio in MDD patients, compared to pretreatment (Zhu et al., 2013). Interestingly, patients who showed poor response to sertraline treatment did not experience changes in these pathways. Thus, antidepressant effects of sertraline appear to be linked to KYN pathway regulation. Mackay et al. (2009) showed a positive correlation between KYN metabolite concentrations and psychiatric rating scores in depressive patients treated for 18 weeks with fluoxetine, an SSRI. Single Nucleotide Polymorphism (SNP) in enzymes involved with KYN metabolism (TPH-2, KMO and KAT) were shown to be altered in patients with depression and BD compared to control populations (Claes et al., 2011). SNPs in the genes IDO1 and IDO2 exhibited an association with citalopram treatment in depressive patients (Cutler et al., 2012). Thus, the genes involved with KYN pathway may play an important role in both pathogenesis and treatment of mood disorders. However, in patients with IFN-α therapy depression and anxiety the symptoms were not linked to variants in the IDO gene (Galvao de Almeida et al., 2011), though these discrepancies could be related to the cross-sectional study design.
Depressed patients stimulated with LPS demonstrated an increase in IFN-γ and IL-10 in blood cultures. However, when the antidepressant imipramine and celecoxib (a cyclooxygenase-2 inhibitor) were added, IL-10 levels were decreased (Krause et al., 2012). Kocki et al. (2012) reported that 24–48 hours of exposure to SSRIs or TCAs stimulated the synthesis of KYNA (neuroprotective) and decreased OHK (neurotoxic) in astroglial cultures.
Studies with rodents have also shown that classical antidepressants act on the KYN pathway and that KYN pathway modulators produce antidepressant effects. Sprague-Dawley rats subjected to unpredictable chronic mild stress (UCMS), an animal model of depression, and QUIN microinjection into the hippocampus exhibited depressive-like behavior, increased levels of glutamate, and altered levels of the glutamate receptors subunit (Chen et al., 2013). On the other hand, Ro61-8048 (a QUIN antagonist) and MK-801 (a glutamate antagonist) reversed depressive-like behavior as well as glutamatergic system alterations (Chen et al., 2013).
In an animal model of depression induced by TRP diet depletion, the depressive-like behavior was associated with increased levels of KYN and reduced levels of KYNA (Franklin et al., 2012). Treatment with the SSRI antidepressant paroxetine reversed these alterations in KYN pathway, but not the depressive behavior (Franklin et al., 2012). Danzhi Xiaoyao San (DXS), which is used in the prevention and treatment of affective disorders in China, was administrated to rats subjected to animal models of unpredictable chronic mild stress (UCMS). Treatment was effective in reversing anhedonic behavior (Zhu et al., 2015). In the same study, DXS decreased IL-6 and TNF-α in the serum, and downregulated IDO activity and KYN production, and upregulated TRP in the hippocampus (Zhu et al., 2015).
The antidepressant citalopram exerted antidepressive-like effects and increased turnover of 5-HT via IDO inhibition in the hippocampus, amygdala and hypothalamus of stressed rats (Ara and Bano, 2012). Fluoxetine administration reduced depressive-like behaviors in tumor-bearing mice, but did not have an effect on KMO mRNA or IDO expression (Norden et al., 2015). On the other hand, in a BV-2 microglial cell line, fluoxetine alone or in combination with acetylsalicylic acid inhibited microglial activation and attenuated LPS-induced production of IL-1β, expression of IDO, and the depletion of 5-HT (Yang et al., 2014). The anti-inflammatory effects of combined treatment with fluoxetine and acetylsalicylic acid were mediated by NF-κB activation and p38 MAPK and ERK1/2 phosphorylation (Yang et al., 2014). In rats with combined exposure of LPS and chronic mild stress (CMS), treatment with the antidepressants imipramine and pentoxyphylline (an anti-TNF-α) were effective in reversing depressive-like behavior and the elevated KYN/TRP ratio and TNF-α gene expression induced by both LPS and CMS in the hippocampus (Elgarf et al., 2014). Agomelatine, a melatonergic antidepressant, was able to reverse LPS-induced IL-1β and IL-6 production in the brain and periphery. Agomelatine also prevented the LPS-dependent increase in KMO (Molteni et al., 2013). Pre-treatment with etanercept, a TNF-α antagonist, partially diminished BCG-induced IDO activation and depressive-like behavior (O’Connor et al., 2009a). Interestingly, IDO activation is seen to mediate depressive behavior following BCG infection. In fact, both IDO inhibitor treated mice and IDO-deficient mice did not exhibit depressive-like behavior after BCG infection (O’Connor et al., 2009b).
Ketamine, an antagonist of NMDA receptors, has been described as a revolutionary new antidepressant drug (Abdallah et al., 2015), and its antidepressant effects seem to be mediated by the MAPK pathway (Réus et al., 2014). Interestingly, ketamine presents antioxidant (Réus et al., 2015a) and anti-inflammatory properties (Réus et al., 2015b) in rodents subjected to the animal model of maternal care deprivation. Prenatal inhibition of the KP alters the expression of proteins involved with glutamatergic signally in rat brains both during development (Forrest et al., 2013a) and adult life (Forrest et al., 2013b). In LPS-induced anhedonic behavior, ketamine produced antidepressant effects (Walker et al., 2013). However, while ketamine abrogated the LPS-induced depressive-like behavior mediated by a-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) in adult rats, and it did not have effects on IDO activity or pro-inflammatory cytokines (Walker et al., 2013). These animal studies suggest a temporal relationship between stress, kynurenine pathway activation, ketamine administration, and the production of depressive-like symptoms in later in life. More studies are warranted to study the effects of ketamine administration during different stages of brain development.
In restraint stress of Sprague-Dawley rats, allopurinol, a TDO inhibitor, attenuated depressive-like behavior and reduced circulating KYN concentrations (Gibney et al., 2014). TDO activity was inhibited and an increase in 5-HT levels in the hippocampus of rats was observed after treatment with tolmetin and sulindac (non-steroidal anti-inflammatory agents) (Dairam et al., 2006). In fractalkine receptor deficient mice (CX3CR1−/−), LPS injection induced an enhancement in mRNA expression of IL-1β, IDO and KMO in microglia in hippocampus and prefrontal cortex at 4 and 24 hours after LPS injection (Corona et al., 2010). In addition, activated microglia in CX3CR1−/− mice was associated with depressive-like behavior (Corona et al., 2010). LPS-induced neuroinflammation and depressive-like behavior in CX3CR1−/− was abrogated by 1-MT, an IDO inhibitor (Corona et al., 2013). Furthermore, 1-MT was able to prevent depressive-like behavior induced by stress (Dobos et al., 2012). Lawson et al. (2013) also demonstrated that genetic deletion or pharmacological inhibition of IDO1 attenuated the anhedonic behavior and duration of immobility time during the tail suspension test, induced by intracerebroventricular infusion of LPS. In LPS-induced depressive behavior, minocycline, which indirectly inhibits IDO and 1-MT, exhibited antidepressant-like action and regulated KYN/TRP ratios in the plasma and brain of LPS treated mice (O’Connor et al., 2009c). Minocycline and 1-MT also normalized the KYN/TRP and 5-HT/TRP ratios and prevented depressive-like behavior induced by an animal model of epilepsy (Xie et al., 2014). 1-MT treatment also reversed anxiety and anhedonic behavior induced by systemic LPS administration in mice, and in contrast administration of l-kynurenine (an enzymatic product of IDO) induced anxiogenic and depressive behavior in mice (Salazar et al., 2012). Therefore, the inhibition of TDO and IDO enzymes, which results in decreased conversion of TRP to KYN, appears to be an important target for antidepressant effects.
4. CONCLUSION
Dysregulation in the kynurenine pathway is evident in depression and has been reported by both animal and human studies. In fact, the kynurenine pathway is associated with not only the diagnosis of MDD but also the severity of depressive symptoms. However, the pathophysiologic mechanisms involved in kynurenine pathway dysfunctions are still not fully understood. Results from human studies are not always reproducible and are sometimes contradictory; the findings are dependent on the stage of depression, age, and brain anatomic area involved. IFN-α therapy may be predictive of the depressive symptoms that are mediated by kynurenine toxic pathway activation. Additionally, the kynurenine pathway could represent the link between MDD and medical conditions such as cancer, pain, and cardiovascular diseases. Thus, future studies are needed to better characterize the role of KYN and its key regulators in the progression of depression. Some classical antidepressants do mediate the kynurenine pathway, decrease inflammation, and reduce toxic KYN metabolites, but there are no kynurenine pathway-specific medications currently available for MDD. Inhibition of IDO could be a therapeutic target for novel MDD treatment based on its central role in the kynurenine pathway. The ability of kynurenine modulators to improve depressive symptoms indicates a new avenue for therapeutic interventions in the treatment of MDD.
Table 1.
Observational and experimental studies with human sample.
| AUTHOR, ANO | SAMPLE | DESIGN | ASSESSMENT | MAIN FINDINGS |
|---|---|---|---|---|
| ALTMAIER, 2013 | n=1502 community sample |
Cross-sectional Population-based study |
Depressive (PHQ-9) and anxious (GAD-7) symptoms | Lower levels of TRP and KYN were associated with type D personality, while no significant associations could be found for depressive and anxious symptoms |
| BAY-RICHTER, 2015 | n=66 (30 patients with suicide attempters and 36 HC) | Cross-sectional study | Cytokines and kynurenine metabolites in CSF; depressive symptoms (MADRS) and symptoms of suicidality over time (SUAS) | QUIN was increased and KA decreased over time in suicidal patients compared to HC Significant association between low KA and severe depressive symptoms, as well as between high OL-6 levels and more severe suicidal symptoms was found |
| BARANYI, 2013 | n=41 patients with chronic HCV |
Clinical study Interferon-α treatment |
TRP, KYN and QUIN were assessed before, during (at 1, 3, 6 and 9 months) and after the end of IFN-α treatment | 53.7% of patients fulfilled the criteria for treatment-related MDD TRP depletion and KYN and QUIN elevated levels in depressive patients |
| CAPURON, 2003 | n=26 patients with malignant melanoma |
Randomized clinical trial, double blind Interventions: placebo or paroxetine, beginning 2 weeks before IFN-alpha treatment | TRP, KYN, Neopterin (a marker of immune activation) and depressive symptoms (HDRS) were assessed at baseline and at 2, 4, and 12 weeks of treatment | IFN-α therapy increased plasma KYN, Neopterin concentrations, and KYN/TRP ratio Antidepressant-free patients lower TRP was correlated with depressive symptoms |
| CLAES, 2011 | n=338 (266 MDD, 72 bipolar depression, and 310 population controls) | Case-control study | TPH2, KMO and KAT SNPs and haplotype association analysis | SNP in enzymes involved with KYN metabolism (TPH-2, KMO and KAT) were shown to be altered in patients with MDD and BD compared to population control |
| CUTLER, 2012 | n=1953 participants enrolled in the Sequenced Treatment Alternatives to Relieve Depression study |
Clinical study | Genotypes corresponding to 94 SNPs in the genes IDO1 and IDO2 were extracted from a larger genome-wide set | SNP in the genes IDO1 and IDO2 exhibited association to citalopram treatment in depressive patients |
| ELOVAINIO, 2011 | n=986 young with cardiovascular risk |
Cohort study | IDO, KYN-TRP ratio and depressive symptoms (BDI) | Positive correlation between IDO activation with depressive symptoms and carotid atherosclerosis in women |
| ELOVAINIO, 2012 | n=986 young with cardiovascular risk |
Cohort study | IDO and depressive symptoms (BDI) | Positive correlation between IDO and depressive symptoms at baseline and follow-up |
| ERHARDT, 2013 | n=100 (64 medication-free suicide attempters and 36 HC) | Clinical study | QUIN and KYNA were assessed in CSF; suicidality (SIS) and severity of depressive symptoms (MADRS) | QUIN and IL-6, but not KYNA, was elevated in patients with suicide attempters. QUIN levels correlated with the total scores on Suicide Intent Scale They verified a significant decrease of QUIN in patients who came for follow-up lumbar punctures within 6 months after the suicide attempt |
| FITZGERALD, 2008 | n=74 females (41 IBS subjects and 33 controls) |
Case-control study | KYN, TRP and IFN-gamma; IDO (KYN/TRP); Depression and anxiety (PHQ) and IBS (IBS self-report ordinal scales) | Individuals with severe IBS exhibited higher levels of IDO, compared to less severe symptoms and controls, and were over twice when linked to depression and anxiety compared to less severe |
| GABBAY, 2010A | n=72 adolescents |
Cross-sectional study | KYN, TRP, KYN/TRP (estimating IDO activity), 3-HAA/KYN (reflecting neurotoxic load), and diagnosis groups by K-SADS | IDO activity were elevated and TRP concentrations were reduced in adolescents with M-MDD compared to non M-MDD and HC |
| GABBAY, 2010B | n=20 adolescents |
Cross-sectional study | KYN, 3-HAA, striatal total choline, and diagnosis groups by K-SADS | In M-MDD patients was found a positive correlation between KYN, 3-HAA, and striatal total choline in the right caudate and the left putamen brain areas |
| GABBAY, 2012 | n=56 adolescents |
Cross-sectional study | KYN, TRP, IDO, MDD (K-SADS) and anhedonia (BDI-II - item 4 and 12; CDRS-R - item 2; and K-SADS-PL - page 8) | IDO activity and anhedonia scores were positively correlated in the group psychotropic medication-free adolescents with MDD and in a combined group of MDD subjects and healthy controls |
| GALVÃO, 2011 | n=277 hepatitis C patients |
Cross-sectional study | IDO SNPs were genotyped (rs3824259; rs10089084 and rs35099072); Current depression and anxiety disorder (MINI) | Current major depression and/or current anxiety disorder was significantly associated with IFN-α-related depression In patients in IFN-α therapy depression and anxiety symptoms were not linked to variants in the IDO gene |
| HUGHES, 2012 | n=78 (39 patients with MDD and 39 HC) | Case-control study | Plasma IL-6, TNF-a, IL-1 b, IFN-c and CRP; TRP, KYN metabolites using HPLC; Depressive symptoms (HDRS) | IDO expression, plasma KYN levels and metabolites levels were not different between MDD patients, when compared to controls A reduction in circulating TRP concentrations was correlated with HDRS score |
| MACKAY, 2009 | n=63 (28 patients treated with fluoxetine 20 mg/day, 12 patients treated with fluoxetine 20 mg/day together with T3, and 23 patients received counselling with no antidepressant therapy) | Clinical study treatment for 18 weeks |
5-HT, 5-HIAA, tryptophan metabolites, BDNF, and IL-2, CRP were measured in peripheral blood, depressive symptoms (BDI, HDRS) | Showed a correlation between KYN metabolites concentration and psychiatric rating scores in depressive patients treated for 18 weeks with fluoxetine, an SSRI |
| MAES, 2011 | n=146 psychiatric inpatients (117 patients and 35 HC) |
Clinical study Inpatient treatment Center for Psychiatric and psychosomatic disorders |
TRP, KYN, KA, IDO (KYN/TRP ratio), KAT (KYN/KA ratio), somatization symptoms (SSI, SOMS), depression (BDI) | TRP is lower in patients than in HC and lower in somatization than in depression KA is lower in patients than in HC, and lower in somatization than in depression The severity of somatization was correlated with KY/TRP and KY/KA (positive) and TRP (negative) KYN and KA were correlated in controls, somatization + depression, and depression, but not in somatization |
| MARTINEZ, 2014 | n=504 HIV-infected |
Cross-sectional study nested a cohort study | TRP, KYN, dietary diversity, and severity of depressive symptom (HSCL-D) | The severity of depressive symptoms in HIV-infected patients was correlated with lower plasma levels of TRP and a higher plasma KYN/TRP ratio |
| MYINT, 2007 | n=247 (56 MDD patients hospitalized during 6-week and 189 HC) | Clinical study | QUIN, TRP, 3-HAA, and MDD (SCID) | Higher levels of TRP breakdown and protective KYN pathway metabolites were decreased in patients with depression compared to controls The neuroprotective ratio increased after treatment in those with first episodes |
| MYINT, 2013 | n=218 (125 MDD patients and 93 HC) | Case-control study with clinical sample | CA-repeat polymorphism in intron 1 of the interferon-γ gene, TRP, KYN, 5HIAA, depressive symptoms (HDRS) | IFN-α gene CA repeat polymorphisms was associated higher KYN concentrations, and increase in TRP breakdown in patients with depression compared to HC |
| PERTL, 2013 | n=61 breast cancer patients prior to chemotherapy |
Clinical study | IFN-γ, IL-6, TNF-α, CRP, KYN; | KYN levels predicted the trajectory of depression and were an important factor associated with depression and fatigue |
| RAISON, 2009 | n=27 HCV patients (16 in treatment group and 11 awaiting therapy) |
Clinical study Therapy with IFN-alpha/ribavirin for 12 weeks |
TRP, KYN, QUIN and KA were measured in cerebrospinal fluid (CSF) and blood; IL-6, sIL6R, sTNFR2, MCP-1, IFN; MDD (SCID) and depressive symptoms (MADRS) | Increased levels of KYN, QUIN and KYNA in CSF were correlated with increase of depressive symptoms after IFN-α treatment |
| SAVITZ, 2015B | n=128 unmedicated subjects (49 MDD, 21 remitted MDD, and 58 HC) |
Cross-sectional study | KYNA, QUIN, 3-hydroxykynurenine, and MDD (SCID) | Showed a reduction in KYNA/QUIN ratio among MDD and remitted MDD patients, compared to HC An inverse correlation between KYNA/QUIN and anhedonia in MDD patients was demonstrated, as well as a negative correlation between number of depressive episodes and KYNA/QUIN, and a positive correlation between the number of months in remission and KYNA/QUIN |
| SAVITZ, 2015C | n=49 unmedicated subjects (29 MDD and 20 HC) |
Cross-sectional study | KYNA, QUIN, KYN, TRP, IL-1, and MDD (SCID) | Interleukin-1 receptor antagonist, QUIN, and KYN were significantly elevated in MDD patients, compared to HC KYNA, TRP, and KYN were positively correlated with hippocampal and amygdala volume in MDD patients |
| SUBLETTE, 2011 | n=61 (14 MDD with history of suicide attempt, 16 MDD, and 31 HC) | Cross-sectional study | KYN, TRP, neopterin, MDD (SCID), depressive symptoms (BDI and HDRS) | Plasma kynurenine levels are elevated in MDD patients with history of suicide attempters, compared to MDD patients without history of suicide attempters and HC |
| SWARDFAGER, 2009 | n=95 patients with coronary artery disease from a cardiac rehabilitation facility |
Cross-sectional study | IDO (KYN/TRP), MDD (SCID), depressive symptoms (CES-D) | IDO activation was correlated with severity of depressive symptoms among patients with coronary artery disease |
| WICHERS, 2005 | n=16 patients with HCV, free of psychiatric disorders |
Clinical study Therapy with IFN-alpha |
TRP, KYN, KA, IDO, KYN/KA, and depressive symptoms (MADRS) All assessments were carried out at baseline and 1, 2, 4, 8, 12 and 24 weeks after treatment was initiated |
MADRS score increased during IFN-alpha treatment as did the IDO activity, and the KYN/KA ratio MADRS score was associated over time with the KYN/KA ratio, but not with the TRP/CAA ratio. |
| ZHU, 2013 | n=75 Outpatients with MDD (35 patients treated with sertraline and 40 placebo) | Randomized clinical trial double-blind 4-week trial |
5-MTPOL, MEL, KYN/MEL, and 3-OHKY/MEL ratios post-treatment compared to pretreatment Depressive symptoms (HDRS) | Antidepressant sertraline produced a reduction in the KYN/MEL ratio and 3-Hydroxykynurenine 3-OHKY/MEL ratio in MDD patients, compared to pretreatment |
| ZOGA, 2014 | n=80 females (40 MDD patients and 40 HC) | Case-control study with clinical sample | IDO, TNF-α, IFN-γ, CRP and 5-HT | Higher levels of IDO and inflammatory markers, and lower levels of 5-HT at baseline of patients Undergoing effective treatment decreased IDO and TNF-α and was positively linked to patient improvement |
Legend:
3-HAA = 3-hydroxyanthranilic acid; 3-OHKY = 3-Hydroxykynurenine; 5-HIAA = 5-hydroxyindoleacetic acid; 5-HT = 5-hydroxytryptamine serotonin; 5-MTPOL = 5-Methoxytryptophol; BD = Bipolar Disorder; BDI = Beck Depression Inventory; CES-D = Center for Epidemiological Studies-Depression Scale; CDRS-R = Children’s Depression Rating Scale-Revised; CSF = cerebrospinal fluid; HC = Healthy Control; HCV = hepatitis C virus; HDRS = Hamilton Depression Rating Scale; HSCL-D = Hopkins Symptom Checklist for Depression; IBS = irritable bowel syndrome; IDO = Indoleamine 2,3 dioxygenase; IL-6 = Interleukin IFN-α = Interferon alpha; KA = Kynurenic acid; KAT = kynurenine amino transferase; KMO = kynurenine 3 monooxygenase; 6; K-SADS-PL = Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version; KYN = L-kynurenine; MADRS = Montgomery-Asberg Depression Rating Scale; MDD = Major Depressive Disorder; M-MDD = MDD with melancholic features; MEL = Melatonin; MCP-1 = Monocyte chemoattractant protein 1; PHQ = 9-item Public Health Questionnaire; QUIN = Metabolized to quinolinic acid; SCID = Structured Clinical Interview for DSM Disorders; sIL6R = Soluble IL-6 receptor; SIS = Suicide Intent Scale; SUAS = Suicide Assessment Scale; SOMS = Somatoform Symptoms; SSI = Somatic Symptom Index; sTNFR2 = Soluble tumor necrosis factor receptor 2; TPH-2 = Tryptophan hydroxylase 2; TRP = Catabolizes L-tryptophan.
Table 2.
Evidences of the kynurenine pathway in depression from animal studies.
| AUTHOR, YEAR | ANIMAL MODEL | MAIN FINDINGS |
|---|---|---|
| GOUBOUT, 2008 | LPS | Advance in age was associated with depressive-like behavior, elevated peripheral and brain IDO, and turnover rate of brain 5-HT |
| MOREAU, 2005; 2008 | BCG | Depressive-like behavior was correlated with increased levels of TNF-α and peripheral IDO activation |
| DOBOS, 2012 | LPS | IDO inhibitor prevented depressive-like behavior |
| LAUGERAY, 2010 | Chronic stress | KYN/TRP ratio in the periphery was linked to the magnitude of depressive and anxiety-like behaviors |
| CHEN, 2013 | UCMS and QUIN microinjection | Depressive-like behavior, increased levels of glutamate and altered levels of glutamate receptors subunit. Glutamate and QUIN antagonists reversed behavior and neurochemical alterations |
| GIBNEY, 2013 | poly I:C | Depression and anxiety-like behavior, elevated expression pro-inflammatory cytokines, and decreased expression of BDNF and it receptor TrkB in hippocampus and frontal cortex |
| FRANKLIN, 2012 | TRP diet depletion | Depressive-like behavior was correlated with increased levels of KYN and reduced levels of KYNA. Paroxetine treatment reversed these alterations in KYN pathway, but not in depressive behavior |
| ARA, 2012 | Chronic stress | Citalopram treatment exerted antidepressive-like behavior and increased turnover of 5-HT via IDO inhibition in brain |
| ELGARF, 2014 | LPS and CMS | Imipramine and pentoxyphylline were effective to reverse depressive-like behavior and the elevated levels in the hippocampal KYN/TRP ratio and TNF-α gene expression induced by both LPS and CMS |
| GIBNEY, 2014 | Restraint stress | TDO inhibitor attenuated depressive-like behavior and reduced circulating KYN concentrations |
| CORONA, 2013 | CX3CR1−/− and LPS | IDO inhibitor reversed neuroinflammation and depressive-like behavior |
| LAWSON, 2013 | LPS | Genetic deletion or pharmacological inhibition of IDO1 attenuated anhedonic behavior |
| O’CONNOR, 2009C | LPS | Minocycline and 1-MT exhibited antidepressant-like behavior and regulated KYN/TRP ratio in the plasma and brain |
| XIE, 2014 | Epilepsy | Minocycline and 1-MT normalized KYN/TRP and 5-HT/TRP ratio and prevented depressive-like behavior |
Legend: 5-HT = 5-hydroxytryptamine serotonin; CMS = Chronic mild stress; CX3CR1−/− = fractalkine receptor deficient; IDO = Indoleamine 2,3 dioxygenase; BCG = Bacillus Calmette-Guérin; BDNF = brain-derived neurotrophic factor; KYNA = kynurenic acid; KYN = kynurenine; LPS = lipopolysaccharide; QUIN = Quinolinic acid; TDO = tryptophan 2,3-dioxygenase; TNF-α = tumor necrosis factor alpha; TRP = Catabolizes L-tryptophan; UCMS = unpredictable chronic mild stress.
Highlights.
Dysregulation in the kynurenine pathway is evident in depression.
Kynurenine pathway is associated the severity of depressive symptoms.
Kynurenine pathway could represent the link between MDD and medical conditions.
Kynurenine modulators could be new therapeutic targets to MDD.
Acknowledgments
Laboratory of Neurosciences (Brazil) is a center within the National Institute for Molecular Medicine (INCT-MM) and a member of the Center of Excellence in Applied Neurosciences of Santa Catarina (NENASC). This research was supported by grants from CNPq (JQ, AFC, KJ, and GZR), FAPESC (JQ), Instituto Cérebro e Mente, UNESC (JQ), and L’Oréal/UNESCO/ABC Brazil Fellowship for Women in Science 2011 (GZR). JQ, KJ, and AFC are CNPq Research Fellows. Center for Translational Psychiatry (USA) is funded by the Department of Psychiatry and Behavioral Sciences, The University of Texas Medical School at Houston.
Footnotes
Contributors
GZR, KJ and SET contributed with writing manuscript. AFC contributes to search strategy. GZR and SET did the figures. GZR and KJ prepared the tables. JQ, AFC, VM and GZR contributed with study design and manuscript revision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdallah CG, Averill LA, Krystal JH. Ketamine as a promising prototype for a new generation of rapid-acting antidepressants. Ann N Y Acad Sci. 2015 doi: 10.1111/nyas.12718. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmaier E, Emeny RT, Krumsiek J, Lacruz ME, Lukaschek K, Häfner S, Kastenmüller G, Römisch-Margl W, Prehn C, Mohney RP, Evans AM, Milburn MV, Illig T, Adamski J, Theis F, Suhre K, Ladwig KH. Metabolomic profiles in individuals with negative affectivity and social inhibition: a population-based study ofType D personality. Psychoneuroendocrinology. 2013;38:1299–309. doi: 10.1016/j.psyneuen.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Anderson G, Kubera M, Duda W, Lasoń W, Berk M, Maes M. Increased IL-6 trans-signaling in depression: focus on the tryptophan catabolite pathway, melatonin and neuroprogression. Pharmacol Rep. 2013;65:1647–54. doi: 10.1016/s1734-1140(13)71526-3. [DOI] [PubMed] [Google Scholar]
- Anderson G, Maes M, Berk M. Inflammation-related disorders in the tryptophan catabolite pathway in depression and somatization. Adv Protein Chem Struct Biol. 2012;88:27–48. doi: 10.1016/B978-0-12-398314-5.00002-7. [DOI] [PubMed] [Google Scholar]
- Ara I, Bano S. Citalopram decreases tryptophan 2,3-dioxygenase activity and brain 5-HT turnover in swim stressed rats. Pharmacol Rep. 2012;64:558–66. doi: 10.1016/s1734-1140(12)70851-4. [DOI] [PubMed] [Google Scholar]
- Baranyi A, Meinitzer A, Stepan A, Putz-Bankuti C, Breitenecker RJ, Stauber R, Kapfhammer HP, Rothenhäusler HB. A biopsychosocial model of interferon-alpha-induced depression in patients with chronic hepatitis C infection. Psychother Psychosom. 2013;82:332–40. doi: 10.1159/000348587. [DOI] [PubMed] [Google Scholar]
- Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Träskman-Bendz L, Guillemin GJ, Erhardt S, Brundin L. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain Behav Immun. 2015;43:110–7. doi: 10.1016/j.bbi.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Berlim MT, Turecki G. Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry. 2007;52:46–54. doi: 10.1177/070674370705200108. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–51. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Bobo WV, Shelton RC. Efficacy, safety and tolerability of Symbyax for acute-phase management of treatment-resistant depression. Expert Rev Neurother. 2010;10:651–70. doi: 10.1586/ern.10.44. [DOI] [PubMed] [Google Scholar]
- Browne CA, O’Brien FE, Connor TJ, Dinan TG, Cryan JF. Differential lipopolysaccharide-induced immune alterations in the hippocampus of two mouse strains: effects of stress. Neuroscience. 2012;225:237–48. doi: 10.1016/j.neuroscience.2012.08.031. [DOI] [PubMed] [Google Scholar]
- Busse M, Busse S, Myint AM, Gos T, Dobrowolny H, Müller UJ, Bogerts B, Bernstein HG, Steiner J. Decreased quinolinic acid in the hippocampus of depressive patients: evidence for local anti-inflammatory and neuroprotective responses? Eur Arch Psychiatry Clin Neurosci. 2014 doi: 10.1007/s00406-014-0562-0. in press. [DOI] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH. Interferon-alpha-induced changes in tryptophan metabolism relationship to depression and paroxetinetreatment. Biol Psychiatry. 2003;54:906–14. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- Carlin JM, Borden EC, Sondel PM, Byrne GI. Biologicresponse-modifier-induced indoleamine 2, 3-dioxygenase activity in human peripheral blood mononuclear cell cultures. J Immunol. 1987;139:2414–8. [PubMed] [Google Scholar]
- Chen HB, Li F, Wu S, An SC. Hippocampus quinolinic acid modulates glutamate and NMDAR/mGluR1 in chronic unpredictable mild stress-induced depression. Sheng Li Xue Bao. 2013;65:577–85. [PubMed] [Google Scholar]
- Chiarugi A, Calvani M, Meli E, Traggiai E, Moroni F. Synthesis and release of neurotoxic kynurenine metabolites by human monocyte-derived macrophages. J Neuroimmunol. 2001;120:190–8. doi: 10.1016/s0165-5728(01)00418-0. [DOI] [PubMed] [Google Scholar]
- Claes S, Myint AM, Domschke K, Del-Favero J, Entrich K, Engelborghs S, De Deyn P, Mueller N, Baune B, Rothermundt M. The kynurenine pathway in major depression: haplotype analysis of three related functional candidate genes. Psychiatry Res. 2011;188:355–60. doi: 10.1016/j.psychres.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Connolly KR, Thase ME. If at first you don’t succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs. 2011;71:43–64. doi: 10.2165/11587620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Conwell Y, Heisel MJ. The elderly. In: Simon RI, Hales RE, editors. The american psychiatric publishing textbook of suicide assessment and management. 2. Arlington, VA: American Psychiatric Publishing; 2012. pp. 367–388. [Google Scholar]
- Corona AW, Norden DM, Skendelas JP, Huang Y, O’Connor JC, Lawson M, Dantzer R, Kelley KW, Godbout JP. Indoleamine 2,3-dioxygenase inhibition attenuates lipopolysaccharide induced persistent microglial activationand depressive-like complications in fractalkine receptor (CX(3)CR1)-deficient mice. Brain Behav Immun. 2013;31:134–42. doi: 10.1016/j.bbi.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona AW1, Huang Y, O’Connor JC, Dantzer R, Kelley KW, Popovich PG, Godbout JP. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J Neuroinflammation. 2010;7:93. doi: 10.1186/1742-2094-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown WH, Finkelstein S, Berndt ER, Ling D, Poret AW, Rush AJ, Russell JM. The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry. 2002;63:963–71. doi: 10.4088/jcp.v63n1102. [DOI] [PubMed] [Google Scholar]
- Cutler JA1, Rush AJ, McMahon FJ, Laje G. Common genetic variation in the indoleamine-2,3-dioxygenase genes and antidepressant treatment outcome in major depressive disorder. J Psychopharmacol. 2012;26:360–7. doi: 10.1177/0269881111434622. [DOI] [PubMed] [Google Scholar]
- Dairam A, Antunes EM, Saravanan KS, Daya S. Non-steroidal anti-inflammatory agents, tolmetin and sulindac, inhibit liver tryptophan 2,3-dioxygenase activity and alter brain neurotransmitter levels. Life Sci. 2006;79:2269–74. doi: 10.1016/j.lfs.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Davey KJ, O’Mahony SM, Schellekens H, O’Sullivan O, Bienenstock J, Cotter PD, Dinan TG, Cryan JF. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology (Berl) 2012;221:155–69. doi: 10.1007/s00213-011-2555-2. [DOI] [PubMed] [Google Scholar]
- DeBattista C. Chapter 30: Antidepressant agents. In: Katusung BG, Masters S, Trevor A, editors. Basic & Clinical Pharmacology. 12. McGraw-Hill; 2012. pp. 521–542. [Google Scholar]
- Delgado PL. Depression: the case for a monoamine deficiency. J Clin Psychiatry. 2006;61:7–11. [PubMed] [Google Scholar]
- Dinel AL, André C, Aubert A, Ferreira G, Layé S, Castanon N. Lipopolysaccharide-induced brain activation of the indoleamine 2,3-dioxygenase and depressive-like behaviorare impaired in a mouse model of metabolic syndrome. Psychoneuroendocrinology. 2014;40:48–59. doi: 10.1016/j.psyneuen.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Dobos N1, de Vries EF, Kema IP, Patas K, Prins M, Nijholt IM, Dierckx RA, Korf J, den Boer JA, Luiten PG, Eisel UL. The role of indoleamine 2,3-dioxygenase in a mouse model of neuroinflammation-induced depression. J Alzheimers Dis. 2012;28:905–15. doi: 10.3233/JAD-2011-111097. [DOI] [PubMed] [Google Scholar]
- Elgarf AS, Aboul-Fotouh S, Abd-Alkhalek HA, El Tabbal M, Hassan AN, Kassim SK, Hammouda GA, Farrag KA, Abdel-tawab AM. Lipopolysaccharide repeated challenge followed by chronic mild stress protocol introduces a combined model of depression in rats: reversibility by imipramine and pentoxifylline. Pharmacol Biochem Behav. 2014;126:152–62. doi: 10.1016/j.pbb.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Elhwuegi AS. Central monoamines and their role in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:435–51. doi: 10.1016/j.pnpbp.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Elovainio M, Hurme M, Jokela M, Pulkki-Råback L, Kivimäki M, Hintsanen M, Hintsa T, Lehtimäki T, Viikari J, Raitakari OT, Keltikangas-Järvinen L. Moderating effect of indoleamine 2,3-dioxygenase (IDO) activation in the association between depressive symptoms and carotid atherosclerosis: evidence from the Young Finns study. J Affect Disord. 2011;133:611–4. doi: 10.1016/j.jad.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Elovainio M, Hurme M, Jokela M, Pulkki-Råback L, Kivimäki M, Hintsanen M, Hintsa T, Lehtimäki T, Viikari J, Raitakari OT, Keltikangas-Järvinen L. Indoleamine 2,3-dioxygenase activation and depressive symptoms: results from the Young Finns Study. Psychosom Med. 2012;74:675–81. doi: 10.1097/PSY.0b013e318266d0f5. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M, Lundberg K, Postolache TT, Träskman-Bendz L, Guillemin GJ, Brundin L. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology. 2013;38:743–52. doi: 10.1038/npp.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Vos T, Whiteford HA. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P, Cassidy Eugene M, Clarke G, Scully P, Barry S, Quigley Eamonn MM, Shanahan F, Cryan J, Dinan Timothy G. Tryptophan catabolism in females with irritable bowel syndrome: relationship to interferon-gamma, severity of symptoms and psychiatric co-morbidity. Neurogastroenterol Motil. 2008;20:1291–7. doi: 10.1111/j.1365-2982.2008.01195.x. [DOI] [PubMed] [Google Scholar]
- Forrest CM, Khalil OS, Pisar M, Darlington LG, Stone TW. Prenatal inhibition of the tryptophan-kynurenine pathway alters synaptic plasticity and protein expression in the rat hippocampus. Brain Res. 2013a;1504:1–15. doi: 10.1016/j.brainres.2013.01.031. [DOI] [PubMed] [Google Scholar]
- Forrest CM, Khalil OS, Pisar M, McNair K, Kornisiuk E, Snitcofsky M, Gonzalez N, Jerusalinsky D, Darlington LG, Stone TW. Changes in synaptic transmission and protein expression in the brains of adult offspring after prenatal inhibition of the kynurenine pathway. Neuroscience. 2013b;254:241–59. doi: 10.1016/j.neuroscience.2013.09.034. [DOI] [PubMed] [Google Scholar]
- Franklin M, Bermudez I, Murck H, Singewald N, Gaburro S. Sub-chronic dietary tryptophan depletion an animal model of depression with improved face and good construct validity. J Psychiatr Res. 2012;46:239–47. doi: 10.1016/j.jpsychires.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Fu X, Zunich SM, O’Connor JC, Kavelaars A, Dantzer R, Kelley KW. Central administration of lipopolysaccharide induces depressive-like behavior in vivo and activates brainindoleamine 2,3 dioxygenase in murine organotypic hippocampal slice cultures. J Neuroinflammation. 2010;7:43. doi: 10.1186/1742-2094-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, Case JA, Khan O, Ricart T, Hanna A, Alonso CM, Gabbay V. The role of the kynurenine pathway in suicidality in adolescent major depressive disorder. Psychiatry Res. 2015 doi: 10.1016/j.psychres.2015.03.031. S0165-1781:00164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Babb J, Liebes L. The possible role of the kynurenine pathway in anhedonia in adolescents. J Neural Transm. 2012;119:253–60. doi: 10.1007/s00702-011-0685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Liebes L, Katz Y, Liu S, Mendoza S, Babb JS, Klein RG, Gonen O. The kynurenine pathway in adolescent depression: preliminary findings from a proton MR spectroscopy study. Prog Neuropsychopharmacol Biol Psychiatry. 2010b;34:37–44. doi: 10.1016/j.pnpbp.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V1, Klein RG, Katz Y, Mendoza S, Guttman LE, Alonso CM, Babb JS, Hirsch GS, Liebes L. The possible role of the kynurenine pathway in adolescent depression with melancholic features. J Child Psychol Psychiatry. 2010a;51:935–43. doi: 10.1111/j.1469-7610.2010.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão-de Almeida A1, Quarantini LC, Sampaio AS, Lyra AC, Parise CL, Paraná R, de Oliveira IR, Koenen KC, Miranda-Scippa A, Guindalini C. Lack of association of indoleamine 2,3-dioxygenase polymorphisms with interferon-alpha-related depression in hepatitis C. Brain Behav Immun. 2011;25:1491–7. doi: 10.1016/j.bbi.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Gibney SM, Fagan EM, Waldron AM, O’Byrne J, Connor TJ, Harkin A. Inhibition of stress-induced hepatic tryptophan 2,3-dioxygenase exhibits antidepressant activity in an animal model of depressive behaviour. Int J Neuropsychopharmacol. 2014;17:917–28. doi: 10.1017/S1461145713001673. [DOI] [PubMed] [Google Scholar]
- Gibney SM1, McGuinness B, Prendergast C, Harkin A, Connor TJ. Poly I:C-induced activation of the immune response is accompanied by depression and anxiety-like behaviours, kynurenine pathway activation and reduced BDNF expression. Brain Behav Immun. 2013;28:170–81. doi: 10.1016/j.bbi.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Godbout JP1, Moreau M, Lestage J, Chen J, Sparkman NL, O’Connor J, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–51. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RS, Naif H, Espinosa M, Kapoor V. IDO induction in IFN-gamma activated astroglia: a role in improving cell viability during oxidative stress. Redox Rep. 2000;5:101–4. doi: 10.1179/135100000101535357. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Kerr SJ, Smythe GA, Smith DG, Kapoor V, Armati PJ, Cruitoru J, Brew BJ. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. 2001;78:842–53. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (lps) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–17. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes MP, Lackner A. Increased cerebrospinal fluid quinolinic acid, kynurenic acid, and L-kynurenine in acute septicemia. J Neurochem. 1990;55:338–41. doi: 10.1111/j.1471-4159.1990.tb08857.x. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Saito K, Major EO, Milstien S, Markey SP, Vickers JH. A mechanism of quinolinic acid formation by brain in inflammatory neurological disease. Attenuation of synthesis from L-tryptophan by 6-chlorotryptophan and 4-chloro-3-hydroxyanthranilate. Brain. 1993;116:1425–50. doi: 10.1093/brain/116.6.1425. [DOI] [PubMed] [Google Scholar]
- Hochstrasser T, Ullrich C, Sperner-Unterweger B, Humpel C. Inflammatory stimuli reduce survival of serotonergic neurons and induce neuronal expression of indoleamine 2,3-dioxygenase in rat dorsal raphe nucleus organotypic brain slices. Neuroscience. 2011;184:128–38. doi: 10.1016/j.neuroscience.2011.03.070. [DOI] [PubMed] [Google Scholar]
- Hughes MM1, Carballedo A, McLoughlin DM, Amico F, Harkin A, Frodl T, Connor TJ. Tryptophan depletion in depressed patients occurs independent of kynurenine pathway activation. Brain Behav Immun. 2012;26:979–87. doi: 10.1016/j.bbi.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Ignácio ZM, Réus GZ, Abelaira HM, Quevedo J. Epigenetic and epistatic interactions between serotonin transporter and brain-derived neurotrophic factor genetic polymorphism: insights in depression. Neuroscience. 2014;275:455–68. doi: 10.1016/j.neuroscience.2014.06.036. [DOI] [PubMed] [Google Scholar]
- Jia HM, Feng YF, Liu YT, Chang X, Chen L, Zhang HW, Ding G, Zou ZM. Integration of 1H NMR and UPLC-Q-TOF/MS for a comprehensive urinary metabonomics study on a rat model ofdepression induced by chronic unpredictable mild stress. PLoS One. 2013;8:e63624. doi: 10.1371/journal.pone.0063624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegel ME, Bhat M, Skogh E, Samuelsson M, Lundberg K, Dahl ML, Sellgren C, Schweiler L, Engberg G, Schuppe-Koistinen I, Erhardt S. Imbalanced kynurenine pathway in schizophrenia. Int J Tryptophan Res. 2014;7:15–22. doi: 10.4137/IJTR.S16800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW1, O’Connor JC, Lawson MA, Dantzer R, Rodriguez-Zas SL, McCusker RH. Aging leads to prolonged duration of inflammation-induced depression-like behavior caused by BacillusCalmette-Guérin. Brain Behav Immun. 2013;32:63–9. doi: 10.1016/j.bbi.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Chen L, Lim G, Sung B, Wang S, McCabe MF, Rusanescu G, Yang L, Tian Y, Mao J. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J Clin Invest. 2012;122:2940–54. doi: 10.1172/JCI61884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JP, Choi DW. Quinolinate neurotoxicity in cortical cell culture. Neuroscience. 1987;23:423–32. doi: 10.1016/0306-4522(87)90066-2. [DOI] [PubMed] [Google Scholar]
- Kocki T, Wnuk S, Kloc R, Kocki J, Owe-Larsson B, Urbanska EM. New insight into the antidepressants action: modulation of kynurenine pathway by increasing the kynurenic acid/3-hydroxykynurenine ratio. J Neural Transm. 2012;119:235–43. doi: 10.1007/s00702-011-0668-8. [DOI] [PubMed] [Google Scholar]
- Krause DL, Riedel M, Müller N, Weidinger E, Schwarz MJ, Myint AM. Effects of antidepressants and cyclooxygenase-2 inhibitor on cytokines and kynurenines in stimulated in vitro blood culture from depressed patients. Inflammopharmacology. 2012;20:169–76. doi: 10.1007/s10787-011-0112-6. [DOI] [PubMed] [Google Scholar]
- Lapin IP, Oxenkrug GF. Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet. 1969:132–6. doi: 10.1016/s0140-6736(69)91140-4. [DOI] [PubMed] [Google Scholar]
- Lardy HA. The role of tryptophan metabolites in regulating gluconeogenesis. Am J Clin Nutr. 1971;24:764–5. doi: 10.1093/ajcn/24.7.764. [DOI] [PubMed] [Google Scholar]
- Laugeray A, Launay JM, Callebert J, Surget A, Belzung C, Barone PR. Evidence for a key role of the peripheral kynurenine pathway in the modulation of anxiety- and depression-like behaviours in mice: focus on individual differences. Pharmacol Biochem Behav. 2011;98:161–8. doi: 10.1016/j.pbb.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Laugeray A, Launay JM, Callebert J, Surget A, Belzung C, Barone PR. Peripheral and cerebral metabolic abnormalities of the tryptophan-kynurenine pathway in a murine model of major depression. Behav Brain Res. 2010;210:84–91. doi: 10.1016/j.bbr.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Laugeray A, Launay JM, Callebert J, Surget A, Belzung C, Barone PR. Evidence for a key role of the peripheral kynurenine pathway in the modulation of anxiety- and depression-like behaviours in mice: focus on individual differences. Pharmacol Biochem Behav. 2011;98:161–8. doi: 10.1016/j.pbb.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Kelley KW, Dantzer R. Intracerebroventricular administration of HIV-1 Tat induces brain cytokine and indoleamine 2,3-dioxygenase expression: a possible mechanism for AIDS comorbid depression. Brain Behav Immun. 2011;25:1569–75. doi: 10.1016/j.bbi.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson MA, Parrott JM, McCusker RH, Dantzer R, Kelley KW, O’Connor JC. Intracerebroventricular administration of lipopolysaccharide induces indoleamine-2,3-dioxygenase-dependent depression-like behaviors. J Neuroinflammation. 2013;10:87. doi: 10.1186/1742-2094-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leklem JE. Quantitative aspects of tryptophan metabolism in humans and other species: a review. Am J Clin Nutr. 1971;24:659–72. doi: 10.1093/ajcn/24.6.659. [DOI] [PubMed] [Google Scholar]
- Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, Samuelsson M, Erdhardt S. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;38:426–32. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XL, Luo L, Liu BB, Li J, Geng D, Liu Q, Yi LT. Ethanol extracts from Hemerocallis citrina attenuate the upregulation of proinflammatory cytokines andindoleamine 2,3-dioxygenase in rats. J Ethnopharmacol. 2014;153:484–90. doi: 10.1016/j.jep.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Lopresti AL, Maker GL, Hood SD, Drummond PD. A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:102–11. doi: 10.1016/j.pnpbp.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Mackay GM1, Forrest CM, Christofides J, Bridel MA, Mitchell S, Cowlard R, Stone TW, Darlington LG. Kynurenine metabolites and inflammation markers in depressed patients treated with fluoxetine or counselling. Clin Exp Pharmacol Physiol. 2009;36:425–35. doi: 10.1111/j.1440-1681.2008.05077.x. [DOI] [PubMed] [Google Scholar]
- Maes M, Galecki P, Verkerk R, Rief W. Somatization, but not depression, is characterized by disorders in the tryptophan catabolite (TRYCAT) pathway, indicating increased indoleamine 2,3-dioxygenase and lowered kynurenine aminotransferase activity. Neuro Endocrinol Lett. 2011;32:264–73. [PubMed] [Google Scholar]
- Mangoni A. The “kynurenine shunt” and depression. Adv Biochem Psychopharmacol. 1974;11:293–8. [PubMed] [Google Scholar]
- Martinez P, Tsai AC, Muzoora C, Kembabazi A, Weiser SD, Huang Y, Haberer JE, Martin JN, Bangsberg DR, Hunt PW. Reversal of the Kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected Ugandans. J Acquir Immune Defic Syndr. 2014;65:456–62. doi: 10.1097/QAI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew J, Beart PM, Walker FR. Astrocyte and microglial control of glutamatergic signaling: a primer on understanding the disruptive role of chronic stress. J Neuroendocrinol. 2015 doi: 10.1111/jne.12273. in press. [DOI] [PubMed] [Google Scholar]
- McInnis OA, Matheson K, Anisman H. Living with the unexplained: coping, distress, and depression among women with chronic fatigue syndrome and/or fibromyalgia compared to an autoimmune disorder. Anxiety Stress Coping. 2014;27:601–18. doi: 10.1080/10615806.2014.888060. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Munn DH. Tryptophan catabolism and t-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469–73. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Multi-target strategies for the improved treatment of depressive states: conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther. 2006;111:135–370. doi: 10.1016/j.pharmthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulated cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;107:25–37. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Molteni R, Macchi F, Zecchillo C, Dell’agli M, Colombo E, Calabrese F, Guidotti G, Racagni G, Riva MA. Modulation of the inflammatory response in rats chronically treated with the antidepressant agomelatine. Eur Neuropsychopharmacol. 2013;23:1645–55. doi: 10.1016/j.euroneuro.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Moreau M, André C, O’Connor JC, Dumich SA, Woods JA, Kelley KW, Dantzer R, Lestage J, Castanon N. Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain Behav Immun. 2008;22:1087–95. doi: 10.1016/j.bbi.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M, Lestage J, Verrier D, Mormede C, Kelley KW, Dantzer R, Castanon N. Bacille Calmette-Guérin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. J Infect Dis. 2005;192(3):537–44. doi: 10.1086/431603. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. The role of the kynurenine metabolism in major depression: evidence of impaired neuroprotection. J Affect Disord. 2007;98:143–51. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Myint AM, Schwarz MJ, Muller N. The role of the kynurenine metabolism in major depression. J Neural Transm. 2012a;119:245–51. doi: 10.1007/s00702-011-0741-3. [DOI] [PubMed] [Google Scholar]
- Myint AM. Kynurenines: from the perspective of major psychiatric disorders. FEBS Journal. 2012b;270:1375–85. doi: 10.1111/j.1742-4658.2012.08551.x. [DOI] [PubMed] [Google Scholar]
- Myint AM1, Bondy B, Baghai TC, Eser D, Nothdurfter C, Schüle C, Zill P, Müller N, Rupprecht R, Schwarz MJ. Tryptophan metabolism and immunogenetics in major depression: a role for interferon-γ gene. Brain Behav Immun. 2013;31:128–33. doi: 10.1016/j.bbi.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Norden DM, Devine R, Bicer S, Jing R, Reiser PJ, Wold LE, Godbout JP, McCarthy DO. Fluoxetine prevents the development of depressive-like behavior in a mouse model of cancer related fatigue. Physiol Behav. 2015 doi: 10.1016/j.physbeh.2014.12.045. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary OF, Dinan TG, Cryan FJ. Faster, better, stronger: towards new antidepressant therapeautic strategies. Eur J Pharmacol. 2014 doi: 10.1016/j.ejphar.2014.07.046. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- O’Brien SM, Scully P, Fitzgerald P, Scott LV, Dinan TG. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatr Res. 2017;41:326–31. doi: 10.1016/j.jpsychires.2006.05.013. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, André C, Wang Y, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW, Dantzer R. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009;29:4200–9. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, André C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, Kelley KW. Induction of IDO by bacille Calmette-Guérin is responsible for development of murine depressive-like behavior. J Immunol. 2009;182:3202–12. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–22. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Fujii T, Koga N, Hori H, Teraishi T, Hattori K, Noda T, Higuchi T, Motohashi N, Kunugi H. Plasma L-tryptophan concentration in major depressive disorder: new data and meta-analysis. J Clin Psychiatry. 2014;75:e906–15. doi: 10.4088/JCP.13r08908. [DOI] [PubMed] [Google Scholar]
- Okuda S, Nishiyama N, Saito H, Katsuki H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J Neurochem. 1998;70:299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–7. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- Pertl MM, Hevey D, Boyle NT, Hughes MM, Collier S, O’Dwyer AM, Harkin A, Kennedy MJ, Connor TJ. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain Behav Immun. 2013;34:108–19. doi: 10.1016/j.bbi.2013.07.177. [DOI] [PubMed] [Google Scholar]
- Pertz H, Back W. Synthesis and resolution of chiral ring opened serotonin analogs of the 5-hydroxykynuramine type. Pharm Acta Helv. 1988;63:128–31. [PubMed] [Google Scholar]
- Qiu HM, Yang JX, Jiang XH, Fei HZ, Liu D, Hu XY, Zhou QX. Upregulating serotonin transporter expression and downregulating monoamine oxidase-A and indoleamine 2, 3-dioxygenase expression involved in the antidepressant effect of sodium valproate in a rat model. Neuroreport. 2014;25:1338–43. doi: 10.1097/WNR.0000000000000269. [DOI] [PubMed] [Google Scholar]
- Quagliariello E, Papa S, Saccone C, Alifano A. Effect of 3-hydroxyanthranilic acid on the mitochondrial respiratory system. Biochem J. 1964;91:137–46. doi: 10.1042/bj0910137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quak J, Doornbos B, Roest AM, Duivis HE, Vogelzangs N, Nolen WA, Penninx BW, Kema IP, de Jonge P. Does tryptophan degradation along the kynurenine pathway mediate the association between pro-inflammatory immune activity and depressive symptoms? Psychoneuroendocrinology. 2014;45:202–10. doi: 10.1016/j.psyneuen.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2009;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–98. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Reeves WC, Strine TW, Pratt LA, Thompson W, Ahluwalia I, Dhingra SS, McKnight-Eily LR, Harrison L, D’Angelo DV, Williams L, Morrow B, Gould D, Safran MA Centers for Disease Control and Prevention (CDC) Mental illness surveillance among adults in the United States. MMWR Surveill Summ. 2011;60(Suppl 3):1–29. [PubMed] [Google Scholar]
- Réus GZ, Carlessi AS, Titus SE, Abelaira HM, Ignácio ZM, da Luz JR, Matias BI, Bruchchen L, Florentino D, Vieira A, Petronilho F, Quevedo J. A single dose of s-ketamine induces long-term antidepressant effects and decreases oxidative stress in adulthood rats following maternal deprivation. Dev Neurobiol. 2015a doi: 10.1002/dneu.22283. in press. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Dos Santos MA, Abelaira HM, Ribeiro KF, Petronilho F, Vuolo F, Colpo GD, Pfaffenseller B, Kapczinski F, Dal-Pizzol F, Quevedo J. Imipramine reverses alterations in cytokines and BDNF levels induced by maternal deprivation in adult rats. Behav Brain Res. 2013;242:40–6. doi: 10.1016/j.bbr.2012.11.044. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Nacif MP, Abelaira HM, Tomaz DB, dos Santos MA, Carlessi AS, da Luz JR, Gonçalves RC, Vuolo F, Dal-Pizzol F, Carvalho AF, Quevedo J. Ketamine ameliorates depressive-like behaviors and immune alterations in adult rats following maternal deprivation. Neurosci Lett. 2015b;584:83–7. doi: 10.1016/j.neulet.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Vieira FG, Abelaira HM, Michels M, Tomaz DB, dos Santos MA, Carlessi AS, Neotti MV, Matias BI, Luz JR, Dal-Pizzol F, Quevedo J. MAPK signaling correlates with the antidepressant effects of ketamine. J Psychiatr Res. 2014;55:15–21. doi: 10.1016/j.jpsychires.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Salazar A, Gonzalez-Rivera BL, Redus L, Parrott JM, O’Connor JC. Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm Behav. 2012;62:202–9. doi: 10.1016/j.yhbeh.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Dantzer R, Wurfel BE, Victor TA, Ford BN, Bodurka J, Bellgowan PS, Teague TK, Drevets WC. Neuroprotective kynurenine metabolite indices are abnormally reduced and positively associated with hippocampal and amygdalar volume in bipolar disorder. Psychoneuroendocrinology. 2015a;52:200–11. doi: 10.1016/j.psyneuen.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Wurfel BE, Ford BN, Bellgowan PS, Victor TA, Bodurka J, Teague TK, Dantzer R. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain Behav Immun. 2015b doi: 10.1016/j.bbi.2015.02.007. S0889–1591(15)00026–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS, Bodurka J, Teague TK, Dantzer R. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology. 2015c;40:463–71. doi: 10.1038/npp.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. J Neuropsychiatry Clin Neurosci. 1965;7:524–33. doi: 10.1176/jnp.7.4.524. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–30. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Whetsell WO, Jr, Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983;219:316–8. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–7. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z, Mawrin C, Brisch R, Bielau H, Meyer zu Schwabedissen L, Bogerts B, Myint AM. Severe depression is associated with increased quinolinic acid immunoreactivity in the dorsal and ventral anterior cingulum: further evidence for an immune modulation of glutamatergic neurotransmission? J Neuroinflammation. 2011;8:94. doi: 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW, Behan WM, MacDonald M, Darlington LG. Possible mediation of quinolinic acid-induced hippocampal damage by reactive oxygen species. Amino Acids. 2000;19:275–281. doi: 10.1007/s007260070059. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, Oquendo MA, Mann JJ, Postolache TT. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav Immun. 2011;25:1272–8. doi: 10.1016/j.bbi.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swardfager W, Herrmann N, Dowlati Y, Oh PI, Kiss A, Walker SE, Lanctôt KL. Indoleamine 2,3-dioxygenase activation and depressive symptoms in patients with coronary artery disease. Psychoneuroendocrinology. 2009;34:1560–6. doi: 10.1016/j.psyneuen.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Takikawa O. Biochemical and medical aspects of the indoeamine 2,3-dioxygenase-initiated l-tryptophan metabolism. Biochem Biophys Res Commun. 2005;338:12–9. doi: 10.1016/j.bbrc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Hollander E, Nutt D, Blier P. Clinical evidence and potential neurobiological underpinnings of unresolved symptoms of depression. J Clin Psychiatry. 2008;69:246–58. doi: 10.4088/jcp.v69n0211. [DOI] [PubMed] [Google Scholar]
- van Praag HM, Korf J. A pilot study of some kinetic aspects of the metabolism of 5-hydroxytrptamine in depressive patients. Biol Psychiatry. 1971;3:105–12. [PubMed] [Google Scholar]
- Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology. 2013;38:1609–16. doi: 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. 2005;10:538–44. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- Willner P, Sampson D, Phillips G, Muscat R. A matching law analysis of the effects of dopamine receptor antagonists. Psychopharmacology (Berl) 1990;101:560–7. doi: 10.1007/BF02244238. [DOI] [PubMed] [Google Scholar]
- Xie W, Cai L, Yu Y, Gao L, Xiao L, He Q, Ren Z, Liu Y. Activation of brain indoleamine 2,3-dioxygenase contributes to epilepsy-associated depressive-like behavior in rats with chronic temporal lobe epilepsy. J Neuroinflammation. 2014;11:41. doi: 10.1186/1742-2094-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JM, Rui BB, Chen C, Chen H, Xu TJ, Xu WP, Wei W. Acetylsalicylic acid enhances the anti-inflammatory effect of fluoxetine through inhibition of NF-κB, p38-MAPK and ERK1/2 activation in lipopolysaccharide-induced BV-2 microglia cells. Neuroscience. 2014;275:296–304. doi: 10.1016/j.neuroscience.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Zhu H, Bogdanov MB, Boyle SH, Matson W, Sharma S, Matson S, Churchill E, Fiehn O, Rush JA, Krishnan RR, Pickering E, Delnomdedieu M, Kaddurah-Daouk R Pharmacometabolomics Research Network. Pharmacometabolomics of response to sertraline and to placebo in major depressive disorder - possible role for methoxyindole pathway. PLoS One. 2013;8:e68283. doi: 10.1371/journal.pone.0068283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Jing L, Chen C, Shao M, Fan Q, Diao J, Liu Y, Lv Z, Sun X. Danzhi Xiaoyao San ameliorates depressive-like behavior by shifting toward serotonin via the downregulation of hippocampal indoleamine 2,3-dioxygenase. J Ethnopharmacol. 2015;160:86–93. doi: 10.1016/j.jep.2014.11.031. [DOI] [PubMed] [Google Scholar]
- Zoga M, Oulis P, Chatzipanagiotou S, Masdrakis VG, Pliatsika P, Boufidou F, Foteli S, Soldatos CR, Nikolaou C, Papageorgiou C. Indoleamine 2,3-dioxygenase and immune changes under antidepressive treatment in major depression in females. In Vivo. 2014;28:633–8. [PubMed] [Google Scholar]