Abstract
Extracellular membrane-bound and secreted heat shock protein 90 (Hsp90) is known to be involved in cell motility and invasion. The mechanism of Hsp90 anchoring to the plasma membrane remains obscure. We showed that treatment of human glioblastoma A-172 and fibrosarcoma HT1080 cells with sodium chlorate, heparinase, and heparin causes a prominent loss of 2 Hsp90 cytosolic isoforms, Hsp90α and Hsp90β, from the cell surface and strongly inhibits the binding of exogenous Hsp90 to cells. We revealed that Hsp90α and Hsp90β are partly colocalized with heparan sulfate proteoglycans (HSPGs) on the cell surface and that this colocalization was sensitive to heparin. The results demonstrate that cell surface HSPGs are involved in the binding/anchoring of Hsp90α and Hsp90β to the plasma membrane.
Keywords: anchoring and binding to plasma membrane, cell surface-bound Hsp90α, and Hsp90β, cell surface HSPGs, extracellular Hsp90, human glioblastoma A-172 and fibrosarcoma HT1080 cells
Introduction
Heat shock protein 90 (Hsp90) is one of the most abundant and ubiquitously expressed proteins.1 There are 2 major cytoplasmic isoforms of Hsp90, Hsp90α (the inducible form) and Hsp90β (the constitutive form), which share a high sequence homology but show some differences in biochemical properties, expression, and function.2 Hsp90 acts inside cells as a chaperone that regulates folding, maturation, transport, and degradation of a diverse set of client proteins, in particular, signaling molecules, steroid receptors, and transcription factors.1,2 In addition, Hsp90 can be found in the extracellular space and at the cell surface membrane. Both Hsp90 cytosolic isoforms, Hsp90α and Hsp90β, were shown to be secreted by many types of normal and cancer cells and expressed at the cell surface.3,4,5 Cell stressors such as heat shock, oxidative stress and hypoxia, as well as growth factors, stimulate Hsp90 translocation to the membrane and/or secretion.6,7,8
The main function of the extracellular Hsp90 is to promote cell motility. It stimulates neurite outgrowth,9 migration processes in the developing nervous system,10 dermal fibroblast migration,3,11 promotes skin wound healing,3,11,12 and stimulates migratory, invasion, and metastatic activity of cancer cells.8,13-16 Extracellular Hsp90 acts as a pro-motility factor via cell surface receptors.4,17 Hsp90α was shown to bind to cell surface LRP1.18 and stimulate cell migration in an LRP1-dependent manner in normal and cancer cells.6,19 Extracellular Hsp90α plays an important role in ErbB-1 and ErbB-2 activation, downstream kinase signaling, and subsequent cell migration.16 Extracellular Hsp90α and Hsp90β can also function as chaperones for some surface-bound or secreted proteins, such as matrix metalloproteinases and extracellular matrix proteins, thus promoting the cell motility.17,20-23
Both Hsp90 isoforms have neither a signal peptide necessary for the secretion via the classic endoplasmic reticulum/Golgi protein secretory pathway nor a recognizable transmembrane sequence necessary for the anchoring to the cell plasma membrane. It was demonstrated that Hsp90 uses an unconventional exosomal way of secretion.3,7,14 The mechanisms of the translocation and anchoring of Hsp90 to the plasma membrane are not elucidated.
Heparan sulfate proteoglycans (HSPGs) are cell-surface and extracellular matrix glycoproteins that comprise a core protein to which heparan sulfate glycosaminoglycan chains are covalently attached.24 Surface HSPGs are involved in a large number of biological processes, including cell growth and division, cell adhesion and motility, and cell signaling.24-26 They primarily function as co-receptors for numerous surface receptors and interact with various protein ligands, enabling their more efficient interaction with respective receptors.24-26
In this study, a line of independent evidence is presented for the first time that cell surface HSPGs are involved in the binding and anchoring of Hsp90α and Hsp90β to the cell plasma membrane.
Results and Discussion
It is known that Hsp90 binds to heparin whose chemical structure is similar to HSPG polysaccharide side chains.27 We also observed that both isoforms of Hsp90, Hsp90α and Hsp90β, specifically bind to heparin-Sepharose (not shown). These data suggested that cell surface HSPGs may play a role in the anchoring of Hsp90 to the plasma membrane. To verify this consideration, we investigated the cell surface expression of Hsp90α and Hsp90β in human glioblastoma A-172 and fibrosarcoma HT1080 cells treated with different reagents impairing the structure of cell surface HSPGs. We revealed that Hsp90α and Hsp90β are expressed on the surface of A-172 and HT1080 human tumor cells. The contribution of cell-surface HSPGs to plasma membrane anchoring of Hsp90α and Hsp90β was estimated using different experimental approaches. First, we determined the cell-surface expression of Hsp90α and Hsp90β after the cultivation of cells in a medium supplemented with sodium chlorate, a substance known to reduce the sulfation of HSPG glycan chains during their biosynthesis.28 After a 24-h incubation of cells with sodium chlorate at a nontoxic concentration of 30 mM, the surface HSPG sulfation detected by heparin/heparan sulfate-specific antibodies strongly decreased (Fig. 1A). The level of cell surface-bound Hsp90 isoforms also severely diminished. For A-172 cells, the amount of surface-associated Hsp90α and Hsp90β decreased to 11.4 ± 5.0 and 7.4 ± 5.8% of the initial level, respectively; for HT1080 cells, the level of cell surface Hsp90α and Hsp90β diminished to 19.1 ± 6.0 and 13.3 ± 5.8%, respectively (Fig. 1A-C; Fig. S1). During a 24-h sodium chlorate treatment, the amounts of total (intracellular and membrane-bound) Hsp90α and Hsp90β remained unaltered (Fig. 1D; Fig. S1), which excludes the possibility that the decrease in the amount of membrane-bound Hsp90α and Hsp90β was due to a respective decrease in the total amounts of Hsp90α and Hsp90β in cells. Then, we showed that the cleavage of sulfated glycan chains of cell surface HSPGs with a heparinase I/III blend strongly reduced the level of cell surface heparan sulfates (Fig. 2A), which was accompanied by a considerable loss of surface-associated Hsp90 isoforms. For A-172 cells, the amount of surface-bound Hsp90α and Hsp90β diminished to 33.0 ± 7.9 and 23.4 ± 6.2% of the initial level, respectively; for HT1080 cells, the level of cell surface Hsp90α and Hsp90β decreased to 22.7 ± 6.5 and 15.1 ± 5.1%, respectively (Fig. 2A-C; Fig. S2). Treatment of cells with heparinase I/III blend did not change the contents of total cellular Hsp90α and Hsp90β(Fig. 2D; Fig. S2).\raster="rgFigKCAM_A_1103421_F0001_B" \raster="rgFigKCAM_A_1103421_F0002_B"
Figure 1.
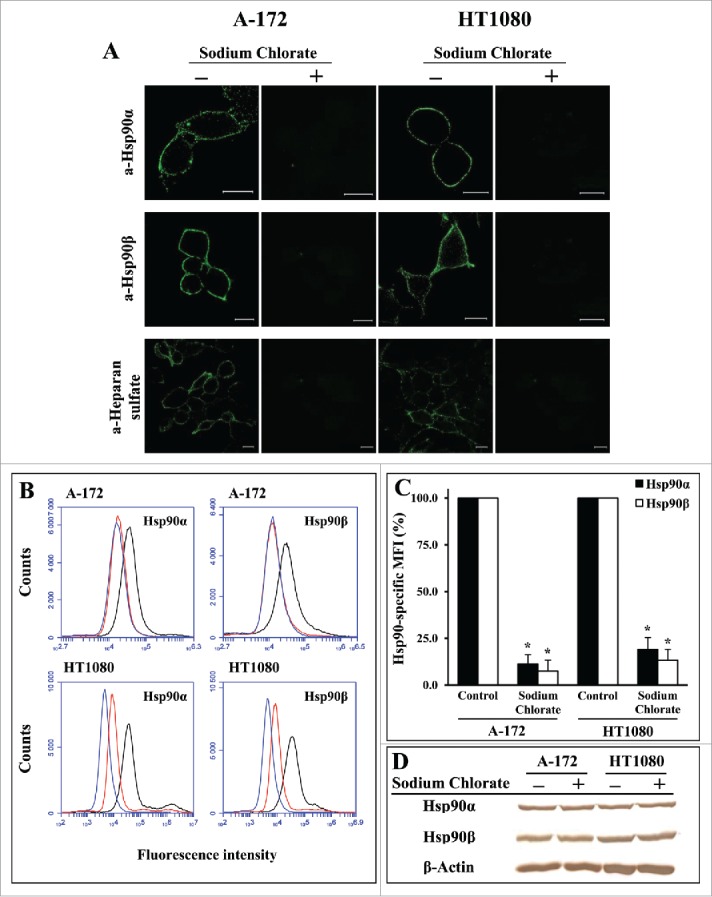
Undersulfation of HSPGs leads to a severe loss of surface-bound Hsp90α and Hsp90β in A-172 and HT1080 cells. Cells were grown in DMEM-FBS containing 30 mM sodium chlorate for 24 h at 37°C, stained with anti-Hsp90α, anti-Hsp90β, and anti-heparan sulfate antibodies, and analyzed by confocal microscopy (A) and flow cytometry (B). (A) Representative confocal microscopy images showing the surface staining with antibodies are presented. Scale bar: 20 μm. (B) Representative flow cytometry histograms for control (black lines) and chlorate-treated (red lines) cells stained with Hsp90-specific antibodies, as well as for cells stained with the negative control rabbit antibody (blue lines) are presented. (C) Flow cytometry-based quantification of membrane-bound Hsp90α and Hsp90β expression after chlorate treatment. The data are presented as the MFI specific for Hsp90α and Hsp90β, expressed in percent. MFI of control cells was taken as 100%. Each bar represents the mean ± SD (n = 4–5). *Statistically significantly different (P < 0.05) from untreated cells. The representative results from 3 independent experiments are shown. (D) Western blot analyses of total (intracellular and cell-surface) levels of Hsp90α, Hsp90β, and β-actin (loading control) in chlorate-treated and control A-172 and HT1080 cells. The representative results from 3 independent experiments are shown.
Figure 2.
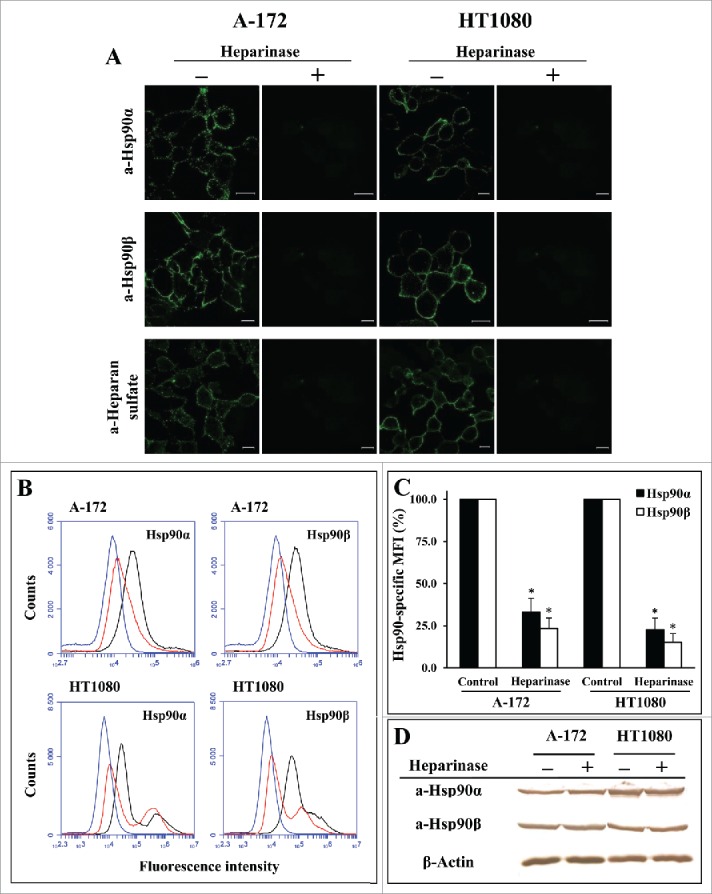
Digestion of cell surface HSPGs with heparinase considerably reduces the level of surface-bound Hsp90α and Hsp90β in A-172 and HT1080 cells. Cells were incubated for 1 h at 37°C with a heparinase I/III blend, stained with anti-Hsp90α, anti-Hsp90β, and anti-heparan sulfate antibodies, and analyzed by confocal microscopy (A) and flow cytometry (B). (A) Representative confocal microscopy images showing the surface staining with antibodies are presented. Scale bar: 20 μm. (B) Representative flow cytometry histograms for control (black lines) and heparinase-treated (red lines) cells stained with Hsp90-specific antibodies, as well as for cells stained with the negative control rabbit antibody (blue lines) are presented. (C) Flow cytometry-based quantification of membrane-bound Hsp90α and Hsp90β expression after heparinase treatment. The data are presented as the MFI specific for Hsp90α and Hsp90β, expressed in percent. MFI of control cells was taken as 100%. Each bar represents the mean ± SD (n = 4–5). *Statistically significantly different (P < 0.05) from untreated cells. The representative results from 3 independent experiments are shown. (D) Western blot analyses of total (intracellular and cell-surface) amounts of Hsp90α, Hsp90β, and β-actin (loading control) in heparinase-treated and control A-172 and HT1080 cells. The representative results from 3 independent experiments are presented.
The third approach involved the evaluation of the influence of heparin, a polysaccharide closely related to heparan sulfate,29 on the attachment of Hsp90α and Hsp90β to the cell surface. For both cell cultures, the treatment of cells at 37°C with heparin at a concentration of 50 μg/ml reduced the amount of cell-associated Hsp90α and Hsp90β by 30–35% and 70–75%, respectively (Fig. 3A, B, D; Fig. S3). At the same time, the heparin treatment of cells did not change the levels of total (intracellular and cell-associated) Hsp90α and Hsp90β in cells, as evidenced by Western blot (Fig. 3C; Fig. S3). The effect of heparin on the cell surface Hsp90 isoforms was concentration-dependent; heparin significantly decreased the levels of both Hsp90 isoforms even at a concentration of 10 μg/ml, reaching a maximum effect at concentrations of 50–100 μg/ml (Fig. 3D). Nevertheless, even at a concentration of 100 μg/ml, heparin did not completely remove surface-bound Hsp90α and Hsp90β, and a significant portion of surface-bound Hsp90 isoforms (65–70% of Hsp90α and 20–30% of Hsp90β) was insensitive to it (Fig. 3D). The effect of heparin on the surface-bound Hsp90 isoforms was time-dependent; the detachment of Hsp90 from cell surface was maximum after a 30-60-min treatment of cells (data not shown).
Figure 3.
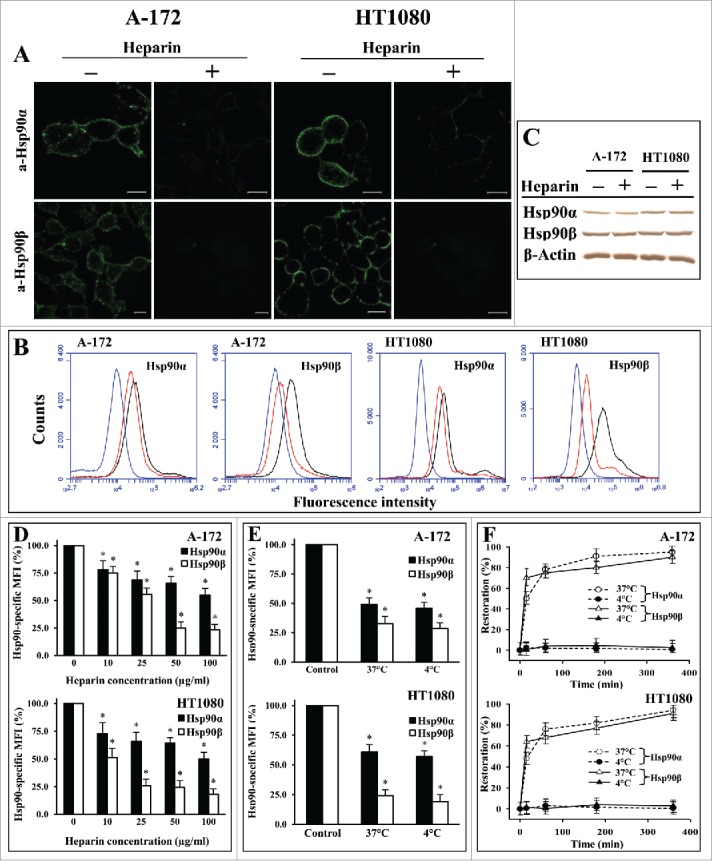
Treatment of A-172 and HT1080 cells with heparin results in a significant loss of membrane-bound Hsp90β and Hsp90α. Cells were treated for 1 h at 37°C (A, B, D, F) or at 37°C and 4°C (E) with heparin at concentrations of 50 μg/ml (A, B, E, F) or 0–100 μg/ml (D), stained with anti-Hsp90α and anti-Hsp90β antibodies, and analyzed by confocal microscopy (A) and flow cytometry (B, D, E, F). (A) Representative confocal microscopy images showing the surface staining with antibodies are presented. Scale bar: 20 μm. (B) Representative flow cytometry histograms for control (black lines) and heparin-treated (red lines) cells stained with Hsp90-specific antibodies, as well as cells stained with the negative control rabbit antibody (blue lines) are presented. (C) Western blot analyses of total (intracellular and cell-surface) levels of Hsp90α, Hsp90β, and β-actin (loading control) in A-172 and HT1080 cells treated with heparin at a concentration of 50 μg/ml for 1 h at 37°C. The representative results from 3 independent experiments are shown. (D, E) The flow cytometry-based quantification of membrane-bound Hsp90α and Hsp90β after treatment with heparin at different concentrations (D) and different temperatures (E). The data are presented as the MFI specific for Hsp90α and Hsp90β, expressed in percent. MFI of control cells was taken as 100%. (F) The percentage of restoration of membrane-bound Hsp90α and Hsp90β is presented. The levels of membrane-bound Hsp90α and Hsp90β were estimated by the respective specific MFI. The levels of membrane-associated Hsp90 in heparin-treated cells and control cells were taken as a 0% restoration and a 100% restoration, respectively. Each bar (D, E) and point (F) represent the mean ± SD (n = 5–6). *Statistically significantly different (P < 0.05) from untreated cells. The representative results from 3 independent experiments are shown.
Some active processes that may occur during treatments of cells with heparin at 37°C, in particular, the appearance of new Hsp90s on the plasma membrane and the internalization of complexes of Hsp90 with cellular receptor(s), may complicate the interpretation of the results. To eliminate these active processes, we evaluated the surface expression of Hsp90α and Hsp90β after treatment of cells at 4°C, a temperature that inhibits the translocation of proteins across the membrane, classical and exosomal secretion, and internalization of receptor-ligand complexes.30-32 We observed that the effect of heparin was independent of temperature and was nearly the same at 37 and 4°C (Fig. 3E).
The incubation of heparin-treated cells in DMEM-FBS without heparin at 37°C resulted in a quick (15 min) restoration of cell surface expression of Hsp90α and Hsp90β up to 50% of the control level (untreated cells) followed by a slower restoration of Hsp90 expression to the control level by 6 h. The restoration of surface-bound Hsp90α and Hsp90β was not observed when the cells were incubated for 1 h at 4°C, a temperature at which proteins are not expected to be translocated across the plasma membrane (Fig. 3F),30-32 which indicates that the restoration of surface-bound Hsp90 is an active process.
Taken together, the data of 3 experimental approaches strongly indicated that cell surface HSPGs are involved in the anchoring of 2 isoforms of Hsp90, Hsp90α and Hsp90β, to the cell plasma membrane.
To support our finding, we analyzed the colocalization of cell surface HSPGs and Hsp90 isoforms. Simultaneous staining of cell surface HSPGs and Hsp90 isoforms with MAb specific to heparin/heparan sulfate and anti-Hsp90α or anti-Hsp90β antibodies revealed a colocalization of a portion of Hsp90α and Hsp90β with HSPGs on the cell surface (yellow dots in Fig. 4D). After the treatment of cells with heparin, a significant decrease in the number of yellow dots was observed, indicating the disturbance of colocalization of Hsp90α and Hsp90β with surface HSPGs. The results are presented for HT1080 cells (Fig. 4D); similar data were obtained for A-172 cells. The results suggest that a portion of cell surface Hsp90s is associated with surface HSPGs and provide additional support for our conclusion about the involvement of HSPGs in the attachment of Hsp90s to the cell surface.
Figure 4.
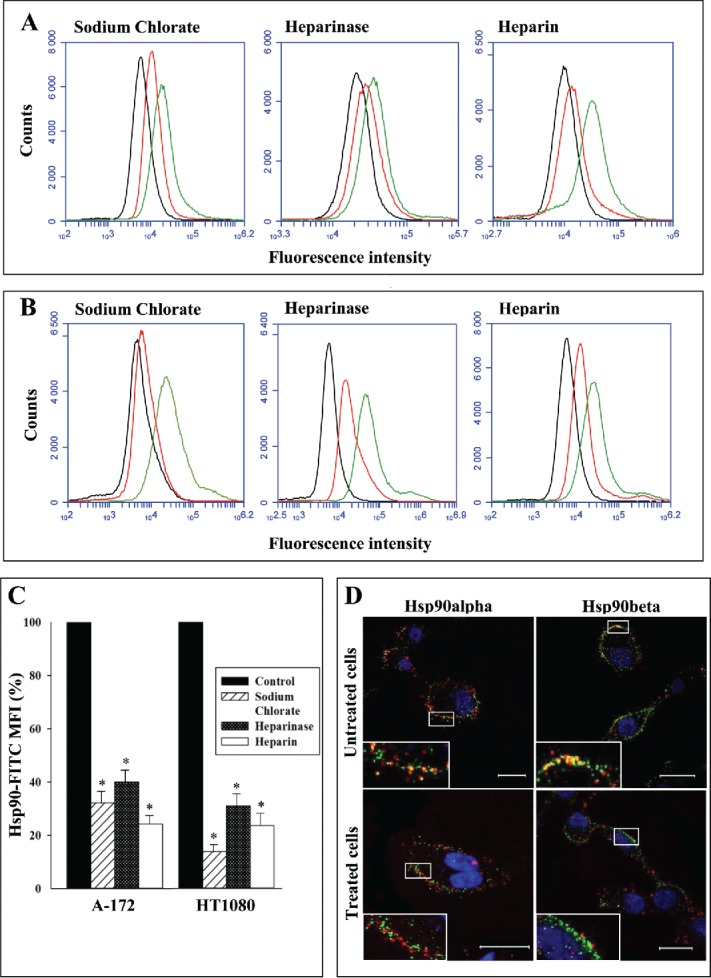
Binding of Hsp90 to cells (A-C) and colocalization of cell surface Hsp90 with cellular HSPGs (D). (A, B) A-172 and HT1080 cells were treated with sodium chlorate or heparinase. A suspension of cells was incubated for 1 h at 4°C with Hsp90-FITC. Untreated cells were incubated with Hsp90-FITC in the presence and absence of heparin (20 μg/ml). Then, the cells were washed, fixed with formaldehyde, and analyzed by flow cytometry. Representative flow cytometry histograms for A-172 (A) and HT1080 (B) cells are presented. Red lines – chlorate- and heparinase-treated cells and cells incubated with heparin; green lines - untreated cells; black lines – control cells (autofluorescence). (C) Flow cytometry-based quantification of Hsp90 binding to cells. The data are presented as the FITC-Hsp90-specific MFI of treated and control cells, expressed in percent. MFI of control cells was taken as 100%. Each bar represents the mean ± SD (n = 4–5). *Statistically significantly different (P < 0.05) from untreated cells. (D) Live untreated cells and cells treated with heparin at a concentration of 50 μg/ml for 1 h at 37°C were stained simultaneously with antibodies specific to Hsp90α or Hsp90β (red fluorescence) and HSPGs (green fluorescence), counterstained with Hoechst 33258, and analyzed by confocal microscopy. Representative confocal microscopy images showing surface staining with antibodies are presented. Inserts are enlarged images of regions of interest. Yellow fluorescence indicates the colocalization of cell-surface Hsp90 and HSPGs. Scale bar: 20 μm.
We also determined the influence of sodium chlorate and heparinase treatment of cells, as well as of heparin, on the direct binding of exogenous Hsp90 to the cell surface. We showed that FITC-labeled native mouse Hsp90 (a mixture of Hsp90α and Hsp90β) efficiently bound to the cell surface at 4°C of untreated cells (Fig. 4A, B, C). Unlabeled Hsp90 inhibited the binding of FITC-labeled Hsp90, suggesting the specificity of the binding of Hsp90 to cells (not shown). The binding of FITC-labeled Hsp90 strongly decreased in chlorate- and heparinase-treated cells as compared to control untreated cells. Heparin at a concentration of 20 μg/ml also considerably diminished the binding of Hsp90-FITC to the surface of untreated cells (Fig. 4A, B, C). Taken together, the results suggest a role of cell-surface HSPGs in the direct binding of extracellular Hsp90 to cells.
To our knowledge, this is the first report demonstrating the role of cell surface HSPGs in the binding/anchoring of 2 Hsp90 cytosolic isoforms, Hsp90α and Hsp90β, to the cell surface. The revelation of heparin-sensitive and heparin-resistant modes of interaction of Hsp90α and Hsp90β with cell surface suggests that cell-surface HSPGs may serve as co-receptors in the binding of Hsp90 to the plasma membrane. It has been shown that cell-surface HSPGs and LRP1 function in a cooperative manner to mediate GRP94 cell surface binding,33 cellular uptake of C4b-binding protein,34 lipid metabolism,35 internalization of apoE/lipoprotein particles,36 and amyloid-β uptake.37 Since LRP1 is considered to be one of the receptors of extracellular Hsp90,6,18,19 these data suggest that cell-surface HSPGs may cooperate with LRP1 in mediating the binding/anchoring of Hsp90α and Hsp90β isoforms to the cell surface.
Heparin is known to affect the migration and invasion of cells and cancer progression by inhibition of heparinase, blocking of P- and L-selectin mediated cell adhesion, and the inhibition of angiogenesis.38-41 We also observed that heparin inhibited the migration and invasion of A-172 and HT1080 cells in vitro, which correlated with the reduction of the levels of cell surface-bound Hsp90α and Hsp90β (in preparation). These results and our data presented above suggest another possible mechanism of action of heparin on tumor cells. Namely, heparin may diminish the level of cell surface-bound Hsp90 and prevent the binding of Hsp90 to the plasma cell membrane, which would inhibit the migration and invasion of tumor cells in vitro and in vivo.
The recovery of the cell-surface expression of Hsp90α and Hsp90β after heparin treatment of cells was biphasic with a relatively fast partial restoration of the level of surface Hsp90. This suggests that either Hsp90 isoforms are quickly translocated directly to the plasma membrane from cytosol by an unknown mechanism or the autocrine secreted Hsp90 isoforms are anchored to the membrane. If Hsp90 isoforms are to be secreted before being anchored to the plasma membrane, autocrine secreted Hsp90 may reach relatively high local concentrations in a close proximity to the plasma membrane, which can drastically increase the rate of the Hsp90 association with receptor(s) and enable the quick emergence of Hsp90 on the cell surface. The binding of paracrine secreted Hsp90 to adjacent or remote cells cannot be excluded, but this process needs a reasonable time for the diffusion of Hsp90 and attainment of appreciable concentrations of Hsp90 in the medium.
Materials and Methods
Cell cultures
Human glioblastoma A-172 and fibrosarcoma HT1080 cells were from the Cell Culture Collection of Vertebrates (St. Petersburg, Russia). The cells were maintained in DMEM (HyClone) supplemented with 10% FBS (HyClone) (DMEM-FBS). The cells were grown till 50–60% confluence in chambered coverglasses (Thermo Scientific) for confocal microscopy analysis or in culture dishes (Corning) for flow cytometry analysis. Each experiment was performed independently at least 3 times, and representative results are shown in the figures.
Antibodies and Hsp90 detection
Rabbit Hsp90α- and Hsp90β-specific antibodies directed to N-terminal fragments of human Hsp90α (PEETQTQDQPM) and Hsp90β (EEVHHGEEEVE) were used (USBiological H1834–55A and H1832–98H). Mouse monoclonal antibody specific to heparin/heparan sulfate (clone T320.11) was from Millipore. For confocal microscopy, the cells grown in chambered coverglasses and treated as described below were washed with cold PBS containing 0.05% NaN3 (PBS-NaN3), incubated with primary specific or control antibodies and, after washing, with Alexa 488-labeled secondary anti-rabbit IgG or anti-mouse IgG conjugates (Jacksons ImmunoResearch). For colocalization experiments, primary rabbit anti-Hsp90α or anti-Hsp90β antibodies were incubated with cells simultaneously with mouse heparin/heparan sulfate antibody. The cells were washed and incubated simultaneously with Alexa 488-labeled anti-mouse IgG and Alexa 647-labeled anti-rabbit IgG conjugates cross-adsorbed against IgG of different species; anti-mouse and anti-rabbit IgG conjugates do not cross-react with rabbit and mouse IgG, respectively. All incubations with antibodies were performed at 4°C in PBS-NaN3 containing 2% BSA (PBS-BSA-NaN3), conditions that preclude the translocation of intracellular Hsp90s to the plasma membrane and the internalization of surface Hsp90s. After washing, the cells were fixed with 0.5% formaldehyde for 15 min at 4°C and counterstained with Hoechst 33258 prior to imaging on a confocal laser-scanning microscope Leica TCS SP5 (Leica Microsystems) fitted with a 63x objective. For intracellular staining, the cells were fixed with 4% formaldehyde for 15 min at 4°C and treated with 0.1% Triton X-100 after which they were incubated with primary antibodies and Alexa 488-labeled anti-rabbit IgG conjugates, and counterstained with Hoechst 33258 prior to imaging. For flow cytometry, treated cells were detached from culture dishes by incubation for 5 min at 37°C with 0.05% Na-EDTA, washed with cold PBS-NaN3, incubated with primary and secondary antibodies, fixed with formaldehyde, and analyzed using an Accuri C6 flow cytometer (Becton Dickinson). Each analysis included at least 50,000 events of gated cells. The mean fluorescence intensity (MFI) was determined using the BD Accuri C6 software. To quantify the flow cytometry data, the levels of surface-bound Hsp90α and Hsp90β were expressed as the respective MFI ± SD of 3 to 6 independent replicates. The statistical significance was determined by the Student's t-test, and p<0.05 was considered to be significant.
Treatment of cells with heparinase I/III and sodium chlorate
To assess the influence of surface heparan sulfate digestion on the cell surface expression of Hsp90α and Hsp90β, A-172 and HT1080 cells were washed with DMEM and incubated for 1 h at 37°C with a heparinase I/III blend from Flavobacterium heparinum (Sigma-Aldrich) diluted in DMEM-FBS (0.03 IU/ml). Thereafter, the cells were either probed with Hsp90α- and Hsp90β-specific antibodies for confocal microscopy or were detached from the surface followed by staining with Hsp90α- and Hsp90β-specific antibodies for flow cytometry. The effect of undersulfation of HSPGs on the expression of cell-surface Hsp90α and Hsp90β was examined by growing the cells in DMEM-FBS supplemented with 30 mM sodium chlorate (Sigma-Aldrich) for 24 h at 37°C. After the treatment, the membrane-bound Hsp90 isoforms were detected using specific antibodies as described above.
Heparin treatment of cells and surface Hsp90 restoration experiments
A-172 and HT1080 cells were treated for different times at 37°C or 4°C with different concentrations of heparin (Sigma or MPBiomedicals) diluted in DMEM-FBS. Then, the cells were either probed with Hsp90α- and Hsp90β-specific antibodies for confocal microscopy or were detached from culture dishes and stained in suspension with Hsp90α- and Hsp90β-specific antibodies for flow cytometry. To evaluate the restoration of membrane expression of Hsp90α and Hsp90β after heparin treatment, the cells were treated with heparin (50 μg/ml) for 1 h at 37°C, washed with DMEM and incubated for different periods of time at 37°C or 4°C in DMEM-FBS. Then, the membrane-bound Hsp90α and Hsp90β were detected using the antibodies as described above.
Binding of Hsp90 to cells
Native mouse Hsp90 was purified as described earlier.42 The purity of Hsp90 was 96–97%, as indicated by SDS-PAGE. Purified Hsp90 was labeled with FITC according to a standard protocol. Cells were treated with sodium chlorate or heparinase I/III blend as described above. Treated and untreated cells were detached from the culture dishes by incubation for 5 min at 37°C with 0.05% Na-EDTA, washed 2 times with ice-cold PBS-NaN3, and incubated for 1 h at 4°C with Hsp90-FITC diluted in PBS-BSA-NaN3. To assess the influence of heparin on Hsp90 binding, untreated cells were incubated with Hsp90-FITC in the presence and absence of heparin (20 μg/ml). Then the cells were washed, fixed with formaldehyde, and analyzed by flow cytometry. The nonspecific binding of Hsp90 to cells was determined by incubation of Hsp90-FITC with cells in the presence of a four- to eightfold excess of unlabeled Hsp90.
Western Blot Analysis
Cells were treated with sodium chlorate and heparinase I/III blend as described above. Cells were treated with heparin at a concentration of 50 μg/ml for 1 h at 37°C. Treated and untreated cells were lysed in PBS containing 1% Nonidet P-40, 0.5% deoxycholate, 0.3% SDS, and 1 mM phenylmethylsulfonyl fluoride. Cell samples were separated by 10% SDS-PAGE and transferred onto a polyvinylidene difluoride membrane. The membrane was blocked in PBS containing 0.05% Tween 20 and 2% BSA (PBS-T-BSA). The membrane was incubated with anti-Hsp90α, anti-Hsp90β, and anti-β-actin (Santa Cruz Biotechnology) antibodies in PBS-T-BSA for 2 h at room temperature, washed, and then incubated with HRP-conjugated secondary anti-rabbit IgG or anti-mouse IgG antibodies in PBS-T-BSA (AbD Serotec, STAR117P and STAR121P) for 1 h. After thorough washing, immunoreactive bands were detected using 3,3'-diaminobenzidine substrate.
Disclosure of Potential Conflicts of Interest
No potential conflict of interests was disclosed.
Acknowledgments
We thank V.A. Yashin and A.O. Shepelyakovskaya for the help with confocal laser-scanning microscopy and flow cytometry.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Johnson JL. Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim Biophys Acta 2012; 1823:607-13; PMID:22008467; http://dx.doi.org/ 10.1016/j.bbamcr.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 2.Li J, Buchner J. Structure, function and regulation of the hsp90 machinery. Biomed J 2013; 36:106-17; PMID:23806880; http://dx.doi.org/ 10.4103/2319-4170.113230 [DOI] [PubMed] [Google Scholar]
- 3.Li W, Li Y, Guan S, Fan J, Cheng C-F, Bright AM, Chinn C, Chen M, Woodley DT. Extracellular heat shock protein-90alpha: linking hypoxia to skin cell motility and wound healing. EMBO J 2007; 26:1221-33; PMID:17304217; http://dx.doi.org/ 10.1038/sj.emboj.7601579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsutsumi S, Beebe K, Neckers L. Impact of heat-shock protein 90 on cancer metastasis. Future Oncol 2009; 5:679-88; PMID:19519207; http://dx.doi.org/ 10.2217/fon.09.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Song X, Zhuo W, Fu Y, Shi H, Liang Y, Tong M, Chang G, Luo Y. The regulatory mechanism of HSP90α secretion and its function in tumor malignancy Proc Natl Acad Sci USA 2009; 106:21288-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng CF, Fan J, Fedesco M, Guan S, Li Y, Bandyopadhyay B, Bright AM, Yerushalmi D, Liang M, Chen M et al.. Transforming growth factor α (TGFalpha)-stimulated secretion of HSP90alpha: using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing. Mol Cell Biol 2008; 28:3344-58; PMID:18332123; http://dx.doi.org/ 10.1128/MCB.01287-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci 2005; 118:3631-8; PMID:16046478; http://dx.doi.org/ 10.1242/jcs.02494 [DOI] [PubMed] [Google Scholar]
- 8.Gopal U, Bohonowych JE, Lema-Tome C, Liu A, Garrett-Mayer E, Wang B, Isaacs JS A novel extracellular Hsp90 mediated co-receptor function for LRP1 regulates EphA2 dependent glioblastoma cell invasion. PLoS ONE. 2011; 6:e17649; PMID:21408136; http://dx.doi.org/ 10.1371/journal.pone.0017649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishimetamoto T, Kamei A, Koyanagi S, Nishide N, Uyeda A, Kasai M, Taguchi T. HSP90 has neurite-promoting activity in vitro for telencephalic and spinal neurons of chick embryos. Biochem Biophys Res Commun 1998; 253:283-7; PMID:9878529; http://dx.doi.org/ 10.1006/bbrc.1998.9701 [DOI] [PubMed] [Google Scholar]
- 10.Sidera K, Samiotaki M, Yfanti E, Panayotou G, Patsavoudi E. Involvement of cell surface HSP90 in cell migration reveals a novel role in the developing nervous system. J Biol Chem 2004; 279:45379-88; PMID:15302889; http://dx.doi.org/ 10.1074/jbc.M405486200 [DOI] [PubMed] [Google Scholar]
- 11.Cheng CF, Sahu D, Tsen F, Zhao Z, Fan J, Kim R, Wang X, O'Brien K, Li Y, Kuang Y, et al.. A fragment of secreted Hsp90α carries properties that enable it to accelerate effectively both acute and diabetic wound healing in mice. J Clin Invest 2011; 121:4348-61; PMID:22019588; http://dx.doi.org/ 10.1172/JCI46475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Tsen F, Sahu D, Bhatia A, Chen M, Multhoff G, Woodley DT. Extracellular Hsp90 (eHsp90) as the actual target in clinical trials: intentionally or unintentionally. Int Rev Cell Mol Biol 2013; 303:203-35; PMID:23445811; http://dx.doi.org/ 10.1016/B978-0-12-407697-6.00005-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker B, Multhoff G, Farkas B, Wild PJ, Landthaler M, Stolz W, Vogt T. Induction of Hsp90 protein expression in malignant melanomas and melanoma metastases. Exp Dermatol 2004; 13:27-32; PMID:15009113; http://dx.doi.org/ 10.1111/j.0906-6705.2004.00114.x [DOI] [PubMed] [Google Scholar]
- 14.McCready J, Sims JD, Chan D, Jay DG. Secretion of extracellular hsp90α via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer 2010; 10:294; PMID:20553606; http://dx.doi.org/ 10.1186/1471-2407-10-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sidera K, Gaitanou M, Stellas D, Matsas R, Patsavoudi E. A critical role for HSP90 in cancer cell invasion involves interaction with the extracellular domain of HER-2. J Biol Chem 2008; 283:2031-41; PMID:18056992; http://dx.doi.org/ 10.1074/jbc.M701803200 [DOI] [PubMed] [Google Scholar]
- 16.Stellas D, El Hamidieh A, Patsavoudi E. Monoclonal antibody 4C5 prevents activation of MMP2 and MMP9 by disrupting their interaction with extracellular HSP90 and inhibits formation of metastatic breast cancer cell deposits. BMC Cell Biol 2010; 11:51; PMID:20602761; http://dx.doi.org/ 10.1186/1471-2121-11-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eustace BK, Jay DG. Extracellular roles for the molecular chaperone, hsp90. Cell Cycle 2004; 3:1098-1100; PMID:15326368; http://dx.doi.org/ 10.4161/cc.3.9.1088 [DOI] [PubMed] [Google Scholar]
- 18.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 2001; 14:303-13; PMID:11290339; http://dx.doi.org/ 10.1016/S1074-7613(01)00111-X [DOI] [PubMed] [Google Scholar]
- 19.Chen JS, Hsu YM, Chen CC, Chen LL, Lee CC, Huang TS. Secreted heat shock protein 90α induces colorectal cancer cell invasion through CD91/LRP-1 and NF-kB-mediated integrin αV expression. J Biol Chem 2010; 285:25458-66; PMID:20558745; http://dx.doi.org/ 10.1074/jbc.M110.139345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Correia AL, Mori H, Chen EI, Schmitt FC, Bissell MJ. The hemopexin domain of MMP3 is responsible for mammary epithelial invasion and morphogenesis through extracellular interaction with HSP90β. Genes Dev 2013; 27:805-17; PMID:23592797; http://dx.doi.org/ 10.1101/gad.211383.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter MC, O'Hagan KL, Kenyon A, Dhanani KC, Prinsloo E, Edkins AL. Hsp90 binds directly to fibronectin (FN) and inhibition reduces the extracellular fibronectin matrix in breast cancer cells. PLoS ONE 2014; 9:e86842; PMID:24466266; http://dx.doi.org/ 10.1371/journal.pone.0086842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song X, Wang X, Zhuo W, Shi H, Feng D, Sun Y, Liang Y, Fu Y, Zhou D, Luo Y. The regulatory mechanism of extracellular Hsp90 on matrix metalloproteinase-2 processing and tumor angiogenesis. J Biol Chem 2010; 285:40039-49; PMID:20937816; http://dx.doi.org/ 10.1074/jbc.M110.181941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki S, Kulkarni AB. Extracellular heat shock protein HSP90beta secreted by MG63 osteosarcoma cells inhibits activation of latent TGF-β 1. Biochem Biophys Res Commun 2010; 398:525-31; PMID:20599762; http://dx.doi.org/ 10.1016/j.bbrc.2010.06.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 2011; 3:a004952; PMID:21690215; http://dx.doi.org/ 10.1101/cshperspect.a004952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 2007; 446:1030-7; PMID:17460664; http://dx.doi.org/ 10.1038/nature05817 [DOI] [PubMed] [Google Scholar]
- 26.Tumova S, Woods A, Couchman JR. Heparan sulfate proteoglycans on the cell surface: versatile coordinators of cellular functions. Int J Biochem Cell Biol 2000; 32:263-384; PMID:10716624; http://dx.doi.org/ 10.1016/S1357-2725(99)00116-8 [DOI] [PubMed] [Google Scholar]
- 27.Menoret A, Bell G. Purification of multiple heat shock proteins from a single tumor sample. J Immunol Methods 2000; 237:119-30; PMID:10725457; http://dx.doi.org/ 10.1016/S0022-1759(00)00137-X [DOI] [PubMed] [Google Scholar]
- 28.Keller KM, Brauer PR, Keller JM. Modulation of cell surface heparan sulfate structure by growth of cells in the presence of chlorate. Biochemistry 1989; 28:8100-7; PMID:2532538; http://dx.doi.org/ 10.1021/bi00446a021 [DOI] [PubMed] [Google Scholar]
- 29.Shriver Z, Capila I, Venkataraman G, Sasisekharan R. Heparin and heparan sulfate: analyzing structure and microheterogeneity. Handb Exp Pharmacol 2012; 207:159-76; PMID:22566225; http://dx.doi.org/ 10.1007/978-3-642-23056-1_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heller-Harrison RA, Morin M, Czech MP. Insulin regulation of membrane-associated insulin receptor substrate 1. J Biol Chem 1995; 270:24442-50; PMID:7592659; http://dx.doi.org/ 10.1074/jbc.270.41.24442 [DOI] [PubMed] [Google Scholar]
- 31.Jang A, Lee HJ, Suk JE, Jung JW, Kim KP, Lee SJ. Non-classical exocytosis of α-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem 2010; 113:1263-74; PMID:20345754 [DOI] [PubMed] [Google Scholar]
- 32.Römisch K, Collie N, Soto N, Logue J, Lindsay M, Scheper W, Cheng CH. Protein translocation across the endoplasmic reticulum membrane in cold-adapted organisms. J Cell Sci 2003; 116:2875-83; PMID:12771183; http://dx.doi.org/ 10.1242/jcs.00597 [DOI] [PubMed] [Google Scholar]
- 33.Jockheck-Clark AR, Bowers EV, Totonchy MB, Neubauer J, Pizzo SV, Nicchitta CV. Re-examination of CD91 function in GRP94 (glycoprotein 96) surface binding, uptake, and peptide cross-presentation. J Immunol 2010; 185:6819-30; PMID:21048103; http://dx.doi.org/ 10.4049/jimmunol.1000448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spijkers PP, Denis CV, Blom AM, Lenting PJ. Cellular uptake of C4b-binding protein is mediated by heparan sulfate proteoglycans and CD91/LDL receptor-related protein. Eur J Immunol 2008; 38:809-17; PMID:18266273; http://dx.doi.org/ 10.1002/eji.200737722 [DOI] [PubMed] [Google Scholar]
- 35.Wilsie LC, Orlando RA. The low density lipoprotein receptor-related protein complexes with cell surface heparan sulfate proteoglycans to regulate proteoglycan-mediated lipoprotein catabolism. J Biol Chem 2003; 278:15758-64; PMID:12598530; http://dx.doi.org/ 10.1074/jbc.M208786200 [DOI] [PubMed] [Google Scholar]
- 36.Mahley RW, Ji ZS. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res 1999; 40:1-16; PMID:9869645 [PubMed] [Google Scholar]
- 37.Kanekiyo T, Zhang J, Liu Q, Liu CC, Zhang L, Bu G. Heparan sulphate proteoglycan and the low-density lipoprotein receptor-related protein 1 constitute major pathways for neuronal amyloid-β uptake. J Neurosci 2011; 31:1644-51; PMID:21289173; http://dx.doi.org/ 10.1523/JNEUROSCI.5491-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bendas G, Borsig L. Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins. Int J Cell Biol 2012; 2012:676731; PMID:22505933; http://dx.doi.org/ 10.1155/2012/676731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci USA. 2001; 98:3352-7; http://dx.doi.org/ 10.1073/pnas.061615598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mousa SA, Petersen LJ. Anti-cancer properties of low-molecular-weight heparin: preclinical evidence. Thromb Haemost 2009; 102:258-67; PMID:19652876 [DOI] [PubMed] [Google Scholar]
- 41.Raman K, Kuberan B. Chemical tumor biology of heparan sulfate proteoglycans. Curr Chem Biol 2010; 4:20-31; PMID:20596243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skarga Y, Vrublevskaya V, Evdokimovskaya Y, Morenkov O. Purification of the 90 kDa heat shock protein (hsp90) and simultaneous purification of hsp70/hsc70, hsp90 and hsp96 from mammalian tissues and cells using thiophilic interaction chromatography. Biomed Chromatogr 2009; 23:1208-16; PMID:19488974; http://dx.doi.org/ 10.1002/bmc.1245 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


