Abstract
OBJECTIVE
To identify and quantify any legacy effect of bariatric surgery on risk of incident microvascular disease in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
We conducted a retrospective observational cohort study (n = 4,683; 40% racial/ethnic minority) of patients with type 2 diabetes who underwent bariatric surgery from 2001 through 2011. The primary outcome measure was incident microvascular disease defined as a composite indicator of the first occurrence of retinopathy, neuropathy, and/or nephropathy. The Cox proportional hazards framework was used to investigate the associations between type 2 diabetes remission/relapse status and time to microvascular disease.
RESULTS
Covariate-adjusted analyses showed that patients who experienced type 2 diabetes remission had 29% lower risk of incident microvascular disease compared with patients who never remitted (hazard ratio [HR] 0.71 [95% CI 0.60, 0.85]). Among patients who experienced a relapse after remission, the length of time spent in remission was inversely related to the risk of incident microvascular disease; for every additional year of time spent in remission prior to relapse, the risk of microvascular disease was reduced by 19% (HR 0.81 [95% CI 0.67, 0.99]) compared with patients who never remitted.
CONCLUSIONS
Our results indicate that remission of type 2 diabetes after bariatric surgery confers benefits for risk of incident microvascular disease even if patients eventually experience a relapse of their type 2 diabetes. This provides support for a legacy effect of bariatric surgery, where even a transient period of surgically induced type 2 diabetes remission is associated with lower long-term microvascular disease risk.
Introduction
Numerous studies have documented the effects that bariatric surgical procedures can have on glycemic control among people with type 2 diabetes (1–5). The magnitude of this effect varies by procedure (1). Our own work has suggested that there is a 5-year type 2 diabetes remission rate of 68% for Roux-en-Y gastric bypass (RYGB) (6,7). Despite the mounting evidence of the impact of bariatric surgery for patients with type 2 diabetes, studies have now begun to show that type 2 diabetes remission after bariatric surgery is often not durable, with rates of relapse as high as 43% after 15 years postsurgery (6,8,9).
Also of importance to patients and providers are the complications that result from type 2 diabetes such as microvascular disease, primarily nephropathy, neuropathy, and retinopathy. A recent review of the literature published since 2011 found that in general, there was strong support for postbariatric reduction in risk for nephropathy, less so for retinopathy, and almost no evidence published for neuropathy (10). Only one study had been performed with a large patient population (>2,500 bariatric patients) representative of current clinical practices in the U.S. (11).
None of these studies examined how type 2 diabetes relapse affected the impact of bariatric surgery on microvascular disease. Of particular interest is whether patients who experience a relapse of their type 2 diabetes after surgery still have lasting protection from the development of microvascular disease. There is evidence in the nonsurgical literature that even if adults with diabetes experience a recurrence of poor glycemic control, transient periods of improved control may confer benefits for long-term incidence of microvascular complications (12,13). The prolonged beneficial effects of improved glycemic control observed in these studies have been termed the “legacy effect” or “metabolic memory.” Whether there is a legacy effect of bariatric surgery has yet to be established.
In this report we present data that tests three specific hypotheses: 1) after bariatric surgery, patients with type 2 diabetes remission would have lower risk of incident microvascular disease compared with patients who did not experience remission, 2) patients who experienced a relapse of their type 2 diabetes after initial remission would have lower risk of incident microvascular disease than those who had never remitted, and 3) the longer a patient spent in remission before experiencing relapse, the lower their risk would be for incident microvascular disease.
Research Design and Methods
Settings
We conducted a retrospective observational cohort study with patients who had bariatric surgery from 2001 to 2011 while enrolled in one of four U.S. integrated health care systems from the Health Care Systems Research Network (formerly the HMO Research Network) (14): Group Health (GH) in Washington state; HealthPartners (HP) in Minnesota; Kaiser Permanente Northern California (KPNC), serving the northern half of California; and Kaiser Permanente Southern California (KPSC), serving the southern half of the state. These health care systems were very diverse in their patient selection and preparation for bariatric surgery as well as in the intensity of their postoperative care and monitoring. For example, some systems required that patients with type 2 diabetes be well controlled at the time of surgery and others did not have this requirement. All study procedures were reviewed and approved by the institutional review boards of all participating sites.
Data Sources
For all health care systems, electronic medical records, insurance claims, and other data systems were organized in a virtual data warehouse to facilitate population-based research (15). These data included enrollment and insurance coverage details; demographics; blood pressure; height; weight; laboratory values; medications dispensed; deaths; outpatient, inpatient, and emergency department use; and the diagnoses and procedures for this health care use.
Participants
The primary population of interest for this study was 20- to 79-year-old adults with severe obesity and type 2 diabetes who had a primary bariatric surgical procedure between 1 January 2001 and 31 December 2011. We used a combination of bariatric registries, chart reviews, International Classification of Diseases (ICD)-9 codes (43.89, 44.31, 44.38, 44.39, 44.68, 44.69, and 44.95), and Current Procedural Terminology procedure codes (43633, 43644, 43645, 43659, 43770, 43775, 43842, 43843, 43844, 43845, 43846, and 43847) to identify bariatric procedures. Patients were classified as having type 2 diabetes if they met at least one of the following two criteria at the time of the primary bariatric procedure: 1) uncontrolled type 2 diabetes, defined as a glycated hemoglobin A1c (HbA1c) ≥6.5%/48 mmol/mol or fasting plasma glucose ≥126 mg/dL at the most recent measurement within 2 years prior to surgery, or 2) medication-treated type 2 diabetes, defined as a current prescription for any oral or injectable diabetes medication at the time of surgery.
Once this population was selected, the following exclusion criteria were applied: 1) less than one full year of continuous enrollment and drug coverage, 2) a history of gastrointestinal surgery for cancer, 3) preexisting neuropathy or retinopathy, 4) gestational diabetes if it was the sole diabetes diagnosis, and 5) metformin as the sole indicator of possible type 2 diabetes (no other type 2 diabetes medications, laboratories, or diagnoses). Application of these eligibility and exclusion criteria yielded a sample of 9,516 patients.
Further exclusions were made for patients who, at the time of surgery, were observed to have 1) a BMI <35 kg/m2, 2) an estimated glomerular filtration rate (eGFR) <60, and/or 3) an HbA1c <6.5%/48 mmol/mol or fasting glucose <126 mg/dL without use of any type 2 diabetes medication, leaving us with 7,237 patients. Finally, for our primary analyses, we excluded 2,554 patients who were missing BMI, HbA1c, and/or serum creatinine measures in the 2 years before surgery. This provided us with a final analytic sample of 4,683 patients. As indicated below, sensitivity analyses were conducted to examine the impact of missing data on our conclusions.
Analyses
Exposure Definition
The primary independent variable of interest was patient type 2 diabetes status during the course of postsurgical follow-up. The patient’s type 2 diabetes status at any point during the postsurgical follow-up was determined as either having type 2 diabetes (beginning at the time of surgery), being in a state of remission after surgery, or being in a state of relapse after experiencing a period of remission. This was calculated as a time-varying quantity that took on a value of zero at baseline and increased as a patient accrued patient-time in remission.
A patient experienced type 2 diabetes remission when they had either 1) an HbA1c <6.5%/48 mmol/mol after being off all type 2 diabetes medications for ≥90 days and/or 2) fasting glucose <126 mg/dL after being off all type 2 diabetes medications for ≥7 days (16). These time frames were chosen because they corresponded with the expected duration of the effects of medications on laboratory indicators of type 2 diabetes status.
If patients experienced type 2 diabetes remission, they were classified as having relapsed if they then had 1) HbA1c ≥6.5%/48 mmol/mol, 2) fasting plasma glucose ≥126 mg/dL, and/or 3) a restart of type 2 diabetes medication. The date of relapse was defined as the first date of an elevated HbA1c, elevated fasting plasma glucose, or a new medication fill. If a patient experienced relapse during or within 30 days after the end of a pregnancy or hospitalization and/or during an active prescription for an oral steroid medication, this was not used for analyses as these would not be attributable to bariatric surgery. Patients could transition back and forth between the states of remission and relapse.
Outcome Definitions
The primary outcome measure was incident microvascular disease, defined as a composite indicator of the first occurrence of retinopathy, neuropathy, or nephropathy. Retinopathy was defined based on ICD-9 diagnosis codes 362.0x (diabetic retinopathy) or 250.5x (diabetes with ophthalmic manifestations). Neuropathy was also identified based on first occurrence of ICD-9 diagnosis codes (250.6 [diabetes with neurological manifestations] or 357.2 [polyneuropathy in diabetes]). Finally, nephropathy was identified based on a patient having at least two eGFR measures <60 mL/min/1.73 m2 separated by ≥90 days without any intervening values ≥60 mL/min/1.73 m2. We estimated the glomerular filtration rate using serum creatinine values and the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation (17), using the nonblack race coefficient for individuals with unknown race. We chose not to use urine protein measures to define incident nephropathy because nearly 25% of our cohort had no baseline urine protein measurements. As indicated below, sensitivity analyses were performed to determine the impact of using this information in addition to eGFR to define nephropathy (and thus excluding 25% of the sample) on our results.
Statistical Models
Cox proportional hazards regression was used to investigate the association between type 2 diabetes status at any point in time after surgery (having type 2 diabetes without remission, being in a state of remission, or being in a state of relapse after remission) and risk of incident microvascular disease among patients with type 2 diabetes at the time of bariatric surgery. Patients were followed from the date of surgery until the first occurrence of either incident microvascular disease or a censoring event (death, disenrollment, cessation of drug coverage, an incident cancer diagnosis, or a period of >13 months without any observed health care use).
Two sets of primary models were fit. The first of these was specified to investigate relative differences in risk of incident microvascular disease, at any given point in time postsurgery, between patients who were in remission and those who had not remitted, as well as between those who had relapsed and those who had not remitted. Toward this, two time-varying dummy variable indicators were used to distinguish patients who had not remitted from those who had remitted and from those who had relapsed. Throughout this report of our findings, these models are referred to as “main effects only” models.
To investigate our central hypothesis of a bariatric surgery legacy effect related to time in remission, we additionally included an interaction between the time-varying relapse indicator and a variable representing the total time a patient spent in remission prior to their relapse. Throughout this report of our findings, these models are referred to as “interaction” models. Supplementary Data provide a detailed overview of these models.
In addition to unadjusted models, we fit adjusted models that included the following covariates at the time of surgery: age, sex, race/ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, or non-Hispanic other), surgery type (RYGB, adjustable gastric banding [AGB], or sleeve gastrectomy [SG]), surgical year, insurance type (commercial, Medicare, Medicaid, or other), BMI, smoking status (current, former, or never), duration of observed diabetes before surgery (defined as first observed diagnosis, laboratory value, or prescription indicating type 2 diabetes), insulin use, oral diabetes medication use, LDL cholesterol ≥100 mg/dL, triglycerides ≥250 mg/dL, uncontrolled blood pressure (defined as either systolic ≥140 or diastolic ≥90 at two consecutive measures on different days), use of antihypertensive medications (ACE inhibitor, angiotensin receptor blocker [ARB], or other), use of cholesterol-lowering medication (statin or other), use of aspirin or other platelet inhibitor, and incident cardiovascular event before surgery (defined based on ICD-9 codes). Because of the variation in surgery eligibility and postsurgical care across the health care sites, study site (GH, HP, KPSC, and KPNC) was adjusted for using stratification of the baseline hazard function.
Sensitivity Analyses
A number of planned sensitivity analyses were also conducted to examine the impact of the following: 1) excluding the largest health care site from analyses to determine its impact on the findings, 2) using both abnormally elevated urine protein and low eGFR as indicators of incident nephropathy, and 3) performing multiple imputation for all missing values of BMI, HbA1c, eGFR, and race/ethnicity. For the last of these, we constructed M = 10 full data sets using multiple imputation by chained equations (18), each of size n = 9,525 corresponding to the bariatric patients identified after applying the first round of inclusion/exclusion criteria. Patients in these full data sets not satisfying BMI, eGFR, and type 2 diabetes eligibility criteria (either on the basis of known values or imputed values) were excluded to form the M = 10 analytic data sets.
Effect Modification
In addition to our main analysis, we investigated several a priori–specified interactions, including age, BMI, race/ethnicity, and procedure type.
Results
Participants
Table 1 presents descriptive statistics for the final analytic sample of patients (n = 4,863). The study sample was primarily middle aged (60% 45–64 years old), female (76%), and had commercial insurance (94%), and 40% were racial/ethnic minorities. Most patients had RYGB (79%) in years 2000–2011, with 15% having undergone SG and 6% AGB. At the time of surgery, 52% of patients had a BMI of 40–49.9 kg/m2, 44% had poor glycemic control (HbA1c ≥7.0%/53 mmol/mol), and 38% had type 2 diabetes for 5 years or more before surgery. Comorbidities were common, with 72% and 75% of patients having diagnosed hypertension or dyslipidemia, respectively. Some patients (11%) had been smokers in the 2 years before surgery. One-, three-, and five-year retention rates for patients in the analytic sample were 93%, 77%, and 69%, respectively.
Table 1.
Baseline characteristics for patients with uncontrolled or medication-controlled type 2 diabetes (n = 4,683)
| Age (years) | 46.7 ± 9.7 |
| Female | 76% (n = 3,580) |
| Race/ethnicity | |
| Hispanic | 18% (n = 841) |
| Non-Hispanic black | 15% (n = 698) |
| Non-Hispanic white | 48% (n = 2,271) |
| Other | 7% (n = 329) |
| Unknown/missing | 12% (n = 544) |
| Health care site | |
| GH | 6% (n = 285) |
| HP | 5% (n = 232) |
| KPNC | 32% (n = 1,478) |
| KPSC | 57% (n = 2,688) |
| Insurance type | |
| Commercial | 94% (n = 4,396) |
| Medicaid | 2% (n = 104) |
| Medicare | 2% (n = 107) |
| Other | 2% (n = 76) |
| Year of surgery | |
| 2001–2002 | 3% (n = 128) |
| 2003–2004 | 7% (n = 330) |
| 2005–2006 | 11% (n = 509) |
| 2007–2008 | 21% (n = 978) |
| 2009–2010 | 33% (n = 1,551) |
| 2011 | 35% (n = 1,187) |
| Type of bariatric surgery | |
| AGB | 6% (n = 285) |
| RYGB | 79% (n = 3,865) |
| SG | 15% (n = 713) |
| BMI (kg/m2) | 45.2 ± 7.3 |
| Serum creatinine (mg/dL) | 0.79 ± 0.17 |
| eGFR (mL/min/1.73 m2) | 96.49 ± 18.04 |
| HbA1c (%) | 7.1 ± 1.3 |
| HbA1c (mmol/mol) | 54 |
| Observed duration of diabetes (years) | 4.5 ± 3.5 |
| Use of oral diabetes medication | 68% (n = 3,186) |
| Use of insulin | 17% (n = 773) |
| Dyslipidemia | |
| LDL cholesterol ≥100 mg/dL | 40% (n = 1,851) |
| Triglycerides ≥150 mg/dL | 48% (n = 2,244) |
| Dyslipidemia diagnosis* | 76% (n = 3,572) |
| Use of a statin | 48% (n = 2,264) |
| Use of other lipid-lowering medications | 5% (n = 251) |
| Hypertension | |
| Uncontrolled hypertension | 7% (n = 314) |
| Hypertension diagnosis* | 72% (n = 3,375) |
| Use of ACE inhibitors or ARB | 53% (n = 2,466) |
| Use of other antihypertensive medications | 41% (n = 1,932) |
| Cardiovascular disease | |
| ≥1 cardiac event* | 2% (n = 71) |
| ≥1 cerebrovascular event* | 1% (n = 45) |
| ≥1 peripheral arterial event* | 2% (n = 79) |
| Use of platelet inhibitor | 1% (n = 50) |
| Smoking status | |
| Current | 11% (n = 528) |
| Former | 29% (n = 1,377) |
| Never | 59% (n = 2,778) |
Values represent characteristics at the time of bariatric surgery or for the 2-year period prior to surgery (as indicated). For categorical variables, counts and percentages are presented; for continuous variables, means ± SDs are presented.
*In the 2 years prior to surgery.
Effect of Type 2 Diabetes Remission and Relapse on Risk for Incident Microvascular Disease
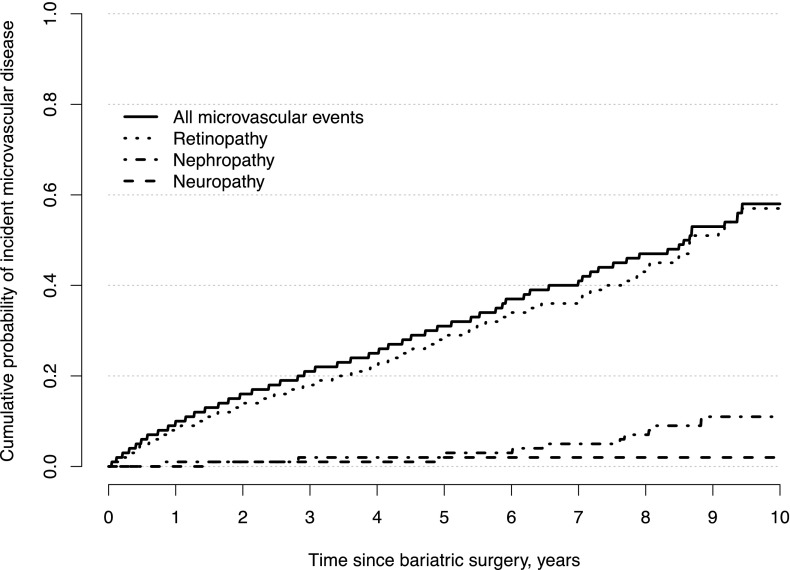
Figure 1 provides estimates of the cumulative probability of incident microvascular disease over time after bariatric surgery, as well as the cumulative probability of each of the indicators of microvascular disease (nephropathy, neuropathy, and retinopathy). The 1-, 3-, 5-, and 7-year rates of incident microvascular disease were 9.5%, 20.9%, 31.1%, and 40.5%, respectively. The rate of incident microvascular disease was primarily due to the incidence of retinopathy, which occurred at a rate of 8.0%, 18.2%, 28.4%, and 36.5% at 1, 3, 5, and 7 years postsurgery. The rates of nephropathy (0.7%, 1.5%, 2.6%, and 4.9%) and neuropathy (0.4%, 1.1%, 1.5%, and 1.9%) were much lower.
Figure 1.
Kaplan-Meier estimates of the cumulative probability of incident microvascular disease over time after bariatric surgery. Separate estimates for nephropathy, neuropathy, and retinopathy are shown, as well as an estimate for incident microvascular disease due to any of the three.
Table 2 shows the key results from the unadjusted and adjusted multivariable Cox models investigating the association between type 2 diabetes status and incident microvascular disease after bariatric surgery; the details for the adjusted models are shown in Supplementary Data. From the adjusted main effects only model, patients who were in a state of remission had significantly lower risk of incident microvascular disease when compared with patients who had not remitted (hazard ratio [HR] 0.71 [95% CI 0.60, 0.85]). Although the estimated HR was <1.0, patients in a state of relapse (after remission) did not have significantly different risk for microvascular disease at any given point in time after surgery than patients who had not remitted (HR 0.87 [95% CI 0.65, 1.16]).
Table 2.
HRs and 95% CIs from unadjusted and adjusted* Cox regression analysis of incident microvascular disease
| Main effects only model |
Interaction model |
|||
|---|---|---|---|---|
| Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Type 2 diabetes status | ||||
| Remission | 0.49 (0.41, 0.57) | 0.71 (0.60, 0.85) | 0.48 (0.41, 0.57) | 0.70 (0.59, 0.84) |
| Relapse | 0.73 (0.55, 0.98) | 0.87 (0.65, 1.16) | 1.02 (0.71, 1.47) | 1.14 (0.79, 1.65) |
| Legacy effect | 0.79 (0.65, 0.95) | 0.81 (0.67, 0.99) | ||
Main effects only models solely consider a patient’s type 2 diabetes status, comparing patients in a state of remission or relapse to those who have not remitted. Interaction models investigate a potential legacy effect by additionally permitting the HR of incident microvascular disease for patients who have relapsed, compared with those who have not remitted, to depend on the cumulative time spent in remission prior to relapsing.
*Stratified by site and adjusted for the following variables described in Table 1: age, sex, surgery type, surgery year, race/ethnicity, insurance type, BMI, diabetes duration, insulin use, oral diabetes medication use, HbA1c, ACE or ARB use, other antihypertensive medication use, uncontrolled blood pressure at baseline, preoperative hypertension diagnosis, statin use, other lipid-lowering medication use, dyslipidemia diagnosis, LDL ≥100 mg/dL, triglycerides ≥100 mg/dL, cardiovascular disease at baseline, cerebrovascular disease at baseline, peripheral vascular disease at baseline, platelet inhibitor use, and smoking status.
Similar to the main effects only model, patients in the interaction model who experienced remission had significantly lower risk of incident microvascular disease when compared with patients who had not remitted (HR 0.70 [95% CI 0.59, 0.84]). Also similar the main effects only model, the interaction model found that the risk for incident microvascular disease in patients who experienced relapse after remission was not significantly different than the risk for those who had not remitted (HR 1.14 [95% CI 0.79, 1.65]). However, unlike the main effects only model, the HR in the interaction model was >1.0. The difference in the HR is likely due to the presence of the interaction and is interpreted as the relative difference in risk between patients who relapse immediately after remitting their type 2 diabetes (i.e., the cumulative time in remission is zero) and those who never remitted.
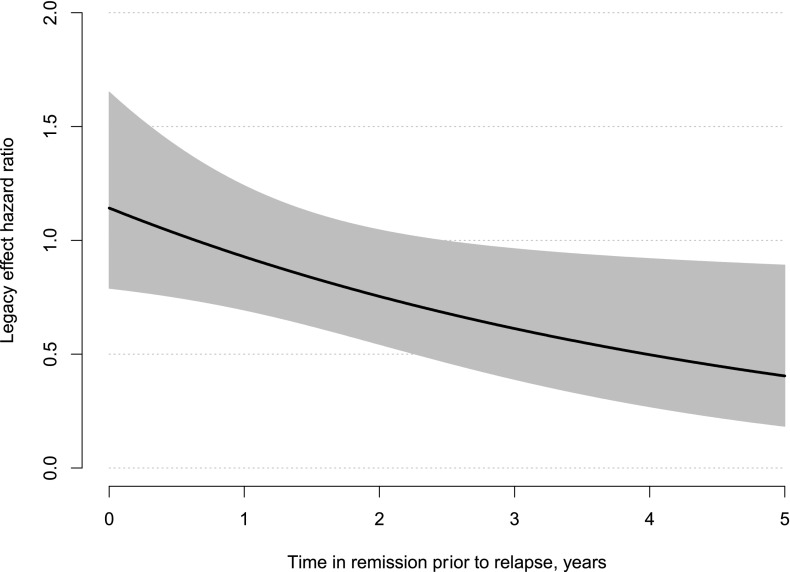
Finally we found support for the legacy effect of bariatric surgery for patients with type 2 diabetes. The more time a patient accrued in remission before experiencing relapse, the less risk they had of incident microvascular disease when compared with patients who never remitted (HR 0.81 [95% CI 0.67, 0.99]). For each additional year of time spent in remission, the HR decreased by 19%. Figure 2 provides a visual representation of the legacy effect HR as a function of time spent in remission prior to relapse. The HR is equal to 1.14 when time spent in remission is zero, with the slope decreasing at a rate of 19% per year.
Figure 2.
Estimated HR and corresponding point-wise 95% CI for the hypothesized legacy effect for incident microvascular disease. The HR compares patients who have experienced a relapse of their type 2 diabetes after some period of remission to patients who have not remitted as a function of the time spent in remission.
Results of the sensitivity analyses are provided in Supplementary Data. Excluding the largest health care site from the interaction analyses did not change the adjusted legacy effect findings (HR 0.78 [95% CI 0.61, 0.98] vs. HR 0.81 [95% CI 0.67, 0.99]). Although adding abnormally elevated urine protein to low eGFR as an indicator of incident nephropathy in the interaction analyses did not change the adjusted legacy effect HR substantially (HR 0.83 vs. 0.81), the legacy effect was no longer statistically significant (95% CI 0.62, 1.09 vs. 0.67, 0.99). Finally, performing multiple imputation for all missing values of BMI, HbA1c, eGFR, and race/ethnicity also did not change the interaction analyses–adjusted legacy effect findings substantially (HR 0.88 vs. 0.81); however, the legacy effect was no longer significant (95% CI 0.76, 1.02 vs. 0.67, 0.99).
Effect of Patient-Level Factors on Risk for Incident Microvascular Disease
Additional factors that might be associated with the risk of incident microvascular disease were also examined. In the detailed fully adjusted model (see Supplementary Data for these findings), age was the strongest factor associated with the risk of developing incident microvascular disease after bariatric surgery. Significant HRs ranged from 6.46 (95% CI 2.03, 20.58) for 45- to 54-year-old adults to 34.31 (95% CI 10.54, 111.70) in 65- to 79-year-old adults when compared with adults aged 18–29 years.
Other factors related to the risk of developing incident microvascular disease after bariatric surgery were 1) having type 2 diabetes for 5 years or more before surgery compared with people with type 2 diabetes for <1 year before surgery (HR 1.46 [95% CI 1.12, 1.89]), 2) being on insulin at the time of surgery (HR 1.41 [95% CI 1.19, 1.68]), and 3) having an HbA1c >6.5%/48 mmol/mol before surgery compared with HbA1c <6%/42 mmol/mol (HR ranged from 1.37 [95% CI 1.04, 1.79] for 6.5–7.0% to 1.56 [95% CI 1.17, 2.08] for >8.0%). Bariatric procedure type, race/ethnicity, and preoperative BMI were not associated with the risk of developing incident microvascular disease after surgery. No significant interactions were observed between type 2 diabetes status and any patient-level factors we examined in our models of effect modification (data not shown).
Conclusions
Many prior studies, including our own (6,7), have demonstrated that a majority of patients who undergo bariatric procedures experience remission of their type 2 diabetes (1–5). However, very little is known about whether type 2 diabetes remission after bariatric surgery prevents the development of incident microvascular disease, and whether this positive effect persists even if a patient experiences relapse of their type 2 diabetes. In the largest multisite study to date in this area (n = 4,683 patients with type 2 diabetes), we found that patients who experienced remission of their type 2 diabetes after surgery experienced a 29% reduction in the risk of incident microvascular disease compared with those who did not experience remission after surgery.
In addition, even if these patients then went on to experience a relapse of their type 2 diabetes, the more time they spent in remission prior to their relapse, the lower their risk was for developing incident microvascular disease. For each additional year of time spent in remission, the HR associated with microvascular disease decreased by 19%. This has previously been referred to as the legacy effect or as metabolic memory. Although the legacy effect has been shown with other diabetes treatment modalities (12,13), this is the first study to show this effect after bariatric surgery.
Although our findings for reduction in incident microvascular disease for postbariatric patients with type 2 diabetes are supported by the literature to date (10,11,19), the rate reported here is less pronounced (29% reduction) compared with rates reported in other studies. For example, the Swedish Obese Subjects (SOS) study found a 56% lower risk of incident microvascular disease postsurgery (19), and a much larger retrospective observational study of 2,580 bariatric patients found an 80% lower risk of incident microvascular disease (11). These differences are likely related to a variety of factors, including the differential methods of determining incident microvascular disease using diagnosis and/or procedure codes alone or in combination with laboratory measures in retrospective observational studies (11), whereas standardized, controlled methods are normally implemented for outcome assessment in prospective studies (19).
Variations in the proportion of racial/ethnic minority patients in these studies may also contribute to the different associations between bariatric surgery and incident microvascular disease. Patients from different racial/ethnic minority groups may have greater disease burden and severity at the time of surgery (20), and thus the effect of surgery on type 2 diabetes remission and subsequent risk for incident microvascular disease may be attenuated. Most studies in this area are 70–90% non-Hispanic white, whereas our sample was 40% racial/ethnic minority. Although an independent association between race/ethnicity and risk of incident microvascular disease was not observed in our study (see Supplementary Data), longer duration of type 2 diabetes and higher HbA1c before surgery was shown to attenuate the effect of bariatric surgery on risk of incident microvascular disease.
Most of the effect of bariatric surgery on incident microvascular disease in this study population was due to a pronounced reduction in risk for retinopathy (36.5% reduction in risk at 7 years postsurgery). There have been a number of much smaller studies that have also shown the impact of bariatric surgery on the reduction in risk of retinopathy in patients with type 2 diabetes (21–25), although the STAMPEDE (Surgical Therapy and Medications Potentially Eradicate Diabetes Efficiently) trial found no improvement in retinopathy outcomes with surgery after 2 years of follow-up (26). Our study is the largest to date supporting the impact of bariatric surgery on reduction in risk for retinopathy up to 7 years postoperatively.
The interpretation of our data is limited by a number of factors. First, the retrospective observational study design precludes causal inference. Randomized controlled trials would be a superior way to evaluate the causal efficacy of bariatric surgery for risk of incident microvascular disease in patients with type 2 diabetes. However, few randomized controlled trials could enroll thousands of patients, as we have done with our study, which may be necessary to detect relatively rare events such as incident microvascular complications in bariatric surgery patients who experience remission of their type 2 diabetes, as accruing patient-time in type 2 diabetes status is difficult.
Second, the data used to define incident microvascular disease (retinopathy, nephropathy, and neuropathy) were collected in the process of routine clinical care across four large, integrated health care systems, so that missing data may have led to misclassification of microvascular complication status for some patients. Our ability to detect the end points in our study was dependent on people receiving care (eye exams, blood tests, and neuropathy assessment) and thus were more subject to misclassification than macrovascular events such as myocardial infarction and stroke, for which individuals are much more likely to seek care.
Finally, although the point estimate for the legacy effect of bariatric surgery was always <1.0 (indicating a consistently beneficial effect of greater time in remission prior to relapse), the CIs crossed 1.0 in two of the sensitivity analyses (addition of proteinuria as a nephropathy end point and multiple imputation). As a result, our legacy effect finding should be interpreted with some caution and replicated in future studies with larger sample sizes and more complete data.
Conclusion
Our results indicate that a large and racially/ethnically diverse population of adults with type 2 diabetes who experienced remission of their type 2 diabetes after bariatric surgery also experienced a 29% reduction in risk of incident microvascular complications up to 7 years after surgery. In addition, even if they relapsed during this period, there was still a 19% reduction in risk for each year they spent in remission before this relapse. To date, this legacy effect on microvascular complications had only been seen in patients undergoing nonsurgical treatment for poor glycemic control. We also found that older age, longer duration of type 2 diabetes before surgery, being on insulin, and having uncontrolled type 2 diabetes at the time of surgery attenuated the effect of bariatric surgery on the risk of developing incident microvascular disease. Taken in combination with our other work (6,7,20), this implies that younger patients with less severe type 2 diabetes may be more likely to experience the maximum benefits from bariatric surgery for remission of their type 2 diabetes and reduction in subsequent risk for microvascular disease.
Supplementary Material
Article Information
Funding. This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (5R01DK092317-04; E.B.S. was supported by 1K23DK099237-01).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.J.C. was responsible for the oversight of the manuscript and its writing. S.H., E.J., A.B., and M.K.T. were responsible for data abstraction, preparation, and analyses as well as the summary and writing of methods and results, including the appendices. D.A. was responsible for overseeing the conduct of the study in general and guiding the hypothesis formation and testing. All authors contributed to the plan of the study, including data abstraction and preparation for analyses, interpretation of analyses, and writing of the manuscript. K.J.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-0194/-/DC1.
References
- 1.Buchwald H, Estok R, Fahrbach K, et al. . Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248–256.e5 [DOI] [PubMed] [Google Scholar]
- 2.Mingrone G, Panunzi S, De Gaetano A, et al. . Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–1585 [DOI] [PubMed] [Google Scholar]
- 3.Dixon JB, O’Brien PE, Playfair J, et al. . Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316–323 [DOI] [PubMed] [Google Scholar]
- 4.Schauer PR, Bhatt DL, Kirwan JP, et al.; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med 2014;370:2002–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikramuddin S, Korner J, Lee WJ, et al. . Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arterburn DE, Bogart A, Sherwood NE, et al. . A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg 2013;23:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arterburn D, Bogart A, Coleman KJ, et al. . Comparative effectiveness of bariatric surgery vs. nonsurgical treatment of type 2 diabetes among severely obese adults. Obes Res Clin Pract 2013;7:e258–e268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chikunguwo SM, Wolfe LG, Dodson P, et al. . Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg Obes Relat Dis 2010;6:254–259 [DOI] [PubMed] [Google Scholar]
- 9.DiGiorgi M, Rosen DJ, Choi JJ, et al. . Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis 2010;6:249–253 [DOI] [PubMed] [Google Scholar]
- 10.Cohen R, Pechy F, Petry T, Correa JL, Caravatto PP, Tzanno-Martins C. Bariatric and metabolic surgery and microvascular complications of type 2 diabetes mellitus. J Bras Nefrol 2015;37:399–409 [DOI] [PubMed] [Google Scholar]
- 11.Johnson BL, Blackhurst DW, Latham BB, et al. . Bariatric surgery is associated with a reduction in major macrovascular and microvascular complications in moderately to severely obese patients with type 2 diabetes mellitus. J Am Coll Surg 2013;216:545–556; discussion 556–558 [DOI] [PubMed] [Google Scholar]
- 12.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 13.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Healthcare Systems Research Network. Available from http://www.hcsrn.org/en/. Accessed 21 January 2016
- 15.Ross TR, Ng D, Brown JS, et al. . The HMO Research Network Virtual Data Warehouse: a public data model to support collaboration. EGEMS (Wash DC) 2014;2:1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buse JB, Caprio S, Cefalu WT, et al. . How do we define cure of diabetes? Diabetes Care 2009;32:2133–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 2012; 307:1941–1951 [DOI] [PMC free article] [PubMed]
- 18.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–399 [DOI] [PubMed] [Google Scholar]
- 19.Sjöström L, Peltonen M, Jacobson P, et al. . Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–2304 [DOI] [PubMed] [Google Scholar]
- 20.Coleman KJ, Huang YC, Koebnick C, et al. . Metabolic syndrome is less likely to resolve in Hispanics and non-Hispanic blacks after bariatric surgery. Ann Surg 2014;259:279–285 [DOI] [PubMed] [Google Scholar]
- 21.Banks J, Adams ST, Laughlan K, et al. . Roux-en-Y gastric bypass could slow progression of retinopathy in type 2 diabetes: a pilot study. Obes Surg 2015;25:777–781 [DOI] [PubMed] [Google Scholar]
- 22.Brynskov T, Laugesen CS, Svenningsen AL, Floyd AK, Sorensen TL. Monitoring of diabetic retinopathy in relation to bariatric surgery: a prospective observational study. Obes Surg 2016;26:1279–1286 [DOI] [PubMed] [Google Scholar]
- 23.Miras AD, Chuah LL, Lascaratos G, et al. . Bariatric surgery does not exacerbate and may be beneficial for the microvascular complications of type 2 diabetes. Diabetes Care 2012;35:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy R, Jiang Y, Booth M, et al. . Progression of diabetic retinopathy after bariatric surgery. Diabet Med 2015;32:1212–1220 [DOI] [PubMed] [Google Scholar]
- 25.Amin AM, Wharton H, Clarke M, Syed A, Dodson P, Tahrani A. The impact of bariatric surgery on retinopathy in patients with type 2 diabetes: A retrospective cohort study. Surg Obes Relat Dis 2016;12:606–612 [DOI] [PubMed]
- 26.Singh RP, Gans R, Kashyap SR, et al. . Effect of bariatric surgery versus intensive medical management on diabetic ophthalmic outcomes. Diabetes Care 2015;38:e32–e33 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.