Abstract
Tissue engineering (TE) has provided promising strategies for regenerating tissue defects, but few TE approaches have been translated for clinical applications. One major barrier in TE is providing adequate oxygen supply to implanted tissue scaffolds, since oxygen diffusion from surrounding vasculature in vivo is limited to the periphery of the scaffolds. Moreover, oxygen is also an important signaling molecule for controlling stem cell differentiation within TE scaffolds. Various technologies have been developed to increase oxygen delivery in vivo and enhance the effectiveness of TE strategies. Such technologies include hyperbaric oxygen therapy, perfluorocarbon- and hemoglobin-based oxygen carriers, and oxygen-generating, peroxide-based materials. Here, we provide an overview of the underlying mechanisms and how these technologies have been utilized for in vivo TE applications. Emerging technologies and future prospects for oxygen delivery in TE are also discussed to evaluate the progress of this field towards clinical translation.
Introduction
TE approaches have the potential to address the worldwide shortage of donor tissues for transplantation. The TE approach for developing implantable tissues involves incorporating cells onto a scaffolding material for structural support, possibly with the addition of mechanical cues or chemical growth factors to elicit particular cell responses. Engineers have developed clinically tested biomaterials to regenerate tissues such as the trachea, nasal alar lobule cartilage, bladder, and vaginal wall; however these tissues are either largely avascular or less than 3 mm in thickness.1–5 One reason for the lack of clinical translation of larger implants is that the standard methods of TE rely on diffusion to transport oxygen throughout the scaffold, which results in higher oxygen concentrations towards scaffold edges and lower concentrations towards the center.
Consistent oxygen supply throughout implantable constructs is of particular importance due to oxygen’s crucial role as a metabolic substrate and signaling molecule. In low oxygen environments, mammalian cells must utilize lactic acid fermentation to produce ATP, which requires 15 times more glucose to produce the same amount of ATP as oxidative phosphorylation. When ATP stores are depleted, as in ischemic tissues, cell necrosis will occur.6 Hypoxia and nutrient deprivation, two hallmarks of ischemic tissue, have also been shown to induce apoptosis in mesenchymal stem cells, further highlighting the need to deliver oxygen along with transplanted cells.7 Oxygen concentration also serves as an important signaling molecule for differentiation. When cultured under hypoxic conditions, several stem cell lineages including human embryonic stem cells, hematopoetic stem cells, and mesenchymal stem cells exhibit reduced differentiation potential and increased maintenance of stem cell markers.8 Hypoxia-induced factors (HIFs) thought to be responsible for altering cell functions such as differentiation and proliferation in response to hypoxia.9 For example, inhibiting HIF-1α increased adipogenesis and decreased chondrogenesis in mesenchymal stem cells.10 As low oxygen tensions can lead to necrosis or apoptosis, but also may be necessary to control stem cell differentiation, steps should be taken to limit oxygen concentration within a range that enables stem cells to differentiate into target cell types, without compromising metabolic activity.
Low oxygen diffusion through scaffolds has been a limiting factor in TE scaffolds for both in vitro and in vivo applications. Within native tissues, oxygen can only diffuse 100–200 µm from capillaries meaning simple diffusion would not be sufficient to maintain cell viability in non-vascularized scaffolds much larger than 1 mm.11 For bone engineering, mineralized tissue characteristic of bone formation was only observed in the edges of scaffolds in vitro, with maximum penetration depths of approximately 200 µm.12,13 When hepatocytes were transplanted with scaffolds in vivo for liver repair, 95–99% of cells died within 7 days; the surviving cells were predominately located near vasculature in the periphery of the scaffolds.14 Due to the unequal distribution of oxygen, cells near the center of non-vascularized scaffolds tend to die, limiting the current effectiveness of tissue engineered scaffolds for large defect repair in vivo.
To overcome oxygen diffusion limitations in vivo, blood vessels from host vasculature must infiltrate implanted scaffolds or integrate with preassembled vasculature in the scaffold. Host-mediated angiogenesis alone is not adequate to combat scaffold center necrosis as blood vessel ingrowth occurs slowly, with full vascular penetration of a 3 mm scaffold taking 1–2 weeks and a 5 mm scaffold taking up to 35 days even when implanted near highly vascularized host tissue in vivo.15,16 Pre-vascularization and blood vessel engineering are methods engineers have been exploring to combat this issue; however these systems will have no oxygen or nutrient supply during the time it takes to integrate with the host vascular network. To support cells with enough oxygen to maintain metabolic activity and provide appropriate signals during the period of vessel ingrowth, incorporation of oxygen releasing materials may be necessary.
Despite the necessity of oxygen for cell survival, there is a need for oxygen release to be tightly controlled for four reasons: (1) hyperoxia has been shown to cause oxidative damage to cells by reactive oxygen species (ROS) production17, (2) oxygen concentration and ROS generation affects cell differentiation,18 (3) ROS acts as a mediator in the natural inflammatory process,19 and (4) moderate hypoxia has been shown to stimulate vascular infiltration20,21. It is crucial to understand the oxygen requirements for particular tissues and the rate of vascularization into particular tissues to ensure that there is enough oxygen to preserve cell viability, but to avoid inhibiting either vascularization or differentiation or causing tissue damage from inflammation or releasing an excess of oxygen.
The primary methods of oxygen delivery fall into three categories: hyperbaric oxygen therapy (HBO2), oxygen carrying materials, and oxygen generating materials. HBO2 has been used clinically to treat decompression sickness in divers since the 1920s, but has only recently been investigated for TE applications.22 Of the oxygen carrying materials, the most heavily investigated methods for TE applications are perfluorocarbon (PFC) technologies and hemoglobin-based oxygen carriers (HbOCs). Oxygen generating materials for TE applications have traditionally been comprised of peroxides and inorganic peroxide salts, taking advantage of the degradation of hydrogen peroxide into water and oxygen. The purpose of this literature review is to review traditional oxygen delivering technologies as well as new emerging technologies that hold promise for in vivo TE applications.
Current Technologies
Hyperbaric Oxygen (HBO2) Therapy
HBO2 has been investigated for many applications including wound healing and TE (Table 1). Patients undergoing hyperbaric oxygen therapy HBO2 breathe 100% O2 while in a chamber at increased atmospheric pressure, increasing oxygen tension in blood and tissues. HBO2 has been shown to increase cellular oxygen levels, which leads to the generation of ROS. Though ROS can be toxic to individual cells and cause systemic damage to the pulmonary and central nervous systems, this toxicity is dependent upon duration of treatment and concentration of ROS.23 In some studies, lower concentrations of ROS generated by the HBO2 have been shown to decrease levels of pro-inflammatory cytokines and increase growth factor and collagen synthesis; however, whether ROS have positive or negative effects on tissues depends on the concentration, intracellular location, and tissue type.24 These potentially therapeutic effects led to the investigation of HBO2 for TE applications such as ligament healing25, cartilage26, bone27,28, and oral mucosa29.
Table 1.
Summary of current O2 delivery technologies.
| Method | Stem Cell and Biomaterials Applications |
Pros | Cons |
|---|---|---|---|
| Hyperbaric oxygen therapy |
|
|
|
| Perfluorocarbon technologies |
|
||
| Peroxide-based treatments |
|
||
| Hemoglobin-based oxygen carriers |
|
HBO2 has been shown to improve TE outcomes in vivo. One study investigated whether HBO2 could improve healing of rat lacerated patellar ligaments. HBO2 was administered for a total of 10 sessions over two weeks. Histological sections, collagen I gene expression, and gross images showed that in all treatment groups, the lacerated patellar ligament had filled with fibroblasts and collagen II weeks after surgery, but this was not observed in the control.25 Another study used HBO2 in conjunction with scaffold implantation to test bone formation and vascularization in a rat calvarial defect model. They reported increased numbers of osteoprogenitor cells and endothelial cells in groups treated with HBO2.27 Similar results were seen in bone diaphyseal defects in rabbits.28 These studies show that increased oxygen tension has potential to improve TE outcomes.
Unfortunately, HBO2 may not be the most effective method for several reasons. As the main method of delivery is through arterial blood, it is unlikely that implanted tissues without any integration into the host vasculature would benefit significantly. Furthermore, HBO2 does not provide oxygen in a sustained manner and the treatment requires specialized equipment, requiring the patient to return to the hospital consistently for any beneficial effect to be observed. Additionally, HBO2 is delivered systemically instead of locally and is known to cause rare but dangerous side effects, such as grand mal seizures, in some individuals.30 Though investigating HBO2 has shown that increasing oxygen partial pressures is likely beneficial to the tissue healing and remodeling process, a sustained delivery method that is sensitive to local partial pressures of oxygen would be preferred.
Hemoglobin-based oxygen carriers (HbOCs)
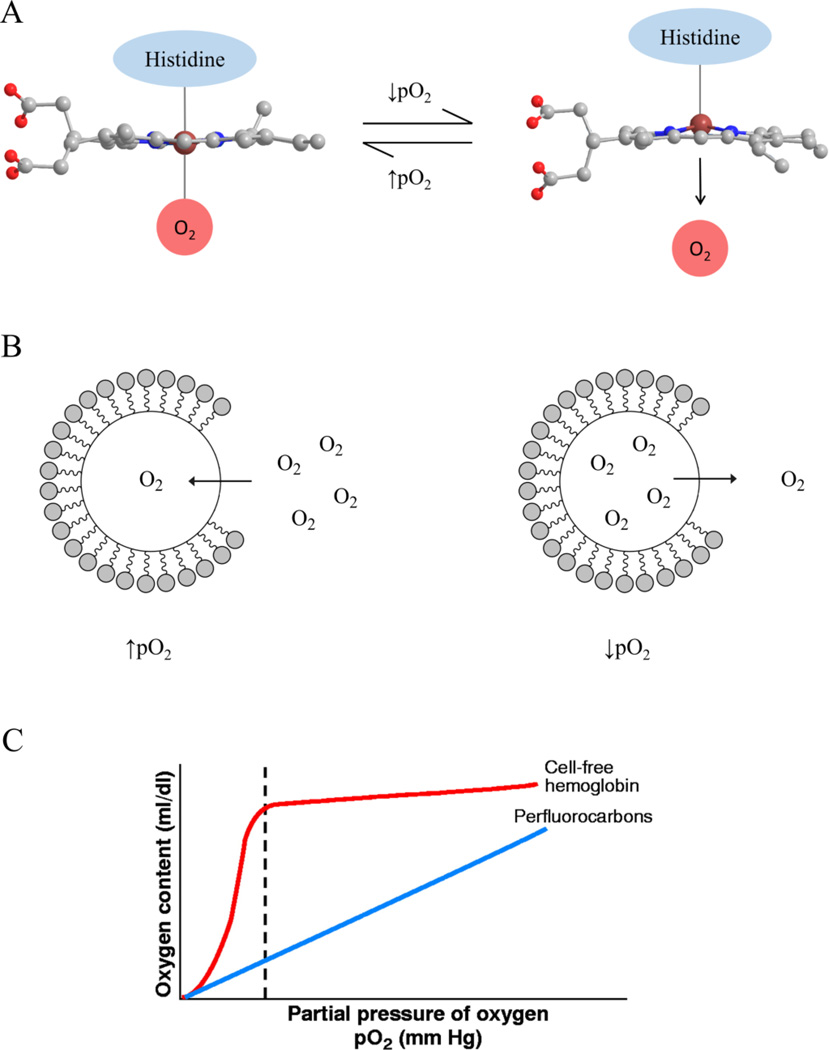
Hemoglobin-based oxygen carriers (HbOCs) use hemoglobin extract from human or mammalian red blood cells red blood cells in order store and delivery oxygen to human tissues. HbOCs work similarly to the hemoglobin in red blood cells; changes in chemical conformation of hemoglobin allows for selective binding and releasing of O2. Binding of hemoglobin to O2 occurs when the pO2 of the environment increases past a given threshold. During this process, the heme molecule within hemoglobin changes its conformation from a “taut” state to a “relaxed” state, allowing it to readily chemically bind to O2 (Figure 1A). Heme returns to its taut conformation and releases O2 when the pO2 decreases below the given threshold. In contrast to the linear relationship of O2 saturation within PFCs to pO2, O2 saturation in HbOCs exhibits a sigmoidal relationship with pO2 (Figure 1C). This would result in O2 release by HbOCs only during hypoxic environments, in which O2 demand is highest.
Figure 1.
O2 loading and release mechanisms of (A) HbOCs and (B) PFC-based oxygen carriers. (C) Relationship of O2 content as a function of pO2 in HbOCs and PFC based oxygen carriers, as reproduced from Ref. 28. Heme molecules in HbOCs chemically bind and unbind to O2 at a specific pO2 range, resulting in the sigmoidal relationship between pO2 and O2 content. In contrast, uptake and release of O2 from PFCs is dictated by Henry’s Law, in which the amount of O2 dissolved in PFCs is linearly related to pO2.
Although some HbOCs have been clinically tested, there remains some safety concerns. Reasons for failure of first-generation HbOCs in clinical trials included hypertension, elevation in pancreatic and liver enzymes, renal and neural toxicity, and oxidative stress31,32. In particular, hypertension was attributed to nitric oxide (NO) extravasation and premature release of O2 in the arterioles, both of which cause vasoconstriction.32,33 NO extravasation also likely contributed to the elevation in liver and pancreatic enzymes in clinical trials.31 Oxidation of hemoglobin to methemoglobin – which releases reactive ferryl ions and radicals – was the primary cause for oxidative stress and tissue toxicity observed with clinically-tested HbOCs.31,34
Due to these safety concerns, new HbOCs are being developed to eliminate the negative side effects of first-generation HbOCs. Several groups have developed HbOCs with specific sizes to minimize cytotoxicity, inflammation, and NO extravasation. In study performed by Lai et. al, cross-linked hemoglobin and albumin microparticles above 9 µm in diameter decreased cellular uptake by macrophages and increased viability of endothelial and liver cells in vitro.35 Moreover, the microparticles – regardless of size – had a higher oxygen retention (P50 = 9.3–9.7 mmHg O2), which could potentially prevent vasoconstriction caused by premature release of O2 in arterioles. Xiong et al. also fabricated cross-linked hemoglobin and albumin particles with a 700 µm diameter to prevent migration through the endothelium.36 When they perfused isolated mice glomeruli with these microparticles in vitro, no significant vasoconstriction occurred throughout an 11 min interval. However, further studies are needed to confirm that these hemoglobin-based particles do not cause vasoconstriction in vivo.
With regards to decreasing oxidation of hemoglobin to metHb, Xiong et al. found that adding ascorbic acid and NaBH4 – both reducing agents – decreased metHb content within their hemoglobin microparticles from 15–32% to 7%.37 This coincided with a decreased phagocytosis rate (less than 5%) relative to the non-reduced groups when the hemoglobin microparticles were incubated with heparinized whole human blood for 30 min.37 Given that oxidative stress was a primary cause for failure of first-generation HbOCs in clinical trials, treating HbOCs with reducing agents could potentially eliminate hemoglobin-induced oxidative stress and help progress this technology towards clinical translation.
In vitro biomaterials applications
Various HbOCs have been utilized as a media supplement in vitro to provide more physiologically relevant levels of O2 and improve cell viability and function in 3D culture systems. For example, Chen et al. used purified bovine hemoglobin-supplemented medium in a hollow fiber, perfusion bioreactor to engineer liver tissue.38 They demonstrated that hemoglobin-supplemented medium increased levels of aerobic respiration and drug metabolism in hepatocytes with respect to the control. However, there was also an upregulation of genes associated with ROS metabolism, which suggests that the hemoglobin oxidation was affecting cell behavior. To prevent oxidative stress, Centis et al. encapsulated cells in fibrin gels to minimize cell-HbOC contact.39 HbOC-supplemented medium increased cell viability and downregulated expression of HIF-1α, a gene associated with cellular response to hypoxia. Given these results, this system could be further adapted to study effects of varying O2 concentration on stem cell differentiation within hydrogels to better inform techniques used in vivo.
Besides the technologies presented in these studies, no significant work has been published that apply HbOCs to TE. One reason for the lack of TE-related studied is that HbOCs have had lesser success in clinical trials than perfluorocarbons, another artificial blood substitute that has been widely studied.40 However, with recent improvements that make them safer, HbOCs could potentially provide benefits over other O2 delivery technologies. HbOCs only release O2 in hypoxic environments, in which cellular oxygen demand is highest. This could be essential for engineering specific tissue types that are negatively affected by higher O2 concentrations, but still need O2 delivered during hypoxia. Moving forward, more studies that use new HbOCs technologies – as opposed to first generation HbOCs – are needed to evaluate their potential for translational TE applications.
Perfluorocarbons (PFCs) technologies
PFCs consist of fluorinated carbon chains that exhibit various properties that make them highly suitable oxygen carrier for biological applications (Figure 1B). First, the strength of C-F bonds within PFCs makes them inert and thus biocompatible with living tissues.41 PFCs are also hydro- and lipophobic and self-assemble in aqueous solution, which contributes to their structural stability in living tissues.42 Lastly, PFCs have a low polarizability and are miscible with non-polar gases such as O2, CO, CO2, and NO.42 This property enables PFCs to dissolve O2 by physically entrapping the molecules via van der Waals forces. The amount of dissolved O2 within a given PFC is defined by Henry’s law, in which the solubility of O2 is linearly dependent upon the partial pressure of O2 (pO2) within the environment (Figure 1C).43 This allows PFCs to store and release O2 based upon the metabolic demands and oxygen availability of a given tissue.
Several PFC emulsions have been clinically tested as artificial blood substitutes with some success. Of the emulsions that have been clinically tested, Oxygent (Alliance Pharmaceutical Corp.), an emulsion containing 58% perflubron, has shown the most promising results in terms of biocompatibility, oxygen carrying capacity, and emulsion stability in Phase I and II trials.44 However, patients in phase III clinical trials experienced increased stroke risk and adverse neurological side effects.40,44 Although, aggressive procedures performed in these trials left it unclear whether Oxygent was the cause of adverse clinical outcomes.40 No PFCs are currently approved by the FDA, but they have overall shown greater clinical potential than other material-based O2 delivery technologies.
In vitro biomaterials applications
Like HbOCs, PFCs have also been used as a media supplement in vitro to deliver O2 to cells in 3D culture systems. For example, in experiments performed by Radisic et al., media supplemented with 11% v/v Oxygent was used to engineer cardiac muscle on a porous, poly(glycerol-sebacate) scaffold.45 The authors found that supplementing the medium with Oxygent improved contractile function, higher cell viability, and increased expression of connexin-43 and cardiac troponin I. Other studies using similar bioreactor setups have also found that supplementing culture medium with PFC emulsions improved cell viability, differentiation, and function for tracheal and hepatic tissue constructs.46,47 However, one of these studies found that adding PFC emulsions to their bioreactor setup decreased chondrogenesis in the cartilaginous layer of tracheal constructs. Thus, supplementing culture medium with PFC emulsions may only benefit specific cell types.46
Another in vitro application of PFCs has utilized the hydrophobicity and high density of PFCs to culture scaffolds between immiscible layers of aqueous medium and PFCs. This approach would allow the aqueous medium on top of the scaffold to provide nutrients to cells, while the PFC layer on bottom would deliver O2. Pilarek et al. used this setup to culture CP5 chondrocytes on electrospun polylactic scaffolds.48 After 7 days of culture, they found that this layered-culture system yielded higher cell viability, proliferation, and ECM deposition on the scaffold in comparison to samples grown in culture medium alone. While this in vitro setup has yet to be used in other TE-specific studies, it could provide a platform for studying cell-biomaterial interactions within O2 levels that are more physiologically relevant, and for investigating the effects of varying O2 levels on stem cell differentiation.
In vivo biomaterials applications
PFCs have been incorporated into biomaterials for a variety of applications, including bone49,50, hepatic51,52, pancreatic53, and neural54,55 TE. PFC-based biomaterials were initially fabricated by encapsulating cells and PFC emulsions into hydrogels. Fabrication of these biomaterials is performed by mixing PFC emulsions with a polymeric solution, adding cells to the solution, and crosslinking the polymer in the solution to yield a gel consistency. As an example, Chin. et al. fabricated alginate hydrogel beads with perfluorooctylbromide (PFOB) emulsions in effort to increase cell viability and metabolic activity in tissue-engineered liver constructs.52 They found that alginate beads with PFOB emulsions had higher cell viability, metabolic activity, and glucose and O2 consumption in comparison to beads without PFOB in 7 days of normoxia. Another group has also shown that incorporating perfluorotributylamine (PFTBA) into alginate enhances human mesenchymal stem cell viability and osteogenic differentiation while decreasing chondrogenic differentiation and expression of hypoxic-related genes.50 Moreover, they demonstrated that alginate and fibrin hydrogels with PFTBA emulsions promote more ectopic bone formation in vivo in comparison to hydrogels without PFTBA.49,50 Fibrin hydrogels with PFTBA emulsions have also shown to enhance Schwann cell differentiation and migration, demonstrating the ability of oxygen delivery to promote tissue formation in a variety of applications.54
While promising results have been demonstrated for hydrogels supplemented with PFC emulsions, some drawbacks have been observed. Goh et al. showed that there was a steep decrease in βTC-tet insulinoma cell viability and metabolic activity when cultured under hypoxic (2% O2) conditions after 4 days, regardless of whether the beads were supplemented with PFTBA.53 The authors attributed these results to the low O2 carrying capacity of PFC emulsions, supporting their explanation with computer simulations that showed that PFTBA-supplemented beads results had similar dissolved O2 concentrations to the control. They claimed that the concentration of PFTBA emulsions needed to significantly raise the O2 would make the alginate beads mechanically unstable and cytotoxic due to increased levels of surfactant. Another study by White et al. showed that adding 10% PFOB to 1% alginate gels with 1% or 2% w/v Pluronic F68 surfactant significantly decreases fracture stress and protein transport in comparison to gels without PFOB.56 These studies demonstrate challenges with PFC emulsion-based hydrogels in engineering tissues that require mechanical strength or protein transport to function.
To mitigate the issues from hydrogels with PFC emulsions, several groups have developed hydrogels functionalized with PFCs.55,57,58 Li et al. fabricated a fluorinated methacrylamide chitosan hydrogel (MACF) with various aliphatic and aromatic PFCs to engineer neural tissue from neural stem/progenitor cells (NSPCs).55 In 8 days of normoxic culture, they found that the NSPCs encapsulated within MACF hydrogels had higher cell viability and neural differentiation in comparison to the MAC control. Moreover, there was no statistical difference between the elastic modulus of the MAC control and MACF hydrogels. Another study by Palumbo et al. showed that functionalized hyaluronic acid (HA) hydrogels with 1,2,4-oxadiazole also resulted in higher cell viability after 7 days of hypoxic culture; although, viability still decreased over the course of the experiment.57 Unlike hydrogels with PFC emulsions, these fluorinated hydrogels can also be tuned to release different levels of O2, which could be beneficial for guiding stem cell differentiation into targeted cell types.
Besides these hydrogels, Seifu et al. developed a solvent-casted, salt-leached poly(carbonate urethane) with fluorinated-zeolite microparticles to overcome issues with PFC emulsions.59 Fluorinated-zeolite microparticles, which consist of a zeolite Y core coated with a monolayer of perfluorodecyltrethoxysilane, on the surface avoid cytotoxicity concerns since they do not require a stabilizing surfactant as in PFC emulsions. With these particles incorporated into the porous scaffold, there was significantly higher proliferation and infiltration of smooth muscle cells into the scaffold after 7 days of normoxic culture. Given that porous, synthetic scaffolds have shown much promise in TE, incorporating PFC-based microparticles into these scaffolds may solve issues associated with cell viability and infiltration at the scaffold center.60
While PFC-based biomaterials have demonstrated an ability to increase cell viability, promote cell differentiation, and maintain cell metabolism for various tissue types, their inability to provide a sustained release of O2 may be a potential barrier for in vivo success. Vascularization of 3–5 mm scaffold has been shown to take 1–3 weeks in vivo even when placed near highly vascularized areas, which means that O2 supply in the construct is depleted for at least the first week post-implantation.15,16 In vitro, PFC-supplemented biomaterials provide significant amounts of O2 to cells for up to 4–8 days in normoxic and 3 days in hypoxic conditions (Figure 2).50,55,57,58 Moreover, most O2 release occurs within 24 hours after cell seeding (Figure 2).50,58 Since minimal in vivo testing has been performed with PFC-based biomaterials, it is unclear whether PFCs would supply enough O2 to sustain the TE scaffold before vascularization occurs.
Figure 2.
O2 release of (A) PFC-enriched fibrin gel in normoxic and hypoxic conditions, as adapted from [71]. Dissolved O2 content within the culture medium was significantly higher than the control for up to 84 hours. (B) Oxygen release over time in 0.5% O2 from PLGA cylinders with incorporated calcium peroxide without cells as reproduced, as adapted from [49].
To address this issue, methods to sustain the O2 release of PFCs should be investigated to further their potential for clinical use. Techniques such as encapsulating PFCs within materials that are less O2 permeable could help extend O2 release. For example, microparticles encapsulating perfluorooctane in a within shell of polycaprolactone provided more O2 release to cells in comparison to the control for at least 14 days in normoxic conditions.61 Incorporating shelled-particles into tissue engineered constructs could potentially sustain O2 release long enough to allow vascularization and robust tissue formation to occur in vivo.
Peroxides
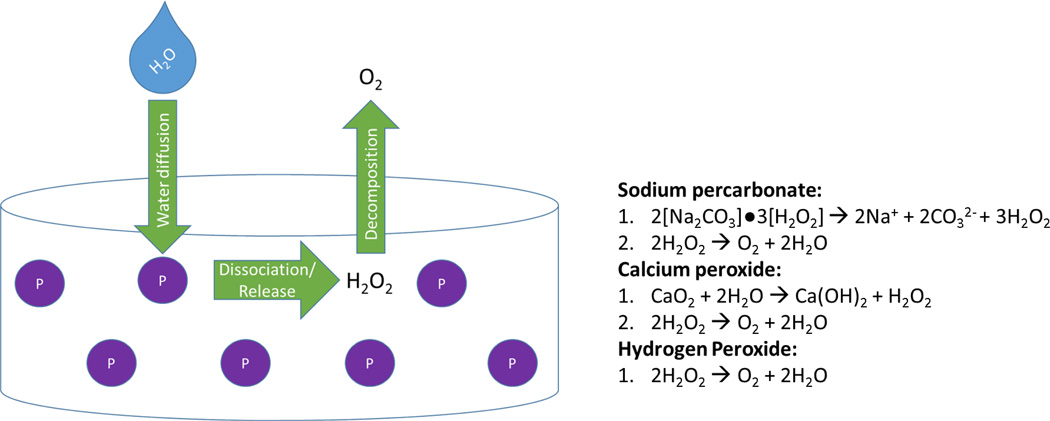
The most heavily investigated oxygen generating biomaterials involve the decomposition of hydrogen peroxide into molecular oxygen directly, in the case of hydrogen peroxide,62–65 or through following dissolution of solid peroxides, in the cases of sodium percarbonate66–68 and calcium peroxide.67,69,70 Sodium percarbonate is a solid adduct of sodium carbonate and hydrogen peroxide that decomposes into ions and hydrogen peroxide in aqueous solution. Calcium peroxide is another solid molecule that releases hydrogen peroxide when exposed to water. In all cases, hydrogen peroxide is then degraded into oxygen and water. The chemical reactions of these species are presented in Figure 2.
For biomaterials applications, peroxides have been incorporated into bulk polymers and hydrogels as depicted in Figure 2. Specific examples are described in detail below.
The use of peroxides as an oxygen delivery technique has promise, because the materials generate oxygen instead of simply delivering it; however, much work was done to ensure that oxygen delivery could occur over a sustained time period. One group measured oxygen release after incorporating sodium percarbonate into PLGA films and found that sodium percarbonate was depleted after 70 hours in vitro.66 The biomaterials protected devascularized skin flaps from necrosis in vivo for 3 days, but as oxygen was depleted before vessels from the host could infiltrate the scaffold, no protective effect was observed at 7 day time point.66 This study highlighted the potential for oxygen generating materials to prevent necrosis, but that effects would be transient if peroxide depletion occurred before the tissue was sufficiently vascularized. Though liquid hydrogen peroxide, sodium percarbonate, and calcium peroxide are all capable of producing oxygen, release rates differ depending upon the peroxide used. For example, oxygen generation from peroxide decomposition is much more rapid in the case of sodium carbonate compared to calcium peroxide.71 If sustained release is the goal, it is likely that the slower release time of calcium peroxide could provide more sustained release than sodium percarbonate.
Subsequent studies of inorganic peroxides for oxygen generation used calcium peroxide, likely because of its longer release time. Of particular note, porous PLGA cylinders containing calcium peroxide sustained oxygen release for 10 days, which falls within the 1–2 week range needed for vascularization of a 1 mm defect (Figure 3).69 This study did not test their materials in vivo, but noted that 3T3 fibroblasts cultured under hypoxic conditions (1% O2) with calcium peroxide scaffolds for 10 days were more metabolically active than cells cultured without calcium peroxide.
Figure 3.
Peroxide-based oxygen generating materials (P) for tissue engineering applications incorporate peroxide salts or liquid hydrogen peroxide within microparticles into bulk polymers or hydrogels. If solid peroxides are used, when water diffuses into the scaffold it causes the sodium percarbonate or calcium peroxide to dissociate into ions and hydrogen peroxide, as in the reactions on the right. Alternatively, liquid hydrogen peroxide can be encapsulated into microspheres and released from the material. In either case, hydrogen peroxide then decomposes into oxygen, which cells can uptake, and water.
Another method, hydrophobic polymers, limit the amount of water that comes into contact with the peroxides and have been used to extend oxygen release times. Calcium peroxide was incorporated within polydimethylsiloxane (PDMS), a biostable, hydrophobic, oxygen permeable polymer. Oxygen release was relatively high for the first two weeks, with some release still occurring at 7 weeks.70 The longer release rates resulted in improved measures of viability for pancreatic islets and β cells in vitro. The main drawback of this technology is that PDMS is not biodegradable and would either remain in the body indefinitely or need to be removed surgically.
The second challenge with using peroxide salts for oxygen generation was the release of cations into solution, which may negatively impact some cell types. For this reason, groups have also investigated whether hydrogen peroxide could be used for oxygen generating biomaterials. In a set of studies, Ng et al. developed a double emulsion solvent evaporation method for encapsulating hydrophilic low molecular weight drugs (such as hydrogen peroxide) within PLGA microparticles and Abdi et al. then tested these particles in vitro within a 3D alginate matrix containing catalase.63,64 Viability experiments showed that culturing cells in hypoxic conditions (1% O2) resulted in only 60–65% viability, but incorporation of the oxygen generating microparticles resulted in nearly 100% viability. The main drawback of these materials is that oxygen generation lasts only 5 hours, which would likely be insufficient to improve cell viability in hypoxic conditions in vivo. Subsequent studies by Mallepally et al.62 and Li et al.65 extended oxygen generation from hydrogen peroxide to 24 hours by using poly(methyl methacrylate) as the encapsulating material and to 14 days by conjugating H2O2 to a high molecular weight polymer which slowly diffuses through a PLGA shell, respectively.
A third challenge with peroxide-based oxygen generating systems is that they produce cytotoxic ROS as byproducts. The method used by many groups highlighted here to overcome this concern is incorporation of catalase, an enzyme used in peroxisomes to convert hydrogen peroxide into oxygen and water without ROS production. One study that found burst release of calcium peroxide from PCL nanofibers caused cytotoxic morphological and proliferative changes in osteoblasts at day 1, but following a media change these effects were reversed.72 This may mean that cell lines resistant to oxidative stress may be able to proliferate and differentiate as normal, but more studies should test how much hydrogen peroxide is released from biomaterials and whether those concentrations effect stem cells morphology, proliferation, and differentiation. Currently, there is still some controversy as to whether peroxide-based oxygen generating biomaterials without catalase would be a safe method for oxygen delivery in vivo, particularly since ROS are biological signaling molecules and effectors for the innate inflammatory response, which can lead to tissue injury.19 In a normal inflammatory process, neutrophils and M1 macrophages produce ROS to elicit a multitude of responses to initiate inflammation including ROS-mediated killing of engulfed bacteria. Changes in endothelial cell structures that allow neutrophils and macrophages to migrate from vasculature into tissues such as decreased strength of occludins proteins in tight junctions, and increased expression of leukocyte-specific cell adhesion molecules on the endothelial cell membrane can also be ROS-induced.19 Finally, hydrogen peroxide within the cell can increase expression of the pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α.19 It is currently unclear whether the use of oxygen-delivering biomaterials will cause or exacerbate inflammation at the implant site and research in this area is of critical importance before these technologies can be translatable.
In vivo biomaterials applications
Groups have explored the use of oxygen generating peroxides to generate oxygen for particular TE applications including wound healing,67 myocardial infarction,65 pancreatic islets and β cells,70 and adipose transplants73. Though the applications are different, biomaterial designs for incorporated peroxides have been very similar between groups: embedding either peroxide salts or liquid peroxide microspheres into synthetic polymers or alginate matrices.
For example, Chandra et al. developed a multi-layered oxygen generating biomaterial for wound healing using peroxide salts within a synthetic polymer.67 The layer of the bandage in contact with skin is made of gelatin, to absorb fluid from the wound. This fluid will seep into the second layer, containing solid calcium peroxide and sodium percarbonate within polyvinyl alcohol, which will release oxygen in an aqueous environment. The third layer quenches hydrogen peroxide by including MgCl and the fourth layer is made of a synthetic polymer with low gas permeability. In a porcine model, groups with the bandages showed faster wound closure and a higher density of new blood vessels formed.
To treat myocardial infarction, Li et al. conjugated H2O2 to a higher molecular weight molecule, PVP to slow peroxide release.65 The PVP/H2O2 conjugates were encapsulated within PLGA microsphere shell, which were subsequently loaded into a thermosensitive injectable hydrogel. Biomechanically, the hydrogels had a similar modulus (40 kPa) to gels shown to induce differentiation of cardiosphere-derived cells into cardiomyocytes. When tested without cells, materials kept oxygen tensions above 15% for 14 days. Peroxide biomaterials improved cell viability and differentiation in hypoxia compared to cells grown without peroxide biomaterials.
Oxygen generating biomaterials using peroxides have overcome several challenges to develop materials that could potentially be used for in vivo tissue applications. Several groups have observed release times longer than the period necessary for vascular infiltration. Peroxide incorporation also does not affect mechanical integrity of scaffolds at concentrations necessary for oxygen delivery. However, the drawbacks to these technologies are that they may produce ROS in concentrations that are cytotoxic to cells or induce local inflammation, the solid peroxides release ions into solution, and the materials are less sensitive to local oxygen concentration than PFCs or HbOCs which could cause hyperoxia.
Emerging Technologies
Photosynthetic Algae
Given that existing O2 delivery techniques have yet to produce promising results in vivo, recent technologies have been developed to improve clinical translation success of tissue engineered constructs. One technology utilizes the photosynthetic abilities of algae to supply a constant supply of O2 within constructs for wound healing. In a study performed by Hopfner et al., 3T3 fibroblasts were co-cultured with photosynthetic C. reinhardtii algae and exposed to continuous light for 22 hours in hypoxic conditions (1% O2).74 Fibroblasts co-cultured with algae had significantly lower expression of HIF-1α relative to those cultured alone, which was correlated with significantly elevated levels of O2 in co-culture medium. In a subsequent study, algae were genetically engineered to secrete vascular endothelial growth factor (VEGF) to promote vascularization as well as provide O2 in tissue constructs.75 When the algae-incorporating collagen gels were implanted subcutaneously into mice, scaffolds with genetically modified algae had longer vessel formation, more CD31 positive cells, and higher levels of VEGF after 7 days post implantation. To further evaluate the regenerative potential of these photosynthetic biomaterials, more work is needed to determine whether the algae induced an inflammatory or immune response in vivo. Moreover, these biomaterials require extended periods of light exposure in order to produce O2, so their application would be limited to skin and some subcutaneous tissues.
Myoglobin-polymer-surfactant complex
Another technology recently reported by Armstrong et al. involved functionalizing stem cells with a myoglobin-polymer-surfactant complex, [Mb_C][S], that would provide each cell with its own O2-delivering molecule.76 To produce the protein-surfactant complex, they conjugated a cationized myoglobin protein with an anionic surfactant, yielding an amphiphilic molecule capable of anchoring to the cell membrane. They combined the [Mb_C][S] with hMSCs and tested the ability of the hMSCs to form cartilage within non-woven polyglycolic acid (PGA) scaffolds. After culturing the modified hMSCs in the scaffolds for 35 days, they found modifying hMSCs with [Mb_C][S] significantly increased the ratio of collagen II/I expression and reduced the size of the necrotic center from 42 ± 24 to 7 ± 6 percent. While this technique requires further evaluation and development, it could provide an effective to alternative to current O2-deliverying biomaterials and be used for a wide variety of TE applications.
Microtanks
Another development has been the utilization of commercially available hollow spheres, termed microtanks, to supply oxygen for TE.77 These polyacrylonitrile spheres have been incorporated into bulk PCL disks and then hyperbarically loaded with 100% oxygen. When incorporated into culture, oxygen diffuses out of the microtanks and into the culture medium. These materials showed release for approximately 24 hours, but release kinetics can be altered by the concentration of microtanks as well as the thickness and permeability of the microtank shell and the bulk polymer. When cultured with cells under anoxia for 6 days, the experimental groups showed similar proliferation and metabolic rates to those cultured in normoxia, showing promise for these materials in TE. Improvements in this area can be made by using a biodegradable material for the microtanks and extending release time.
Endoperoxides
One drawback of peroxide based oxygen generating materials is the potential cytotoxicity of peroxide/catalase mixtures or uncontrolled release of sodium and calcium ions. To address this concern, Benz et al. incorporated endoperoxides into small organic molecules for oxygen release.78 When in contact with water, these molecules release oxygen via a retro Diels-Alder reaction. The endoperoxides were added into solution at various concentrations, achieving oxygen release for 8–13 hours. It is likely that this release time would improve by covalent binding to a synthetic polymer. Cytotoxicity was mitigated by addition of vitamin C, which can quench singlet oxygen. Under anoxic conditions, 3T3 fibroblast and smooth muscle cell cytotoxicity was negligible when vitamin C was included. It would be enlightening to see these materials incorporated into biomaterials to test for effects on differentiation in the future.
MIcrobubbles
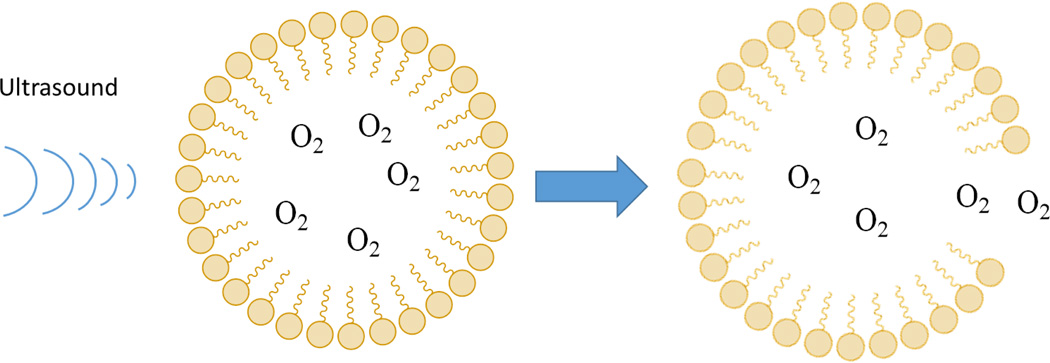
Another interesting technology that has extensive use clinically, but has never been used for TE involves microbubbles. These consist of gaseous cores surrounded by thin shells that have been composed of lipids, polymers, surfactants, dextran, and chitosan.79 Microbubbles have been used primarily as ultrasound contrast agents, but recently have also been used to deliver oxygen transiently to hypoxic tumor sites to improve the effectiveness of radiation therapy.80 The unique characteristic of microbubbles is that they can be burst locally using ultrasound in vivo, releasing loaded oxygen to a particular area of interest (Figure 4). Though further work must be done to improve stability in vivo, these molecules may be useful for TE applications as well.
Figure 4.
Microbubbles filled with O2 or other therapeutic gases can be burst released locally in vivo by ultrasound waves, as depicted in this schematic.
Conclusion
O2 delivery methods in TE are essential for tissue survival and function, especially in large-scale grafts where O2 diffusion at the center is limited. While various methods have been investigated to deliver O2 to TE scaffolds in vivo, no specific technologies have demonstrated clear promise in translating TE therapies to the clinic. PFC-based biomaterials could be advantageous in directing stem cell differentiation of various cells types since their O2 release is sensitive by cell demand, while peroxides release O2 by a chemical reaction independent of the environmental O2 concentration. Moreover, PFCs have fewer biocompatibility concerns than peroxides, which produce ROS as a by-product of their chemical reactions. However, peroxide-based biomaterials provide a more sustained release of O2 than PFC-based biomaterial while maintaining their mechanical integrity.
Emerging technologies, such as photosynthetic biomaterials and cell priming with Mb-protein-surfactant complexes, could provide tunable O2 delivery without compromising the mechanical integrity of a tissue construct. However, achieving FDA approval for these technologies could be difficult given the complex biological techniques they use. Additionally, photosynthetic biomaterials can only deliver O2 when exposed to light, which would limit their application to skin and some subcutaneous tissues. Newly developed HbOCs also have potential to provide safe, controlled O2 delivery in TE scaffolds, but they have yet to be incorporated into biomaterials.
In order to progress O2 delivery technologies to the clinic, there are several avenues of investigation that could guide further development. For in vitro studies, consistent methods for measuring oxygen release should be established to allow scientists to discern which methods could be the most clinically relevant for particular TE applications. This would provide a direct means of comparing different O2 delivery technologies prior to in vivo testing. Additionally, for HbOCs and peroxides, tests should be performed to measure reactive oxygen species generation from their materials to ensure cell health and viability. More studies should also be performed to evaluate the range of oxygen that should be delivered to a particular tissue to avoid damage from hypoxic or hyperoxic conditions and to maximize release of vasculogenic signals in vivo.
Alongside these in vitro studies, defining parameters, such as scaffold geometry and cell type, that affect the time for vascularization to occur in vivo will help determine O2 demand of specific tissue types. Finally, more in vivo studies are needed to determine whether currently available materials, such as the ones discussed in this review, could supply sufficient oxygen to tissues as functional vasculature is developing. Such studies may be used to construct more targeted specifications for O2-delivering biomaterials and determine which technologies are most suitable for clinical translation.
Acknowledgments
This work was supported by an NIH Biomedical Engineering Training Grant (ALF and ANR), NSF Graduate Research Fellowships (ALF and ANR), and an R03 grant from the National Institute of Biomedical Imaging and Bioengineering (WLG).
References
- 1.Haykal S, Salna M, Waddell TK, Hofer SO. Plast. Reconstr. Surg. Glob. Open. 2014;2:e178. doi: 10.1097/GOX.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, Dickinson SC, Hollander AP, Mantero S, Conconi MT, Birchall MA. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 3.Fulco I, Miot S, Haug MD, Barbero A, Wixmerten A, Feliciano S, Wolf F, Jundt G, Marsano A, Farhadi J, Heberer M, Jakob M, Schaefer DJ, Martin I. Lancet (London, England) 2014;384:337–346. doi: 10.1016/S0140-6736(14)60544-4. [DOI] [PubMed] [Google Scholar]
- 4.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 5.Raya-Rivera AM, Esquiliano D, Fierro-Pastrana R, López-Bayghen E, Valencia P, Ordorica-Flores R, Soker S, Yoo JJ, Atala A. Lancet. 2014;384:329–336. doi: 10.1016/S0140-6736(14)60542-0. [DOI] [PubMed] [Google Scholar]
- 6.Boutilier RG, St-Pierre J. Comp. Biochem. Physiol. Part A. 2000;126:481–490. doi: 10.1016/s1095-6433(00)00234-8. [DOI] [PubMed] [Google Scholar]
- 7.Zhu W, Chen J, Cong X, Hu S, Chen X. Stem Cells. 2006;24:416–425. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]
- 8.Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Malladi P, Xu Y, Chiou M, Giaccia AJ, Michael T, Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Am. J. Physiol. Cell Physiol. 2006;94305:1139–1146. [Google Scholar]
- 11.Thomlinson RH, Gray LH. Br. J. Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. J. Biomed. Mater. Res. 1997;36:17–28. doi: 10.1002/(sici)1097-4636(199707)36:1<17::aid-jbm3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Ishaug-Riley SL, Crane-Kruger GM, Yaszemski MJ, Mikos AG. Biomaterials. 1998;19:1405–1412. doi: 10.1016/s0142-9612(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 14.Mooney DJ, Sano K, Kaufmann PM, Majahod K, Schloo B, Vacanti JP, Langer R. J. Biomed. Mater. Res. 1997;37:413–420. doi: 10.1002/(sici)1097-4636(19971205)37:3<413::aid-jbm12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Nomi M, Atala A, De Coppi P, Soker S. Mol. Aspects Med. 2002;23:463–483. doi: 10.1016/s0098-2997(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 16.Mikos AG, Sarakinos G, Lyman MD, Ingber DE, Vacanti JP, Langer R. Biotechnol. Bioeng. 1993;42:716–723. doi: 10.1002/bit.260420606. [DOI] [PubMed] [Google Scholar]
- 17.Zhou D, Shao L, Spitz DR. Adv. Cancer Res. 2014;122:1–67. doi: 10.1016/B978-0-12-420117-0.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atashi F, Modarressi A, Pepper MS. Stem Cells Dev. 2015;24:1150–1163. doi: 10.1089/scd.2014.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis DM, Abaci HE, Xu Y, Gerecht S. Biofabrication. 2015;7:45010. doi: 10.1088/1758-5090/7/4/045010. [DOI] [PubMed] [Google Scholar]
- 21.Lam GC, V Sefton M. Tissue Eng. Part A. 2015;21:803–816. doi: 10.1089/ten.tea.2014.0315. [DOI] [PubMed] [Google Scholar]
- 22.Acott C. 1999 [Google Scholar]
- 23.Clark JM. Am. Rev. Respir. Dis. 1974;110:40–50. doi: 10.1164/arrd.1974.110.6P2.40. [DOI] [PubMed] [Google Scholar]
- 24.Thom SR. J. Appl. Physiol. 2009;106:988–995. doi: 10.1152/japplphysiol.91004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishii Y, Ushida T, Tateishi T, Shimojo H, Miyanaga Y. J. Orthop. Res. 2002;20:353–356. doi: 10.1016/S0736-0266(01)00094-8. [DOI] [PubMed] [Google Scholar]
- 26.Cherng J-H, Chang S-C, Chen S-G, Hsu M-L, Hong P-D, Teng S-C, Chan Y-H, Wang C-H, Chen T-M, Dai N-T. Ann. Plast. Surg. 2012;69:650–655. doi: 10.1097/SAP.0b013e3182745f95. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen TO, Xing Z, Finne-Wistrand a, Hellem S, Mustafa K. Int. J. Oral Maxillofac. Surg. 2013;42:907–914. doi: 10.1016/j.ijom.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Grassmann J, Schneppendahl J, Hakimi A, Herten M, Betsch M, Lögters T, Thelen S, Sager M, Wild M, Windolf J, Jungbluth P, Hakimi M. J. Orthop. Res. 2015;33:513–520. doi: 10.1002/jor.22805. [DOI] [PubMed] [Google Scholar]
- 29.Tra WMW, Spiegelberg L, Tuk B, Hovius SER, Perez-Amodio S. Tissue Eng. Part A. 2014;20:1523–1530. doi: 10.1089/ten.TEA.2012.0629. [DOI] [PubMed] [Google Scholar]
- 30.Hampson N, Atik D. Undersea Hyperb. Med. 2003;30:147–153. [PubMed] [Google Scholar]
- 31.Alayash AI. Trends Biotechnol. 2014;32:177–185. doi: 10.1016/j.tibtech.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao Z, Ghoroghchian PP. Trends Biotechnol. 2014;32:466–473. doi: 10.1016/j.tibtech.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Winslow RM. J. Intern. Med. 2003;253:508–517. doi: 10.1046/j.1365-2796.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 34.Buehler PW, D’Agnillo F, Schaer DJ. Trends Mol. Med. 2010;16:447–457. doi: 10.1016/j.molmed.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Lai Y-T, Ohta S, Akamatsu K, Nakao S, Sakai Y, Ito T. Biotechnol. Prog. 2015:n/a–n/a. doi: 10.1002/btpr.2170. [DOI] [PubMed] [Google Scholar]
- 36.Xiong Y, Liu ZZ, Georgieva R, Smuda K, Steffen A, Sendeski M, Voigt A, Patzak A, Bäumler H. ACS Nano. 2013;7:7454–7461. doi: 10.1021/nn402073n. [DOI] [PubMed] [Google Scholar]
- 37.Xiong Y, Ste A, Andreas K, Mu S, Sternberg N, Georgieva R. 2012 [Google Scholar]
- 38.Chen G, Palmer AF. Tissue Eng. Part A. 2010;16:3231–3240. doi: 10.1089/ten.tea.2010.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centis V, Proulx P, Vermette P. Biochem. Eng. J. 2011;55:162–168. [Google Scholar]
- 40.Castro CI, Briceno JC. Artif. Organs. 2010;34:no–no. doi: 10.1111/j.1525-1594.2009.00944.x. [DOI] [PubMed] [Google Scholar]
- 41.Riess JG, Krafft MP. Biomaterials. 1998;19:1529–1539. doi: 10.1016/s0142-9612(98)00071-4. [DOI] [PubMed] [Google Scholar]
- 42.Riess JG. Artif. Cells. Blood Substit. Immobil. Biotechnol. 2005;33:47–63. doi: 10.1081/bio-200046659. [DOI] [PubMed] [Google Scholar]
- 43.Squires JE. Science (80-.) 2002;295:1002–1005. doi: 10.1126/science.1068443. [DOI] [PubMed] [Google Scholar]
- 44.Cohn CS, Cushing MM. Crit. Care Clin. 2009;25:399–414. doi: 10.1016/j.ccc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Tissue Eng. 2006;12:2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 46.Tan Q, El-Badry AM, Contaldo C, Steiner R, Hillinger S, Welti M, Hilbe M, Spahn DR, Jaussi R, Higuera G, van Blitterswijk CA, Luo Q, Weder W. Tissue Eng. Part A. 2009;15:2471–2480. doi: 10.1089/ten.tea.2008.0461. [DOI] [PubMed] [Google Scholar]
- 47.Shi G, Coger RN. 2013 [Google Scholar]
- 48.Pilarek M, Grabowska I, Senderek I, Wojasiński M, Janicka J, Janczyk-Ilach K, Ciach T. Bioprocess Biosyst. Eng. 2014;37:1707–1715. doi: 10.1007/s00449-014-1143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kimelman-Bleich N, Pelled G, Sheyn D, Kallai I, Zilberman Y, Mizrahi O, Tal Y, Tawackoli W, Gazit Z, Gazit D. Biomaterials. 2009;30:4639–4648. doi: 10.1016/j.biomaterials.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 50.Benjamin S, Sheyn D, Ben-David S, Oh A, Kallai I, Li N, Gazit D, Gazit Z. Tissue Eng. Part A. 2013;19:748–758. doi: 10.1089/ten.TEA.2012.0298. [DOI] [PubMed] [Google Scholar]
- 51.Khattak SF, Chin K, Bhatia SR, Roberts SC. Biotechnol. Bioeng. 2007;96:156–166. doi: 10.1002/bit.21151. [DOI] [PubMed] [Google Scholar]
- 52.Chin K, Khattak SF, Bhatia SR, Roberts SC. Biotechnol. Prog. 2008;24:358–366. doi: 10.1021/bp070160f. [DOI] [PubMed] [Google Scholar]
- 53.Goh F, Gross JD, Simpson NE, Sambanis A. J. Biotechnol. 2010;150:232–239. doi: 10.1016/j.jbiotec.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma T, Wang Y, Qi F, Zhu S, Huang L, Liu Z, Huang J, Luo Z. Biomaterials. 2013;34:10016–10027. doi: 10.1016/j.biomaterials.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 55.Li H, Wijekoon A, Leipzig ND. Ann. Biomed. Eng. 2014;42:1456–1469. doi: 10.1007/s10439-013-0925-0. [DOI] [PubMed] [Google Scholar]
- 56.White JC, Stoppel WL, Roberts SC, Bhatia SR. J. Biomed. Mater. Res. A. 2013;101:438–446. doi: 10.1002/jbm.a.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palumbo FS, Di Stefano M, Palumbo Piccionello a, Fiorica C, Pitarresi G, Pibiri I, Buscemi S, Giammona G. RSC Adv. 2014;4:22894. [Google Scholar]
- 58.Wijekoon A, Fountas-Davis N, Leipzig ND. Acta Biomater. 2013;9:5653–5664. doi: 10.1016/j.actbio.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 59.Seifu DG, Isimjan TT, Mequanint K. Acta Biomater. 2011;7:3670–3678. doi: 10.1016/j.actbio.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 60.O’Brien F. Mater. Today. 2011;14:88–95. [Google Scholar]
- 61.Lee H-Y, Kim H-W, Lee JH, Oh SH. Biomaterials. 2015;53:583–591. doi: 10.1016/j.biomaterials.2015.02.117. [DOI] [PubMed] [Google Scholar]
- 62.Mallepally RR, Parrish CC, Mc Hugh MAM, Ward KR. Int. J. Pharm. 2014;475:130–137. doi: 10.1016/j.ijpharm.2014.08.052. [DOI] [PubMed] [Google Scholar]
- 63.Ng S-M, Choi J-Y, Han H-S, Huh J-S, Lim JO. Int. J. Pharm. 2010;384:120–127. doi: 10.1016/j.ijpharm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Abdi SIH, Ng SM, Lim JO. Int. J. Pharm. 2011;409:203–205. doi: 10.1016/j.ijpharm.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 65.Li Z, Guo X, Guan J. Biomaterials. 2012;33:5914–5923. doi: 10.1016/j.biomaterials.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 66.Harrison BS, Eberli D, Lee SJ, Atala A, Yoo JJ. Biomaterials. 2007;28:4628–4634. doi: 10.1016/j.biomaterials.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 67.Chandra PK, Ross CL, Smith LC, Jeong SS, Kim J, Yoo JJ, Harrison BS. Wound Repair Regen. 2015:n/a–n/a. doi: 10.1111/wrr.12324. [DOI] [PubMed] [Google Scholar]
- 68.Ward CL, Corona BT, Yoo JJ, Harrison BS, Christ GJ. PLoS One. 2013;8:e72485. doi: 10.1371/journal.pone.0072485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS. Biomaterials. 2009;30:757–762. doi: 10.1016/j.biomaterials.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 70.Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Proc. Natl. Acad. Sci. U. S. A. 2012;109:4245–4250. doi: 10.1073/pnas.1113560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Waite A, Bonner J, Autenrieth R. Environ. Eng. Sci. 1999;16:187–199. [Google Scholar]
- 72.Wang J, Zhu Y, Bawa HK, Ng G, Wu Y, Libera M, van der Mei HC, Busscher HJ, Yu X. ACS Appl. Mater. Interfaces. 2011;3:67–73. doi: 10.1021/am100862h. [DOI] [PubMed] [Google Scholar]
- 73.Jung D-W, Kim Y-H, Kim TG, Lee JH, Chung KJ, Lim JO, Choi JY. Ann. Plast. Surg. 2015;75:463–470. doi: 10.1097/SAP.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 74.Hopfner U, Schenck T-L, Chávez M-N, Machens H-G, Bohne A-V, Nickelsen J, Giunta R-E, Egaña J-T. Acta Biomater. 2014;10:2712–2717. doi: 10.1016/j.actbio.2013.12.055. [DOI] [PubMed] [Google Scholar]
- 75.Chávez MN, Schenck TL, Hopfner U, Centeno-Cerdas C, Somlai-Schweiger I, Schwarz C, Machens H-G, Heikenwalder M, Bono MR, Allende ML, Nickelsen J, Egaña JT. Biomaterials. 2015;75:25–36. doi: 10.1016/j.biomaterials.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 76.Armstrong JPK, Shakur R, Horne JP, Dickinson SC, Armstrong CT, Lau K, Kadiwala J, Lowe R, Seddon A, Mann S, Anderson JLR, Perriman AW, Hollander AP. Nat. Commun. 2015;6:7405. doi: 10.1038/ncomms8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cook Ca, Hahn KC, Morrissette-McAlmon JBF, Grayson WL. Biomaterials. 2015;52:376–384. doi: 10.1016/j.biomaterials.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benz S, Nötzli S, Siegel JS, Eberli D, Jessen HJ. J. Med. Chem. 2013;56:10171–10182. doi: 10.1021/jm4016137. [DOI] [PubMed] [Google Scholar]
- 79.Fix SM, Borden MA, Dayton PA. J. Control. Release. 2015;209:139–149. doi: 10.1016/j.jconrel.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 80.Eisenbrey JR, Albala L, Kramer MR, Daroshefski N, Brown D, Liu J-B, Stanczak M, O’Kane P, Forsberg F, Wheatley MA. Int. J. Pharm. 2014;478:361–367. doi: 10.1016/j.ijpharm.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]