Abstract
OBJECTIVE
The shape of the glucose response curve during an oral glucose tolerance test (OGTT), monophasic versus biphasic, identifies physiologically distinct groups of individuals with differences in insulin secretion and sensitivity. We aimed to verify the value of the OGTT-glucose response curve against more sensitive clamp-measured biomarkers of type 2 diabetes risk, and to examine incretin/pancreatic hormones and free fatty acid associations in these curve phenotypes in obese adolescents without diabetes.
RESEARCH DESIGN AND METHODS
A total of 277 obese adolescents without diabetes completed a 2-h OGTT and were categorized to either a monophasic or a biphasic group. Body composition, abdominal adipose tissue, OGTT-based metabolic parameters, and incretin/pancreatic hormone levels were examined. A subset of 106 participants had both hyperinsulinemic-euglycemic and hyperglycemic clamps to measure in vivo insulin sensitivity, insulin secretion, and β-cell function relative to insulin sensitivity.
RESULTS
Despite similar fasting and 2-h glucose and insulin concentrations, the monophasic group had significantly higher glucose, insulin, C-peptide, and free fatty acid OGTT areas under the curve compared with the biphasic group, with no differences in levels of glucagon, total glucagon-like peptide 1, glucose-dependent insulinotropic polypeptide, and pancreatic polypeptide. Furthermore, the monophasic group had significantly lower in vivo hepatic and peripheral insulin sensitivity, lack of compensatory first and second phase insulin secretion, and impaired β-cell function relative to insulin sensitivity.
CONCLUSIONS
In obese youth without diabetes, the risk imparted by the monophasic glucose curve compared with biphasic glucose curve, independent of fasting and 2-h glucose and insulin concentrations, is reflected in lower insulin sensitivity and poorer β-cell function, which are two major pathophysiological biomarkers of type 2 diabetes in youth.
Introduction
Fasting and 2-h glucose concentrations during an oral glucose tolerance test (OGTT) are used either to diagnose type 2 diabetes or to capture the heightened risk for future type 2 diabetes (i.e., prediabetes) (1). However, there is a growing interest in finding novel biomarkers and/or models that can identify early metabolic risk abnormalities (2,3). One such marker is the shape of the glucose response curve during an OGTT, either monophasic or biphasic, which is proposed to harbor metabolic information not captured by the level of glycemia alone (4) and is a potential predictor for type 2 diabetes in adults (5).
Youth prediabetes and type 2 diabetes have emerged as major consequences of the obesity epidemic imposing a serious public health burden (6,7). Considering that insulin resistance, impaired β-cell function, and impaired incretin effect constitute the pathophysiological mechanisms of youth prediabetes and type 2 diabetes (8–10), there is increasing interest in identifying simple biomarkers that can detect impairment in β-cell function heralding type 2 diabetes. Among these are the fasting (11), 1-h (12), and 2-h glucose concentrations during an OGTT, which signal β-cell failure, measured by the clamp-derived disposition index (DI), and presage progression to type 2 diabetes (8,13). Additionally, however, recent cross-sectional studies (14–16) demonstrated that the shape of the OGTT-glucose response curve can differentiate type 2 diabetes risk in Latino and white adolescents without diabetes. Youth with a monophasic glucose response curve manifest an increased risk for type 2 diabetes markers measured by OGTT-derived surrogate indices of insulin sensitivity (IS) and β-cell function. However, because the shape of the glucose response curve was drawn by plotting the time-course change of glucose concentrations during an OGTT, it is likely to be correlated with the various OGTT-derived surrogate estimates of IS and insulin secretion. Furthermore, it remains unknown whether incretin hormones, through augmenting insulin secretion and regulating the rate of gastric emptying, might play a role in the shape of the OGTT-glucose response curve. Last, it has not been investigated whether there might be a relationship between free fatty acid (FFA) concentrations during the OGTT and the glucose response curve despite the well-known lipotoxic phenomenon of elevated FFAs in impairing IS and insulin secretion.
Therefore, the purposes of this study in obese adolescents without diabetes were as follows: 1) to verify the utility of the OGTT-glucose response curve, monophasic versus biphasic, against more sensitive clamp-measured biomarkers of type 2 diabetes; 2) to examine whether there are differences in incretin and pancreatic hormone responses between monophasic and biphasic OGTT-glucose response curves; and 3) to assess whether there are differences in FFA concentration, a marker of lipotoxicity, between monophasic and biphasic groups.
Research Design and Methods
Participants
A total of 287 obese adolescents without diabetes (138 African American [AA], 140 American white [AW], and 9 biracial; age 10 to <20 years old; BMI ≥85th percentile for age and sex; Tanner stages II–V), who participated in our National Institutes of Health–funded K24 grant of Childhood Insulin Resistance (9–13,17–20) from January 2004 through October 2012, were examined. Of them, data from 277 subjects (134 AA, 134 AW, and 9 biracial) were used in the present analysis, whereas 10 subjects were excluded because their glucose responses over the 2-h OGTT could not be classified as either monophasic or biphasic. There were 208 subjects with normal glucose tolerance (NGT) and 69 subjects with prediabetes (15 with impaired fasting glucose [IFG], 44 with impaired glucose tolerance [IGT], and 10 with combined glucose intolerance). Participants were recruited through newspaper advertisements, flyers posted on the medical campus, city bus routes, and the outpatient clinics in the Weight Management and Wellness Center and the Division of Pediatric Endocrinology. The study was approved by the institutional review board of the University of Pittsburgh, and written informed parental consent and child assent were obtained from all participants before any research procedures were conducted, in accordance with the ethical guidelines of Children’s Hospital of Pittsburgh.
Procedures
All procedures were performed at the Pediatric Clinical and Translational Research Center of Children’s Hospital of Pittsburgh. All participants gave a medical history, and underwent a physical examination, and hematologic and biochemical tests. Height and weight were assessed to the nearest 0.1 cm and 0.1 kg, respectively, and were used to calculate BMI. Pubertal development was assessed using Tanner criteria (21). Body composition was evaluated with DEXA by the measurement of total body fat mass, fat-free mass, and percentage of body fat. Abdominal total adipose tissue (TAT), subcutaneous adipose tissue (SAT), and visceral adipose tissue (VAT) were assessed by either computed tomography (CT) or MRI at the L4–5 intervertebral space (10,22). The switch from CT to MRI was imposed by the study section during the competitive grant renewal process. Note that Klopfenstein et al. (23) demonstrated strong correlation (r = 0.89–0.95) and good agreement (i.e., very small average difference) between CT and MRI for the measurement of abdominal adipose tissue.
Metabolic Studies
After 10–12 h of overnight fasting, participants underwent a 2-h OGTT (1.75 g/kg, maximum 75 g) (19,24). Blood samples were obtained at −15, 0, 30, 60, 90, and 120 min for the measurement of glucose, insulin, C-peptide, glucagon, total glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), pancreatic polypeptide (PP), and FFA. Fasting blood samples were obtained for determination of the lipid profile, and levels of HbA1c and adiponectin.
Of the total 277 participants, a subset of 106 participants (70 with NGT and 36 with prediabetes [7 with IFG, 24 with IGT, and 5 with combined glucose intolerance]) were admitted twice within a 1-to 4-week period to the Pediatric Clinical and Translational Research Center for a hyperinsulinemic-euglycemic clamp to assess in vivo IS, and a hyperglycemic clamp to assess insulin secretion, which were performed in random order and synchronized with the OGTT (19,25–27). Each clamp evaluation was performed after a 10- to 12-h overnight fast. Fasting hepatic glucose production was measured before the start of the hyperinsulinemic-euglycemic clamp, with a primed (2.2 μmol/kg) constant infusion of [6,6-2H2] glucose at 0.22 μmol/kg/min for a total of 2 h, as described previously (19,25–27). After the 2-h baseline isotope infusion period, in vivo IS was evaluated during a 3-h hyperinsulinemic (80 mU/m2/min)-euglycemic clamp (19,25–27). First- and second-phase insulin secretion was assessed during a 2-h hyperglycemic (225 mg/dL) clamp, as described before (19,25–27). The plasma glucose concentration was increased rapidly to 225 mg/dL by a bolus infusion of dextrose and was maintained at that level by a variable rate infusion of 20% dextrose for 2 h, with frequent measurement of glucose and insulin concentrations.
Biochemical Measurements
During the 2-h OGTT, blood was collected in chilled aprotinin/EDTA tubes for insulin, C-peptide, and glucagon measurements. Dipeptidyl peptidase-4 inhibitor (10 µL; catalog #DPP4; Millipore, St. Charles, MO) was added before sampling to the aprotinin/EDTA tubes to prevent the enzymatic degradation of total GLP-1, GIP, and FFA. Blood samples were immediately separated in a refrigerated centrifuge. Plasma samples were divided into aliquots and stored at −80°C until analysis. Consistent assay protocols were used for biochemical measurements over the study period. Plasma glucose level was determined by the glucose oxidase method using a glucose analyzer (Yellow Springs Instrument Co., Yellow Springs, OH), and insulin, C-peptide, and glucagon levels were determined by a commercially available radioimmunoassay (Milllipore), as previously reported (24). Total GLP-1 was measured on a microplate reader (BioTek, Winooski, VT) using a multispecies total GLP-1 ELISA kit (catalog #EZGLP1T-36 K; Millipore). GIP and PP were measured on the 200 IS System (Luminex, Austin, TX) using a two-plex human gut hormone MILLIPLEX Kit (catalog #HGT-68K-02; Millipore), as described before (10). FFA was determined using enzymatic colorimetric methods with a (nonesterified fatty acids) NEFA-HR Test Kit (Wako, Osaka, Japan). Adiponectin was measured using a commercially available radioimmunoassay kit (LINCO Research Inc., St Charles, MO). HbA1c level was measured by high-performance liquid chromatography (Tosoh Medics).
Calculations
Glucose response curve phenotype (i.e., monophasic or biphasic) was defined based on plotting glucose concentrations during the 2-h OGTT. A monophasic response curve was determined by a gradual increase in glucose concentrations until a peak was reached followed by a subsequent decrease in glucose of ≥4.5 mg/dL (4,14). A biphasic response curve was defined by a second rise in glucose concentrations of ≥4.5 mg/dL after the decline in glucose (4,14). An unclassified response curve was determined when the glucose concentrations after glucose ingestion continuously increased until 120 min.
The OGTT area under the curve (AUC) for glucose, insulin, C-peptide, glucagon, total GLP-1, GIP, PP, and FFA was calculated with the use of the trapezoidal method. IS was estimated by the HOMA-IS. Early-phase insulin (i.e., insulinogenic index) and C-peptide secretion during the OGTT were calculated as ∆ insulin (or C-peptide) (0–30 min)/∆ glucose (0–30 min), as previously described (18). The oral DI (oDI), which represents β-cell function relative to IS, was calculated as the product of HOMA-IS × insulinogenic index (18).
Fasting hepatic glucose production was calculated during the last 30 min of the 2-h isotope infusion (−30 to 0 min) according to steady-state tracer dilution equations (25,27). Hepatic IS was calculated as the inverse of the product of hepatic glucose production and fasting plasma insulin concentration (27,28). The insulin-stimulated glucose disposal rate was calculated to be equal to the rate of exogenous glucose infusion during the final 30 min of the hyperinsulinemic-euglycemic clamp. Peripheral IS was calculated by dividing the glucose disposal rate by the steady-state clamp insulin concentration multiplied by 100 (25). During the hyperglycemic clamp, first- and second-phase insulin concentrations were calculated as described previously (25,27). β-Cell function relative to IS (i.e., the DI), was calculated as the product of IS, measured by the hyperinsulinemic-euglycemic clamp, and first-phase insulin secretion measured during the hyperglycemic clamp (25,27).
Statistical Analysis
Independent-samples t tests and χ2 analyses were used to compare the two glucose response curve groups (monophasic vs. biphasic). ANCOVA was used to compare phenotypes after adjusting for the potential confounding effects (age, Tanner stage, race, sex, glycemic status, BMI, and fat mass) on phenotypes based on each analytical model. Data that did not meet the assumptions for normality were log10 transformed; untransformed data are presented for ease of interpretation. Data were analyzed using the PASW version 22.0 statistical software package and were presented as the mean ± SEM, unless otherwise specified, with significance set at P ≤ 0.05.
Results
Classification Based on Glucose Response Curve
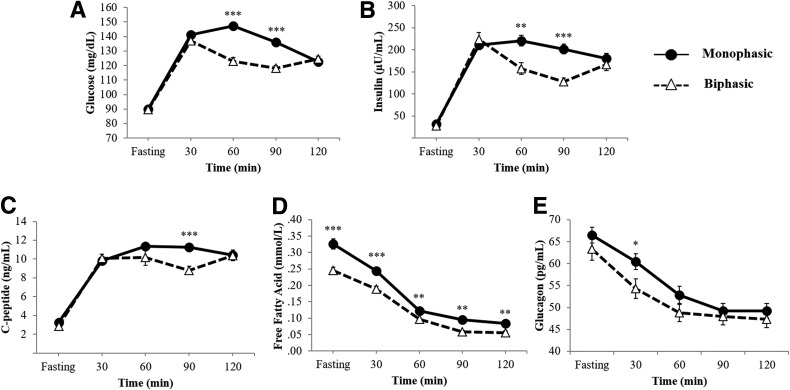
Based on the glucose response curve, 163 participants were classified as monophasic and 114 were categorized as biphasic. Ten participants exhibiting a gradual increase in plasma glucose levels after glucose ingestion without a corresponding fall (i.e., unclassified) were excluded from the present analysis. Figure 1A–E displays the mean glucose curve (Fig. 1A), and the corresponding mean insulin (Fig. 1B), C-peptide (Fig. 1C), FFA (Fig. 1D), and glucagon (Fig. 1E) curves in each of the monophasic and biphasic groups.
Figure 1.
Glucose (A), insulin (B), C-peptide (C), FFA (D), and glucagon (E) response curves during a 2-h OGTT in monophasic (filled circles and solid lines) and biphasic (open triangles and dashed lines) groups. *P < 0.05; **P < 0.01; ***P < 0.001.
Physical and Metabolic Characteristics of the Total OGTT Cohort (n = 277) With Monophasic Versus Biphasic Response Curve
Physical and metabolic characteristics of all participants categorized into monophasic versus biphasic groups are presented in Table 1. The two groups were not different in age, race, sex, Tanner stage, and glycemic status (NGT vs. prediabetes). The monophasic group had higher BMI and total body fat mass, and lower adiponectin concentrations, with no differences in BMI percentile, percentage body fat, and abdominal adiposity. There were no differences in HbA1c and fasting lipid levels (Table 1).
Table 1.
Physical and metabolic characteristics of 277 participants with monophasic versus biphasic OGTT-glucose response curve
| Variables | Monophasic group (n = 163) | Biphasic group (n = 114) | P value |
|---|---|---|---|
| Physical characteristics | |||
| Age (years) | 14.7 ± 0.1 | 15.0 ± 0.2 | NS |
| Race (AA/AW/biracial) | 72 (44)/85 (52)/6 (4) | 62 (54)/49 (43)/3 (3) | NS |
| Sex (male/female) | 60 (37)/103 (63) | 41 (36)/73 (64) | NS |
| Tanner stage (II/III/IV/V), n | 4/23/20/116 | 3/8/16/87 | NS |
| BMI (kg/m2) | 36.0 ± 0.6 | 34.1 ± 0.6 | 0.035 |
| BMI percentile | 97.9 ± 0.2 | 97.5 ± 0.3 | NS |
| Total body fat mass (kg) | 43.2 ± 1.1 | 39.7 ± 1.2 | 0.037 |
| Body fat (%) | 43.6 ± 0.6 | 42.3 ± 0.7 | NS |
| VAT (cm2) | 67.9 ± 2.9 | 64.6 ± 3.3 | NS |
| SAT (cm2) | 508.4 ± 17.4 | 476.5 ± 19.6 | NS |
| TAT (cm2) | 576.4 ± 19.3 | 539.1 ± 21.3 | NS |
| Metabolic characteristics | |||
| Glycemic status (NGT/prediabetes) | 122 (75)/41 (25) | 86 (75)/28 (25) | NS |
| HbA1c (%) | 5.47 ± 0.05 | 5.41 ± 0.06 | NS |
| Adiponectin (μg/mL) | 7.03 ± 0.29 | 8.36 ± 0.45 | 0.015*#¶ |
| Cholesterol (mg/dL) | 150.9 ± 2.5 | 153.0 ± 3.0 | NS |
| Triglyceride (mg/dL) | 102.9 ± 4.8 | 92.9 ± 4.4 | NS |
| HDL (mg/dL) | 48.7 ± 2.0 | 49.0 ± 2.2 | NS |
| LDL (mg/dL) | 83.6 ± 2.4 | 87.3 ± 2.9 | NS |
| VLDL (mg/dL) | 20.3 ± 0.9 | 18.6 ± 0.9 | NS |
| OGTT-based parameters | |||
| Glucose AUC (mg · dL−1 · h−1) | 15,878.8 ± 161.9 | 14,504.0 ± 175.5 | <0.0001*#¶ |
| Insulin AUC (µU · mL−1 · h−1) | 22,248.7 ± 1,085.2 | 17,727.4 ± 1,111.0 | 0.002*#¶ |
| C-peptide AUC (ng · mL−1 · h−1) | 1,173.2 ± 32.7 | 1,055.1 ± 50.6 | 0.005*#¶ |
| FFA AUC (mmol · L−1 · h−1) | 20.0 ± 0.7 | 15.4 ± 0.6 | <0.0001*#¶ |
| Glucagon AUC (pg · mL−1 · h−1) | 6,664.1 ± 191.3 | 6,227.0 ± 230.7 | 0.078 |
| Total GLP-1 AUC (pmol · L−1 · h−1) | 1,725.0 ± 103.4 | 1,672.7 ± 96.0 | NS |
| GIP AUC (pg · mL−1 · h−1) | 21,386.8 ± 745.9 | 20,952.0 ± 693.7 | NS |
| PP AUC (pg · mL−1 · h−1) | 6,672.6 ± 445.7 | 6,436.8 ± 550.6 | NS |
| HOMA-IS | 0.20 ± 0.01 | 0.25 ± 0.02 | 0.003*#¶ |
| Early-phase insulin secretion | 3.7 ± 0.2 | 4.4 ± 0.4 | NS |
| Early-phase C-peptide secretion | 0.14 ± 0.01 | 0.17 ± 0.01 | 0.008*#¶ |
| oDI (HOMA-IS × insulinogenic index) | 0.61 ± 0.05 | 0.79 ± 0.05 | 0.001*#¶ |
Values are reported as the mean ± SEM or n (%), unless otherwise indicated. Prediabetes, IFG and/or IGT. VAT, SAT, and TAT data were available on 229 patients (130 vs. 99). Glucagon is missing in 3 participants (2 in monophasic group and 1 biphasic group). Total n for total GLP-1 is 221 (126 vs. 95), for GIP and PP is 230 (133 vs. 97), for FFA is 250 (148 vs. 102), and for adiponectin is 246 (145 vs. 101).
*P < 0.05 after adjusting for BMI.
#P < 0.05 after adjusting for fat mass in addition to BMI.
¶P < 0.05 after adjusting for race in addition to BMI and fat mass.
Despite similar fasting and 2-h glucose and insulin concentrations between the two groups (Fig. 1A and B), participants with a monophasic response curve exhibited higher glucose, insulin, C-peptide, and FFA AUCs (Table 1). No differences in total GLP-1, GIP, and PP AUCs were found, with a tendency for higher glucagon levels in the monophasic group. The monophasic group had significantly lower HOMA-IS and oDI compared with the biphasic group. All significant differences were independent of BMI, fat mass, and race (Table 1).
When the OGTT-glucose response curve was analyzed within each sex, both boys and girls with a monophasic versus a biphasic response had significantly lower oDI (boys 0.52 ± 0.04 vs. 0.76 ± 0.06; girls 0.67 ± 0.08 vs. 0.81 ± 0.08), higher glucose AUC (boys 15,829.3 ± 218.6 vs. 15,055.4 ± 262.4; girls 15,907.6 ± 223.1 vs. 14,194.3 ± 224.3 mg/dL/h), and higher FFA AUC (boys 20.2 ± 1.6 vs. 15.7 ± 1.0; girls 19.9 ± 0.8 vs. 15.3 ± 0.7 mmol/L/h) (all P < 0.05). Further, girls with a monophasic response had significantly lower adiponectin levels (6.63 ± 0.35 vs. 8.79 ± 0.56 μg/mL) and HOMA-IS (0.19 ± 0.02 vs. 0.25 ± 0.02), and higher insulin AUC (23,951.9 ± 1,480.4 vs. 17,966.6 ± 1,473.6 μU/mL/h) and C-peptide AUC (1,259.0 ± 41.1 vs. 1,110.7 ± 70.9 ng/mL/h) (all P < 0.05) before and after adjusting for BMI and fat mass.
Clamp-Measured In Vivo IS, Insulin Secretion, and β-Cell Function Relative to IS in Monophasic Versus Biphasic Curve Groups
Of the 106 participants who had synchronized and complete OGTT, hyperinsulinemic-euglycemic clamp, and hyperglycemic clamp data, 66 were classified into the monophasic group and 40 into the biphasic group. Subgroup analyses for physical and metabolic characteristics, and OGTT-based parameters were similar to that of the total cohort analyses, with the exception of the younger age in the monophasic group (Table 2). After adjusting for age, Tanner stage, race, sex, and glycemic status, participants with a monophasic glucose response curve exhibited significantly higher glucose, insulin, and FFA AUC, and lower adiponectin concentrations, HOMA-IS, and oDI (Table 2).
Table 2.
Physical and metabolic characteristics of 106 participants who had synchronized OGTT and hyperinsulinemia-euglycemic, and hyperglycemic clamps, with monophasic versus biphasic OGTT-glucose response curve
| Variables | Monophasic group (n = 66) | Biphasic group (n = 40) | P value | Adjusted P value* |
|---|---|---|---|---|
| Physical characteristics | ||||
| Age (years) | 14.8 ± 0.2 | 15.7 ± 0.3 | 0.014 | |
| Race (AA/AW/biracial) | 26 (39)/36 (55)/4 (6) | 16 (40)/21 (53)/3 (7) | NS | |
| Sex (male/female) | 31 (47)/35 (53) | 19 (47)/21 (53) | NS | |
| Tanner stage (III/IV/V) | 9 (14)/7 (10)/50 (76) | 3 (7)/5 (13)/32 (80) | NS | |
| BMI (kg/m2) | 36.1 ± 0.8 | 35.0 ± 0.9 | NS | NS |
| BMI percentile | 97.8 ± 0.3 | 97.4 ± 0.5 | NS | |
| Total body fat mass (kg) | 43.4 ± 1.5 | 42.0 ± 1.9 | NS | NS |
| Body fat (%) | 43.2 ± 0.7 | 43.8 ± 1.0 | NS | NS |
| VAT (cm2) | 72.4 ± 3.3 | 68.3 ± 5.0 | NS | NS |
| SAT (cm2) | 528.0 ± 23.4 | 518.2 ± 29.0 | NS | NS |
| TAT (cm2) | 600.4 ± 25.3 | 580.7 ± 29.9 | NS | NS |
| Metabolic characteristics | ||||
| Glycemic status (NGT/prediabetes) | 46 (70)/20 (30) | 24 (60)/16 (40) | NS | |
| HbA1c (%) | 5.41 ± 0.06 | 5.36 ± 0.07 | NS | NS |
| Adiponectin (μg/mL) | 6.21 ± 0.34 | 7.02 ± 0.47 | NS | 0.036 |
| Cholesterol (mg/dL) | 149.8 ± 4.0 | 157.9 ± 5.1 | NS | NS |
| Triglyceride (mg/dL) | 118.3 ± 7.9 | 109.5 ± 9.1 | NS | NS |
| HDL (mg/dL) | 38.8 ± 1.2 | 40.3 ± 1.2 | NS | NS |
| LDL (mg/dL) | 87.6 ± 3.7 | 95.8 ± 4.4 | NS | NS |
| VLDL (mg/dL) | 22.6 ± 1.3 | 21.9 ± 1.8 | NS | NS |
| OGTT-based parameters | ||||
| Glucose AUC (mg · dL−1 · h−1) | 16,157.5 ± 257.7 | 14,952.6 ± 323.0 | 0.005 | <0.001 |
| Insulin AUC (µU · mL−1 · h−1) | 24,477.1 ± 1,935.7 | 17,089.2 ± 1,397.9 | 0.010 | 0.004 |
| C-peptide AUC (ng · mL−1 · h−1) | 1,173.6 ± 51.7 | 1,025.3 ± 60.0 | NS | NS |
| FFA AUC (mmol · L−1 · h−1) | 19.3 ± 0.9 | 17.0 ± 0.9 | 0.076 | 0.018 |
| Glucagon AUC (pg · mL−1 · h−1) | 6,251.9 ± 333.7 | 6,487.3 ± 375.1 | NS | NS |
| Total GLP-1 AUC (pmol · L−1 · h−1) | 1,445.9 ± 113.2 | 1,706.0 ± 143.0 | NS | NS |
| GIP AUC (pg · mL−1 · h−1) | 22,039.7 ± 1,015.1 | 22,427.3 ± 1,167.5 | NS | NS |
| PP AUC (pg · mL−1 · h−1) | 7,759.5 ± 751.2 | 5,559.9 ± 496.2 | 0.081 | NS |
| HOMA-IS | 0.18 ± 0.01 | 0.25 ± 0.03 | 0.040 | 0.026 |
| Early-phase insulin secretion | 3.93 ± 0.35 | 3.89 ± 0.42 | NS | NS |
| Early-phase C-peptide secretion | 0.13 ± 0.01 | 0.15 ± 0.02 | NS | NS |
| oDI (HOMA-IS × insulinogenic index) | 0.57 ± 0.05 | 0.72 ± 0.08 | 0.080 | 0.014 |
Values are reported as the mean ± SEM or n (%), unless otherwise indicated. VAT, SAT, and TAT data were available on 100 patients (61 vs. 39). Total n for glucagon is 104 (65 vs. 39), for total GLP-1 is 105 (65 vs. 40), for GIP is 104 (64 vs. 40), for PP is 103 (65 vs. 38), for FFA is 106 (66 vs. 40), and for adiponectin is 92 (57 vs. 35).
*Difference after adjusting for age, Tanner stage, race, sex, and glycemic status.
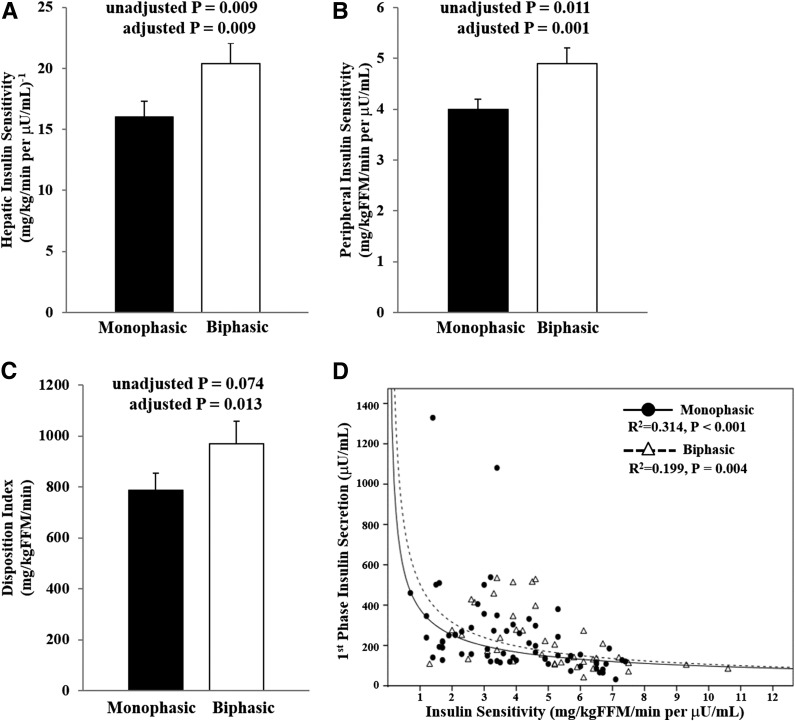
When the monophasic and biphasic groups were compared with respect to clamp-based parameters after controlling for age, Tanner stage, race, sex, and glycemic status (Fig. 2), participants with a monophasic glucose response had significantly lower hepatic and peripheral IS. β-cell function relative to IS (i.e., DI) was significantly lower in the monophasic group compared with the biphasic group (Fig. 2C), with lack of compensation of first- and second-phase insulin secretion (first phase 240.4 ± 25.8 vs. 228.0 ± 23.4 µU/mL; second phase 296.4 ± 22.7 vs. 284.6 ± 31.6 µU/mL, all P > 0.05). The best-fit hyperbolic relationship between IS and first-phase insulin secretion shows a shift to the left toward the origin in the monophasic compared with the biphasic groups (Fig. 2D).
Figure 2.
In vivo hepatic IS (A), peripheral IS (B), β-cell function relative to IS (DI) (C), and the hyperbolic relationship between IS and first-phase insulin secretion (D) in the monophasic group (filled circles and solid line) vs. the biphasic group (open triangles and dashed line). Hyperbolic curve is the nonlinear fitted regression line derived from the individual data points of the monophasic (R2 = 0.314, P < 0.001) vs. the biphasic (R2 = 0.199, P = 0.004) groups. Adjusted P value is for the difference after adjusting for age, Tanner stage, race, sex, and glycemic status.
Conclusions
The present investigation reveals that adolescents with a monophasic versus a biphasic glucose response curve during an OGTT, despite having similar fasting and 2-h glucose and insulin concentrations, manifest heightened risk for type 2 diabetes characterized by the following: 1) lower in vivo hepatic and peripheral IS; 2) impaired β-cell function relative to IS; 3) lower adiponectin concentrations; and 4) higher FFA concentrations during the OGTT despite higher prevailing insulinemia.
Studies in adults established the concept of the OGTT-glucose response curve (i.e., monophasic vs. biphasic) and subsequently supported that the shape of the monophasic response represents an increased risk for type 2 diabetes through cross-sectional studies (4,29–31). The findings from these cross-sectional studies were further confirmed longitudinally, showing that over a 7- to 8-year follow-up period the conversion rate to type 2 diabetes in prediabetic adults with a monophasic glucose response was nearly double those with a biphasic glucose response (5). However, there is a trickle of cross-sectional studies describing the shape of the OGTT-glucose response curve in youth. Kim et al. (14) showed that during a 2-h OGTT Latino youth without diabetes with a biphasic glucose response compared with those with a monophasic glucose response have a lower OGTT glucose AUC, a higher Matsuda index, a higher insulinogenic index, and better oDI, all of which are surrogate indices estimated from the OGTT. Another study in white obese adolescents demonstrated that the shape of the monophasic glucose response compared with the more complex shapes represented a high risk for type 2 diabetes in terms of altered OGTT-derived surrogate estimates of glucose homeostasis (15). Another study (16) divided obese girls, based on a nonstandard threshold of 2 mg/dL instead of the accepted 4.5 mg/dL, into the following four types of OGTT-glucose response curves: monophasic, biphasic, triphasic, and unclassified. Consistent with previous findings, the monophasic glucose response category showed abnormalities in OGTT-based metabolic parameters, including lower insulinogenic index and oDI compared with the groups of biphasic and triphasic glucose responses. Despite consistent observations in youth, a common limitation is that the main outcome measures were based on the results of an OGTT, which is also the same test from which the glucose response pattern is plotted, hence allowing for autocorrelation and bias using the same parameters of glucose and insulin from a single test.
In the present investigation, we not only support the previous findings of monophasic versus biphasic glucose response, but expand the study to verify the utility of the OGTT-glucose response curve against in vivo evaluation of IS and β-cell function. In the subset of 106 obese adolescents without diabetes who completed all three tests (the OGTT, the hyperinsulinemic-euglycemic clamp, and the hyperglycemic clamp), we demonstrated that adolescents with a monophasic glucose response curve had lower hepatic and peripheral IS with inadequate compensation in first- and second-phase insulin secretion when compared with those with a biphasic response, despite having similar fasting and 2-h glucose and insulin concentrations. This altered balance between insulin action and secretion led participants with a monophasic glucose response to have lower β-cell function relative to IS (i.e., DI), which is considered the strongest metabolic predictor for the development of future type 2 diabetes (32). Thus, the risk imparted by the monophasic glucose response curve during an OGTT, independent of fasting and 2-h glucose and insulin concentrations, is mirrored in lower in vivo IS and impaired β-cell function, two major pathophysiological biomarkers of the heightened risk of type 2 diabetes in youth. Furthermore, when the data set was restricted to only those participants with NGT, youth with a monophasic response had significantly lower HOMA-IS and oDI (in the total cohort), and lower hepatic IS and higher glucose, insulin, and FFA AUCs (in the NGT subset that completed both clamps) compared with those with a biphasic response (data not shown).
Of the total cohort in the current study, 59% of obese adolescents were classified as having a monophasic glucose response, whereas 41% were categorized as having a biphasic response. Previous studies reported that the shape of the monophasic response is the dominant phenotype, with prevalence ranging (after excluding the unclassified group) from 57% to 84% in adults (4,5,29–31) and from 35% to 69% in youth (14–16). Based on our present study and other pediatric studies (15) showing, in general, higher biphasic response curve rates than those in adults, together with publications concerning adults, that a biphasic response is associated with younger age compared with the monophasic response (4), it could be postulated that the aging process may have a negative impact on the glucose response curve. Although there were no differences in sex, ethnicity (AA vs. AW), and glycemic status (NGT vs. prediabetes) between the two glucose curve groups, we further scrutinized our analyses concerning the possibility of race-specific disparities in type 2 diabetes risk in youth (6). Our data showed that even after adjusting for these variables the OGTT-glucose response curve continued to discern a risk for type 2 diabetes.
Our data showed that adolescents with a monophasic response curve compared with a biphasic response curve had a higher FFA AUC despite having a higher insulin AUC, which is suggestive of defective regulation of lipolysis by insulin or insulin resistance of lipid metabolism (33). This is in line with the lower in vivo IS of glucose metabolism and lower adiponectin concentrations in the monophasic versus the biphasic group. Adiponectin, being an antidiabetic and antiatherogenic marker with low levels associated with obesity-related metabolic disorders in youth (34) and adults (35), might be a biomarker of a monophasic glucose response curve. With respect to the elevated FFA concentrations, it is tempting to hypothesize that the lower β-cell function in the monophasic group, whether derived from the OGTT results or measured during the clamp, might be consequent to lipotoxicity. We previously demonstrated (36) that in otherwise healthy, even normal-weight children, short periods of ∼5 h of exposure to elevated FFA concentrations results in a significant decline in β-cell function relative to IS that is consistent with an acute lipotoxic effect. Results were similar in overweight/obese adolescents (37). Thus, we postulate that youth with a monophasic OGTT-glucose response curve manifest worse β-cell function secondary to lipotoxicity consequent to an inherent insulin resistance that involves both the glucose and lipid metabolism and manifested in lower adiponectin levels.
Further, we attempted for the first time to examine whether the different glucose response curves are driven by differences in incretin concentrations and pancreatic hormones. Our data did not show differences in total GLP-1, GIP, and PP responses during the OGTT between the monophasic and the biphasic groups. However, a tendency for ∼7% higher glucagon in the monophasic group compared with the biphasic group was observed. It could be postulated that the inadequate suppression of the glucagon secretion may contribute to the monophasic response through the failure of a rapid fall in hepatic glucose production after the oral glucose load (38). Additionally, it remains unresolved whether the incretin effect might be different between the two groups despite similar incretin concentrations.
The strengths of the present investigation include the following: 1) a first-time comparison of the OGTT-glucose response curve phenotypes to clamp-measured in vivo IS, insulin secretion, and β-cell function relative to IS; 2) the comprehensive assessment of pancreatic and incretin hormones in relation to the glucose response curve, which to our knowledge has not been reported previously in adults or adolescents; and 3) the balanced representation of white and black youth. Potential perceived limitations would be that we have no data on OGTT glycerol turnover, which could shed light on the mechanisms responsible for the observed relationship between FFA-glucose response curve through altered lipolysis. In addition, the OGTT-glucose response curve shape was determined by the results of a single OGTT, which may have limited reproducibility in youth (39). A recent study in adults (40) examined the reproducibility of the various OGTT-based biomarkers of type 2 diabetes risk, including the shape of the OGTT-glucose response curve, and found poor reproducibility. Our preliminary analysis of the previous data set (n = 51), in which participants underwent repeated OGTTs (39), shows 57% agreement on the shape classification (i.e., monophasic vs. biphasic) between two OGTTs (unpublished data). Further investigations of whether consistent patterns or shapes of the OGTT-glucose response curves can be observed in an OGTT duplicates in obese youth might shed light on whether the underlying pathophysiological mechanisms fluctuate or not. Most importantly, however, prospective longitudinal studies are needed to investigate the predictive value of the shape of the OGTT-glucose response curve with respect to progression to prediabetes or type 2 diabetes in obese youth. Further, it remains imperative to perform intervention/prevention trials in obese youth to examine whether any lifestyle or pharmacological approaches could shift the OGTT-glucose response curve from monophasic to biphasic.
In summary, the current study verified the shape of the OGTT-glucose response curve against more sensitive clamp measures of type 2 diabetes risk in AA and AW obese adolescents. Our data reveal that the monophasic glucose response curve portends all of the risk biomarkers of youth type 2 diabetes, including poor β-cell function relative to IS, insulin resistance, low adiponectin concentrations, and altered glucose and FFA metabolism, which cannot be captured by the level of glycemia alone.
Article Information
Acknowledgments. The authors thank all of the children who participated in this study and their parents, without whom science would not advance; Nancy Guerra for her assistance; Resa Stauffer for her laboratory expertise; and the nursing staff of the Pediatric Clinical and Translational Research Center for their outstanding care of the participants and their meticulous attention to the research.
Funding. This study was supported by National Institute of Child Health and Human Development grants K24-HD01357 and R01-HD27503 to S.A., National Center for Advancing Translational Sciences Clinical and Translational Science Award UL1TR000005, and National Center for Research Resources grant RR024153 to the General Clinical Research Center.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.Y.K. and S.A. designed the study, analyzed the data, and wrote the manuscript. S.F.M., A.N., S.L., H.T., T.H., K.S.H., and F.B. collected the data and reviewed the manuscript. All authors approved the manuscript in its final version. S.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(Suppl. 1):S81–S90 [DOI] [PubMed] [Google Scholar]
- 2.Stern MP, Williams K, Haffner SM. Identification of persons at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test? Ann Intern Med 2002;136:575–581 [DOI] [PubMed] [Google Scholar]
- 3.Reinehr T, Wabitsch M, Kleber M, de Sousa G, Denzer C, Toschke AM. Parental diabetes, pubertal stage, and extreme obesity are the main risk factors for prediabetes in children and adolescents: a simple risk score to identify children at risk for prediabetes. Pediatr Diabetes 2009;10:395–400 [DOI] [PubMed] [Google Scholar]
- 4.Tschritter O, Fritsche A, Shirkavand F, Machicao F, Häring H, Stumvoll M. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care 2003;26:1026–1033 [DOI] [PubMed] [Google Scholar]
- 5.Abdul-Ghani MA, Lyssenko V, Tuomi T, Defronzo RA, Groop L. The shape of plasma glucose concentration curve during OGTT predicts future risk of type 2 diabetes. Diabetes Metab Res Rev 2010;26:280–286 [DOI] [PubMed] [Google Scholar]
- 6.Dabelea D, Bell RA, D’Agostino RB Jr, et al.; Writing Group for the SEARCH for Diabetes in Youth Study Group . Incidence of diabetes in youth in the United States. JAMA 2007;297:2716–2724 [DOI] [PubMed] [Google Scholar]
- 7.Li C, Ford ES, Zhao G, Mokdad AH. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005-2006. Diabetes Care 2009;32:342–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannini C, Weiss R, Cali A, et al. Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes 2012;61:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacha F, Lee S, Gungor N, Arslanian SA. From pre-diabetes to type 2 diabetes in obese youth: pathophysiological characteristics along the spectrum of glucose dysregulation. Diabetes Care 2010;33:2225–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michaliszyn SF, Mari A, Lee S, et al. β-cell function, incretin effect, and incretin hormones in obese youth along the span of glucose tolerance from normal to prediabetes to type 2 diabetes. Diabetes 2014;63:3846–3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tfayli H, Lee S, Arslanian S. Declining beta-cell function relative to insulin sensitivity with increasing fasting glucose levels in the nondiabetic range in children. Diabetes Care 2010;33:2024–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tfayli H, Lee SJ, Bacha F, Arslanian S. One-hour plasma glucose concentration during the OGTT: what does it tell about β-cell function relative to insulin sensitivity in overweight/obese children? Pediatr Diabetes 2011;12:572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns SF, Bacha F, Lee SJ, Tfayli H, Gungor N, Arslanian SA. Declining β-cell function relative to insulin sensitivity with escalating OGTT 2-h glucose concentrations in the nondiabetic through the diabetic range in overweight youth. Diabetes Care 2011;34:2033–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JY, Coletta DK, Mandarino LJ, Shaibi GQ. Glucose response curve and type 2 diabetes risk in Latino adolescents. Diabetes Care 2012;35:1925–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nolfe G, Spreghini MR, Sforza RW, Morino G, Manco M. Beyond the morphology of the glucose curve following an oral glucose tolerance test in obese youth. Eur J Endocrinol 2012;166:107–114 [DOI] [PubMed] [Google Scholar]
- 16.Bervoets L, Mewis A, Massa G. The shape of the plasma glucose curve during an oral glucose tolerance test as an indicator of Beta cell function and insulin sensitivity in end-pubertal obese girls. Horm Metab Res 2015;47:445–451 [DOI] [PubMed] [Google Scholar]
- 17.George L, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. J Clin Endocrinol Metab 2011;96:2136–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sjaarda LG, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Oral disposition index in obese youth from normal to prediabetes to diabetes: relationship to clamp disposition index. J Pediatr 2012;161:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sjaarda LA, Michaliszyn SF, Lee S, et al. HbA1c diagnostic categories and β-cell function relative to insulin sensitivity in overweight/obese adolescents. Diabetes Care 2012;35:2559–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sjaarda L, Lee S, Tfayli H, Bacha F, Bertolet M, Arslanian S. Measuring β-cell function relative to insulin sensitivity in youth: does the hyperglycemic clamp suffice? Diabetes Care 2013;36:1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanner JM. Growth and maturation during adolescence. Nutr Rev 1981;39:43–55 [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Kuk JL, Kim Y, Arslanian SA. Measurement site of visceral adipose tissue and prediction of metabolic syndrome in youth. Pediatr Diabetes 2011;12:250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klopfenstein BJ, Kim MS, Krisky CM, Szumowski J, Rooney WD, Purnell JQ. Comparison of 3 T MRI and CT for the measurement of visceral and subcutaneous adipose tissue in humans. Br J Radiol 2012;85:e826–e830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tfayli H, Bacha F, Gungor N, Arslanian S. Islet cell antibody-positive versus -negative phenotypic type 2 diabetes in youth: does the oral glucose tolerance test distinguish between the two? Diabetes Care 2010;33:632–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab 2001;86:66–71 [DOI] [PubMed] [Google Scholar]
- 26.Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care 2009;32:100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tfayli H, Ulnach JW, Lee S, Sutton-Tyrrell K, Arslanian S. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab 2011;96:1311–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, DeFronzo RA. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 2002;283:E1135–E1143 [DOI] [PubMed] [Google Scholar]
- 29.Kanauchi M, Kimura K, Kanauchi K, Saito Y. Beta-cell function and insulin sensitivity contribute to the shape of plasma glucose curve during an oral glucose tolerance test in non-diabetic individuals. Int J Clin Pract 2005;59:427–432 [DOI] [PubMed] [Google Scholar]
- 30.Trujillo-Arriaga HM, Román-Ramos R. Fitting and evaluating the glucose curve during a quasi continuous sampled oral glucose tolerance test. Comput Biol Med 2008;38:185–195 [DOI] [PubMed] [Google Scholar]
- 31.Tura A, Morbiducci U, Sbrignadello S, Winhofer Y, Pacini G, Kautzky-Willer A. Shape of glucose, insulin, C-peptide curves during a 3-h oral glucose tolerance test: any relationship with the degree of glucose tolerance? Am J Physiol Regul Integr Comp Physiol 2011;300:R941–R948 [DOI] [PubMed] [Google Scholar]
- 32.Utzschneider KM, Prigeon RL, Faulenbach MV, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009;32:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Periwal V, Chow CC, Bergman RN, Ricks M, Vega GL, Sumner AE. Evaluation of quantitative models of the effect of insulin on lipolysis and glucose disposal. Am J Physiol Regul Integr Comp Physiol 2008;295:R1089–R1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell function. Diabetes Care 2004;27:547–552 [DOI] [PubMed] [Google Scholar]
- 35.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001;7:941–946 [DOI] [PubMed] [Google Scholar]
- 36.Michaliszyn SF, Bonadonna RC, Sjaarda LA, Lee S, Farchoukh L, Arslanian SA. β-Cell lipotoxicity in response to free fatty acid elevation in prepubertal youth: African American versus Caucasian contrast. Diabetes 2013;62:2917–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hughan KS, Bonadonna RC, Lee S, Michaliszyn SF, Arslanian SA. β-Cell lipotoxicity after an overnight intravenous lipid challenge and free fatty acid elevation in African American versus American white overweight/obese adolescents. J Clin Endocrinol Metab 2013;98:2062–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 2012;122:4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab 2008;93:4231–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kramer CK, Vuksan V, Choi H, Zinman B, Retnakaran R. Emerging parameters of the insulin and glucose response on the oral glucose tolerance test: reproducibility and implications for glucose homeostasis in individuals with and without diabetes. Diabetes Res Clin Pract 2014;105:88–95 [DOI] [PubMed] [Google Scholar]