Abstract
There have been significant advances in cancer treatment over the past several years through the use of chemotherapy, radiation therapy, molecularly targeted therapy, and immunotherapy. Despite these advances, treatments such as monotherapy or monomodality have significant limitations. There is increasing interest in using these strategies in combination; however, it is not completely clear how best to incorporate molecularly targeted and immune-targeted therapies into combination regimens. This is particularly pertinent when considering combinations with immunotherapy, as other types of therapy may have significant impact on host immunity, the tumor microenvironment, or both. Thus, the influence of chemotherapy, radiation therapy, and molecularly targeted therapy on the host anti-tumor immune response and the host anti-host response (ie, autoimmune toxicity) must be taken into consideration when designing immunotherapy-based combination regimens. We present data related to many of these combination approaches in the context of investigations in patients with melanoma and discuss their potential relationship to management of patients with other tumor types. Importantly, we also highlight challenges of these approaches and emphasize the need for continued translational research.
ANTIGEN RECOGNITION AND T-CELL ACTIVATION
Antigen Presentation
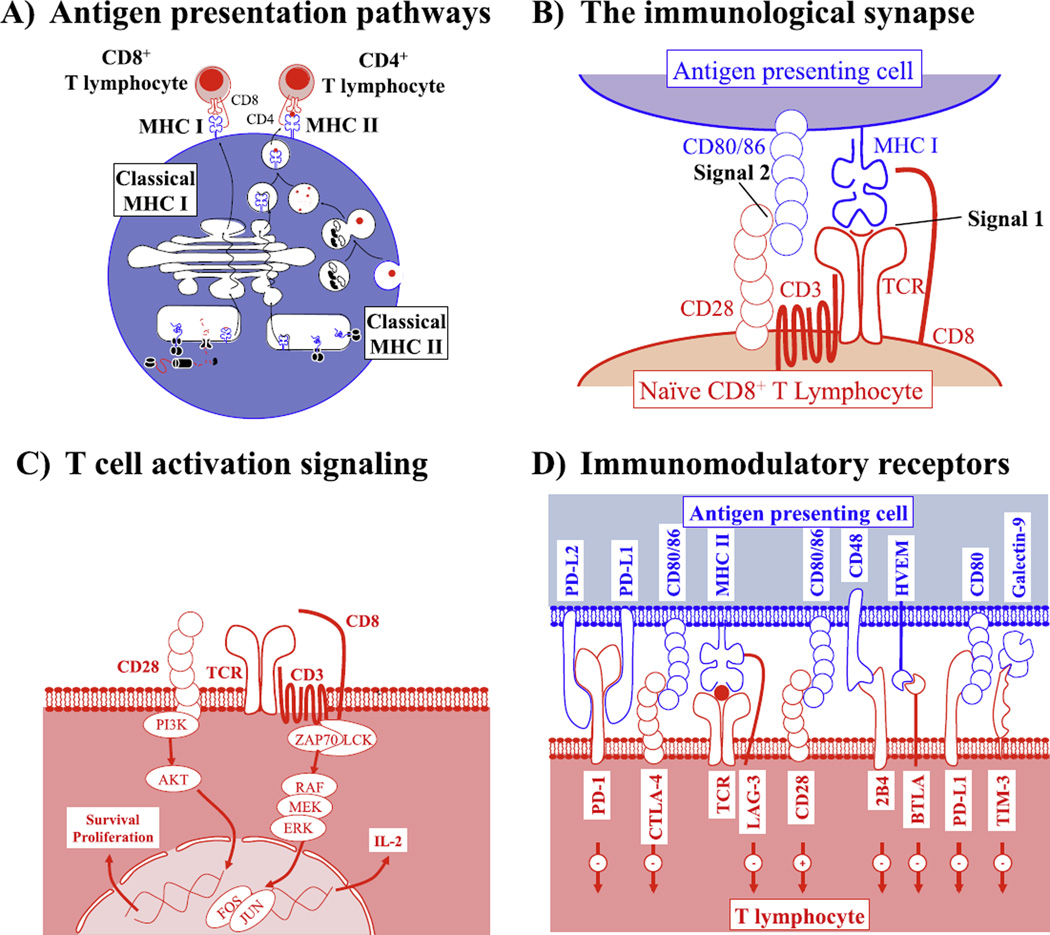
Antigen presentation is a process allowing presentation at the cell surface of peptides reflecting the current state of the cell for recognition by the immune system. These peptides may be presented on major histocompatibility class I (MHC I) molecules by all nucleated cells to CD8+ T lymphocytes,1 or by the MHC II molecules exclusively expressed by antigen-presenting cells (APCs) such as macrophages, B lymphocytes, and dendritic cells to CD4+ T lymphocytes.1 Classically, MHC I molecules present antigens derived from the intra-cellular space, whether it be self-proteins in healthy cells, viral proteins in infected cells, or malignant and mutated proteins in cancer cells. On the other hand, MHC II molecules classically present peptides derived from the digestion of extracellular necrotic cells and cell debris and therefore paint a picture of the state of the immediate microenvironment rather than of the APC itself. Alternately, APCs are capable of cross-presentation, a process through which they may present exogenously derived antigens on MHC I molecules for recognition by CD8+ T lymphocytes2 (Figure 1A).
Figure 1.
T-cell activation is regulated by antigen presentation, as well as stimulatory and inhibitory co-receptors. (A) Overview of the MHC I and II antigen presentation and processing pathways. Canonical pathways of MHC I antigen presentation allow display of intracellular proteins following processing and transport to circulating CD8+ T lymphocytes. Canonical MHC II antigen presentation allows display of antigens derived from the extracellular environment to circulating CD4+ T lymphocytes. (B) Signals required for proper T-cell activation. Both CD4+ and CD8+ T cells are activated following interaction with signal 1 provided through presentation of MHC I or II/peptide complexes at the cell surface with the T-cell receptor (TCR), as well as signal 2 supplied by interaction of CD80/CD86 costimulation molecules expressed on antigen-presenting cells (APCs) with CD28 expressed on T cells. Only in presence of both signals will naive T cells be activated. Following activation, T cells may recognize and kill cells presenting only signal 1 (MHC/peptide). (C) Signaling pathways engaged following interaction of T cells with signals 1 and 2. T cells stimulated through the TCR engage MAPK pathway signaling, which results in production of cytokines such as IL-2. Signal 2 targets PI3K/AKT signaling, through which T-cell survival and proliferation result. Both signals are crucial for initial T-cell activation. (D) Overview of the receptors and ligands expressed on T cells and APCs, which may influence T-cell activation upon interaction. Several of these receptors may be expressed on T cells following T-cell activation as a mechanism to inhibit T-cell activation and prevent autoimmune disease.
The Immunological Synapse
The interface between APC and T cells is complex, requiring the proximity of multiple ligands to trigger proper T-cell activation. This interface is termed the immunological synapse,3 and is comprised of the T-cell receptor (TCR), which binds to the MHC I or II molecule on APCs in unison with the CD8 (MHC I) or CD4 (MHC II) co-receptors, as well as the interaction between the T-cell–expressed CD28 molecule and its CD80/CD86 co-stimulation ligand on APCs.4 It is crucial that both signal 1 (TCR–MHC interaction) and signal 2 (CD28–CD80/CD86 interaction)5 be engaged for proper initial T-cell priming by APCs, although subsequent activation of T cells may occur in absence of signal 2.6,7 Proper activation of T cells results in lysis of infected (or otherwise targeted) cells through production of cytotoxic proteases such as granzyme B and perforin at the immunological synapse,8 as well as through interaction between Fas and Fas-ligand, which results in apoptosis of targeted cells9 (Figure 1B).
T-Cell Signaling
T-cell signaling occurs upon formation of the immunological synapse, and binding of the TCR to the peptide-presenting MHC molecule expressed on the APC. Recruitment of the CD8 or CD4 co-receptor associated to the intracellular Lck kinase promotes CD3ζ phosphorylation at the TCR and subsequent ZAP-70 phosphorylation.10 In turn, this results in recruitment of the linker for activation of T cells (LAT),11 which promotes downstream signaling through the mitogen activated protein kinase (MAPK) pathway,12 T-cell activation, and cytokine production. Furthermore, ligation of the CD28 co-stimulation molecule by CD80/ CD86 on APC results in phosphoinositide-3-kinase (PI3K) signaling and in subsequent survival and proliferation of T cells13 (Figure 1C).
Contraction of T-Cell Responses
A prolonged T-cell response could have devastating consequences, causing damage to healthy cells and organs following pathogen clearance and eventually resulting in persistent auto-immunity. Accordingly, upon T-cell activation, the inflammatory environment generated by the immune response results in induction of expression of immunomodulatory proteins such as programmed cell death protein 1 (PD-1),14 cytotoxic T-lymphocyte–associated protein-4 (CTLA-4),15 lymphocyte activation gene-3 (LAG-3),16 and T-cell immunoglobulin mucin-3 (Tim-3),17 which cause inhibition of T-cell function and subsequent T-cell anergy and contraction upon ligation. Accordingly, the number of antigen-reactive T cells drastically decreases following pathogen clearance, thereby decreasing chances of damage due to an overdrawn immune response. Importantly, a certain limited number of antigen-specific T cells remain in circulation following contraction in order to ensure T-cell memory and future responses to the same infectious agent (Figure 1D).
RATIONALE FOR COMBINATION STRATEGIES WITH IMMUNOTHERAPY IN CANCER THERAPY
Though monotherapy regimens for cancer have yielded some success, there are significant limitations with regard to response rates and duration of therapy.18 Based on these limitations and some provocative preclinical evidence for potential synergy of immunotherapy with other treatment modalities,19 there is now tremendous enthusiasm for combination strategies in cancer therapy. However, rational design of these combination strategies requires a deep understanding of the effects of each therapy alone (and in combination) on host antitumor immunity.
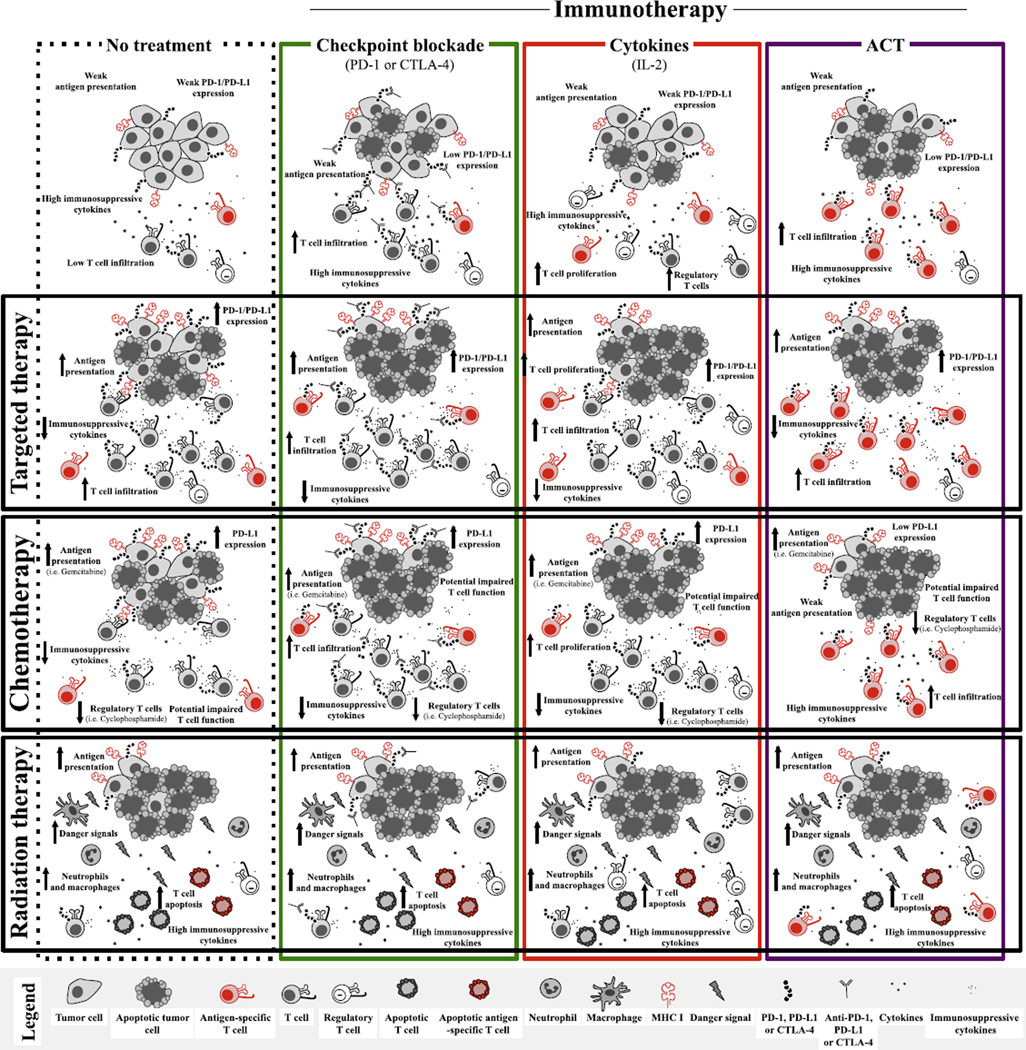
Given the growing success of immunotherapy regimens across cancer types, there is significant interest in combining immunotherapeutic approaches with standard and novel agents to exploit potential synergy. The premise behind this is that several treatments may make a tumor more immunogenic, thus enhancing the effects of immunotherapy when these strategies are given in combination. Immunogenicity of tumors may be enhanced by increased antigen and MHC class I expression on tumors, but may also be enhanced by favorable changes to the tumor microenvironment (such as by increasing vascular permeability and immune infiltrate, or by positively modulating cytotoxic T lymphocytes [CTLs] within the tumor) (Figure 2). A number of therapies are currently being investigated in combination with immunotherapy including radiation therapy, chemotherapy, and a new wave of vaccines; although cytotoxic chemotherapy is the prototype.
Figure 2.
Impact of immunotherapy, targeted chemotherapy, and radiation therapy on tumors and the immune system as monotherapy and in combination. The top row depicts known impacts of immunotherapies through targeting checkpoint inhibitors, cytokine treatment, or adoptive cell therapy on tumor mass, and immune cell function in comparison to without treatment (top left). The left column depicts the known effects of other forms of therapy, such as targeted therapy, chemotherapy, and radiation therapy, on tumor mass and immune cell function in comparison to without treatment (top left). Intersections between forms of treatment represent the extrapolated effect on combining immunotherapies with other forms of therapy, based on known effects of regimens individually.
CLINICAL EVIDENCE OF IMMUNE EFFECTS OF CHEMOTHERAPY
The concept of combining immunotherapeutic approaches with conventional chemotherapy is highlighted in the treatment of melanoma, where a number of different regimens have been tested. One of these regimens, termed “biochemotherapy”, has shown promise in single-center studies. Specifically, the combination of cisplatin, vinblastine, and dacarbazine (CVD) was given with interleukin-2 (IL-2) and interferon (IFN)-α and was associated with response rates approaching 50%.20–22 However, when randomized, multicenter studies were performed, response rates were lower and outcomes, namely overall survival, were not superior to either combination or single-agent chemotherapy23,24 Newer regimens, including nab-paclitaxel, are now being studied25 (NCT00970996).
COMBINATIONS OF CHEMOTHERAPY WITH IMMUNE CHECKPOINT INHIBITORS
With the discovery of therapeutic immune checkpoint inhibitors, efforts to combine these agents with chemotherapy were pursued very early in their clinical development. At the same time, preclinical work continued to describe the effects of various cytotoxic agents on the immune system generally and on the tumor immune microenvironment. For example, chemotherapy with an agent such as gemcitabine, was associated with apoptosis that increased tumor antigen presentation and “cross-priming” of CTLs.26 Additionally, gemcitabine was shown to selectively target and suppress humoral immune elements, but have very little to no effect on cytotoxic immunity, while cyclophosphamide efficiently led to the depletion of regulatory T cells.27,28 These data would predict that chemotherapy may enhance the effects of anti-CTLA-4 antibodies, and this was, in fact, shown to be the case in vivo. Several groups have shown synergy with a variety of combination chemotherapy-immunotherapy regimens in preclinical models with multiple tumor types.29–31
There have also been a number of clinical trials combining ipilimumab (monoclonal antibody targeting CTLA-4) with chemotherapy. The largest was one of the two registration trials of ipilimumab comparing the combination of ipilimumab with dacarbazine versus dacarbazine alone in treatment-na¨ıve patients with unresectable or metastatic melanoma.32–34 While the response rates were similar in the patients receiving combination versus single-agent therapy (15% v 10%), the hazard ratios for overall survival (OS) were 0.72 (P <.001), and 0.76 (P <.001) for progression-free survival (PFS), both favoring ipilimumab plus dacarbazine.34 Unfortunately, there were no data in this trial comparing the combination versus single-agent ipilimumab; though despite this, the results of this trial helped support the approval of single-agent ipilimumab for the treatment of patients with stage IV melanoma. Another large, randomized phase II study enrolled 204 chemotherapy-naïve patients with non-small cell lung cancer (NSCLC) to receive six cycles of carboplatin and paclitaxel (CT), four doses of ipilimumab plus six cycles of CT (commenced concurrently), or two cycles of CT followed by four cycles of CT plus ipilimumab (phased ipilimumab arm). The phased ipilimumab-containing arm had the highest immune-related PFS (irPFS) and immune-related best overall response rate (irBORR), although this antitumor activity did not appear to be associated with a significant OS benefit.35 Additionally, a phase II study randomized 130 patients with chemotherapy-naïve extensive-stage disease small cell lung cancer (SCLC) to CT, concurrent ipilimumab plus CT, or phased ipilimumab.36 As in the NSCLC study, treatment with phased ipilimumab was associated with improvement in irPFS. Several non-randomized studies have been performed with ipilimumab and chemotherapy and are summarized in Table 1.
Table 1.
Ongoing Clinical Trials of Combined Molecularly Targeted Therapy and Immunotherapy
| Target Therapy and Checkpoint Blockade |
Targeted Therapy and Cytokine Therapy |
Targeted Therapy and Adoptive Cell Therapy |
|---|---|---|
| Dabrafenib +/− trametinib + ipilimumab (NCT01767454; NCT01940809) |
Vemurafenib + high-dose IL-2 (NCT01754376; NCT 01683188) |
Vemurafenib + tumor-infiltrating lymphocytes (NCT00338377; NCT01585415; NCT01659151) |
| Vemurafenib + anti–PD-L1 (MPDL3280) (NCT01656642) |
Vemurafenib + IL-2 (infusional 96 hour) + IFN-α (NCT01603212) |
|
| Dabrafenib + trametinib + anti–PD-1 (MK-3475) (NCT02130466) |
Vemurafenib + pegylated IFN (NCT01959633) |
|
| Trametinib +/− dabrafenib + anti–PD- L1 (MEDI4736) (NCT02027961) |
Vemurafenib + high-dose IFN-α2b (NCT01943422) |
|
| Dabrafenib + trametinib followed by ipilimumab + nivolumab or vice versa (NCT02224781) |
||
| Anti-PD-L1 (MPDL3280A) +/− bevacizumab versus sunitinib in advanced RCC. (NCT01984242) |
||
| Nivolumab plus sunitinib, pazopanib, or ipilimumab in subjects with metastatic RCC (NCT01472081) |
||
| Pembrolizumab plus axitinib in advanced RCC (NCT02133742) |
Combination studies of anti–PD-1 inhibitors and chemotherapy have also recently commenced and early data are beginning to emerge. For example, a phase I dose–de-escalation trial of nivolumab with multiple regimens (gemcitabine-cisplatin, pemetrexed-cisplatin, and CT) in chemotherapy-na¨ıve patients with NSCLC was recently presented at the 2014 American Society of Clinical Oncology (ASCO) annual meeting and showed that response rates across four cohorts ranged from 33%–47% without unexpected toxicities.37 A number of other trials are ongoing evaluating the combination of PD-1/PD-L1 inhibitors with chemotherapy.
TOXICITY OF COMBINED CHEMOTHERAPY AND IMMUNOTHERAPY
With the introduction of immune checkpoint inhibitors to the clinic, a new set of toxicities, specifically, immune-related adverse events (irAEs), have emerged. Side effects of these irAEs range from minimal to lethal and require a completely different management approach. Ipilimumab, in particular, is associated with grade 3–5 toxicity in 10%-45% of patients, depending on dose, whether maintenance therapy was allowed, the clinical setting (adjuvant V previously treated metastatic disease), and whether it was given as a single agent or in combination with other immune therapies, chemotherapies, or molecularly targeted therapies.34,38
As a single agent, the dominant toxicities are dermatologic (pruritus, rash, or Stevens Johnson Syndrome), gastrointestinal (ranging from mild diarrhea to frank and severe colitis associated with intestinal perforation), hepatic (typically elevated transaminases, very rarely hepatic failure), endocrine (hypophysitis, thyroiditis), neurologic (mononeuritis such as facial nerve palsy, Guillian-Barre syndrome), and renal (nephritis).33,39,40 Additionally, in most clinical trials, fatal toxicity was seen in approximately 1%-2% and most often from inflammatory colitis with associated intestinal perforation.38 However, one of the more remarkable findings from studies of CTLA-4 antibodies has been the change in toxicity pattern when combined with other agents compared to single-agent ipilimumab or tremelimumab.
The aforementioned phase III trial of dacarbazine with or without ipilimumab offers an interesting example of how the distribution of toxicities may be shifted when CTLA-4 inhibitors are combined with chemotherapy.34 As a single agent, the rate of grade 3–5 ipilimumab-related gastrointestinal toxicity (diarrhea or colitis) is approximately 8%-23%,33 but in this trial it was 4%.34 In contrast, the rate of hepatic toxicity was much greater in this clinical trial with 20%-30% of patients experiencing a grade 3 or 4 elevation of their alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) (eg, hepatitis) with the combination, compared to the usual rate of grade 3/4 ipilimumab-related hepatitis of 3%-7%. A phase II trial of ipilimumab plus fotemustine corroborated the pattern that chemotherapy augments hepatic toxicity but attenuates colonic toxicity. Specifically, the most common irAE was hepatitis (~38%) with 14% being grade 3 or 4, while the rate of grade 3/4 diarrhea/colitis was only 5%.41 Interestingly, the phase II trials of ipilimumab plus CT in patients with either NSCLC or SCLC showed different rates of hepatic toxicity in the combination arm when compared across trials, and similarly low rates of colitis in each arm of both trials. In the SCLC study, the rate of grade 3/4 hepatitis was 43% (18/42) in the combination arm, 18% (7/42) in the phased arm, and 0% in the chemotherapy alone arm.36 In the NSCLC study, the rate of grade 3/4 hepatitis was low across all arms (approximately 3% in each arm).35
CUTTING EDGE: TARGETED THERAPY EFFECTS ON IMMUNE MICROENVIRONMENT
Over the past 15–20 years, numerous oncogenic mutations have been described in cancer that contribute to their malignant potential through increased growth and invasiveness, resistance to apoptosis, and increased angiogenesis.42 Treatment of cancers with pharmacologic agents targeting these mutations represents one of the most significant advances in cancer therapy in decades, and these forms of therapy may demonstrate high response rates but are often limited by a relatively short duration of response.43 This is highlighted in melanoma, where pharmacologic inhibition of the BRAF oncogene (which is mutated in tumors from approximately half of patients with advanced melanoma) results in responses in the vast majority of patients.44,45 However, responses to therapy are of limited duration, with most patients progressing on therapy within a year. Numerous molecular mechanisms of response and resistance to BRAF-targeted therapy have been identified,39,46–56 and there is now growing evidence that there may be immune mechanisms of response and resistance57–60 as well, providing a potential avenue to improve responses by combining molecularly targeted therapy with immunotherapeutic approaches.
In Vitro Evidence
In vitro studies have demonstrated that hyper-activation of the MAPK pathway in melanoma is associated with down-regulation of melanoma anti-gens,61 which is likely due to transcriptional repression of microopthalmia-associated transcription factor (MITF). Additional in vitro studies showed that exposure of melanoma cell lines and fresh tumor digests to a BRAF inhibitor was associated with a significant increase in melanoma antigen expression (up to 100-fold).62 Critically, this increase in antigen expression was associated with an enhanced recognition by antigen-specific T lymphocytes. Another key feature to these studies is that enhanced antigen expression and reactivity of T cells was also seen when BRAF–wild-type melanoma cells were treated with a MEK inhibitor, although culturing T lymphocytes in the presence of a MEK inhibitor completely abrogated the response and inhibited T-cell proliferation.62 Conversely, selective BRAF inhibitors have no detrimental effect on T cell function. The findings of the deleterious effects of MEK inhibition on T-cell function have been corroborated in other studies,63 and have important implications when considering combining MEK inhibitor–containing regimens with immunotherapeutic agents. Interestingly, selective BRAF inhibitors may actually potentiate T-cell function through paradoxical MAPK signaling.64
Evidence of an Immune Response in Patients on Targeted Therapy
Based on this early in vitro evidence, there was intense interest in studying the immune effects of BRAF inhibitors in early phase trials in patients with metastatic melanoma. To do this, investigators performed tumor biopsies pretreatment and shortly after initiating treatment with a BRAF inhibitor, and again at time of progression (when feasible). In these studies, treatment with a BRAF inhibitor was associated with a significant increase in melanoma antigen expression in tumors within 2 weeks of starting therapy,57 corroborating the in vitro findings. In addition, biopsies demonstrated a dramatic increase in CD8+ T-cell infiltrates within 10–14 days of initiating treatment with a BRAF inhibitor.57,65 This increase in T-cell infiltrate was associated with a more favorable tumor microenvironment, a decrease in immunosuppressive cytokines and vascular endothelial growth factor (VEGF), and an increase in markers of cytotoxicity.57–59 There was also a concurrent increase in the expression of immunomodulatory molecules (such as PD-1, Tim-3, and PD-L1)57 in the tumor microenvironment, which was likely related to IFN-γ release from the infiltrating T cells.66 Alternatively, this increase in immunomodulatory molecule expression may represent an immune-mediated mechanism of resistance to BRAF-targeted therapy.67,68 Sorting out the nature and kinetics of the T-cell infiltrate that develops following BRAF inhibitor therapy is highly clinically relevant, and may provide the key to development of rational regimens for combining immune checkpoint inhibitors with molecularly targeted agents in the treatment of patients with advanced melanoma.
The mechanism through which BRAF inhibitors elicit an immune response is not completely clear, though this is an area of intense study. A key question is whether this represents an antigen-specific response versus a non-specific immune infiltration into a dying tumor mass. There is evidence suggesting an active, antigen-specific immune response in the setting of BRAF inhibition. Specifically, TCR sequencing studies have demonstrated that the T-cell infiltrate is polyclonal before treatment and more clonal during therapy,69 suggesting a narrowing of the immune repertoire in response to BRAF-targeted therapy. An intriguing finding in these studies was that patients who had a good response to BRAF inhibitor therapy seemed to have a pre-existing set of dominant immune clones, which were not present in patients who had a poor response to therapy.69 Although melanoma antigen expression is increased in tumors, the T-cell response is not likely to be solely directed against these shared melanoma antigens. Studies are underway to evaluate the ability of infiltrating T cells induced by molecularly targeted therapy to recognize tumor specific neoantigens.
Preclinical Models Exploring Synergy of Targeted Therapy and Immunotherapy
Initial studies exploring potential synergy of targeted therapy and immunotherapy were performed in murine models. Several different combination strategies were used in these studies, including combining BRAF-targeted therapy with either adoptive cellular therapy59,70 or immune checkpoint blockade.19 The majority of studies showed synergy with enhanced responses to therapy,19,59,70,71 although one study did not support this interpretation.72
Through these murine studies, we have gained additional insights into the immune mechanisms of response to BRAF-targeted therapy. The immune infiltrate seems to be related to a decrease in VEGF, which is a direct consequence of inhibiting mutated BRAF and the MAPK pathway.59 Perhaps the strongest evidence for the role of an immune response to BRAF-targeted therapy is an absolute requirement for CD8+ T cells to achieve a response. Two independent studies demonstrated that depletion of CD8+ T cells completely abrogated the response to BRAF-targeted therapy,19,71 suggesting that an immune infiltrate was not only present, but was required for an adequate response to therapy.
Interestingly, recent studies exploring synergy with targeted therapy and immune checkpoint blockade demonstrated that although there are infiltrating T cells in the setting of treatment with BRAF-targeted therapy, these T cells are not completely functional.19 However, the addition of immune checkpoint blockade to a backbone of BRAF-targeted therapy is associated with a dramatic increase in infiltrating CD8+ T cells which demonstrate an activated and cytolytic phenotype.19 Together, these data provide provocative evidence for synergy of targeted therapy and immunotherapy, and these concepts are being investigated in ongoing clinical trials both in patients with melanoma and other tumor types.
Clinical Trials Exploring Synergy of Targeted Therapy and Immunotherapy
Based on the growing evidence for potential synergy of targeted therapy and immunotherapy in preclinical and murine studies, clinical trials are currently underway to evaluate these combination regimens. Early trials focused on combining BRAF-targeted therapy with cytokine-based therapy (NCT01754376, NCT01683188, NCT01603212); however, there are now several trials combining BRAF inhibitor-based targeted therapy with immune checkpoint blockade (NCT01767454, NCT01940 809, NCT01656642, NCT02130466, NCT02027961, NCT02224781) (Table 2). Though response data are not mature on these trials and it is unclear if synergy will be seen, important information has been gained already. Namely, we have learned that complexities exist in combining these strategies, as highlighted by a clinical trial combining vemurafenib (a BRAF inhibitor) with ipilimumab (a monoclonal antibody targeting the CTLA-4 molecule). In this trial, the first cohort of patients received full dose vemurafenib at 960 mg orally twice daily for one month as a single agent prior to administration of ipilimumab at 3 mg/kg intravenously. Dose-limiting toxicity (DLT) of grade 3 transaminase elevations was noted in four of six patients within 5 weeks after the first dose of ipilimumab.73 A second cohort of patients was then enrolled and treated with a lower dose of vemurafenib (720 mg orally twice daily) with ipilimumab at 3 mg/ kg. Hepatotoxicity was again observed, and the trial was closed to accrual. Of note, all hepatic adverse events were asymptomatic and were reversible either with temporary discontinuation of both study drugs or with administration of corticosteroids.73
Table 2.
Results of Clinical Trials Evaluating the Combination of Cytotoxic Chemotherapy and Ipilimumab in Melanoma and Lung Cancer
| Regimen | Disease | No. Enrolled |
Response Rate (%) |
Median PFS (mo) |
Median OS (mo) |
Reference |
|---|---|---|---|---|---|---|
| DTIC | Melanoma | 502 | 10.3 | ~2.8 | 9.1 | 34 |
| DTIC plus IPI | 15.2 | ~2.8 | 11.2 | |||
| CT | Extensive stage, SCLC |
130 | 53 | 5.3 | 9.9 | 36 |
| CT plus IPI | 47 | 5.7 | 9.1 | |||
| Phased IPI | 71 | 6.4 | 12.9 | |||
| CT | NSCLC | 204 | 14 | 4.2 | 8.3 | 35 |
| CT plus IPI | 21 | 4.1 | 9.7 | |||
| Phased IPI | 32 | 5.1 | 12.2 | |||
| IPI | Melanoma | 59 | 33 | n/a | n/a | 33 |
| IPI plus DTIC | 33 | |||||
| IPI plus CT | 28 | |||||
| Fotemustine plus IPI |
Melanoma | 86 | 29 | 5.3 | 13.3 | 41 |
| IPI | Melanoma | 72 | 5.4 | 11.4 | 32 | |
| IPI plus DTIC | 14.3 | 14.3 |
Abbreviations: PFS, progression-free survival; OS, overall survival; DTIC, dacarbazine; IPI, ipilimumab; CT, carboplatin and paclitaxel; SCLC, small cell lung cancer; NSCLC, non-.small cell lung cancer.
There is also an ongoing trial combining dabrafenib (a BRAF inhibitor) with or without a trametinib (a MEK inhibitor) in combination with ipilimumab for patients with BRAF-mutant melanoma (NCT01767454). Data regarding this trial were recently reported at the annual ASCO meeting in June 2014, with seven patients enrolled on dabrafenib + trametinib + ipilimumab and 12 patients enrolled on dabrafenib + ipilimumab. Of note, there was no DLT in the patients receiving dabrafenib + ipilimumab, but two cases of colitis with perforation were noted in the cohort treated with dabrafenib + trametinib + ipilimumab (leading to suspension of accrual in this cohort).74 Trials sequencing these therapies are currently underway, as are additional studies combining different targeted therapy and immunotherapy regimens (Table 2). Taken together, these results highlight the potential for additive to synergistic anti-tumor effects and the complexity in combining these regimens.
Evidence of an Immune Response in Targeted Therapy for Other Cancers
Investigators are now exploring immune effects of targeted therapy for other cancer types. The hypothesis behind this stems from the fact that oncogenic mutations may affect anti-tumor immunity in other cancers as well. An example of this is in gastrointestinal stromal tumors (GIST), where up to 80% of tumors harbor a mutation in c-kit.75 Oncogenic mutations in c-kit lead to signaling down the MAPK and PI3K pathways, with downstream effects similar to those mediated by BRAF mutations.75
The immune effects of targeted therapy for GIST have been studied by a group from Memorial Sloan Kettering Cancer Center, which demonstrated that successful treatment with imatinib (a c-kit inhibitor) in a murine model was dependent on the presence of CD8+ T cells.76 The group also showed that the addition of immune checkpoint blockade to imatinib enhanced immune infiltrates in tumors as well as survival in the same model.76 This concept is now being studied in clinical trials for patients with c-kit mutant tumors, where imatinib treatment is being combined with ipilimumab immunotherapy (NCT0 1738139). The effects of other agents are being studied in other histologies, and clinical trials are either in development or are underway.
Combinations of immune and angiogenesis targeting agents have also been studied for the better part of a decade. Initial studies were principally in patients with renal cell cancer where agents targeting vascular endothelial growth factor (VEGF), such as bevacizumab, and VEGF receptor tyrosine kinase inhibitors such as sorafenib, sunitinib, pazopanib, and axitinib, have shown sufficient activity to lead to FDA approval. In fact, the phase III trial that led to the approval of bevacizumab in renal cell carcinoma (RCC) randomized patients to interferon plus bevacizumab versus interferon alone as part of the Cancer and Leukemia Group B (CALGB) 90206 study. The trial demonstrated improved PFS and response rate with the combination.77,78 However, a subsequent phase II trial of the combination of bevacizumab plus high-dose IL-2 in patients with RCC showed no apparent increase in response rate or durable response rate compared to historical data with single-agent IL-2.79
More recently, the combination of immune checkpoint inhibitors with VEGF pathway blockers has been evaluated primarily in patients with either melanoma or RCC with more compelling results. In melanoma, the combination of ipilimumab with bevacizumab in a dose-escalation phase I trial was associated with a 17% response rate, a 67% disease control rate, and a 25-month median OS.80 Based on these results, a randomized phase II intergroup trial is now accruing patients (NCT01950390). In kidney cancer, the results from a phase I trial of nivolumab plus either sunitinib or pazopanib, were presented at the 2014 ASCO annual meeting and showed a response rate of approximately 50% in each arm (17/33 treated with nivolumab plus sunitinib, 9/20 treated with pazopanib plus nivolumab). However, similar to what had been observed with chemotherapy, the addition of VEGF receptor inhibitors to PD-1–based blockade was associated with enhanced hepatotoxicity. Nonetheless, building on this encouraging anti-tumor data, there are now several trials looking at anti–PD-1/PD-L1 treatment plus anti-VEGF therapy, (NCT02210117, NCT02133742, NCT01984242) and an interest in exploring more selective VEGF pathway inhibitors such as bevacizumab and axitinib in the hopes of reducing hepatotoxicity.
RADIATION THERAPY, THE IMMUNE SYSTEM, AND THE ABSCOPAL EFFECT
Combining immunotherapy with radiotherapy is another area of great interest. The foundation of this approach rests on the premise that localized radiotherapy will promote tumor antigen release, enhancing tumor-specific targeting by the adaptive immune system.81 Although durable responses to radiotherapy are rare, most patients derive some measurable benefit from this treatment.
Immune Effects of Radiation Therapy
Radiotherapy effectiveness is thought to occur through numerous biological mechanisms. These include the creation of significant levels of clustered DNA damage, including complex double-strand breaks (DSB), which helps slow or kill tumor cells by limiting DNA damage repair.82,83 Radiotherapy has long been viewed as immunosuppressive, in part due to lymphocyte sensitivity to direct radiation.84–86 However, the radiation therapy-induced pro-inflammatory response, which includes the promotion of immunity through the release of damage associated molecular patterns (DAMPs) and toll-like receptors, more recently has been described and provides evidence to the contrary.87–92 Additionally, sublethal irradiation of tumors may result in increased expression of MHC class I antigen or tumor-associated antigens in melanoma cells and gastric adenocarcinoma cells, respectively.93–95
Preclinical studies have also demonstrated the important role the immune system plays in treatment response to radiotherapy. Lee et al demonstrated that the efficacy of high-dose ablative radiotherapy was mediated by CD8+ T cells.96 Additionally, Perez and colleagues suggested that ex vivo irradiated melanomas demonstrated an increase in tumoral CD8+ T-cell infiltrate and dendritic cell-mediated phagocytosis that was responsible for the decrease in metastatic disease observed in mice with irradiated tumors.97
Clinical Evidence of the Immune Effects of Radiation Therapy
Anecdotal reports of patients treated with radiotherapy support the existence of both local and systemic radiation-induced immune-modulatory effects. The term “abscopal effect” was coined in 195398 and refers to the ability of radiation therapy to produce effects at sites distant to the radiation field. In 1975, Kingsley et al published a case report of a 28 year old man with melanoma and diffuse nodal disease who received 14.4 Gy in 12 fractions to right inguinal involvement and subsequently experienced a complete and durable response in all nodal chains.99 Similar effects have been reported in patients with melanoma,40,99–102 NSCLC,103 Merkel cell carcinoma,104 hepatocellular carcinoma,105 and RCC.106,107 The abscopal effect can also be observed in mouse models. However, T-cell–deficient or CD8+ T-cell–depleted mice lack the abscopal effect, supporting the concept that radiation-induced distant effects may be mediated by immune cells and the two treatment approaches might work synergistically.91,108,109
In the treatment of melanoma, radiation-induced immune modulation has been described after depigmentation following irradiation within the target area as well as in non-irradiated areas and was associated with durable disease regression.110,111 Immune analysis of the peripheral blood, depigmented skin, and metastasis demonstrated the presence of specific CD8+ T and B cells that could respond to melanocyte-derived antigens (MDA–MART-1 or gp100).110 Interestingly, depigmentation (aka, vitiligo) has been suggested to be a sign of effective radiotherapy and/or immunotherapy.110,112,113
Clinical Evidence of Synergy With Radiation Therapy and Immunotherapy
Immune checkpoint inhibitors such as anti–PD-1 and anti-CTLA4 have been successful in inducing effective and durable anti-tumor immunity. However, the response has been limited to a subset of patients.38,114 Early clinical evidence suggests that responses to immune checkpoint blockade may be augmented through combination with radiation therapy. Postow and colleagues reported a case of a patient who was being treated with maintenance ipilimumab and exhibiting slow disease progression. The patient experienced regression of metastatic melanoma following the initiation of concurrent radiotherapy. Following three 9.5-Gy fractions to an area near the spine, an abscopal effect was observed in distant splenic lesions and lymph nodes leading to near complete regression of disease.100 The authors also demonstrated a temporal association of antibody response to NY-ESO-1 antigen, peripheral blood immune cells and an increase in antibody responses to other antigens after radiation initiation.101 This anecdotal evidence is corroborated by a case reported by Hiniker et al in which the authors treated a patient with melanoma with ipilimumab and concurrent radiotherapy (54 Gy in three fractions), leading to a complete regression in all metastatic lesions.115 This enhanced response to radiation therapy may even be seen after patients exhibit disease progression on immune checkpoint blockade. Grimaldi et al demonstrated an abscopal response in 11 of the 21 patients with melanoma; nine had partial responses (43%) and two maintained stable disease (10%).102
Clinical Trials Combining Radiation Therapy and Immunotherapy
There is now ongoing interest in combining immune checkpoint blockade with radiotherapy in other cancers. In a phase I/II study in metastatic prostate cancer, 50 men were given 4- to 10-mg/kg doses of ipilimumab plus 8-Gy fractions to each metastatic lesion for 3 weeks.116 This led to one complete response and six cases with disease stabilization. This approach is being tested in a randomized phase II trial (NCT01689974) in patients with prostate cancer and in other tumor types. Studies combining anti–PD-1 antibodies and radiotherapy are also underway, though it is too early to comment on the efficacy of this approach.
Cytokine therapy has also been tested in combination with radiation with mixed results.117–119 Intralesional injections of 3–5 million units of IFN-β three times weekly preceding radiotherapy (5 days a week for a total of 40–60 Gy) demonstrated complete (70%) or partial remission in all 21 patients with metastatic melanoma and a median survival of 10 months.120
The combination of IL-2 and radiotherapy is also being explored. The earliest results from the National Cancer Institute demonstrated tolerability of 10–20 Gy of radiotherapy before IL-2 but no increase in clinical efficacy.121 More recently, Seung and colleagues initiated a trial of stereotactic body radiation therapy (SBRT) followed by high-dose IL-2 in patients with metastatic melanoma or RCC.122 In this phase I trial, patients received one, two, or three doses of SBRT (20 Gy per fraction), with the last dose administered 3 days before starting the standard high-dose IL-2 regimen. Eight of the 12 enrolled patients had a Response Evaluation Criteria in Solid Tumors (RECIST)-defined anti-tumor response (one complete and seven partial responses) in non-irradiated target lesions. Blood-based immune monitoring showed an increase in CD4+ memory effector cells.122 Further trials are underway in patients with either renal cell carcinoma or melanoma in which three daily doses of 6–12 Gy will be given concurrently with high-dose IL-2 with a primary objective of studying immunological effects.123
PUTTING IT TOGETHER: NOVEL COMBINATIONS WITH IMMUNE TARGETED THERAPY
Prior to 2010, there were no randomized, phase III trials in patients with melanoma showing an improvement of OS. Over the past 5 years there have been single-agent studies of immune checkpoint inhibitors (ipilimumab), BRAF inhibitors (vemurafenib, dabrafenib), MEK inhibitors (trametinib), chemotherapy (nab-paclitaxel), and vaccines (TVEC) showing improvements in overall survival versus a control arm. Over this same period, combination strategies have also shown remarkable benefit (OS, PFS) compared to single agents, approved agents, or contemporary controls including ipilimumab plus dacarbazine (DTIC) versus DTIC, high-dose IL-2 plus gp100 vaccine versus high-dose IL-2, ipilimumab plus nivolumab (compared to contemporary rates of 1- and 2-year OS), vemurafenib plus cobimetinib versus vemurafenib, dabrafenib plus trametinib versus dabrafenib (and versus single-agent vemurafenib), and ipilimumab plus TVEC (high response rate, limited toxicity). Needless to say, it has been a half-decade of dramatic advances that have transformed the treatment of melanoma patients and provided hope to a patient demographic that historically was afforded little. The major questions are no longer “How can we trigger immune responses in more patients?”, “How do we sensitize patients to chemotherapy?”, or “How can we effectively target melanoma?”, but rather “How can we safely combine effective therapies?” and “How can we best sequence cytotoxic treatments (either via molecularly targeting or with traditional chemotherapy) with immune therapy?” These questions, while specifically being asked in reference to melanoma, are appropriate for almost every disease where immunotherapy is proving to be effective. With expected approvals of anti-PD-1 inhibitors in NSCLC and with emerging data in treating head and neck cancer, bladder cancer, kidney cancer, triple-negative breast cancer, and Hodgkin disease with PD-1/PD-L1 pathway inhibitors, the approach to sequencing standard cytotoxic or molecularly targeted therapies with immune therapies or the development of treatment regimens that incorporate combinations of immune therapies and standard therapies now being extensively explored in patients with melanoma will be applicable to these diseases as well.
The Case for Sequencing
An alternative to combining other treatment modalities with immunotherapy is the administration of the distinct modalities in sequence. Theoretically, this could be both simpler and less toxic in practice, this may not be the case. When evaluating the merits of sequencing two treatment modalities, it is imperative to take into consideration toxicity of a particular sequence in addition to determining its effectiveness. While predicting toxicity of combination therapy is fraught with challenges, predicting the toxicity of a sequence of therapies is equally after high-dose IL-2 is associated with similar toxicity of ipilimumab not given after high-dose IL-2 and is as likely to be effective.38,124 Conversely, high-dose IL-2 given after ipilimumab is associated with a markedly increased risk of intestinal perforation (3/22 patients) than either single-agent high-dose IL-2 or ipilimumab (8/1797 and 4/198, P =0.002 and .024, respectively).125 This example highlights the concept that a previous treatment may prime an individual to have a more (or less) robust reaction (either efficacy or toxicity) to a subsequent therapy.
With a number of immune and molecular targeted therapies entering the clinic, it is important to determine if there is an ideal sequence for these two modalities. In melanoma, two distinct datasets showed that outcome appeared to be better if ipilimumab was given before single-agent BRAF inhibitor or combined BRAF-MEK inhibitor therapy as opposed to ipilimumab after BRAF-targeted therapy.126,127 While selection bias may explain this difference in part (patients with rapidly progressing disease are more likely to be offered a BRAF inhibitor than ipilimumab and are more likely to have a worse outcome than patients with slower progressing disease), there are data suggesting that this phenomenon may be related to changes in the tumor-immune microenvironment. Namely, at the time of progression on BRAF inhibitors, the presumably immunologically favorable effects on the tumor microenvironment (increased antigen expression, infiltration of CD8+ T cells, etc) are gone.67 Another explanation for these data is that patients exhibiting disease progression on molecularly targeted therapy do not have sufficient time to respond to immune therapy.128 Whether differences in sequencing will be seen with PD-1/PD-L1 inhibitors and BRAF-targeted therapy in BRAF mutant melanoma or with PD-1/PD-L1 inhibitors and chemotherapy or oncogene-targeted (eg, EGFR, ALK) therapy in NSCLC or VEGFR tyrosine kinase inhibitors therapy in patients with RCC requires further exploration.
The Case for Combinations
One of the key principles in oncology and microbiology is that therapeutic resistance more commonly occurs with single-agent therapy than with multi-drug regimens. In fact, the development of chemotherapy regimens with non-overlapping dose limiting toxicities for the treatment of childhood leukemia still stands as the most important development in oncology; serving as the exemplar in the chemotherapy age that led to the development of curative regimens in both adult and childhood acute leukemia, Hodgkin and non-Hodgkin lymphoma, and testicular cancer, as well as leading to adjuvant therapy regimens improving surgical cure rates in colon cancer, breast cancer, and lung cancer. It is conceivable that in the future development of immune-immune targeted, or immune-oncogene targeted, or immune-targeted chemotherapy regimens will become standards of care. The results from the first combined checkpoint inhibitor study, ipilimumab plus nivolumab, highlight both the promise and challenge of these types of studies.129
Concluding Thoughts
The approach to incorporating novel immunotherapy regimens into the treatment armamentarium for various cancers will likely be different across malignancies and even across subgroups of patients with a specific malignancy. Needless to say, there is work to be done, and medical and radiation oncologists treating a wide spectrum of diseases will be carrying out trials to sort this out over the next several years and decades. What is clear is that efforts must be made to collect tissue and blood, both to determine which patients are most likely to benefit from specific treatment paths and to understand the effects of these therapies on tumor-immune microenvironment with the ultimate aim of gaining insight as to why they do or do not work. This is a large job that will require collaboration among investigators, surgeons, pathologists, bench researchers, pharmaceutical companies, and regulatory bodies to successfully carry it out. However, given the transformative potential of combined modality, immunotherapy-based treatment regimens, this is a task worthy or pursuit.
Acknowledgments
J.A.W. acknowledges NIH grants 1K08CA160692-01A1, U54CA163125-01, and the generous philanthropic support of several families whose lives have been affected by melanoma.
Footnotes
Conflicts of interest: J.A.W. has honoraria from speakers’ bureau of Dava Oncology and is an advisory board member for GlaxoSmithKline and Roche/Genentech. RJ.S. is a consultant for Astex. No conflicts of interest were disclosed by the other authors.
REFERENCES
- 1.Madden DR. The three-dimensional structure of peptide-MHC complexes. Ann Rev Immunol. 1995;13:587–622. doi: 10.1146/annurev.iy.13.040195.003103. [DOI] [PubMed] [Google Scholar]
- 2.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Natu Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 3.Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, et al. The immunological synapse. Ann Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 4.Yokosuka T, Saito T. The immunological synapse, TCR microclusters, and T-cell activation. Curr Top Microbiol Immunol. 2010;340:81–107. doi: 10.1007/978-3-642-03858-7_5. [DOI] [PubMed] [Google Scholar]
- 5.Bernard A, Lamy, Alberti I. The two-signal model of T-cell activation after 30 years. Transplantation. 2002;73:S31–S35. doi: 10.1097/00007890-200201151-00011. [DOI] [PubMed] [Google Scholar]
- 6.Caux C, Vanbervliet B, Massacrier C, et al. B70/B7-2 is identical to CD86 and is the major functional ligand for CD28 expressed on human dendritic cells. J Exp Med. 1994;180:1841–1847. doi: 10.1084/jem.180.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caux C, Massacrier C, Vanbervliet B, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lancki DW, Hsieh CS, Fitch FW. Mechanisms of lysis by cytotoxic T lymphocyte clones. Lytic activity and gene expression in cloned antigen-specific CD4+ and CD8+ T lymphocytes. J Immunol. 1991;146:3242–3249. [PubMed] [Google Scholar]
- 9.Berke G. The CTL’s kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 10.Huse M. The T-cell-receptor signaling network. J Cell Sci. 2009;122:1269–1273. doi: 10.1242/jcs.042762. [DOI] [PubMed] [Google Scholar]
- 11.Paz PE, Wang S, Clarke H, Lu X, Stokoe D, Abo A. Mapping the Zap-70 phosphorylation sites on LAT (linker for activation of T cells) required for recruitment and activation of signalling proteins in T cells. Biochem J. 2001;356:461–471. doi: 10.1042/0264-6021:3560461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy K, Chaudhri G. Activation and signal transduction via mitogen-activated protein (MAP) kinases in T lymphocytes. Immunol Cell Biol. 1997;75:528–545. doi: 10.1038/icb.1997.84. [DOI] [PubMed] [Google Scholar]
- 13.Harada Y, Tanabe E, Watanabe R, et al. Novel role of phosphatidylinositol 3-kinase in CD28-mediated costimulation. J Biol Chem. 2001;276:9003–9008. doi: 10.1074/jbc.M005051200. [DOI] [PubMed] [Google Scholar]
- 14.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruniquel D, Borie N, Hannier S, Triebel F. Regulation of expression of the human lymphocyte activation gene-3 (LAG-3) molecule, a ligand for MHC class II. Immunogenetics. 1998;48:116–124. doi: 10.1007/s002510050411. [DOI] [PubMed] [Google Scholar]
- 17.Sakuishi K, Ngiow SF, Sullivan JM, et al. TIM3FOXP3 regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology. 2013;2:e23849. doi: 10.4161/onci.23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDermott D, Lebbe C, Hodi FS, et al. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat Rev. 2014;40:1056–1064. doi: 10.1016/j.ctrv.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Cooper ZA, Juneja VR, Sage PT, et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol Res. 2014;2:643–654. doi: 10.1158/2326-6066.CIR-13-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legha SS, Ring S, Bedikian A, et al. Treatment of metastatic melanoma with combined chemotherapy containing cisplatin, vinblastine and dacarbazine (CVD) and biotherapy using interleukin-2 and interferon-alpha. Ann Oncol. 1996;7:827–835. doi: 10.1093/oxfordjournals.annonc.a010762. [DOI] [PubMed] [Google Scholar]
- 21.Legha SS, Ring S, Eton O, et al. Development of a biochemotherapy regimen with concurrent administration of cisplatin, vinblastine, dacarbazine, interferon alfa, and interleukin-2 for patients with metastatic melanoma. J Clin Oncol. 1998;16:1752–1759. doi: 10.1200/JCO.1998.16.5.1752. [DOI] [PubMed] [Google Scholar]
- 22.McDermott DF, Mier JW, Lawrence DP, et al. A phase II pilot trial of concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin 2, and interferon alpha-2B in patients with metastatic melanoma. Clin Cancer Res. 2000;6:2201–2208. [PubMed] [Google Scholar]
- 23.Bajetta E, Del Vecchio M, Nova P, et al. Multicenter phase III randomized trial of polychemotherapy (CVD regimen) versus the same chemotherapy (CT) plus subcutaneous interleukin-2 and interferon-alpha2b in metastatic melanoma. Ann Oncol. 2006;17:571–577. doi: 10.1093/annonc/mdl007. [DOI] [PubMed] [Google Scholar]
- 24.Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2008;26:5748–5754. doi: 10.1200/JCO.2008.17.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alrwas A, Papadopoulos NE, Cain S, et al. Phase I trial of biochemotherapy with cisplatin, temozolomide, and dose escalation of nab-paclitaxel combined with interleukin-2 and interferon-alpha in patients with metastatic melanoma. Melanoma Res. 2014;24:342–348. doi: 10.1097/CMR.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowak AK, Lake RA, Marzo AL, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 27.Nowak AK, Robinson BW, Lake RA. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy. Cancer Res. 2002;62:2353–2358. [PubMed] [Google Scholar]
- 28.van der Most RG, Currie AJ, Mahendran S, Prosser A, Darabi A, Robinson BW, et al. Tumor eradication after cyclophosphamide depends on concurrent depletion of regulatory T cells: a role for cycling TNFR2-expressing effector-suppressor T cells in limiting effective chemotherapy. Cancer Immunol Immunother. 2009;58:1219–1228. doi: 10.1007/s00262-008-0628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jure-Kunkel M, Masters G, Girit E, et al. Synergy between chemotherapeutic agents and CTLA-4 blockade in preclinical tumor models. Cancer Immunol Immunother. 2013;62:1533–1545. doi: 10.1007/s00262-013-1451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lesterhuis WJ, Salmons J, Nowak AK, et al. Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity. PloS One. 2013;8:e61895. doi: 10.1371/journal.pone.0061895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu L, Yun Z, Tagawa T, Rey-McIntyre K, de Perrot M. CTLA-4 blockade expands infiltrating T cells and inhibits cancer cell repopulation during the intervals of chemotherapy in murine mesothelioma. Mol Cancer Ther. 2012;11:1809–1819. doi: 10.1158/1535-7163.MCT-11-1014. [DOI] [PubMed] [Google Scholar]
- 32.Hersh EM, O’Day SJ, Powderly J, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Invest New Drugs. 2011;29:489–498. doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]
- 33.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 34.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 35.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 36.Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24:75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- 37.Antonia SJ, Brahmer JR, Gettingger SN, et al. Nivolu-mab (anti-PD-1; BMS-936558, ONO-4538) in combination with platinum-based doublet chemotherapy (PT-DC) in advanced non-small cell lung cancer (NSCLC); Chicago, IL. ASCO Annual Meeting.2014. [Google Scholar]
- 38.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan RJ, Lawrence DP, Wargo JA, Oh KS, Gonzalez RG, Piris A. Case records of the Massachusetts General Hospital. Case 21-2013. A 68-year-old man with metastatic melanoma. N Engl J Med. 2013;369:173–183. doi: 10.1056/NEJMcpc1302332. [DOI] [PubMed] [Google Scholar]
- 41.Di Giacomo AM, Ascierto PA, Pilla L, et al. Ipilimu-mab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol. 2012;13:879–886. doi: 10.1016/S1470-2045(12)70324-8. [DOI] [PubMed] [Google Scholar]
- 42.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Griewank KG, Scolyer RA, Thompson JF, Flaherty KT, Schadendorf D, Murali R. Genetic alterations and personalized medicine in melanoma: progress and future prospects. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/djt435. djt435. [DOI] [PubMed] [Google Scholar]
- 44.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emery CM, Vijayendran KG, Zipser MC, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A. 2009;106:20411–20416. doi: 10.1073/pnas.0905833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montagut C, Sharma SV, Shioda T, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res. 2008;68:4853–4861. doi: 10.1158/0008-5472.CAN-07-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Connell MP, Marchbank K, Webster MR, et al. Hypoxia induces phenotypic plasticity and therapy resistance in melanoma via the tyrosine kinase receptors ROR1 and ROR2. Cancer Disc. 2013;3:1378–1393. doi: 10.1158/2159-8290.CD-13-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabbatino F, Wang Y, Wang X, et al. PDGFRalpha up-regulation mediated by sonic hedgehog pathway activation leads to BRAF inhibitor resistance in melanoma cells with BRAF mutation. Oncotarget. 2014;5:1926–1941. doi: 10.18632/oncotarget.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Allen EM, Wagle N, Sucker A, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Disc. 2014;4:94–109. doi: 10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villanueva J, Vultur A, Lee JT, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol. 2011;29:3085–3096. doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagle N, Van Allen EM, Treacy DJ, et al. MAP kinase pathway alterations in BRAF-mutant melanoma patients with acquired resistance to combined RAF/ MEK inhibition. Cancer Disc. 2014;4:61–68. doi: 10.1158/2159-8290.CD-13-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Straussman R, Morikawa T, Shee K, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frederick DT, Piris A, Cogdill AP, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khalili JS, Liu S, Rodriguez-Cruz TG, et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin Cancer Res. 2012;18:5329–5340. doi: 10.1158/1078-0432.CCR-12-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu C, Peng W, Xu C, Lou Y, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res. 2013;19:393–403. doi: 10.1158/1078-0432.CCR-12-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith MP, Sanchez-Laorden B, O’Brien K, et al. The immune microenvironment confers resistance to MAPK pathway inhibitors through macrophage-derived TNFalpha. Cancer Disc. 2014;4:1214–1229. doi: 10.1158/2159-8290.CD-13-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boni A, Cogdill AP, Dang P, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 63.Vella LJ, Pasam A, Dimopoulos N, et al. MEK inhibition, alone or in combination with BRAF inhibition, affects multiple functions of isolated normal human lymphocytes and dendritic cells. Cancer Immunol Res. 2014;2:351–360. doi: 10.1158/2326-6066.CIR-13-0181. [DOI] [PubMed] [Google Scholar]
- 64.Callahan MK, Masters G, Pratilas CA, et al. Paradoxical activation of T cells via augmented ERK signaling mediated by a RAF inhibitor. Cancer Immunol Res. 2014;2:70–79. doi: 10.1158/2326-6066.CIR-13-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilmott JS, Long GV, Howle JR, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18:1386–1394. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 66.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Translat Med. 2013;5 doi: 10.1126/scitranslmed.3006504. 200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frederick DT, Ahmed Z, Cooper ZA, et al. Society For Melanoma Congress. Philadelphia, PA: 2013. Stromal fibroblasts contribute to the up-regulation of PD-L1 in melanoma after BRAF inhibition; pp. 950–951. [Google Scholar]
- 68.Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, Hodi FS. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res. 2013;19:598–609. doi: 10.1158/1078-0432.CCR-12-2731. [DOI] [PubMed] [Google Scholar]
- 69.Cooper ZA, Frederick DT, Juneja VR, et al. BRAF inhibition is associated with increased clonality in tumor-infiltrating lymphocytes. Oncoimmunology. 2013;2:e26615. doi: 10.4161/onci.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koya RC, Mok S, Otte N, et al. BRAF inhibitor vemurafenib improves the antitumor activity of adoptive cell immunotherapy. Cancer Res. 2012;72:3928–3937. doi: 10.1158/0008-5472.CAN-11-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knight DA, Ngiow SF, Li M, et al. Host immunity contributes to the anti-melanoma activity of BRAF inhibitors. J Clin Invest. 2013;123:1371–1381. doi: 10.1172/JCI66236. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Hooijkaas A, Gadiot J, Morrow M, Stewart R, Schumacher T, Blank CU. Selective BRAF inhibition decreases tumor-resident lymphocyte frequencies in a mouse model of human melanoma. Oncoimmunology. 2012;1:609–617. doi: 10.4161/onci.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368:1365–1366. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 74.Puzanov I, Callahan M, Linette GP, et al. Phase I study of the BRAF inhibitor dabrafenib (D) ± the MEK inhibitor trametinib (T) in combination with ipilimumab (I) for V600E/K mutation-positive unresectable or metastatic melanoma (MM) 2014 ASCO Annual Meeting; Chicago, IL. 2014. [Google Scholar]
- 75.Corless CL, Heinrich MC. Molecular pathobiology of gastrointestinal stromal sarcomas. Ann Rev Pathol. 2008;3:557–586. doi: 10.1146/annurev.pathmechdis.3.121806.151538. [DOI] [PubMed] [Google Scholar]
- 76.Balachandran VP, Cavnar MJ, Zeng S, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–1100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol. 2010;28:2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dandamudi UB, Ghebremichael M, Sosman JA, et al. A phase II study of bevacizumab and high-dose interleukin-2 in patients with metastatic renal cell carcinoma: a Cytokine Working Group (CWG) study. J Immunother. 2013;36:490–495. doi: 10.1097/CJI.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 80.Hodi FS, Lawrence D, Lezcano C, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2:632–642. doi: 10.1158/2326-6066.CIR-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huguenin PU, Kieser S, Glanzmann C, Capaul R, Lutolf UM. Radiotherapy for metastatic carcinomas of the kidney or melanomas: an analysis using palliative end points. Int J Radiat Oncol Biol Phys. 1998;41:401–405. doi: 10.1016/s0360-3016(98)00021-2. [DOI] [PubMed] [Google Scholar]
- 82.Brenner DJ, Ward JF. Constraints on energy deposition and target size of multiply damaged sites associated with DNA double-strand breaks. Int J Radiat Biol. 1992;61:737–748. doi: 10.1080/09553009214551591. [DOI] [PubMed] [Google Scholar]
- 83.Lobrich M, Cooper PK, Rydberg B. Non-random distribution of DNA double-strand breaks induced by particle irradiation. Int J Radiat Biol. 1996;70:493–503. doi: 10.1080/095530096144680. [DOI] [PubMed] [Google Scholar]
- 84.Cole S. Long-term effects of local ionizing radiation treatment on Langerhans cells in mouse footpad epidermis. J Invest Dermatol. 1986;87:608–612. doi: 10.1111/1523-1747.ep12455853. [DOI] [PubMed] [Google Scholar]
- 85.James RF, Lake SP, Chamberlain J, et al. Gamma irradiation of isolated rat islets pretransplantation produces indefinite allograft survival in cyclosporine-treated recipients. Transplantation. 1989;47:929–933. doi: 10.1097/00007890-198906000-00001. [DOI] [PubMed] [Google Scholar]
- 86.Wasserman J, Blomgren H, Rotstein S, Petrini B, Hammarstrom S. Immunosuppression in irradiated breast cancer patients: in vitro effect of cyclooxygenase inhibitors. Bull NY. Acad Med. 1989;65:36–44. [PMC free article] [PubMed] [Google Scholar]
- 87.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 88.Golden EB, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC. The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol. 2012;2:88. doi: 10.3389/fonc.2012.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burnette B, Weichselbaum RR. Radiation as an immune modulator. Semin Radiat Oncol. 2013;23:273–280. doi: 10.1016/j.semradonc.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 90.El-Saghire H, Michaux A, Thierens H, Baatout S. Low doses of ionizing radiation induce immune-stimulatory responses in isolated human primary monocytes. Int J Mol Med. 2013;32:1407–1414. doi: 10.3892/ijmm.2013.1514. [DOI] [PubMed] [Google Scholar]
- 91.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 92.McBride WH, Chiang CS, Olson JL, et al. A sense of danger from radiation. Radiat Res. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- 93.Hauser SH, Calorini L, Wazer DE, Gattoni-Celli S. Radiation-enhanced expression of major histocompatibility complex class I antigen H-2Db in B16 melanoma cells. Cancer Res. 1993;53:1952–1955. [PubMed] [Google Scholar]
- 94.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hareyama M, Imai K, Kubo K, et al. Effect of radiation on the expression of carcinoembryonic antigen of human gastric adenocarcinoma cells. Cancer. 1991;67:2269–2274. doi: 10.1002/1097-0142(19910501)67:9<2269::aid-cncr2820670910>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 96.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perez CA, Fu A, Onishko H, Hallahan DE, Geng L. Radiation induces an antitumour immune response to mouse melanoma. Int J Radiat Biol. 2009;85:1126–1136. doi: 10.3109/09553000903242099. [DOI] [PubMed] [Google Scholar]
- 98.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 99.Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br J Radiol. 1975;48:863–866. doi: 10.1259/0007-1285-48-574-863. [DOI] [PubMed] [Google Scholar]
- 100.Postow MA, Callahan MK, Barker CA, et al. Immuno-logic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013;85:293–295. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grimaldi AM, Simeone E, Giannarelli D, et al. Absco-pal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780. doi: 10.4161/onci.28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1:365–372. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cotter SE, Dunn GP, Collins KM, et al. Abscopal effect in a patient with metastatic Merkel cell carcinoma following radiation therapy: potential role of induced antitumor immunity. Arch Dermatol. 2011;147:870–872. doi: 10.1001/archdermatol.2011.176. [DOI] [PubMed] [Google Scholar]
- 105.Okuma K, Yamashita H, Niibe Y, Hayakawa K, Nakagawa K. Abscopal effect of radiation on lung metastases of hepatocellular carcinoma: a case report. J Med Case Rep. 2011;5:111. doi: 10.1186/1752-1947-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fairlamb DJ. Spontaneous regression of metastases of renal cancer: A report of two cases including the first recorded regression following irradiation of a dominant metastasis and review of the world literature. Cancer. 1981;47:2102–2106. doi: 10.1002/1097-0142(19810415)47:8<2102::aid-cncr2820470833>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 107.MacManus MP, Harte RJ, Stranex S. Spontaneous regression of metastatic renal cell carcinoma following palliative irradiation of the primary tumour. Irish J Med Sci. 1994;163:461–463. doi: 10.1007/BF02940567. [DOI] [PubMed] [Google Scholar]
- 108.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 109.Liang H, Deng L, Chmura S, et al. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J Immunol. 2013;190:5874–5881. doi: 10.4049/jimmunol.1202612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Teulings HE, Tjin EP, Willemsen KJ, et al. Radiation-induced melanoma-associated leucoderma, systemic antimelanoma immunity and disease-free survival in a patient with advanced-stage melanoma: a case report and immunological analysis. Br J Dermatol. 2013;168:733–738. doi: 10.1111/bjd.12136. [DOI] [PubMed] [Google Scholar]
- 111.Abood A, Saleh DB, Watt DA. Malignant melanoma and vitiligo: can radiotherapy shed light on the subject? J Plastic Reconstruct Aesthet Surg. 2009;62:e119–e120. doi: 10.1016/j.bjps.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 112.Yee C, Thompson JA, Roche P, et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of t cell-mediated vitiligo. J Exp Med. 2000;192:1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Teulings HE, Overkamp M, Ceylan E, et al. Decreased risk of melanoma and nonmelanoma skin cancer in patients with vitiligo: a survey among 1307 patients and their partners. Br J Dermatol. 2013;168:162–171. doi: 10.1111/bjd.12111. [DOI] [PubMed] [Google Scholar]
- 114.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hiniker SM, Chen DS, Reddy S, et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Translat Oncol. 2012;5:404–407. doi: 10.1593/tlo.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in meta-static castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hazard LJ, Sause WT, Noyes RD. Combined adjuvant radiation and interferon-alpha 2B therapy in high-risk melanoma patients: the potential for increased radiation toxicity. Int J Radiat Oncol Biol Phys. 2002;52:796–800. doi: 10.1016/s0360-3016(01)02700-6. [DOI] [PubMed] [Google Scholar]
- 118.Nguyen NP, Levinson B, Dutta S, et al. Concurrent interferon-alpha and radiation for head and neck melanoma. Melanoma Res. 2003;13:67–71. doi: 10.1097/00008390-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 119.Conill C, Jorcano S, Domingo-Domenech J, et al. Toxicity of combined treatment of adjuvant irradiation and interferon alpha2b in high-risk melanoma patients. Melanoma Res. 2007;17:304–309. doi: 10.1097/CMR.0b013e3282c3a6ed. [DOI] [PubMed] [Google Scholar]
- 120.Paul E, Muller I, Renner H, Bodeker RH, Cochran AJ. Treatment of locoregional metastases of malignant melanomas with radiotherapy and intralesional beta-interferon injection. Melanoma Res. 2003;13:611–617. doi: 10.1097/01.cmr.0000056285.15046.ff. [DOI] [PubMed] [Google Scholar]
- 121.Lange JR, Raubitschek AA, Pockaj BA, et al. A pilot study of the combination of interleukin-2-based immunotherapy and radiation therapy. J Immunother. 1992;12:265–271. doi: 10.1097/00002371-199211000-00007. [DOI] [PubMed] [Google Scholar]
- 122.Seung SK, Curti BD, Crittenden M, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2— tumor and immunological responses. Sci Translat Med. 2012;4 doi: 10.1126/scitranslmed.3003649. 137ra74. [DOI] [PubMed] [Google Scholar]
- 123.Ridolfi L, de Rosa F, Ridolfi R, et al. Radiotherapy as an immunological booster in patients with metastatic melanoma or renal cell carcinoma treated with high-dose interleukin-2: evaluation of biomarkers of immunologic and therapeutic response. J Translat Med. 2014;12:262. doi: 10.1186/s12967-014-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Joseph RW, Sullivan RJ, Harrell R, et al. Correlation of NRAS mutations with clinical response to high-dose IL-2 in patients with advanced melanoma. J Immun-other. 2012;35:66–72. doi: 10.1097/CJI.0b013e3182372636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smith FO, Goff SL, Klapper JA, et al. Risk of bowel perforation in patients receiving interleukin-2 after therapy with anti-CTLA 4 monoclonal antibody. J Immunother. 2007;30:130. doi: 10.1097/01.cji.0000211334.06762.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ackerman A, Klein O, McDermott DF, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120:1695–1701. doi: 10.1002/cncr.28620. [DOI] [PubMed] [Google Scholar]
- 127.Ascierto PA, Simeone E, Sileni VC, et al. Sequential treatment with ipilimumab and BRAF inhibitors in patients with metastatic melanoma: data from the Italian cohort of the ipilimumab expanded access program. Cancer Invest. 2014;32:144–149. doi: 10.3109/07357907.2014.885984. [DOI] [PubMed] [Google Scholar]
- 128.Chan MM, Haydu LE, Menzies AM, et al. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer. 2014;120:3142–3153. doi: 10.1002/cncr.28851. [DOI] [PubMed] [Google Scholar]
- 129.Wolchok JD, Kluger H, Callahan MK, et al. Nivolu-mab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]