Introduction
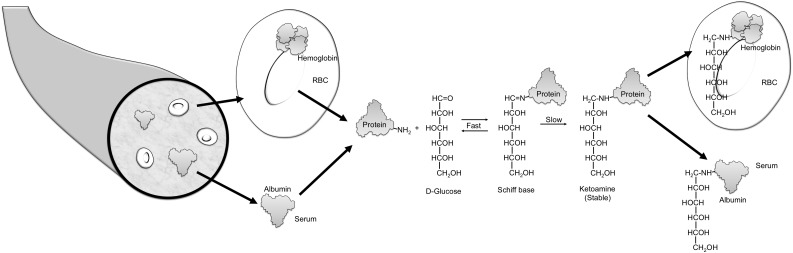
Blood oligosaccharides are attached to many proteins after translation, forming glycoproteins. Glycosylation refers to an enzyme-mediated modification that alters protein function, for example, their life span or their interactions with other proteins (1). By contrast, glycation refers to a monosaccharide (usually glucose) attaching nonenzymatically to the amino group of a protein. Glycated hemoglobin is formed by the condensation of glucose with select amino acid residues, commonly lysine, in hemoglobin to form an unstable Schiff base (aldimine, pre-HbA1c) (Fig. 1). The Schiff base may dissociate or may undergo an Amadori rearrangement to form a stable ketoamine.
Figure 1.
Formation of glycated protein. A reversible interaction between a primary amino group (depicted as NH2) of a protein and the carbonyl group of d-glucose yields a labile intermediate, called a Schiff base. This can undergo a slow and spontaneous Amadori rearrangement to form a stable ketoamine. HbA1c is formed if glucose attaches to the N-terminal valine of the β-chain of hemoglobin. If the glucose attaches to proteins in the plasma, fructosamine or glycated albumin results. RBC, red blood cell.
Glycated hemoglobin, particularly HbA1c, has for decades been widely incorporated into the management (and, more recently, the diagnosis) of patients with diabetes. An important attribute is that glycation occurs continuously over the lifetime of the protein, so the concentration of the glycated protein reflects the average blood glucose value over a period of time. This contrasts with the measurement of blood glucose, which reveals the glucose concentration at the instant blood is sampled and which is acutely altered by multiple factors such as hormones, illness, food ingestion, and exercise (2). While HbA1c is by far the most extensively used—and studied—glycated protein (2–4), other glycated proteins that have been evaluated in clinical studies include fructosamine, glycated albumin, and advanced glycation end products (AGEs).
Hemoglobin A1c
HbA1c is glycated hemoglobin in which glucose is attached to the N-terminal valine residue of each β-chain of hemoglobin A (HbA). Glucose can also be attached at other amino acids, predominantly lysine, in either the α- or β-chain of hemoglobin (5). However, modern methods that measure HbA1c do not report these other glycated hemoglobin species. The extent of hemoglobin glycation is influenced by the concentration of glucose in the blood. Since the life span of erythrocytes is ∼120 days, HbA1c reflects the average glucose concentration over the preceding 8–12 weeks (3).
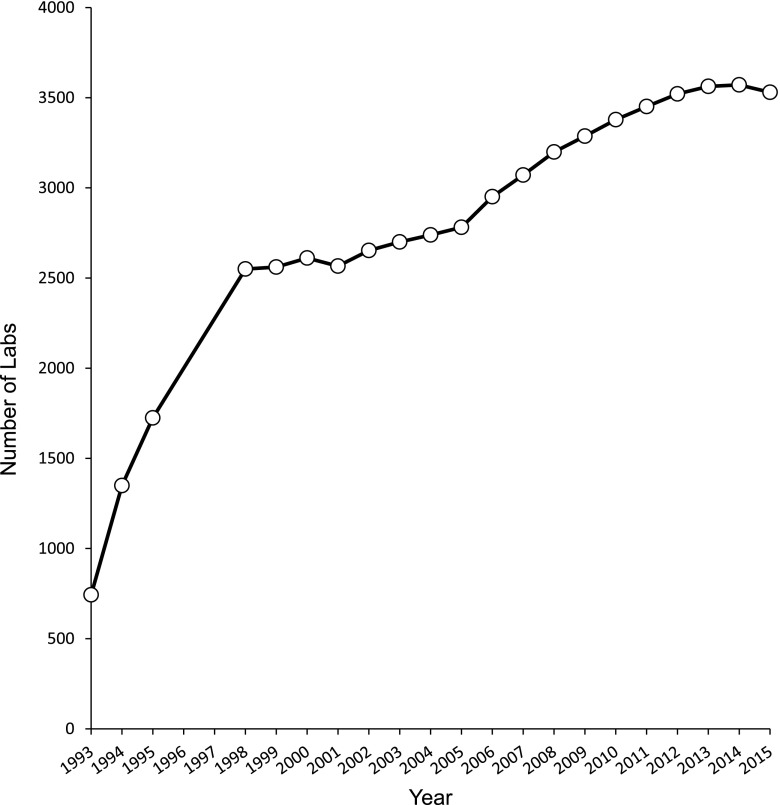
HbA1c has been recommended by the American Diabetes Association since 1988 for routine monitoring of patients with diabetes (6). Although the association of chronic hyperglycemia with the risk of chronic complications of diabetes was suspected for many years, landmark trials such as the Diabetes Control and Complications Trial (DCCT) in type 1 diabetes (7) and the UK Prospective Diabetes Study (UKPDS) in type 2 diabetes (8) and their follow-up studies (9,10) confirmed that lowering mean glucose, as measured by HbA1c, significantly reduced the onset and progression of complications. This led to the development of treatment goals for HbA1c and the use of HbA1c as a performance measure. The increasing use of HbA1c in patient management is evident from the increase in the number of clinical laboratories that are enrolled in proficiency testing surveys conducted by the College of American Pathologists (Fig. 2). Note the large (more than threefold) increase in participants during the 4 years after the publication of the DCCT results in 1993.
Figure 2.
Progressive increase in HbA1c testing over time. The number of clinical laboratories enrolled in proficiency testing surveys from the College of American Pathologists from 1993 to 2015 is depicted. Data used with permission from the College of American Pathologists.
HbA1c was recently included as a diagnostic criterion for diabetes by the American Diabetes Association (11), European Association for the Study of Diabetes, International Diabetes Federation, and World Health Organization (12). This recommendation was motivated by improvements in the measurement of HbA1c and by the certain advantages of its measurement over that of glucose, such as the convenience of not requiring the patient to fast and the reduced intraindividual variability compared with fasting or glucose measurements after loading (11).
HbA1c can be measured by immunoassays, high-performance liquid chromatography (HPLC) (the two most commonly used methods in the U.S. and many other developed countries), affinity chromatography, capillary electrophoresis, and enzymatic assays (13). Standardization of methods by the NGSP (formerly called the National Glycohemoglobin Standardization Program) (14,15) and the International Federation of Clinical Chemistry and Laboratory Medicine (16) has yielded highly consistent HbA1c results for a blood sample, regardless of the method used (provided the method is certified by NGSP).
Interference
There are numerous published reports of conditions that change HbA1c independent of glucose (reviewed in refs. 17 and 18). Based on the nature of the interference, these can be conveniently divided into two groups: conditions that influence interpretation (i.e., change HbA1c concentration in ways unrelated to changes in glucose) and conditions that interfere with HbA1c measurement (i.e., analytic interferences) (Table 1).
Table 1.
Nonglycemic factors that may influence HbA1c
| Factors that may influence interpretation of HbA1c |
|---|
| 1. Physiological (e.g., age, race) |
| 2. Chronic renal failure |
| 3. Iron-deficiency anemia |
| 4. Erythrocyte life span |
| 5. Glycation “phenotypes” |
| 6. Drugs (e.g., dapsone, antiretroviral) |
| 7. Other (e.g., vitamin C, vitamin E) |
| Factors that may interfere with HbA1c measurement |
| 1. Uremia |
| 2. Hemoglobin variants |
| 3. Drugs (e.g., opiates) |
| 4. Other (e.g., bilirubin, triglyceride, alcohol) |
Factors That Influence HbA1c Interpretation
Physiological Factors.
HbA1c concentrations increase by ∼0.1% per decade after 30 years of age (19). It is not known whether this gradual increase reflects an effect of age on the relationship of mean glycemia to HbA1c or merely the higher prevalence of prediabetes and diabetes with aging (a true increase in mean glycemia). There is contention surrounding the influence of race on HbA1c concentrations. Herman (20) posits that African Americans have higher HbA1c for any given level of mean glycemia, whereas Selvin (21) argues that the increased mean HbA1c is a reflection of truly higher mean glycemia in African Americans.
Chronic Renal Failure.
Chronic renal failure (CRF) is a common complication of diabetes, and diabetes is the leading cause of end-stage renal disease (22). Red blood cell survival is reduced in CRF, decreasing HbA1c. In addition, many patients with CRF are treated with erythropoietin to stimulate erythropoiesis. The subsequent increase in the number of young erythrocytes further reduces the HbA1c. Therefore the HbA1c concentration in patients with diabetes and with CRF may not accurately indicate glycemic control.
Iron-Deficiency Anemia.
Iron deficiency and iron-deficiency anemia occur frequently. Some studies, generally with small sample sizes, have reported increased HbA1c in individuals with iron deficiency. Two recent systematic reviews reached opposite conclusions regarding the effects of iron deficiency on HbA1c. The first, a meta-analysis and systematic review, concluded that there was no statistically significant difference in HbA1c measured by HPLC in the presence of iron deficiency or iron-deficiency anemia (23). By contrast, another assessment determined that iron deficiency, with or without anemia, increased HbA1c (24). This discrepancy is likely due to the differences in the studies selected and the method of analysis. Several studies included in both meta-analyses of HbA1c in iron deficiency were limited by their small sample sizes and the heterogeneity of the methods. Two large investigations of the National Health and Nutrition Examination Survey (NHANES) data have been conducted. Kim et al. (25) evaluated 6,666 female NHANES participants without diabetes from 1999 to 2006 and concluded that iron deficiency was associated with an increase in HbA1c from <5.5% to 5.5–6.0%; however, this association was not apparent at higher HbA1c concentrations. A second investigation of NHANES data from 1999 to 2002 included 8,296 patients with and without diabetes and found an adjusted increase in HbA1c from 5.46 to 5.56% in the presence of iron deficiency (26). Thus, while HbA1c seems to increase slightly with iron deficiency, the clinical significance of this finding remains to be determined. We agree with Ford et al. (26) that caution should be exercised in diagnosing prediabetes and diabetes when HbA1c is near the decision threshold in patients with iron deficiency.
Erythrocyte Life Span.
A change in erythrocyte survival alters HbA1c. For example, assume HbA1c is 7.0% (53 mmol/mol), with a normal erythrocyte life span of 120 days. If the red blood cell life span is 10 days shorter or longer, the corresponding HbA1c values would be 6.4% (46 mmol/mol) and 7.6% (60 mmol/mol), respectively. HbA1c does not accurately reflect average blood glucose concentration if erythrocyte survival is significantly altered, as in, for example, hemolytic anemia or severe β-thalassemia. Since measurement of red blood cell life span is extremely difficult, one cannot easily solve this problem by, for example, applying a correction factor for erythrocyte age.
Variable Glycation.
Intraindividual variability of HbA1c is very low. Nevertheless, interindividual variation occurs and has been ascribed by some to differences in glycation rates (27,28). This postulate is contentious (29,30) because the data validating significantly different rates of glycation are minimal and no mechanism for differences in this nonenzymatic process has been documented. Moreover, a recent analysis, although indirect, reveals that even the rate of glycation of hemoglobin variants S, C, D, E, J, and G is not significantly different from that of HbA (31), undermining the premise of variable rates of glycation of HbA. There has been speculation that the rate of deglycation (i.e., the removal of glucose from HbA1c) might vary among individuals, resulting in different HbA1c concentrations despite similar average glycemia. Although at least three groups of deglycating enzymes have been identified, only one, fructosamine 3-kinase, is found in humans. Importantly, fructosamine 3-kinase has no effect on valine-1 of the β-chain of hemoglobin (32), the residue where glucose is attached in HbA1c, and it cannot deglycate HbA1c. Thus the concept of variable glycation remains to be validated.
Factors That Interfere With Measurement
Numerous publications have described interferences in HbA1c measurement, but many reports had small numbers of subjects and described changes that were small and unlikely to have clinical significance (33–35). Furthermore, improvements in analytic methods have eliminated interferences from some factors (e.g., aspirin, bilirubin, and triglycerides) that affected older methods. While the possible interference of all substances in each modern method has not been rigorously investigated, it is likely that few drugs or other factors interfere significantly in current HbA1c assays.
Uremia.
Isocyanic acid, derived from urea, is covalently attached to proteins. The nonenzymatic process, termed carbamylation, increases when blood urea concentrations are high, yielding increased carbamylation of circulating proteins, including on lysine or arginine residues of the N-terminus of hemoglobin. Carbamylated hemoglobin altered HbA1c values in some early methods (36), but uremia has no significant effect on HbA1c analysis with most contemporary methods (23,37,38).
Hemoglobin Variants.
Over 1,200 hemoglobin variants have been identified; the β gene is involved in ∼70% of these (39). While the vast majority are uncommon or rare, certain hemoglobin variants, particularly HbAS, HbAC, HbAD, and HbAE, occur at relatively high frequencies in some populations. One cannot measure HbA1c in individuals who are homozygous for these common variants or who have HbSC disease (36) because they have no HbA. While total glycated hemoglobin can be determined using borate affinity methods in patients with these homozygous hemoglobin variants, there is no convincing clinical evidence that these values can reliably be used to monitor glycemia and predict complications, particularly since some patients may have reduced erythrocyte life span because of hemolytic anemia. Most interferences are method-specific (36). Manufacturers of HbA1c methods have considerably reduced analytic interference from variant hemoglobin. Therefore HbA1c can be measured accurately in the presence of the overwhelming majority of variant hemoglobins, provided a suitable assay is used (40). Since common heterozygous variants rarely alter erythrocyte life span, accurate and reliable HbA1c values can be obtained in heterozygous individuals.
Glycated Serum Proteins
Glucose attaches nonenzymatically to amino groups of proteins other than hemoglobin to form ketoamines (Fig. 1). Measures of several glycated serum proteins, including fructosamine and glycated albumin, have been proposed as markers of glycemia that might complement or replace HbA1c in select patient populations. Serum proteins turn over more rapidly than erythrocytes; for example, albumin (the protein found in the highest concentration in serum) has a circulating half-life of about 14–20 days. Therefore the concentration of fructosamine or glycated serum albumin reflects mean glucose over a period of 2–3 weeks. Additionally, glycated serum proteins are not influenced by changes in erythrocyte life span or hemoglobin variants such as homozygous HbS. Glycated serum proteins have therefore been proposed as measures of more rapid changes in glycemia and to monitor glycemic control in patients with conditions that alter the normal relationship of HbA1c to mean glucose (e.g., hemolysis, blood transfusion).
Fructosamine
Fructosamine is the common name for 1-amino-1-deoxy fructose and the generic name for plasma protein ketoamines (41,42). All glycated serum proteins are fructosamines, and since albumin is the most abundant serum protein, measurement of fructosamine is thought to largely reflect the concentration of glycated albumin, though this has been questioned (43). The fructosamine assay is readily automated and is less expensive than measurement of HbA1c. There is disagreement as to whether fructosamine results are independent of serum protein concentrations (absent significant alterations in the latter) or whether fructosamine values need to be corrected for the concentration of serum proteins (44). Most agree, however, that fructosamine is not valid when serum albumin is <30 g/L.
The first commercial method to measure fructosamine suffered from several problems, particularly a lack of specificity and interference by other reducing substances in the serum, such as urates (43,45). Thus many early studies of fructosamine generated confusion regarding its clinical value, with reviews (covering many of the same studies) leading to conflicting conclusions as to whether fructosamine is a reliable test for routine clinical use (41,46). The assay was extensively modified in 1991, which markedly improved the specificity of fructosamine (47). Strong correlations with HbA1c, prognostic value for the development of diabetes and microvascular complications, and good precision have been demonstrated for fructosamine using modern assays on automated platforms (48,49).
There is interest in the role of fructosamine in special populations for whom HbA1c may not provide an accurate assessment of glycemic status. One such potential use of fructosamine is the diagnosis of gestational diabetes mellitus (GDM). Hyperglycemia develops relatively quickly with the onset of GDM, and red cell turnover may be altered in pregnancy, precluding the use of HbA1c to diagnose this form of diabetes. Studies evaluating this use of fructosamine (50) were generally small and used various fructosamine thresholds and diagnostic criteria for GDM. Measurement of fructosamine is not currently recommended to screen for GDM (50).
Other conditions for which fructosamine has shown a potential role in monitoring glycemic status include end-stage renal disease, certain types of anemia, and transfusion (49). Combining HbA1c with fructosamine has been used as a screening strategy to identify patients with prediabetes; however, the combination was not statistically significantly better than the use of HbA1c alone (51). A major limitation of the fructosamine assay is the lack of an evidence base linking the test to long-term complications of diabetes. Hence, unlike HbA1c, there are no generally accepted treatment targets for fructosamine.
Glycated Albumin
Albumin comprises almost two-thirds of total serum protein and accounts for over 80% of total glycated serum proteins (52). HPLC tandem mass spectrometry of human plasma using [13C6]glucose labeling has identified 35 glycation sites on albumin (53). Analogous to HbA1c, which is most commonly reported as a percentage of total hemoglobin, glycated albumin is usually expressed as a percentage of total albumin in the blood. A number of glycated albumin assays are commercially available, but these lack standardization and values vary widely among methods (54). Specifically, the reference intervals have considerable variation depending on the method and range from 0.8–1.4% to 18–22% (52,54). A U.S. Food and Drug Administration–approved method for glycated albumin measurement manufactured by Diazyme Laboratories (Poway, CA) is commercially available (55). A glycated albumin assay developed by Asahi Kasei in Japan (56) is the method most widely used globally and most extensively evaluated in clinical studies.
Values of glycated albumin in blacks are significantly higher than in whites, for reasons that are unclear (54). Factors that influence albumin metabolism may alter glycated albumin independent of glycemia. These factors include the nephrotic syndrome, cirrhosis, thyroid disease, hyperuricemia, hypertriglyceridemia, and smoking (57). As with fructosamine, glycated albumin concentrations can be affected by altered protein levels that occur with liver, thyroid, and renal disease (58). The clinical use of glycated albumin is limited by the same caveats that apply to fructosamine—namely, a paucity of evidence relating it to clinical outcomes, specifically the chronic complications of diabetes. As is the case with fructosamine, further studies are required to determine its clinical utility in the management of diabetes (48,59).
A recent investigation by Sumner et al. (51) identified a potential role for glycated albumin in the diagnosis of prediabetes in African immigrants to the U.S. Using the oral glucose tolerance test as the gold standard, the combination of HbA1c with glycated albumin detected 78% of African immigrants with prediabetes, compared with only 50% detected with HbA1c alone and 72% with HbA1c paired with fructosamine (51). An investigation of 302 adults in Japan found that HbA1c or glycated albumin could diagnose patients at risk for developing diabetes; fructosamine was considered unsuitable as a screening test (60). Glycated albumin is used extensively as a screening test for diabetes among blood donors in Japan, identifying patients who are at risk for the disease (56).
Intriguing data are emerging suggesting that glycated albumin may be a better test than HbA1c for diabetes screening in nonobese patients. Koga et al. (61) found a negative correlation between glycated albumin and BMI in Japanese individuals; this finding has been replicated in other Asian populations (62,63). Similarly, in the study by Sumner et al. (51), the African immigrants whose prediabetes was identified by glycated albumin, but not HbA1c, were more likely to have a lower BMI. The converse implication of these data is the potential for glycated albumin to underestimate glycemic status in the obese.
AGEs
Glycation of tissue proteins may contribute to the link between hyperglycemia and the chronic complications of diabetes. Nonenzymatic attachment of glucose to proteins, lipids, or nucleic acids produces stable Amadori products, which can undergo further modifications to form AGEs (64,65). Irreversible rearrangements of Amadori products occur via both oxidative and nonoxidative pathways, or via condensation of the side chains of lysine, arginine, or cysteine, forming reactive dicarbonyl compounds such as glyoxal, methylglyoxal, and deoxyglucosones that ultimately form irreversible AGEs by forming cross-links between many proteins, altering their structure and function (65). For example, glyoxal can form N-(carboxymethyl)lysine (CML), glyoxal-derived lysyl dimer, or N-(carboxymethyl)arginine, whereas methylglyoxal may induce the formation of methylglyoxal-derived lysyl dimer, argpyrimidine, N-(carboxyethyl)lysine (CEL), and others (65). The most common cross-linked AGE is glucosepane, formed by a mechanism of action that has not yet been fully elucidated (65). More than 20 AGEs have been identified (66,67). These products do not return to normal, even when hyperglycemia is corrected, so they accumulate continuously over the life span of the protein; AGEs also accumulate as an individual ages. Hyperglycemia accelerates the formation of protein-bound AGEs, and patients with diabetes have more AGEs than age-matched subjects without diabetes. There is evidence that AGEs in the diet contribute to AGE accumulation in tissues (68).
Through their heterogeneous effects on the functions of proteins and extracellular matrix, AGEs may contribute to the chronic microvascular and cardiovascular complications of diabetes (69,70). Plasma concentrations of CEL, CML, and pentosidine were correlated with incident, but not prior, cardiovascular outcomes in patients with type 2 diabetes (71,72). AGEs have also been linked to other diabetic complications including nephropathy, retinopathy, and neuropathy (73–78). There is significant heterogeneity among these studies in the specific AGEs evaluated and the method of AGE measurement. Of potential interest, certain publications reported no correlation between serum AGE concentrations and HbA1c (71–73). Levels of AGEs in the skin biopsies of patients from the DCCT were found to be a better predictor of retinopathy and nephropathy progression than HbA1c (74,77). Collectively, these results raise the possibility that AGEs may provide additional independent information to predict microvascular diabetic complications. Thus AGE burden may explain why only a subset of patients with poor glycemic control develop complications and why some patients with good glycemic control also develop certain diabetic complications.
Several methods have been proposed to measure AGEs. Some AGE products fluoresce, which has led to the development of noninvasive measurement of skin autofluorescence to estimate the burden of AGEs in tissues. A meta-analysis of seven studies showed that skin autofluorescence was positively associated with mortality, neuropathy, nephropathy, and cardiovascular events (79). Certain studies found that skin autofluorescence predicted microvascular and macrovascular complications of diabetes independent of HbA1c (80,81), whereas others found that adjustment for HbA1c rendered these associations nonsignificant (82). These discrepant findings are possibly accounted for by differences in the patient population and statistical methods. The utility of skin autofluorescence measurements is limited by several factors. First, most AGEs are not fluorescent, specifically CML and CEL, which have been shown to be important in predicting cardiovascular outcomes (67). Second, skin fluorescence is not specific; numerous skin proteins fluoresce with spectra that overlap the spectra of AGEs (83). Furthermore, skin autofluorescence does not correlate directly with AGE burden.
There is considerable interest in the measurement of AGEs in the circulation as a biomarker to monitor the risk of diabetes complications, given the numerous studies correlating AGEs with various diabetic complications (66). Assays to determine total AGE fluorescence have been used in selected studies, but these methods have limitations similar to those of skin autofluorescence, namely, the most important AGEs are not fluorescent and many other serum proteins interfere. Methods for measuring specific AGEs have been developed, many of which use immunoassays. However, heterogeneity of the structures (ranging from single molecules to complex cross-linked compounds) and composition of AGEs have resulted in assay variability. Questions have been raised regarding antibody specificity (AGEs such as CML and CEL share certain common epitopes), the use of excess blocking proteins that have oxidized and glycated fragments, and the high temperature and pH of the assay (67). Furthermore, the lack of immunoassay standardization has yielded variable results (84).
Isotope dilution analysis liquid chromatography–tandem mass spectrometry (LC-MSMS), with careful preanalytic sample preparation, is a promising technique for circumventing the problems of immunoassays and fluorescence-based methods (67). Analytes are first separated by HPLC from related compounds that have not been oxidized or glycated; then they are detected based on a specific chromatographic retention time, molecular ion mass-to-charge ratio, and fragment ion mass-to-charge ratio, rendering this technique highly specific for the desired analyte. LC-MSMS quantitatively analyzes the modification of proteins by glycation, nitration, and oxidation, as well as free adducts, using a small sample volume. This technique has been applied to a number of clinically important AGEs including pentosidine, CML, CEL, 3-deoxyglucosone, and methylglyoxal hydroimidazolones, and it has aided in the discovery of new candidate AGE products (67,85,86). A limitation of LC-MSMS is the need for specialized (and expensive) equipment and highly trained personnel. Furthermore, isotope-labeled standards are not commercially available for the full range of analytes (67,86), preventing assay standardization.
Certain AGEs activate the receptor for AGE (RAGE), inducing intracellular signaling that results in the production of proinflammatory cytokines and increased oxidative stress (66,69). RAGE is expressed on the surface of several cells, including endothelial and renal cells, raising intriguing hypotheses about the role of RAGE in the pathophysiology of specific diabetes complications. Nevertheless, some studies cast doubt on whether AGE-modified proteins activate RAGE (87). Proteolysis of RAGE leads to a truncated soluble form of RAGE (sRAGE) (66), which is found in serum and can be measured by a commercially available ELISA. There is evidence of clinical value of sRAGE. In a case-cohort study of 3,763 patients with type 2 diabetes, both AGE and sRAGE plasma values predicted decreasing renal function and all-cause mortality, but hazard ratios were only 1.1 to 1.2 (88). There is, however, controversy over the associations between sRAGE concentrations and diabetes complications; some studies show a positive association (89) and others an inverse one (90). The associations between sRAGE and health outcomes remain unresolved. Differences in studied populations and genetic mechanisms have been suggested as a cause of the discrepancies (91,92).
Inhibitors of AGE formation, such as aminoguanidine, prevented signs of microvascular complications of diabetes in animal models, although initial clinical trials in humans failed to show a significant benefit (66). Nonetheless, anti-AGE therapy remains an area of active research. Of interest, patients with type 2 diabetes taking metformin had lower AGE levels than those not receiving metformin (93). Promising studies of the use of recombinant sRAGE in animals suggest the potential of future therapies in humans to reduce the risk of diabetes complications. The recent total synthesis of the lysyl-arginine cross-link glucosepane (94), the main in vivo cross-link in AGEs (95), is likely to permit the generation of relevant reagents (e.g., specific antibodies) to enhance our comprehension of the role of AGEs in disease.
Future Directions
The development and standardization of HbA1c measurement have revolutionized research and clinical care in the field of diabetes (2–4). The role of HbA1c in diabetes has been extensively studied in large, prospective trials with long-term follow-up (9,10), which has extensively validated the value of HbA1c in predicting many diabetic complications. Additionally, diagnostic thresholds for using HbA1c to diagnose prediabetes and diabetes have been established (11,96). Yet despite the documented utility of HbA1c in diabetes research and care, controversies remain. As argued from opposing perspectives by Herman (20) and Selvin (21) in this issue, whether there are clinically significant differences in the relationship between HbA1c and average glucose in different racial groups remains contested, and similar questions exist about age groups. If there are differences in what HbA1c “means” in different groups, what are the implications for the diagnosis and management of diabetes? Considerable progress has been made in reducing interference from hemoglobin variants and other factors in HbA1c assays and in achieving high levels of standardization of the assay in developed countries. We need to continue to overcome the barriers to worldwide standardization of HbA1c assays, particularly in developing countries.
Since the discovery of HbA1c, other potentially useful additional or adjunct measures of protein glycation, glycated serum proteins, and AGEs have emerged. It is unlikely, however, that the newer measures of glycated proteins will be studied as markers of diabetic complications in the same thorough manner as HbA1c because of limited funding for long-term clinical trials with large numbers of patients. We need to develop innovative strategies to establish the evidence base for the link between other glycated proteins and clinical outcomes, so that treatment targets or diagnostic thresholds can be developed. Furthermore, glycated albumin and AGE assays need to undergo standardization, as has been done for HbA1c, to enable comparison among studies and decrease imprecision (15).
AGEs have the potential to identify—independent of HbA1c—a subset of patients who develop cardiovascular and microvascular complications of diabetes. It is important to determine whether AGEs are a cause or consequence of the pathophysiology of diabetes. Because the term comprises a large group of diverse compounds, future studies of AGEs will require detailed knowledge of the specific compound(s) being studied. Although AGEs may go beyond simple biomarkers into pathophysiology, substantial research needs to be done to use AGE-related measures to improve the prediction of risk for diabetes complications or to ultimately develop risk-reduction therapies based on these pathways.
Acknowledgments.
The authors thank Dr. Andrew Hedman (National Institutes of Health) for expertly preparing figures.
Funding. Work in the laboratory of D.B.S. is supported by the National Institutes of Health Clinical Center Intramural Program.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
References
- 1.Dalziel M, Crispin M, Scanlan CN, Zitzmann N, Dwek RA. Emerging principles for the therapeutic exploitation of glycosylation. Science 2014;343:1235681. [DOI] [PubMed] [Google Scholar]
- 2.Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care 2011;34:518–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care 2004;27:1761–1773 [DOI] [PubMed] [Google Scholar]
- 4.Saudek CD, Derr RL, Kalyani RR. Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA 2006;295:1688–1697 [DOI] [PubMed] [Google Scholar]
- 5.Shapiro R, McManus MJ, Zalut C, Bunn HF. Sites of nonenzymatic glycosylation of human hemoglobin A. J Biol Chem 1980;255:3120–3127 [PubMed] [Google Scholar]
- 6.Standards of medical care for patients with diabetes mellitus. Diabetes Care 1989;12:365–368 [DOI] [PubMed] [Google Scholar]
- 7.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 8.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 9.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 10.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mbanya JC, Henry RR, Smith U. Presidents’ statement on WHO recommendation on HbA1c for diabetes diagnosis. Diabetes Res Clin Pract 2011;93:310–311 [DOI] [PubMed] [Google Scholar]
- 13.Sacks DB. Diabetes mellitus. In Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 6th ed. Rifai N, Horvath AR, Wittwer CT, Eds. St. Louis, Elsevier Saunders, in press [Google Scholar]
- 14.Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE; NGSP Steering Committee . The National Glycohemoglobin Standardization Program: a five-year progress report. Clin Chem 2001;47:1985–1992 [PubMed] [Google Scholar]
- 15.Little RR, Rohlfing CL, Sacks DB; National Glycohemoglobin Standardization Program (NGSP) Steering Committee . Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem 2011;57:205–214 [DOI] [PubMed] [Google Scholar]
- 16.Weykamp C, John WG, Mosca A, et al. The IFCC Reference Measurement System for HbA1c: a 6-year progress report. Clin Chem 2008;54:240–248 [DOI] [PubMed] [Google Scholar]
- 17.Dagogo-Jack S. Pitfalls in the use of HbA₁(c) as a diagnostic test: the ethnic conundrum. Nat Rev Endocrinol 2010;6:589–593 [DOI] [PubMed] [Google Scholar]
- 18.Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes 2009;1:9–17 [DOI] [PubMed] [Google Scholar]
- 19.Pani LN, Korenda L, Meigs JB, et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001-2004. Diabetes Care 2008;31:1991–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herman WH. Are there clinical implications of racial differences in HbA1c? Yes, to not consider can do great harm! Diabetes Care 2016;39:1458–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selvin E. Are there clinical implications of racial differences in HbA1c? A difference, to be a difference, must make a difference. Diabetes Care 2016;39:1462–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 2014;37:2864–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavagnolli G, Pimentel AL, Freitas PA, Gross JL, Camargo JL. Factors affecting A1C in non-diabetic individuals: Review and meta-analysis. Clin Chim Acta 2015;445:107–114 [DOI] [PubMed] [Google Scholar]
- 24.English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, John WG. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia 2015;58:1409–1421 [DOI] [PubMed] [Google Scholar]
- 25.Kim C, Bullard KM, Herman WH, Beckles GL. Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999–2006. Diabetes Care 2010;33:780–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ford ES, Cowie CC, Li C, Handelsman Y, Bloomgarden ZT. Iron-deficiency anemia, non-iron-deficiency anemia and HbA1c among adults in the US. J Diabetes 2011;3:67–73 [DOI] [PubMed] [Google Scholar]
- 27.Cohen RM, Smith EP. Frequency of HbA1c discordance in estimating blood glucose control. Curr Opin Clin Nutr Metab Care 2008;11:512–517 [DOI] [PubMed] [Google Scholar]
- 28.McCarter RJ, Hempe JM, Gomez R, Chalew SA. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care 2004;27:1259–1264 [DOI] [PubMed] [Google Scholar]
- 29.Sacks DB, Nathan DM, Lachin JM. Gaps in the glycation gap hypothesis. Clin Chem 2011;57:150–152 [DOI] [PubMed] [Google Scholar]
- 30.Lachin JM, Genuth S, Nathan DM, Rutledge BN. The hemoglobin glycation index is not an independent predictor of the risk of microvascular complications in the Diabetes Control and Complications Trial. Diabetes 2007;56:1913–1921 [DOI] [PubMed] [Google Scholar]
- 31.Weykamp C, Kemna E, Leppink S, Siebelder C. Glycation rate of haemoglobins S, C, D, E, J and G, and analytical interference on the measurement of HbA1c with affinity chromatography and capillary electrophoresis. Clin Chem Lab Med 2015;53:e207–e210 [DOI] [PubMed] [Google Scholar]
- 32.Delpierrre G, Vertommen D, Communi D, Rider MH, Van Schaftingen E. Identification of fructosamine residues deglycated by fructosamine-3-kinase in human hemoglobin. J Biol Chem 2004;279:27613–27620 [DOI] [PubMed] [Google Scholar]
- 33.Camargo JL, Stifft J, Gross JL. The effect of aspirin and vitamins C and E on HbA1c assays. Clin Chim Acta 2006;372:206–209 [DOI] [PubMed] [Google Scholar]
- 34.Schnedl WJ, Lahousen T, Krause R, Wallner SJ, Piswanger-Soelkner C, Lipp RW. Evaluation of conditions associated with glycated hemoglobin values below the reference range. Clin Lab 2007;53:179–181 [PubMed] [Google Scholar]
- 35.Serratrice J, Granel B, Swiader L, et al. Interference of dapsone in HbA1c monitoring of a diabetic patient with polychondritis. Diabetes Metab 2002;28:508–509 [PubMed] [Google Scholar]
- 36.Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem 2001;47:153–163 [PubMed] [Google Scholar]
- 37.Little RR, Rohlfing CL, Tennill AL, et al. Measurement of Hba(1C) in patients with chronic renal failure. Clin Chim Acta 2013;418:73–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Z, Basilio J, Hanson S, Little RR, Sumner AE, Sacks DB. Evaluation of hemoglobin A1c measurement by Capillarys 2 electrophoresis for detection of abnormal glucose tolerance in African immigrants to the United States. Clin Chim Acta 2015;446:54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patrinos GP, Giardine B, Riemer C, et al. Improvements in the HbVar database of human hemoglobin variants and thalassemia mutations for population and sequence variation studies. Nucleic Acids Res 2004;32:D537–D541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.NGSP. HbA1c assay interferences [Internet], 2016. Available from http://www.ngsp.org/interf.asp. Accessed 20 April 2016
- 41.Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem 1987;33:2153–2163 [PubMed] [Google Scholar]
- 42.Hill RP, Hindle EJ, Howey JE, Lemon M, Lloyd DR. Recommendations for adopting standard conditions and analytical procedures in the measurement of serum fructosamine concentration. Ann Clin Biochem 1990;27:413–424 [DOI] [PubMed] [Google Scholar]
- 43.Schleicher ED, Mayer R, Wagner EM, Gerbitz KD. Is serum fructosamine assay specific for determination of glycated serum protein? Clin Chem 1988;34:320–323 [PubMed] [Google Scholar]
- 44.Baker JR, O’Connor JP, Metcalf PA, Lawson MR, Johnson RN. Clinical usefulness of estimation of serum fructosamine concentration as a screening test for diabetes mellitus. Br Med J (Clin Res Ed) 1983;287:863–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benjamin RJ, Sacks DB. Glycated protein update: implications of recent studies, including the diabetes control and complications trial. Clin Chem 1994;40:683–687 [PubMed] [Google Scholar]
- 46.Windeler J, Köbberling J. The fructosamine assay in diagnosis and control of diabetes mellitus scientific evidence for its clinical usefulness? J Clin Chem Clin Biochem 1990;28:129–138 [PubMed] [Google Scholar]
- 47.Baker J, Metcalf P, Scragg R, Johnson R. Fructosamine Test-Plus, a modified fructosamine assay evaluated. Clin Chem 1991;37:552–556 [PubMed] [Google Scholar]
- 48.Parrinello CM, Selvin E. Beyond HbA1c and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr Diab Rep 2014;14:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Selvin E, Rawlings AM, Grams M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol 2014;2:279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Virally M, Laloi-Michelin M. Methods for the screening and diagnosis of gestational diabetes mellitus between 24 and 28 weeks of pregnancy. Diabetes Metab 2010;36:549–565 [DOI] [PubMed] [Google Scholar]
- 51.Sumner AE, Duong MT, Aldana PC, et al. A1C combined with glycated albumin improves detection of prediabetes in Africans: the Africans in America study. Diabetes Care 2016;39:271–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen MP. Clinical, pathophysiological and structure/function consequences of modification of albumin by Amadori-glucose adducts. Biochim Biophys Acta 2013;1830:5480–5485 [DOI] [PubMed] [Google Scholar]
- 53.Priego-Capote F, Scherl A, Müller M, Waridel P, Lisacek F, Sanchez JC. Glycation isotopic labeling with 13C-reducing sugars for quantitative analysis of glycated proteins in human plasma. Mol Cell Proteomics 2010;9:579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohzuma T, Yamamoto T, Uematsu Y, Shihabi ZK, Freedman BI. Basic performance of an enzymatic method for glycated albumin and reference range determination. J Diabetes Sci Technol 2011;5:1455–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez-Capote K, Tovell K, Holmes D, Dayton J, Higgins TN. Analytical evaluation of the Diazyme glycated serum protein assay on the siemens ADVIA 1800: comparison of results against HbA1c for diagnosis and management of diabetes. J Diabetes Sci Technol 2015;9:192–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Araki T, Ishikawa Y, Okazaki H, et al. Japanese Red Cross GA Research Group . Introduction of glycated albumin measurement for all blood donors and the prevalence of a high glycated albumin level in Japan. J Diabetes Investig 2012;3:492–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen RM, Sacks DB. Comparing multiple measures of glycemia: how to transition from biomarker to diagnostic test? Clin Chem 2012;58:1615–1617 [DOI] [PubMed] [Google Scholar]
- 58.Schleicher ED, Olgemöller B, Wiedenmann E, Gerbitz KD. Specific glycation of albumin depends on its half-life. Clin Chem 1993;39:625–628 [PubMed] [Google Scholar]
- 59.Sacks DB, Arnold M, Bakris GL, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2011;57:e1–e47 [DOI] [PubMed] [Google Scholar]
- 60.Shima K, Abe F, Chikakiyo H, Ito N. The relative value of glycated albumin, hemoglobin A1c and fructosamine when screening for diabetes mellitus. Diabetes Res Clin Pract 1989;7:243–250 [DOI] [PubMed] [Google Scholar]
- 61.Koga M, Matsumoto S, Saito H, Kasayama S. Body mass index negatively influences glycated albumin, but not glycated hemoglobin, in diabetic patients. Endocr J 2006;53:387–391 [DOI] [PubMed] [Google Scholar]
- 62.Huh JH, Kim KJ, Lee BW, et al. The relationship between BMI and glycated albumin to glycated hemoglobin (GA/A1c) ratio according to glucose tolerance status. PLoS One 2014;9:e89478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miyashita Y, Nishimura R, Morimoto A, Matsudaira T, Sano H, Tajima N. Glycated albumin is low in obese, type 2 diabetic patients. Diabetes Res Clin Pract 2007;78:51–55 [DOI] [PubMed] [Google Scholar]
- 64.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia 2001;44:129–146 [DOI] [PubMed] [Google Scholar]
- 65.Vistoli G, De Maddis D, Cipak A, Zarkovic N, Carini M, Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic Res 2013;47(Suppl. 1):3–27 [DOI] [PubMed] [Google Scholar]
- 66.Manigrasso MB, Juranek J, Ramasamy R, Schmidt AM. Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol Metab 2014;25:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thornalley PJ, Rabbani N. Detection of oxidized and glycated proteins in clinical samples using mass spectrometry–a user's perspective. Biochim Biophys Acta 2014;1840:818–829 [DOI] [PubMed] [Google Scholar]
- 68.Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc 2010;110:911–916.e912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goh SY, Cooper ME. Clinical review: the role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab 2008;93:1143–1152 [DOI] [PubMed] [Google Scholar]
- 70.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813–820 [DOI] [PubMed] [Google Scholar]
- 71.Hanssen NM, Beulens JW, van Dieren S, et al. Plasma advanced glycation end products are associated with incident cardiovascular events in individuals with type 2 diabetes: a case-cohort study with a median follow-up of 10 years (EPIC-NL). Diabetes 2015;64:257–265 [DOI] [PubMed] [Google Scholar]
- 72.Hanssen NM, Engelen L, Ferreira I, et al. Plasma levels of advanced glycation endproducts Nε-(carboxymethyl)lysine, Nε-(carboxyethyl)lysine, and pentosidine are not independently associated with cardiovascular disease in individuals with or without type 2 diabetes: the Hoorn and CODAM studies. J Clin Endocrinol Metab 2013;98:E1369–E1373 [DOI] [PubMed] [Google Scholar]
- 73.Fosmark DS, Torjesen PA, Kilhovd BK, et al. Increased serum levels of the specific advanced glycation end product methylglyoxal-derived hydroimidazolone are associated with retinopathy in patients with type 2 diabetes mellitus. Metabolism 2006;55:232–236 [DOI] [PubMed] [Google Scholar]
- 74.Genuth S, Sun W, Cleary P, et al.; DCCT Skin Collagen Ancillary Study Group . Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 2005;54:3103–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jack M, Wright D. Role of advanced glycation endproducts and glyoxalase I in diabetic peripheral sensory neuropathy. Transl Res 2012;159:355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miura J, Yamagishi Si, Uchigata Y, et al. Serum levels of non-carboxymethyllysine advanced glycation endproducts are correlated to severity of microvascular complications in patients with Type 1 diabetes. J Diabetes Complications 2003;17:16–21 [DOI] [PubMed] [Google Scholar]
- 77.Monnier VM, Bautista O, Kenny D, et al. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes 1999;48:870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakamura N, Hasegawa G, Obayashi H, et al. Increased concentration of pentosidine, an advanced glycation end product, and interleukin-6 in the vitreous of patients with proliferative diabetic retinopathy. Diabetes Res Clin Pract 2003;61:93–101 [DOI] [PubMed] [Google Scholar]
- 79.Bos DC, de Ranitz-Greven WL, de Valk HW. Advanced glycation end products, measured as skin autofluorescence and diabetes complications: a systematic review. Diabetes Technol Ther 2011;13:773–779 [DOI] [PubMed] [Google Scholar]
- 80.Gerrits EG, Lutgers HL, Kleefstra N, et al. Skin autofluorescence: a tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care 2008;31:517–521 [DOI] [PubMed] [Google Scholar]
- 81.Noordzij MJ, Mulder DJ, Oomen PH, et al. Skin autofluorescence and risk of micro- and macrovascular complications in patients with type 2 diabetes mellitus-a multi-centre study. Diabet Med 2012;29:1556–1561 [DOI] [PubMed] [Google Scholar]
- 82.Orchard TJ, Lyons TJ, Cleary PA, et al.; DCCT/EDIC Research Group . The association of skin intrinsic fluorescence with type 1 diabetes complications in the DCCT/EDIC study. Diabetes Care 2013;36:3146–3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Na R, Stender IM, Ma L, Wulf HC. Autofluorescence spectrum of skin: component bands and body site variations. Skin Res Technol 2000;6:112–117 [DOI] [PubMed] [Google Scholar]
- 84.Mitsuhashi T, Vlassara H, Founds HW, Li YM. Standardizing the immunological measurement of advanced glycation endproducts using normal human serum. J Immunol Methods 1997;207:79–88 [DOI] [PubMed] [Google Scholar]
- 85.Beisswenger PJ, Howell SK, Russell GB, Miller ME, Rich SS, Mauer M. Early progression of diabetic nephropathy correlates with methylglyoxal-derived advanced glycation end products. Diabetes Care 2013;36:3234–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lopez-Clavijo AF, Duque-Daza CA, Romero Canelon I, et al. Study of an unusual advanced glycation end-product (AGE) derived from glyoxal using mass spectrometry. J Am Soc Mass Spectrom 2014;25:673–683 [DOI] [PubMed] [Google Scholar]
- 87.Valencia JV, Mone M, Koehne C, Rediske J, Hughes TE. Binding of receptor for advanced glycation end products (RAGE) ligands is not sufficient to induce inflammatory signals: lack of activity of endotoxin-free albumin-derived advanced glycation end products. Diabetologia 2004;47:844–852 [DOI] [PubMed] [Google Scholar]
- 88.Thomas MC, Woodward M, Neal B, et al.; ADVANCE Collaborative Group . Relationship between levels of advanced glycation end products and their soluble receptor and adverse outcomes in adults with type 2 diabetes. Diabetes Care 2015;38:1891–1897 [DOI] [PubMed] [Google Scholar]
- 89.Nin JW, Jorsal A, Ferreira I, et al. Higher plasma soluble receptor for advanced glycation end products (sRAGE) levels are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12-year follow-up study. Diabetes 2010;59:2027–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Selvin E, Halushka MK, Rawlings AM, et al. sRAGE and risk of diabetes, cardiovascular disease, and death. Diabetes 2013;62:2116–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schalkwijk CG, Stehouwer CD. Comment on: Selvin et al. sRAGE and risk of diabetes, cardiovascular disease, and death. Diabetes 2013;62:2116–2121. Diabetes 2013;62:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Selvin E, Coresh J, Halushka MK. Response to comments on: Selvin et al. sRAGE and risk of diabetes, cardiovascular disease, and death. Diabetes 2013;62:2116–2121. Diabetes 2013;62:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rabbani N, Chittari MV, Bodmer CW, Zehnder D, Ceriello A, Thornalley PJ. Increased glycation and oxidative damage to apolipoprotein B100 of LDL cholesterol in patients with type 2 diabetes and effect of metformin. Diabetes 2010;59:1038–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Draghici C, Wang T, Spiegel DA. Concise total synthesis of glucosepane. Science 2015;350:294–298 [DOI] [PubMed] [Google Scholar]
- 95.Biemel KM, Friedl DA, Lederer MO. Identification and quantification of major maillard cross-links in human serum albumin and lens protein. Evidence for glucosepane as the dominant compound. J Biol Chem 2002;277:24907–24915 [DOI] [PubMed] [Google Scholar]
- 96.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]