Abstract
Purpose
Ipilimumab (Ipi), a monoclonal antibody against cytotoxic T-lymphocyte antigen-4, has been shown to improve survival in patients with metastatic melanoma. In this single-institution study, we investigate the safety and efficacy of stereotactic radiosurgery (SRS) for patients with melanoma brain metastases (BMs) who also received Ipi.
Methods
From 2005 to 2011, 46 patients with melanoma received Ipi and underwent single fraction SRS for BMs. A total of 113 BMs (91% intact, 9% post-operative) were treated with median dose 21Gy (15-24Gy). Ipi was given at 3mg/kg (54%) or 10mg/kg (46%) for a median of 4 doses (1-21). Adverse events were recorded using CTCAE 3.0. Kaplan-Meier methods were used to estimate survival and Cox regression was used to investigate associations.
Results
Fifteen patients received SRS during Ipi, 19 received SRS before Ipi, and 12 received SRS after Ipi. Overall survival (OS) was significantly associated with timing of SRS/Ipi (p=0.035) and melanoma-specific graded prognostic assessment (p=0.013). Patients treated with SRS during or before Ipi had better OS and less regional recurrence (RR) than those treated with SRS after Ipi (1-yr OS 65% vs. 56% vs. 40%, p=0.008; 1-yr RR 69% vs. 64% vs. 92%, p=0.003). SRS during Ipi also yielded a trend toward less local recurrence (LR) than SRS before or after Ipi (1-yr LR 0% vs. 13% vs. 11%, p=0.21). On MRI, an increase in BM diameter to >150% was seen in 50% of patients treated during or before Ipi but only 13% of patients treated after Ipi. Grade 3-4 toxicities were seen in 20% of patients.
Conclusion
Overall, the combination of Ipi and SRS appears to be well tolerated. Concurrent delivery of Ipi and SRS is associated with favorable locoregional control and possibly longer survival. It may also cause a temporary increase in tumor size, possibly due to enhanced immunomodulatory effect.
Keywords: melanoma, brain, ipilimumab, immunotherapy, radiosurgery, antibody, CTLA-4
INTRODUCTION
Ipilimumab (Ipi), a human monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4 (CTLA-4), allows for T cell activation and proliferation, thereby enhancing immune response to cancer. In patients with metastatic melanoma, Ipi has been shown to improve overall survival in two phase III trials, one in comparison with the cancer vaccine gp100 and the other in combination with dacarbazine.1,2 These trials led to FDA approval of ipilimumab in 2011.
Up to 60% of patients with metastatic melanoma will develop brain metastases (BMs), and those with relatively good prognosis and few brain metastases often undergo treatment with stereotactic radiosurgery (SRS).3-7 The rationale for combining Ipi and SRS is based on potential activity of ipilimumab in the brain, as demonstrated by Margolin et al. in a phase II trial, as well as possible abscopal effects of SRS that may enhance the systemic response to Ipi.8-14 Several series have reported promising preliminary results with the combination of SRS and Ipi, including a study by Knisely et al. showing median overall survival of 21.3 mo in 27 patients.5,15-18 Given our large institutional experience with ipilimumab and SRS, we conducted a retrospective study to investigate safety and efficacy of this combination for treatment of melanoma BMs.
METHODS AND MATERIALS
Using an institutional melanoma database, 46 patients were identified who received ipilimumab and underwent single fraction SRS for melanoma BMs between 2005 and 2011. Most of these patients (85%) received Ipi as part of a research protocol. Ipi was delivered intravenously every 3 weeks for 4 doses during the induction phase. After induction, 13 patients (28%) received maintenance therapy every 3 months.
A gadolinium-enhanced T1-weighted MRI with 3-mm slices was obtained prior to SRS. On the day of treatment, patients were immobilized using a stereotactic frame. A contrast-enhanced simulation CT with 2-mm slices was obtained, and the BrainLAB system was used for treatment planning. Radiation dose (15-24Gy) was prescribed based on size of the lesion and proximity to other structures. Typically 10 non-coplanar static beams were delivered. Dose was prescribed to the 80% isodose line. Quality criteria and plan evaluation were completed according to RTOG guidelines.19
Toxicities were recorded using the common terminology criteria for adverse events (CTCAE 3.0). During routine follow-up, patients were assessed with MRI 6-8 weeks after SRS, then every 3 months thereafter. All MRIs were evaluated for tumor size (maximum axial diameter), hemorrhage, and recurrence. The melanoma-specific graded prognostic assessment score (mGPA) was calculated for each patient based on Karnofsky performance status and the number of BMs. This is a validated prognostic score of 0 to 4 (best) that predicts survival of patients with melanoma BMs (see Table 2).4
Table 2.
Median survival by melanoma-specific GPA for patients with brain metastases receiving SRS plus ipilimumab (n = 46). Expected median survival for patients with melanoma brain metastases is based on Sperduto et al.4
| Melanoma GPA score | Expected median survival | Observed median survival |
|---|---|---|
| 0-1 | 3.4 mo | 5.9 mo |
| 2 | 4.7 mo | 7.6 mo |
| 3 | 8.8 mo | 11.0 mo |
| 4 | 13.2 mo | Not reached |
| All | 6.7 mo | 12.4 mo |
GPA = graded prognostic assessment score.
Kaplan-Meier methods were used to estimate overall survival (OS), local control, and regional control. OS was analyzed per patient, from date of diagnosis of BMs to date of death or last patient contact. Local and regional recurrence-free survival were analyzed per treated lesion, from date of SRS to date of recurrence or last MRI exam. Local recurrence (LR) was defined as brain recurrence within the SRS field, and regional recurrence (RR) was defined as brain recurrence outside the SRS field. Cox regression was used to investigate the association of variables with outcomes. Three groups were compared based on timing of therapies: patients who received SRS before the first dose of Ipi (“SRS before Ipi”), patients who received SRS between doses of Ipi or <1 month after the last dose of Ipi (“SRS during Ipi”), and patients who received SRS >1 month after the last dose of Ipi (“SRS after Ipi”).
RESULTS
Patient and tumor characteristics
This study included 46 patients with metastatic melanoma who received ipilimumab and SRS for BMs between 2005 and 2011. Patient, tumor, and treatment characteristics are shown in Table 1. Median age was 57 years (24-76 yrs), and male:female ratio was 1.4:1. Median mGPA was 3 out of 4, as most patients had KPS 90% and 1-2 BMs.4 Only 37% of patients had elevated LDH. Almost all patients had other non-brain metastases and underwent prior systemic therapy, including temozolomide in 46%, interleukin-2 in 15%, and cisplatin, vinblastine, and temozolomide (CVT) in 37%. No patients received vemurafenib.
Table 1.
Patient, tumor, and treatment characteristics (n = 46).
| Patient and Tumor Characteristics | Median (range) or No. (%) |
|---|---|
| Female | 19 (41%) |
| Male | 27 (59%) |
| Age at diagnosis of BMs | 57yrs (24-76) |
| Melanoma-specific GPA score | 3 (1-4) |
| Karnofsky performance status | 90% (70-90%) |
| Median no. of BMs treated | 2 (1-6) |
| BM size (max axial) | 0.8cm (0.2-2.9) |
| No. with non-brain metastases | 41 (89%) |
| No. with prior systemic therapy | 40 (87%) |
| No. with elevated LDH | 17 (37%) |
| Treatment Characteristics | |
| Stereotactic radiosurgery dose | 21Gy (15-24) |
| No. with surgery prior to SRS | 4 (9%) |
| No. with whole brain RT after SRS | 1 (2%) |
| No. with whole brain RT after recurrence | 9 (20%) |
| Ipilimumab dose | |
| 3 mg/kg | 25 (54%) |
| 10 mg/kg | 21 (46%) |
| Median no. of ipilimumab doses | 4 (1-21) |
BMs = brain metastases. GPA = graded prognostic assessment score. LDH = lactate dehydrogenase.
Most of the BMs were small and asymptomatic, with median axial diameter of 0.8 cm (0.2-2.9 cm). The median interval from diagnosis of BMs to date of SRS was <1 month. Only 9% of patients had brain surgery prior to SRS. Only 1 patient had whole brain radiation (WBRT) immediately after SRS, whereas 9 had WBRT after locoregional recurrence. A total of 113 BMs were treated with a median dose of 21Gy (15-24Gy). Most patients (83%) received a short course of prophylactic steroids with SRS (typically 4mg dexamethasone for 2 days).
Ipilimumab dosing was 3 mg/kg in 54% and 10 mg/kg in 46% with a median total of 4 doses (typical induction). Fifteen patients received “SRS during Ipi”, including 8 between induction doses, 4 within 1 month after last induction dose, and 3 between doses of protocol-allowed maintenance. Nineteen patients received “SRS before Ipi” with a median of 3 months between SRS and first dose of Ipi (1-39 mo). Twelve patients received “SRS after Ipi” with a median of 2 months between last dose of Ipi and SRS (1-32 mo). Notably, there were no significant differences among these timing groups for any patient characteristics in Table 1.
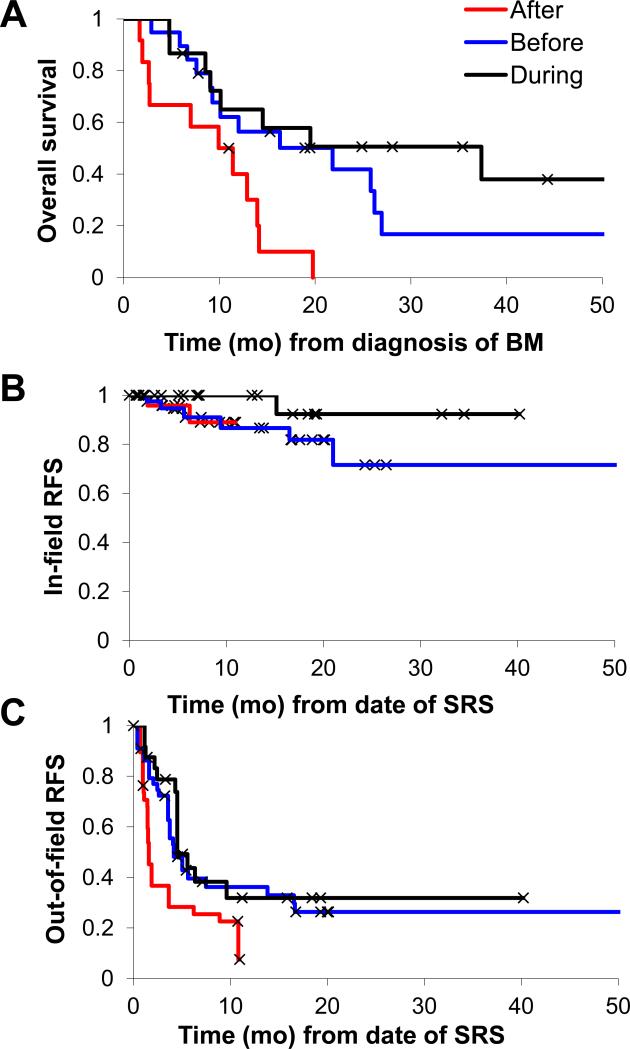
Median OS for all patients was 12.4 months (2-89 mo). Twelve of 46 patients are still alive at the time of analysis, with median follow-up time for survivors of 22 months (6-89 mo). The survivors include 6 patients in the SRS during Ipi group, 4 in the SRS before Ipi group, and 1 in the SRS after Ipi group. On multivariate Cox regression analysis, OS was associated with timing of SRS and Ipi (p = 0.035) and melanoma-specific GPA (p = 0.013) but not age, KPS, LDH, or dose of Ipi. On univariate analysis, patients treated with SRS during or before Ipi had better OS than those treated with SRS after Ipi (Figure 1a; 1-yr OS 65% vs. 56% vs. 40%; p=0.008). Patients with higher mGPA also had improved OS, and median survival of patients in the present cohort was longer than those in the Sperduto et al. study of mGPA (Table 2).4 Notably, the following variables were also tested but were not significantly associated with OS on univariate analysis: SRS dose, number of Ipi treatments, number of BMs, prior systemic therapy, and prior surgical resection.
Figure 1.
Results based on timing of SRS and ipilimumab (Ipi). Overall survival (a) was significantly worse in the SRS after Ipi cohort (p=.008). In-field (b) recurrence-free survival (RFS) was high for all groups (p=.21), but out-of-field RFS (c) was worse in the SRS after Ipi cohort (p=.003). Comparisons were made by Cox regression analysis.
Local control (within the SRS field) was high in all groups (Figure 1b), as expected based on previous studies of melanoma BMs treated with SRS alone.6 There was a trend toward improved local control in the SRS during Ipi group (1-yr LR 0%) compared to the SRS before Ipi (13%) or after Ipi (11%) groups (p=0.21). The size of BMs was not associated with local control in this series. Out-of-field (regional) brain recurrences occurred in almost all patients receiving SRS after Ipi (1-yr RR 92%), compared to significantly less patients receiving SRS during Ipi (69%) or before Ipi (64%; Figure 1c; p=0.003).
Table 3 shows adverse events according to CTCAE 3.0, with emphasis on patients receiving SRS during Ipi. Grade 3-4 adverse events were present in only 20% of patients, and there were too few events to analyze statistically. SRS did not exacerbate the typical systemic immune-related adverse events associated with ipilimumab such as enterocolitis, pruritus, and hepatitis. Central nervous system toxicities were slightly more frequent in patients receiving SRS during Ipi, as discussed below. However, adverse events did not interfere with completion of planned Ipi in any patients.
Table 3.
Adverse events of patients receiving SRS plus ipilimumab (n = 46).
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||||
|---|---|---|---|---|---|---|---|---|
| SRS before/ after Ipi |
SRS during Ipi |
SRS before/ after Ipi |
SRS during Ipi |
SRS before/ after Ipi |
SRS during Ipi |
SRS before/ after Ipi |
SRS during Ipi |
|
| Diarrhea/ nausea | 11 (35%) | 8 (53%) | 3 (10%) | 4 (27%) | 0 | 0 | 0 | 0 |
| Pruritus/ rash | 13 (42%) | 6 (40%) | 5 (16%) | 4 (27%) | 0 | 1 (7%) | 0 | 0 |
| Cardiopulmonary | 6 (19%) | 1 (7%) | 0 | 2 (13%) | 0 | 0 | 0 | 1 (7%) |
| Hepatitis | 0 | 0 | 1 (3%) | 0 | 2 (6%) | 1 (7%) | 0 | 0 |
| Fatigue | 16 (52%) | 8 (53%) | 3 (10%) | 3 (20%) | 0 | 0 | 0 | 0 |
| Headache | 1 (3%) | 3 (20%) | 1 (3%) | 1 (7%) | 0 | 0 | 0 | 0 |
| CNS bleeding (from treated BM) | 2 (6%) | 1 (7%) | 1 (3%) | 3 (20%) | 2 (6%) | 2 (13%) | 1 (3%) | 0 |
| Seizure | 0 | 0 | 2 (6%) | 1 (7%) | 0 | 2 (13%) | 0 | 0 |
| Cognitive change | 2 (6%) | 2 (13%) | 0 | 1 (7%) | 0 | 0 | 0 | 0 |
| Neurologic dysfunction | 2 (6%) | 2 (13%) | 0 | 0 | 0 | 0 | 0 | 0 |
SRS = stereotactic radiosurgery. Ipi = ipilimumab. CNS = central nervous system. BM = brain metastasis.
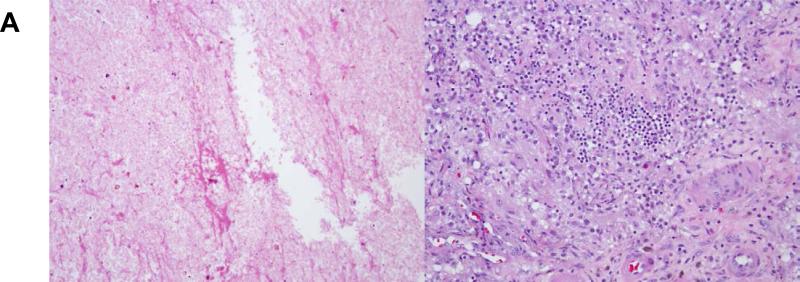
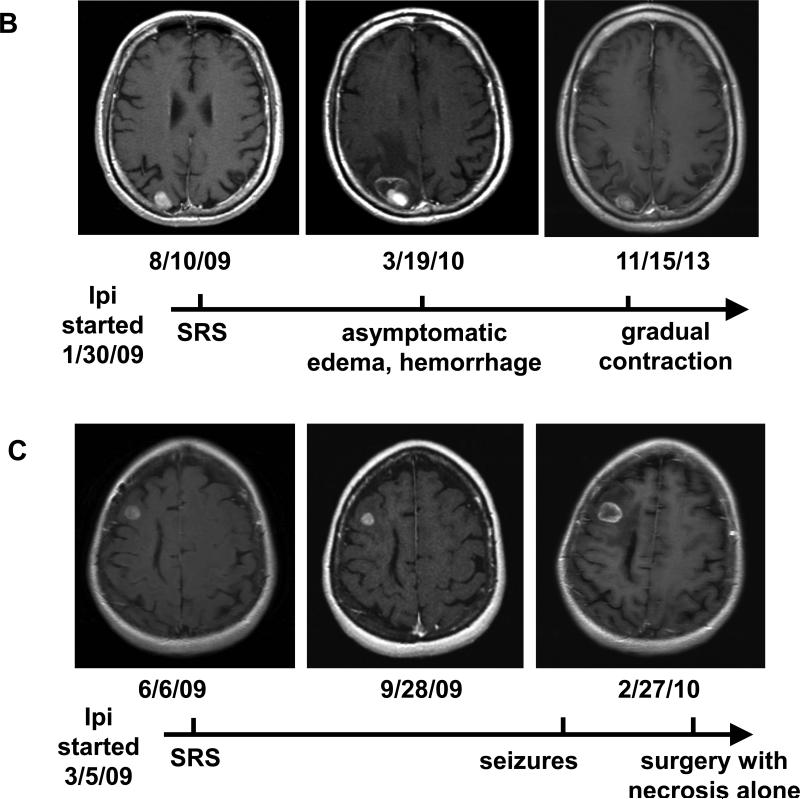
On follow-up MRIs, the maximum axial diameter of treated tumors increased to >150% of pre-SRS size in 50% of patients treated with SRS during or before Ipi, compared with only 13% of those treated with SRS after Ipi. Notably, for patients treated with SRS before Ipi, tumors did not increase in size until Ipi was started, often months after completion of SRS. Imaging findings were consistent with hemorrhagic products and/or edema in 82% of these patients and were concerning for local recurrence in only 18%. The presence of hemorrhagic products was particularly common after SRS during Ipi (40%), and some of these patients experienced headache, seizure, and/or temporary cognitive or neurologic change, as reflected in Table 3. However, the majority of patients were asymptomatic. Overall, only 11 patients (24%) required steroid treatment for more than 2 weeks, including 5 patients in the SRS during Ipi group (31%). Most of the lesions subsequently contracted without further treatment, but 11 were resected for suspected recurrence or progression. Surprisingly, in 5 of these cases, pathology showed complete necrosis and lymphocytic/histiocytic inflammation with no viable tumor (Figure 2a). The remaining lesions showed components of viable melanoma admixed with necrosis and/or inflammation.
Figure 2.
Pathologic and radiologic findings after treatment with SRS during ipilimumab. (a) Micrographs at 200X of a brain lesion resected 5 months after treatment, demonstrating no viable tumor but (left) cellular effacement and granular eosinophilic debris characteristic of necrosis and (right) lymphocytic and histiocytic infiltration. (b) and (c) Brain MRI findings of two patients who received SRS during ipilimumab.
Figure 2 also shows examples of post-contrast T1-weighted MR images from two patients treated with SRS during Ipi. Patient B is a 53 year-old man who started Ipi in early 2009. He was treated with SRS 6 months later for a single BM. In March 2010, a routine MRI showed increased size of the lesion with hemorrhage and edema (asymptomatic). He was observed, and the lesion gradually contracted and remained stable at 4 years post-SRS. The patient continued Ipi until December 2013 and remains alive with no active melanoma in the brain or systemically as of August 2014 by PET/CT. Patient C is a 51year-old woman who began treatment with Ipi in March 2009. Three months later, she was treated with SRS for two BMs. Her initial follow-up scans showed stable lesions. However, 8 months after SRS, she presented with seizures and was found to have increased size of the treated right frontal lesion with hemorrhage and edema. Due to suspicion for recurrence, she underwent surgical resection. Pathology showed complete necrosis with no viable tumor. She continued Ipi and had excellent control of melanoma in the brain and systemically until she died of pancreatic cancer in 2012.
Previous reports have suggested a possible abscopal effect of stereotactic body radiation inducing an enhanced systemic response to Ipi.11-14 This study was not designed to investigate whether brain SRS may induce an abscopal effect in combination with Ipi. However, anecdotally there was one patient with possible abscopal effect after receiving SRS during Ipi. The patient began treatment with Ipi in late 2008 but had continued progression of disease in the pelvis and lungs. One year later, she received brain SRS and subsequently had a gradual durable response in the pelvis and lungs. She continued Ipi through late 2011 and did not receive any other systemic therapies, and she remains alive at the time of analysis.
DISCUSSION
This retrospective, single-institution study is the largest series to date investigating the combination of brain SRS and ipilimumab immunotherapy for patients with melanoma brain metastases (n = 46 patients). Our results suggest several important hypotheses. First, delivery of SRS during or before Ipi may yield comparatively favorable survival and regional control compared to delivery of SRS after Ipi. These effects clearly need to be investigated further. Second, SRS during or before Ipi may cause a temporary increase in tumor size due to local inflammation or hemorrhage. Finally, the combination of Ipi and SRS appears to be safe and well tolerated in patients with melanoma BMs.
Our study demonstrated favorable OS (median 12.4 months) when compared to previous studies of patients with melanoma BMs treated with SRS alone or ipilimumab alone, with caveats noted for cross-trial comparison. Sperduto et al. recently validated the melanoma-specific GPA as a prognostic tool for patients with melanoma BMs (n = 481; treated in 1993-2010).4 For the subset of patients who received SRS alone (n = 221), Sperduto reported a median OS of 7.3 mo (compared to 2.9 mo for WBRT alone and 6.7 mo for all patients). Our results confirmed the importance of mGPA as a prognostic factor but showed consistently longer survival for each mGPA score than expected (Table 2). In a recent phase II trial by Margolin et al., patients with melanoma BMs were treated with ipilimumab alone (n = 72; treated in 2008-2009), with only 8% receiving SRS and 33% receiving WBRT.8 The median survival for asymptomatic patients who received Ipi was 7.0 mo, again less than found in our study. Notably, this multi-institutional phase II study included patients from our institution treated in the same time period. Several other series have reported promising preliminary results with the combination of SRS and Ipi, including a study by Knisely et al. showing extended survival in 27 patients receiving SRS plus Ipi (21.3 mo) versus 50 patients receiving SRS alone (4.9 mo).5,15-18
Patients with melanoma BMs typically have high rates of regional (brain) recurrence and thus high risk of neurologic death.20-22 SRS and surgery have excellent rates of local control but high rates of RR requiring subsequent courses of treatment.5,21,22 In the current study, patients in the SRS after Ipi group had almost 100% RR but those in the SRS during Ipi or before Ipi groups had only 69% and 64% RR, respectively. This suggests that Ipi may have regional immunomodulatory effects in the brain. It is possible that antibodies such as Ipi may be able to penetrate melanoma BMs if the blood-brain barrier is disrupted, but even if Ipi is unable to penetrate, previous reports have shown infiltration of activated T cells in BMs after treatment with Ipi.23
The results of this study suggest that the combination of SRS and Ipi may yield increased BM size, possibly due to inflammation. This phenomenon of lesion expansion during immunotherapy has been well-described.24 Indeed, systemic Ipi responses are often characterized by initial stable or increased lesions followed by delayed contraction, prompting the need for new immune-related response criteria.25 In our study, imaging frequently showed a temporary increase in size of lesions after treatment with SRS during Ipi, often associated with hemorrhage or edema, followed by gradual contraction suggestive of subacute inflammatory response. In patients receiving SRS before Ipi, there was a similar temporary increase in BM size but this did not occur until after Ipi was started, often months after completion of SRS. This timing suggests that local inflammation was not just a response to SRS, but might reflect the local immunomodulatory effects of Ipi. Without Ipi, Huber et al. reported transient increases in BM size after SRS in only 12% of patients.26 The appearance of these treated lesions on MRI may raise suspicion for recurrence, but in many cases close observation may be appropriate rather than surgical resection. Indeed, we found that 5 out of 11 lesions that were resected for suspected recurrence showed 100% necrosis on pathology (Figure 2a).
Many of the above patients with local inflammation or bleeding after SRS were asymptomatic, but several had associated headaches, seizures, or temporary cognitive/neurologic change (Table 3). Other commonly observed side effects from Ipi included immune-mediated effects such as pruritus/rash, enterocolitis, and fatigue.27 These findings highlight the unique spectrum of toxicities seen with immunotherapies, as well as the importance of caution when administering them in combination with other therapies. Overall, the combination of Ipi and SRS appeared to be well tolerated and safe, with grade 3-4 toxicities in only 20% of patients, but this needs to be verified in a larger prospective study. Only 11% of patients required steroid treatment for more than 2 weeks, and the authors no longer recommend prophylactic steroids for patients with small asymptomatic lesions, especially since steroids could potentially counteract the effectiveness of Ipi.8
An important remaining question is whether single fraction brain SRS may induce an abscopal effect enhancing the systemic response to Ipi. The current study was not designed to answer this question, and though a single case showed timing suggestive of an abscopal effect, there were no immunologic correlates to support this hypothesis. However, the improved overall survival and regional brain control seen in patients receiving SRS before and during Ipi, as well as the increased local inflammation, do suggest an interaction between radiation and Ipi. Although speculative, the release of antigens from dying melanoma cells after SRS may help Ipi to prime the immune response. When SRS is given long after Ipi, these effects would not be expected. An increasing number of researchers are now investigating this interaction (especially with large radiation dose per fraction), and preliminary results show possible mediation by activated T cells and/or anti-tumor antibodies.9-11,28,29
The limitations of this study include its retrospective nature and small number of patients, though it is the largest series investigating the combination of SRS and ipilimumab for melanoma BMs. Our relatively favorable results with prolonged OS may be partly due to the fact that most of these patients are on protocol and have screening MRIs and close follow-up, which could result in lead-time bias. There could also be selection bias affecting the comparison of SRS timing groups, since the patients receiving SRS after Ipi may have had previous progression or lack of response to Ipi, predisposing them to worse outcomes. Additionally, three patients in the SRS during Ipi group received SRS during maintenance ipilimumab. Since only patients with stable disease or response are generally candidates for maintenance, this may have contributed to the favorable overall survival in this group. Overall, these data must be considered as hypothesis-generating rather than hypothesis-testing. However, in the absence of larger prospective studies, the results may inform patient care by suggesting that the combination of Ipi and SRS is relatively safe. To further investigate safety, several institutions are currently conducting prospective studies of Ipi and brain radiation for patients with melanoma BMs (NCT01703507 and NCT01950195).
CONCLUSION
This largest-to-date single-institution retrospective study investigated the safety and efficacy of SRS in 46 patients with melanoma BMs who also received ipilimumab immunotherapy. We found that delivery of SRS during or before Ipi was associated with comparatively favorable survival and regional control compared to delivery of SRS after Ipi. However, SRS during or before Ipi may also be associated with a temporary increase in size or hemorrhage of the irradiated lesion. Overall, the combination of Ipi and SRS appears to be safe and well tolerated.
SUMMARY.
We investigated the safety and efficacy of SRS for 46 patients with melanoma BMs who also received Ipi. Patients treated with SRS during or before Ipi had better overall survival (p=0.008) and less regional recurrence (p=0.003) than those treated with SRS after Ipi. Many also had a temporary increase in tumor size, possibly due to enhanced immunomodulatory effect. Overall, the combination of Ipi and SRS was well tolerated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: none.
REFERENCES
- 1.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 Aug 19;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011 Jun 30;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 3.Gupta T. Stereotactic radiosurgery for brain oligometastases: good for some, better for all? Ann Oncol. 2005 Nov;16(11):1749–1754. doi: 10.1093/annonc/mdi392. [DOI] [PubMed] [Google Scholar]
- 4.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012 Feb 1;30(4):419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knisely JP, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VL. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg. 2012 Aug;117(2):227–233. doi: 10.3171/2012.5.JNS111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liew DN, Kano H, Kondziolka D, et al. Outcome predictors of Gamma Knife surgery for melanoma brain metastases. Clinical article. J Neurosurg. 2011 Mar;114(3):769–779. doi: 10.3171/2010.5.JNS1014. [DOI] [PubMed] [Google Scholar]
- 7.Samlowski WE, Watson GA, Wang M, et al. Multimodality treatment of melanoma brain metastases incorporating stereotactic radiosurgery (SRS). Cancer. 2007 May 1;109(9):1855–1862. doi: 10.1002/cncr.22605. [DOI] [PubMed] [Google Scholar]
- 8.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012 May;13(5):459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 9.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004 Mar 1;58(3):862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009 Sep 1;15(17):5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012 Mar 8;366(10):925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013 Dec;1(6):365–372. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiniker SM, Chen DS, Knox SJ. Abscopal effect in a patient with melanoma. N Engl J Med. 2012 May 24;366(21):2035. doi: 10.1056/NEJMc1203984. author reply 2035-2036. [DOI] [PubMed] [Google Scholar]
- 14.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013 Feb 1;85(2):293–295. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoenfeld J, Dyer M, Alexander B, et al. Melanoma Brain Metastases Treated With Brain-directed Radiation Followed by Ipilimumab.. Int J Rad Onc Bio Phys; 54th Annual Meeting of the American Society for Radiation Oncology; Boston: 2012. p. S287. [Google Scholar]
- 16.Mathew M, Tam M, Ott PA, et al. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res. 2013 Jun;23(3):191–195. doi: 10.1097/CMR.0b013e32835f3d90. [DOI] [PubMed] [Google Scholar]
- 17.Tazi K, Chiuzan C, Shirai K. Survival of melanoma patients with brain metastases treated with ipilimumab combined with stereotactic radiosurgery.. J Clin Oncol; ASCO Annual Meeting; 2013. 2013. abstr e20019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoukat S, Marcus D, Rizzo M, Lawson D, Liu Y, Khan M. Outcome with stereotactic radiosurgery (SRS) and ipilimumab (Ipi) for malignant melanoma brain metastases.. J Clin Oncol; ASCO Annual Meeting; 2013. 2013. abstr 3032. [Google Scholar]
- 19.Shaw E, Kline R, Gillin M, et al. Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys. 1993 Dec 1;27(5):1231–1239. doi: 10.1016/0360-3016(93)90548-a. [DOI] [PubMed] [Google Scholar]
- 20.Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011 Apr 15;117(8):1687–1696. doi: 10.1002/cncr.25634. [DOI] [PubMed] [Google Scholar]
- 21.Fife KM, Colman MH, Stevens GN, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004 Apr 1;22(7):1293–1300. doi: 10.1200/JCO.2004.08.140. [DOI] [PubMed] [Google Scholar]
- 22.Sampson JH, Carter JH, Jr., Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998 Jan;88(1):11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 23.Hodi FS, Oble DA, Drappatz J, et al. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nat Clin Pract Oncol. 2008 Sep;5(9):557–561. doi: 10.1038/ncponc1183. [DOI] [PubMed] [Google Scholar]
- 24.Hoos A, Eggermont AM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010 Sep 22;102(18):1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009 Dec 1;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 26.Huber PE, Hawighorst H, Fuss M, van Kaick G, Wannenmacher MF, Debus J. Transient enlargement of contrast uptake on MRI after linear accelerator (linac) stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2001 Apr 1;49(5):1339–1349. doi: 10.1016/s0360-3016(00)01511-x. [DOI] [PubMed] [Google Scholar]
- 27.Weber JS, Amin A, Minor D, Siegel J, Berman D, O'Day SJ. Safety and clinical activity of ipilimumab in melanoma patients with brain metastases: retrospective analysis of data from a phase 2 trial. Melanoma Res. 2011 Dec;21(6):530–534. doi: 10.1097/CMR.0b013e32834d3d88. [DOI] [PubMed] [Google Scholar]
- 28.Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012;2:153. doi: 10.3389/fonc.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilones KA, Kawashima N, Yang AM, Babb JS, Formenti SC, Demaria S. Invariant natural killer T cells regulate breast cancer response to radiation and CTLA-4 blockade. Clin Cancer Res. 2009 Jan 15;15(2):597–606. doi: 10.1158/1078-0432.CCR-08-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]