Abstract
Cells respond to fluid shear stress through dynamic processes involving changes in actomyosin and other cytoskeletal stresses, remodeling of cell adhesions, and cytoskeleton reorganization. In this study we simultaneously measured focal adhesion dynamics and cytoskeletal stress and reorganization in MDCK cells under fluid shear stress. The measurements used co-expression of fluorescently labeled paxillin and force sensitive FRET probes of α-actinin. A shear stress of 0.74 dyn/cm2 for 3 hours caused redistribution of cytoskeletal tension and significant focal adhesion remodeling. The fate of focal adhesions is determined by the stress state and stability of the linked actin stress fibers. In the interior of the cell, the mature focal adhesions disassembled within 35-40 min under flow and stress fibers disintegrated. Near the cell periphery, the focal adhesions anchoring the stress fibers perpendicular to the cell periphery disassembled, while focal adhesions associated with peripheral fibers sustained. The diminishing focal adhesions are coupled with local cytoskeletal stress release and actin stress fiber disassembly whereas sustaining peripheral focal adhesions are coupled with an increase in stress and enhancement of actin bundles. The results show that flow induced formation of peripheral actin bundles provides a favorable environment for focal adhesion remodeling along the cell periphery. Under such condition, new FAs were observed along the cell edge under flow. Our results suggest that the remodeling of FAs in epithelial cells under flow is orchestrated by actin cytoskeletal stress redistribution and structural reorganization.
Keywords: cytoskeletal force, focal adhesions, FRET, MDCK cells, mechanotransduction
Introduction
Mechanical forces provide key signals that regulate functions of mammalian cells and provide guidance for the tissue development.1 Epithelial cells in kidney tubules experience a range of shear stresses from urinary flow and cells use these mechanical signals to activate adaptation processes involving changes in actomyosin contractility, reorganization of actin cytoskeleton and reassembly of intercellular junctions and cell adhesions to extracellular matrix (ECM).1-5 This adaptation process appears essential to the maintenance of epithelial integrity. It has been shown that exposure of renal epithelial cells to chronic flow results in reorganization of actin cytoskeleton and an increase in the formation of cell-cell junctions.6,7 Shear stress also modifies the expression of tissue plasminogen resulting in a change of phenotype.7
Cells respond to long-term shear stress through cell type-dependent processes, involving cytoskeleton assembly/disassembly, focal adhesion (FA) displacement, and lamellipodia formation.8-10 In endothelial cells, exposure to shear stress causes the peripheral actin bundles on the basal side of the cell to disassociate. This is followed by the formation of actin networks close to the apical membrane allowing the alignment of cells with the flow.11,12 Shear stress also causes rapid formation of lamellipodia and focal adhesions, and lateral displacement of stress fibers in endothelial cells.8 Epithelial cells respond to shear stress differently. In epithelial cells, shear stress causes disassociation of the thick and randomly aligned actin bundles at the basement cortex, followed by the formation of peripheral structure that does not affect the overall cell shape.7,13 There are limited studies on FA remodeling and force transduction in epithelial cells under flow, and these cells might use different mechanisms for force transduction than endothelial cells.
We have shown that the cytoskeletal adaptation of epithelial cells to fluid shear stress spans a wide range of time scales. Pulsatile shear stress causes reversible changes in α-actinin tension, with no apparent rearrangement of cytoskeleton.14 Prolonged shear stress produces an inward contraction of F-actin network, followed by disassociation of actin stress fibers and formation of actin bundles along the cell periphery.15 This reorganization of cytoskeleton is accompanied with multiple phases of cytoskeletal stress variations leading to a net reduction in average cell tension.15 Due to the physical connection between the cytoskeleton and integrin via cross-linking proteins at focal adhesion complexes, this shear-induced cytoskeletal dynamics could cause focal adhesion remodeling in concert.
Although an intact actin cytoskeleton appears essential for adhesion growth, through which myosin-II mediated contractile force is applied to FAs,16-19 the role of cytoskeletal forces and its reorganization on FA remodeling under flow is unclear. Stress fibers could provide a structural template for FA growth without the involvement of forces.20 Recently, we have shown that an increase in α-actinin tension near FAs and recruitment of α-actinin to the FA sites is necessary for the growth of FAs in static condition, suggesting that FA remodeling is a force dependent process.21 Since shear stress causes multiphase redistribution of cytoskeletal stresses in epithelial cells, it can alter FA dynamics in a force dependent fashion under flow. In addition, shear stress induces Rho activation associated with force-activated signaling cascades including Rho GTPases that regulates actin polymerization and cell contractility.22,23
In this study, we analyzed shear induced FA remodeling in epithelial cells and correlated it with simultaneously measured local and global cytoskeletal (α-actinin) tension under fluid shear stress using a microfluidic flow chamber. FAs were labeled with paxillin-mApple and cytoskeletal tension was measured by the force-sensitive FRET sensor, actinin-sstFRET that has been previously characterized.14,24 We show that under flow, the dynamics of FAs depends on the stability of the linked stress fibers that is regulated by local cytoskeletal tension. FAs linked to radial stress fibers at the cell edge or in the middle of the cell disintegrate as the actin stress fibers disassociate under flow; FAs linked to peripheral actin fibers sustain or grow as the associated fibers are enhanced under shear.
Materials and Methods
Actinin-sstFRET sensor
The force sensitive FRET probe has Cerulean as the donor and Venus as the acceptor, linked by a spectrin repeat domain, and the probe is termed spectrin repeat stretch sensitive FRET sensor (sstFRET). The sstFRET probe is inserted near the middle of α-actinin (at amino acid position 300). The linker length is chosen to be equal to the Forster distance of this particular FRET pair, so the sensor operates at optimum sensitivity and linearity. The details of the construction are described previously.24 The force sensitivity of the probe has been calibrated using DNA springs, using a previously described method.25 The calibration shows that our probe (sstFRET) is sensitive to forces on the scale of 2-10 pN.24 The force probe has been extensively characterized and it has been shown that the chimeric constructs behave as endogenous proteins and the labeling does not interfere with physiological functions.24,26,27 Paxillin-mApple was a gift from Michael W. Davidson laboratory at Florida State University. The details of construction of paxillin-mApple are published.28
Flow experiments
The microfluidic chip consists of PDMS channel between 2 parallel glass coverslips and is fabricated using soft lithography. The channel is 1000 µm wide, 100 µm high and 15 mm long. Holes through the PDMS form the inlet and outlet. The chamber was placed in a stage-top incubator system (INUB-ZILCSD-F1-LU, Tokai Hit Co., Ltd, Japan) maintained at 37°C and 5% CO2 for long term experiments. Isotonic solution was perfused through the channel using a syringe pump (PHD2000, Harvard Apparatus). The flow shear stress (τ) was calculated as 6 µQ/wh2, where µ = 1.0 × 10−3 Pa s is the dynamic viscosity of the solution, Q is the volume flow rate, w and h are the width and the height of the channel, respectively.
Cell culture and transfection
The microfluidic channel was coated with fibronectin and a suspension of Madin-Darby Canine Kidney (MDCK) cells (ATCC) was perfused into the channel and kept for 40 min for cells to attach/adhere. Cells were then cultured in Dulbecco's Modified Eagle Medium (DMEM) having 10% fetal bovine serum and 1% penicillin and streptomycin; the culture media was changed every 24 hours. Cells were transfected with actinin-sstFRET and paxillin-mApple after 48 hours of culture, at ∼60% confluency, using Effectene (0.2 µg DNA each; 1:50, DNA to Effectene ratio) by incubating in solution for next 16 hours. The media was changed and experiments were conducted at 80–90% confluency in the channel on the third day of the culture. Transfected cells in a confluent region were chosen for the experiments. Isotonic solution was used for flow experiments to avoid background fluorescence.
Imaging and FRET analysis
Fluorescence images were obtained using an inverted microscope (Axiovert 200M, Zeiss) with 63× oil immersion objective and an EM-CCD camera (ImagEM C9100-13, Hamamatsu, Japan). CFP and YFP images were acquired using 2 filter sets (Ex: 436/20, Em: 480/40 and Ex: 500/20, Em: 535/30, respectively), and paxillin-mApple images were taken using red fluorescent protein (RFP) filter set (Ex: 550/25, Em: 605/70). Zeiss software (AxioVision, Zeiss) was used to record time lapse images of the 3 channels.
FRET energy transfer efficiency was calculated as the acceptor to donor intensity ratio (FRET A/D ratio), where the donor (CFP channel) and acceptor (YFP channel) fluorescence was measured with donor and acceptor excitation respectively.24 The acceptor to donor ratio represents quenching of the donor fluorescence due to energy transfer and is monotonically inverse to the tension in α-actinin. CFP and YFP images were processed using Image-J (NIH) following previously published methods.24 Briefly, the images were aligned, and processed to subtract the background and calculate the acceptor to donor intensity ratio; the result was displayed as a 16-color map where warm colors (red) indicate higher FRET ratio equivalent to lower tension and cool colors (blue) indicate a lower FRET ratio (higher tension). For regional FRET comparison (interior region versus cell edges) within a cell, the FRET ratio images were normalized by average FRET value of the cell at each time point and mean FRET was calculated for each region. To measure FRET variation for whole cell, or at FAs, the FRET ratio was obtained for the region of interest (ROI), and estimated FRET baseline shift of respective cell was subtracted from the data. The change in stresses was calculated by normalizing the FRET ratio with the value at time zero (right before starting the flow). Paxillin-RFP images were used to estimate the dimensions of the focal adhesion regions. The length of the FA was defined as the major axis of an ellipse fit to focal adhesions. FA diminishing/reducing in the text implies reduction in FA length. To get the average tension for multiple cells and the average length variation of multiple FAs over multiple experiments, the normalized data for each measurement was fit with a polynomial of order 5 (to get the data with same time interval) and the mean was calculated from the analytic form. Each measurement used a fresh cell culture in a fresh flow chamber. The statistics are calculated as mean ± s.e.m. of n measurements.
Results
Co-transfection of MDCK with actinin-sstFRET and paxillin-mApple
The cytoskeleton is linked to the ECM through FA protein complexes containing cross-linking proteins such as α-actinin, vinculin, filamin, and talin.29 The FA complex is a multilayered structure, with integrin-mediated signaling proteins such as paxillin at the basal layer, cytoskeletal adaptors such as vinculin and talin in the middle, and actin-regulatory proteins such as α-actinin on the apical cortex.30 The bottom and top layers are separated by ∼40 nm.30 This unique structure enables us to image paxillin and α-actinin simultaneously without fluorescence crosstalk (Fig. 1A), the former is used to analyze the focal adhesion remodeling and the latter is used to measure the forces in α-actinin as well as the assembly/disassembly of actin stress fibers.
Figure 1.
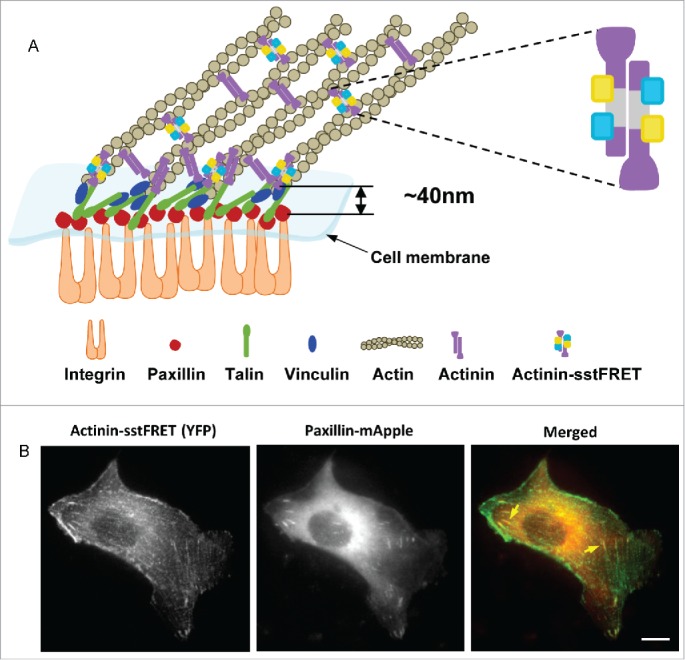
Co-transfection of MDCK cell with actinin-sstFRET and paxillin-mApple. (A) Schematic of a focal adhesion complex, showing that the FRET force sensor (actinin-sstFRET) and paxillin-mApple are located in separate layers of the adhesion complex. (B) Actinin-sstFRET (YFP channel, green), paxillin-mApple (RFP channel, red) and merged images of a co-transfected MDCK cell. Yellow arrows indicate FAs at the ends of the stress fibers. Scale bar =10 μm.
To measure the cytoskeletal force variation, we inserted FRET probes into actin cross-linking protein, α-actinin, that is located in both FAs and along F-actin filaments (Fig. 1A). By expressing actinin-sstFRET and subsequently staining the actin with phalloidin, we have also shown that actinin-sstFRET and F-actin co-localize along the actin network in cells.14,15 Thus, actinin-sstFRET probes (YFP channel) can also be used as labels for F-actin to monitor F-actin in real time.
To correlate time-dependent FA remodeling and cytoskeletal stress and reorganization, cells were co-transfected with paxillin-mApple and actinin-sstFRET sensor. Figure 1B shows actinin-sstFRET (YFP channel), paxillin-mApple (RFP channel) and merged images of a transfected cell in static condition, showing FAs at the ends of the stress fibers (yellow arrows), and co-localization of α-actinin and paxillin at the FAs. We observed different characteristic behaviors related to FA dynamics under shear stress, depending on the orientation of linked actin stress fibers and progression of their stresses.
Fluid shear stress causes heterogeneous distribution of cytoskeletal tension
Epithelial cells in static condition have randomly arranged stress fibers; most radial fibers run across the cell body and terminate at FAs perpendicular to the cell edge or at FAs in the cell interior. These fibers bear high tension in the static condition. Under flow, the reorganization results in disassembly of radial fibers across the cells and the formation of actin bundles along cell edge. The reorganization leads to a net reduction in tension when averaged over an entire cell, as shown previously,15 but the heterogeneous actin redistribution suggests that the changes in cytoskeletal tension could be non-uniform within a cell. Using actinin-sstFRET probes, we analyzed regional cytoskeletal tension, in the interior and at the edge of the cell, under flow. Confluent MDCK cells expressing actinin-sstFRET were subjected to steady fluid shear stress of 0.74 dyn/cm2, a level of shear stress experienced by renal epithelial cells under physiological conditions.31 Figure 2A shows F-actin visualized with actinin-sstFRET (YFP channel) and stress distribution in a pair of MDCK cells before (left panels) and after exposure to shear stress (right panels). Blue color indicates lower FRET ratio, a higher tension, and red color indicates higher FRET ratio, a lower tension. Cytoskeletal stress decreased in the interior region but increased along the edge of the cell under fluid shear stress for 3 hrs. As shown in Fig. 2A, actin stress fibers disassociated and actin bundles formed at the cell periphery under shear stress. This is seen as higher actin intensity at the cell periphery (the region within red-dashed outlines in Fig 2A, upper panel). An increase in tension is shown at the cell edges from FRET measurement (Fig. 2A, lower panel), this could be the result of an increase in tension in the existing actin fibers and recruitment of new fibers to the cell edge. Time course of FRET ratio in the 2 regions is shown in Fig. 2B, showing deviation of stresses between the interior region (red curve in Fig. 2B) and at the edges (blue curve in Fig. 2B) under flow. Figure 2C shows average interior and edge stress variations from 7 cells under shear. The previously reported reduction of averaged stress over the whole cell mainly reflects the interior stress due to disassembly of stress fibers across cells.15 This data shows that the redistribution of cellular tension under shear stress is non-uniform reflecting the heterogeneous and anisotropic nature of the cytoskeleton that leads to regional reorganization.
Figure 2.
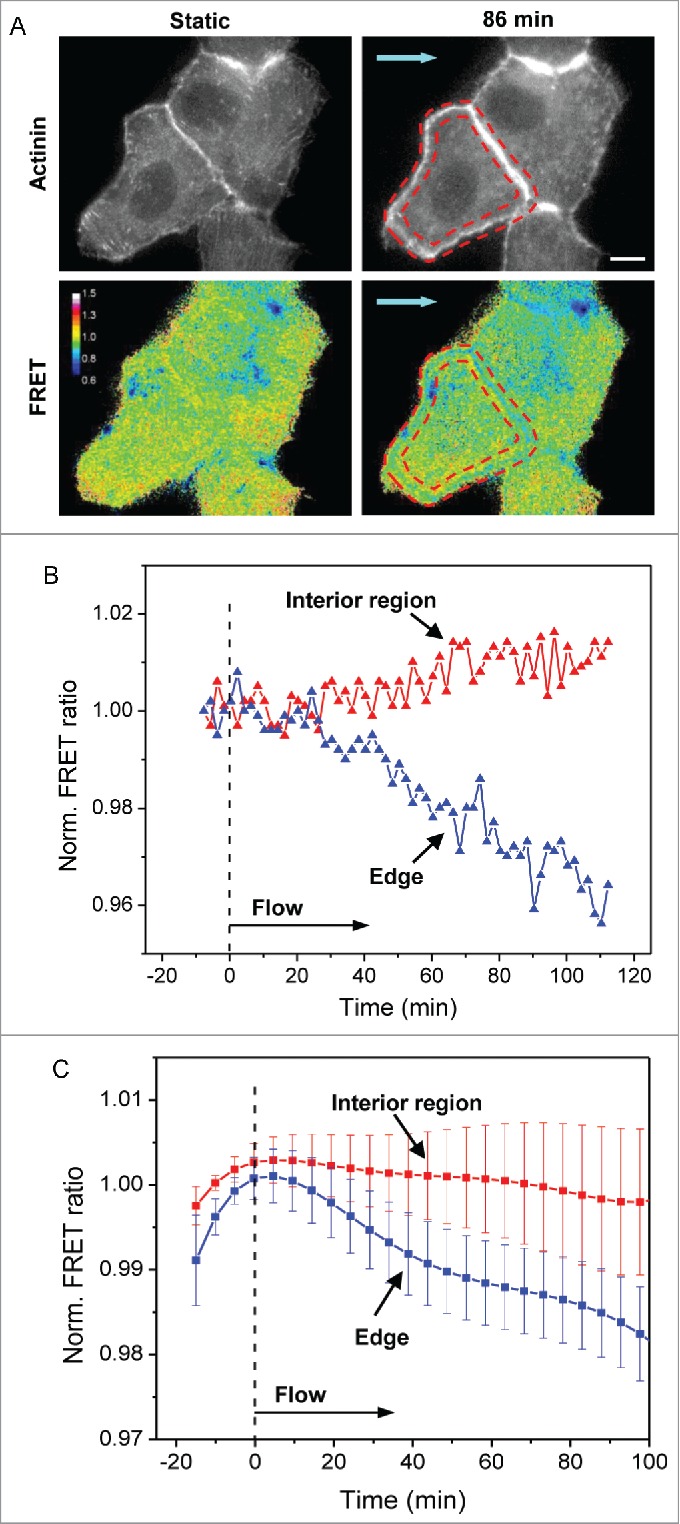
Redistribution of cytoskeletal tension under shear stress. (A) Images of actinin-sstFRET (YFP channel, indicating F-actin) (upper panel) and FRET ratio in color map (lower panel) of cells in static condition and after 86 min of shear stress. The cool colors (blue) in FRET ratio images indicate higher tension whereas warm colors (red) indicate lower tension. The cell periphery is marked by the red-dash outlines, showing a higher actin density and tension under shear. Blue arrows indicate the direction of flow. (B) Time course of the FRET ratio in the interior region of the cell (red curve) and at the cell edges (blue curve) for the cell shown in (A), showing that the tension in the interior region decreases, whereas the tension at the cell edges increases under flow. (C) Time dependent changes in interior and edge tension under flow averaged over multiple cells (n = 7). The error bars indicate s.e.m. Scale bar = 10 μm.
FAs that anchor tensional stress fibers diminish under flow
To correlate the cytoskeletal stresses with the FA remodeling, we simultaneously measured forces and FA dynamics in cells co-expressing actinin-sstFRET and paxillin-mApple. FAs exhibited 2 distinguishable characteristics, the FAs connected with the radial stress fibers diminish under flow, while the FAs connected with peripheral actin fibers persist under flow. We first analyzed dynamic interaction between the diminishing FAs perpendicular to the cell edge and associated radial actin bundles under flow and found that the disassembly of the FA coincided with reduction of local stress and disassociation of radial stress fibers. The dynamic change of 2 representative FAs is shown in Fig. 3A. Both selected FAs diminish under shear (Fig. 3A, upper panel), and this is accompanied with the disruption of actin stress fibers (Fig. 3A, lower panel). The time course of changes in FA length (FA1 in Fig. 3A) and local force (averaged over the marked window in Fig. 3A) is shown in Fig. 3B, showing that disassembly of FA occurred after 30 min under flow and the length of FA was reduced more than 50% within 60 min. Note that the significant stress release occurred ∼10 min after the onset of shear stress, which precedes the FA disassociation (Fig. 3B). The statistics of the focal adhesion length of 14 FAs from 7 experiments under flow is shown in Fig. 3C (p = 0.00001, paired sample t-test before and after 36 min of shear). For comparison, the statistics of FRET ratio from multiple diminishing FAs is shown later in Fig. 6C (red curve). This result suggests that reduction in local stress during disassembly of linked actin stress fiber may lead to the reduction of FAs. The radial actin bundles experience higher tension under static condition, which provides the required environment for FA growth at leading edge during cell migration.21 Exposure to fluid shear stress causes reduction in tension and disassociation of radial actin bundles, which significantly reduces the stability of the connected FAs. In confluent cell culture, most of the cells retract their protrusions and FAs in the protrusions break down under flow. We did not observe new active protrusions under shear. This is consistent with our previous observation that long-term exposure to fluid shear stress reduces cell mobility.15
Figure 3.
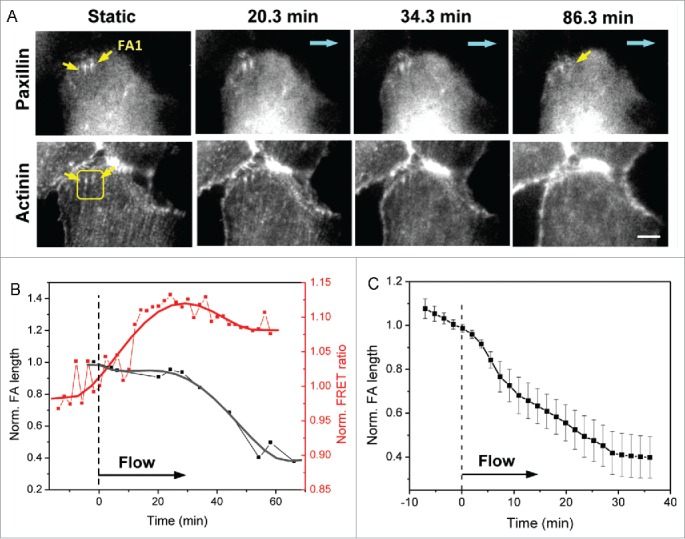
Dynamics of FAs linked with radial stress fibers under flow. (A) Images of paxillin-mApple and actinin-sstFRET (YFP channel) in a ROI near cell edge, showing FAs with actin normal to the cell edge (indicated by yellow arrows) decreasing in size under shear stress (upper panel) as the associated actin stress fibers disintegrate (lower panel). Blue arrows indicate the flow direction. (B) Time dependence of length of FA1 (indicated by yellow arrow in (A) and regional FRET ratio (yellow box in A), showing that FA disassembly is associated with stress release. (C) Averaged length variation of multiple FAs linked to radial stress fibers (n = 14, p = 0.00004, paired sample t-test before and after 36 min of shear). Error bars indicate s.e.m. Scale bar = 5 μm.
Figure 6.
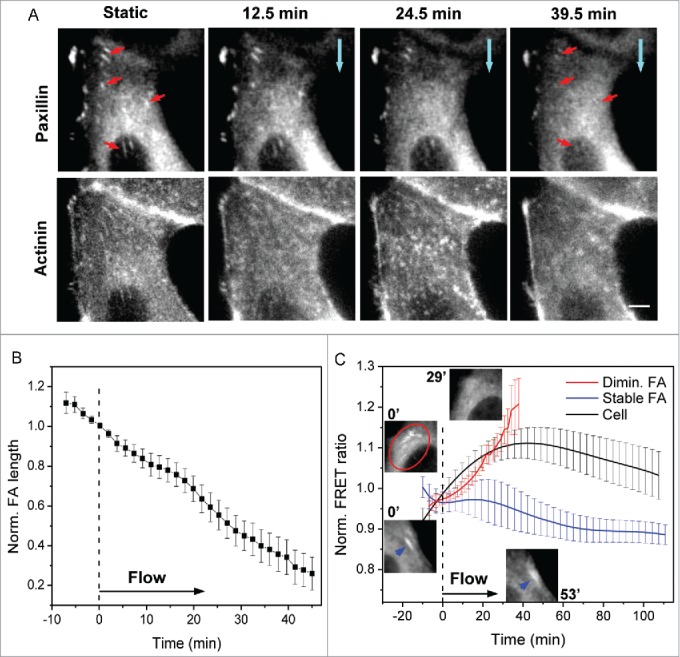
Dynamic changes of interior focal adhesions under flow. (A) Images of paxillin-mApple and actinin-sstFRET (YFP channel) of interior region of a cell in static condition and at various times during application of flow (blue arrows show the direction of flow). Multiple FAs in the interior region (indicated by red arrows) shown under static condition diminish after ~40 min of shear stress (upper panel). This is associated with disassembly of linked actin stress fibers (lower panel). (B) Averaged length variation of multiple FAs in the interior region (n = 22, p = 1 × 10−7, paired sample t-test for FA lengths before and after 31 min of shear). (C) Time course of FRET ratio for diminishing FAs (red curve, n = 17, p = 0.0005, paired sample t-test for stress before and after 18 min of shear) and stable FAs along the cell periphery (blue curve, n = 6, p = 0.14, paired sample t-test for stress before and after 110 min of shear), showing a stress release in diminishing FAs. The two top insets show interior FAs before and after 29 min of shear stress; bottom two insets show a peripheral FA (indicated by blue arrows) persisting under flow. The black curve shows time variation of average FRET ratio for entire cell (n = 7) for comparison. The error bars indicate s.e.m. Scale bar = 5 μm.
FAs associated with peripheral stress fibers enhance under flow
We then examined the FAs that are linked with actin stress fibers parallel to the cell perimeter, and found that these FAs remain stable and some of them are enhanced in length under flow, as the associated actin fiber is incorporated into the peripheral actin ring. We followed a typical FA along the periphery (FA1, in Figs. 4A, B), and show that the length and orientation of the FA remain unchanged under the same flow condition (0.74 dyn/cm2). The associated actin fibers also persist as shown in Fig. 4B. As expected, the α-actinin tension at FA1 increased under flow, and the stress increase coincides with a subtle increase in the FA length (Fig. 4C). The length variation of 7 such FAs from 6 experiments is shown in Fig. 4D. The statistical increase in stress associated with peripheral FAs is shown later in Fig. 6C (blue curve). The continued existence of FA is largely determined by the stress state and the stability of the associated actin fibers. The occurrence of sustained peripheral FAs is small since the majority of FAs at cell edge are linked with radial stress fibers in static condition.
Figure 4.
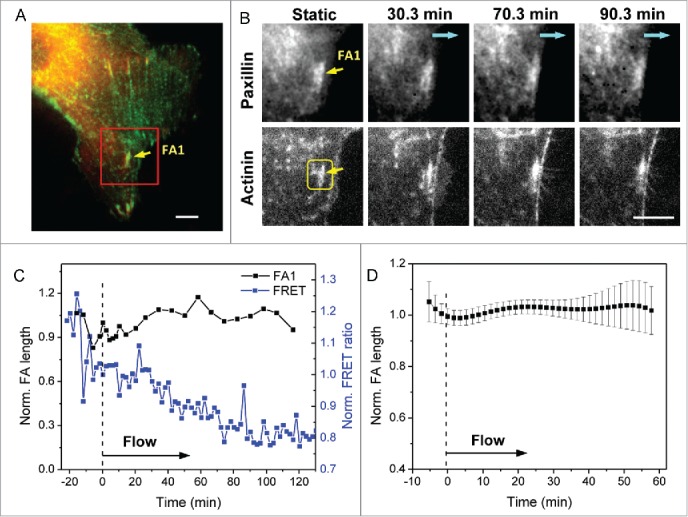
Dynamics of FAs associated with peripheral stress fibers under flow. (A) Merged paxillin-mApple and actinin-sstFRET images of a cell under static condition. (B) Zoom-in view of the box in (A) at various times under flow, showing that the FA linked with actin parallel to the cell edge remains under flow (upper panel) and the associated actin fiber remains (lower panel). Blue arrows indicate the flow direction. (C) Time dependent changes in FA1 length and FRET ratio measured from the box in (B) showing an increase in local α-actinin stress as the FA persists under flow. (D) Averaged length variation of multiple parallel FAs (n = 7). Error bars indicate s.e.m. Scale bar = 5 μm.
With increase in peripheral tension and formation of peripheral actin bundle, new FAs can form and grow along the cell edge. An example of such FA formation is shown in Fig 5A (red arrows indicate newly formed FAs). The increase in paxillin fluorescence intensity coincides with the increase in α-actinin intensity as shown in Fig. 5B illustrating an increase in the density of these proteins. FAs near the edge can also reorganize along with the associated actin fiber to align with the newly formed peripheral actin bundle. An example of such a FA near the cell edge is shown in Figure S1 (supplemental materials), showing disassembly of a FA in first 40 min of flow followed by reassembly along the cell periphery. These observations further support that flow-induced formation of actin bundles along cell periphery create local mechanical environments that favor FA growth along the cell edges.
Figure 5.
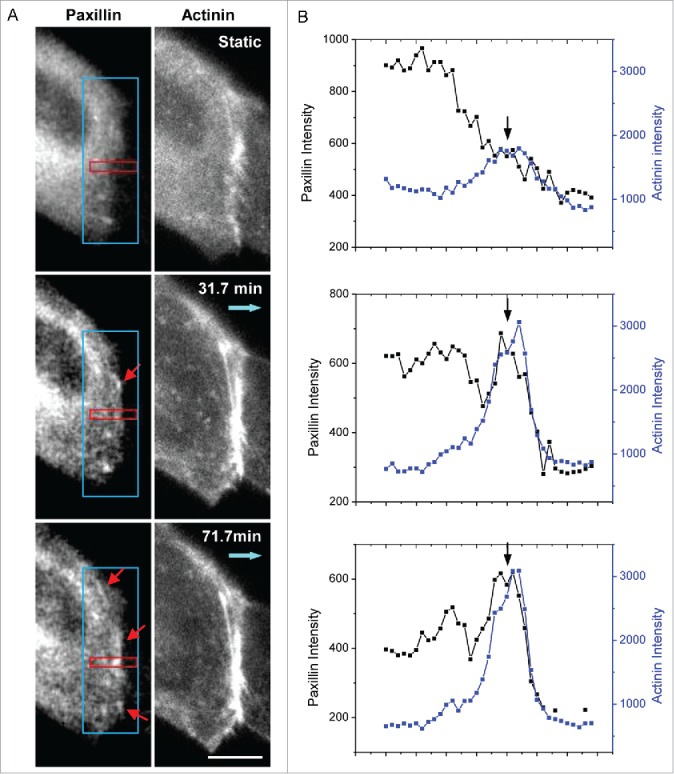
Formation of new peripheral FAs under shear. (A) Images of paxillin-mApple (left column) and actinin-sstFRET (right column) at various times under flow showing new FAs forming at the cell periphery (indicated by red arrows). Blue arrows show the flow direction. (B) Fluorescence intensity of paxillin and α-actinin along the red window (the intensity is averaged across the width of the window) at the selected times shown in panel (A). The plots show that both proteins peaked at 71.7 min (indicated by arrows), indicating the growth of new FA. Scale bar = 10 μm.
Interior focal adhesions diminish under flow
Most of the FAs in the cell interior are linked to the stress fibers under tension and serve as an anchor between the stress fibers and ECM. These FAs disassembled under flow along with the reduction in interior cytoskeletal tension and disassembly of actin stress fibers. Figure 6A shows a sequence of paxillin-mApple images of a cell before and during application of shear stress of 0.74 dyn/cm2. There are multiple FAs in the cell interior that are linked with stress fibers (indicated by red arrows in Fig. 6A), and most of the mature FAs disassemble within 40 min under flow (Fig. 6A, upper panel). Actin stress fibers linked with these FAs simultaneously disassemble (Fig. 6A, lower panel). There is no new FA formation in the interior region of cell under shear. Statistical analysis over multiple cells shows that the interior FAs reduced to less than 50% of their original length within 40 min of application of flow (Fig. 6B, n = 22 FAs from 8 cells in 7 experiments, p = 1 × 10-7, paired sample t-test for FA lengths before and after 31 min of shear). We measured local α-actinin stress for the diminishing FAs anchoring the radial stress fibers both in the cell interior and near the cell edge and show that a reduction of α-actinin stress coincides with diminishing of FAs (Fig. 6C, red curve, n = 17, p = 0.0005, paired sample t-test for stress before and after 18 min of shear). The local stress release at diminishing FAs is temporally coherent with average cell stress reduction (Fig. 6C, red and black curves compared) indicating that FA disassembly is synchronized with cytoskeleton disorganization and global stress release. FAs of different configurations in a single cell are analyzed and their dynamic changes under flow is shown in Figure S2 (supplemental materials). For comparison, we plotted α-actinin stress at FAs linked with peripheral actin fibers, showing a stress increase during the same time period (Fig. 6C, blue curve, n = 6, p = 0.14, paired sample t-test for stress before and after 110 min of shear). The local stress at persistent FAs is coherent with the average tension in peripheral actin bundles (compared with Fig. 2C, blue curve).
The role of cytoskeletal forces is further shown in the remodeling of interior FAs that are occasionally linked with persistent actin bundles. Figure S3 (supplemental materials) shows an interior actin bundle persisting after 45 min of flow, unlike the surrounding bundles, and the FA linked with the intact actin bundle resides (Fig. S3). This emphasizes the heterogeneity of cytoskeletal structures and supports the argument that the remodeling of FAs under flow is coupled with actin tension and actin reorganization. This result shows that the fate of FAs is determined by the tension state and stability of associated stress fibers regardless of their physical locations.
Discussion
Cultured MDCK cells have abundant FAs in protrusions and at the ends of stress fibers in the interior region. The FAs are linked to the actin cytoskeleton via actin binding proteins, such as α-actinin, at adhesion complexes mediating cell migration and changes in cell shape. Fluid shear regulates cytoskeletal stress and cytoskeleton reorganization, resulting in an overall reduction of cellular tension, disassociation of stress fibers and strengthening of the cytoskeleton along cell perimeter. Using force sensitive FRET probes for α-actinin and fluorescently labeled probes for paxillin, we report FA remodeling in epithelial cells under shear and show that FA dynamics are coupled with actin (α-actinin) stress as a part of the flow-induced dynamic loop, redistribution of cytoskeletal tension and the formation of peripheral ring structures.
Although most FAs undergo changes when cells are subjected to flow, the fate of FAs depends on the stress state and the stability of the associated actin stress fibers. The FAs that anchor tensional stress fibers disassemble under flow, accompanied by the disassembly of actin bundles. As a result, majority of FAs connected with radial actin bundles at cell edges and most of FAs in the middle of the cell disintegrate as the actin stress fibers disassociate under shear (Figs. 3 and 6). The disassembly of FAs is accompanied with local actinin stress release (Fig. 3 and 6). In contrast, FAs associated with peripheral actin fibers sustain as the peripheral actin fibers and the regional tension increases under shear (Fig. 4). In addition, new FAs may also form along the cell edge as the peripheral actin bundle organizes (Fig. 5).
It has been reported that stress fiber is required for the formation of new FAs, which provides a structural template for FA growth.20 We have also shown that an increase in α-actinin tension and correlated recruitment of α-actinin to an active adhesion coincide with FA growth,21 suggesting that FA remodeling is a force sensitive process. Long-term fluid shear stress leads to multiphase changes of cytoskeletal tension and reorganization, this cytoskeletal dynamics could transmit the fluid shear force to FAs over the entire cell orchestrating coherent FA dynamics. In fact, our results show a strong coherence of FA dynamics with local and global actin stress variations and remodeling. Moreover, only the FAs linked with peripheral actin bundles at the cell edges are promoted during flow, and these fibers show an increase in tension under flow. It suggests that the actin stress fibers not only serve as template for the FAs, but also provide the mechanical forces for regulating FA remodeling under flow. It has been reported that adherence of MDCK cell to collagen substratum is mainly mediated by peripheral actin filaments at adhesion complexes, but not by stress fibers in kidney.32 Our results show that under flow condition, the cells remain adherent with the substratum despite the absence of FAs in the middle, also indicating that the adherence of MDCK cells relies on the peripheral adhesions under flow. Epithelial cells cultured under flow more closely mimic cells under in vivo conditions.
We summarize the processes of FA remodeling in epithelial cells in a schematic model (Fig. 7). Under long-term shear stress, cytoskeletal tension across the cells reduces, and this is accompanied by disintegration of actin stress fibers. FAs that are linked to these bundles (FA-1, 2, Fig. 7) diminish under flow. Subsequent formation of actin bundles along the cell perimeter promotes the formation and maintenance of FAs parallel to cell periphery (FA-3, 5, Fig. 7). The data suggests that FA remodeling under flow is driven by the cytoskeletal stress changes and reorganization. In conclusion, our results show that MDCK cells adapt to chronic shear stress by redistributing the cytoskeletal stress (relaxing the middle and strengthening the periphery) through cytoskeletal reorganization and simultaneous remodeling of focal adhesions. Focal adhesion dynamics (assembly or disassembly) is determined by stress state and stability of the associated actin cytoskeleton under flow.
Figure 7.
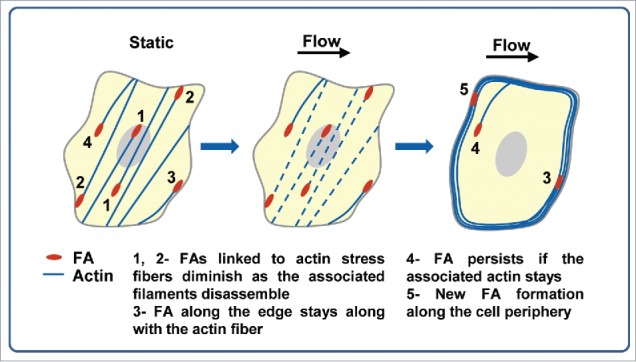
The schematic model of FA dynamics correlating with actin dynamics in epithelial cells under shear stress.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was partly supported by National Institutes of Health grant NS085517 (S.Z.H.) and an NIH grant to F.S. D.V. was partly supported by National Science Foundation Grant DMR1309712.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. Faseb J 2006; 20:811-27; PMID:16675838; http://dx.doi.org/ 10.1096/fj.05-5424rev [DOI] [PubMed] [Google Scholar]
- 2.Weinbaum S, Duan Y, Satlin LM, Wang T, Weinstein AM. Mechanotransduction in the renal tubule. Am J Physiol 2010; 299:F1220-36; PMID:20810611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev 2011; 12:308-19; PMID:21508987; http://dx.doi.org/ 10.1038/nrm3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nature Rev Mol Cell Biol 2006; 7:265-75; http://dx.doi.org/ 10.1038/nrm1890 [DOI] [PubMed] [Google Scholar]
- 5.Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci 2006; 119:508-18; PMID:16443749; http://dx.doi.org/ 10.1242/jcs.02760 [DOI] [PubMed] [Google Scholar]
- 6.Duan Y, Gotoh N, Yan Q, Du Z, Weinstein AM, Wang T, Weinbaum S. Shear-induced reorganization of renal proximal tubule cell actin cytoskeleton and apical junctional complexes. Proc Natl Acad Sci U S A 2008; 105:11418-23; PMID:18685100; http://dx.doi.org/ 10.1073/pnas.0804954105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Essig M, Terzi F, Burtin M, Friedlander G. Mechanical strains induced by tubular flow affect the phenotype of proximal tubular cells. Am J Physiol 2001; 281:F751-62; PMID:11553522 [DOI] [PubMed] [Google Scholar]
- 8.Mott RE, Helmke BP. Mapping the dynamics of shear stress-induced structural changes in endothelial cells. Am J Physiol Cell Physiol 2007; 293:C1616-26; PMID:17855768; http://dx.doi.org/ 10.1152/ajpcell.00457.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a "bumper-car" model. Proc Natl Acad Sci U S A 2004; 101:16483-8; PMID:15545600; http://dx.doi.org/ 10.1073/pnas.0407474101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loufrani L, Retailleau K, Bocquet A, Dumont O, Danker K, Louis H, Lacolley P, Henrion D. Key role of alpha(1)beta(1)-integrin in the activation of PI3-kinase-Akt by flow (shear stress) in resistance arteries. Am J Physiol Heart Circ Physiol 2008; 294:H1906-13; PMID:18245559; http://dx.doi.org/ 10.1152/ajpheart.00966.2006 [DOI] [PubMed] [Google Scholar]
- 11.Franke RP, Grafe M, Schnittler H, Seiffge D, Mittermayer C, Drenckhahn D. Induction of human vascular endothelial stress fibres by fluid shear stress. Nature 1984; 307:648-9; PMID:6537993; http://dx.doi.org/ 10.1038/307648a0 [DOI] [PubMed] [Google Scholar]
- 12.Galbraith CG, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil Cytoskeleton 1998; 40:317-30; PMID:9712262; http://dx.doi.org/ 10.1002/(SICI)1097-0169(1998)40:4%3c317::AID-CM1%3e3.0.CO;2-8 [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Heo J, Hua SZ. Spatially resolved shear distribution in microfluidic chip for studying force transduction mechanisms in cells. Lab on a Chip 2010; 10:235-9; PMID:20066252; http://dx.doi.org/ 10.1039/B914874D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahimzadeh J, Meng F, Sachs F, Wang J, Verma D, Hua SZ. Real-time observation of flow-induced cytoskeletal stress in living cells. Am J Physiol Cell Physiol 2011; 301:C646-52; PMID:21653900; http://dx.doi.org/ 10.1152/ajpcell.00099.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma D, Ye N, Meng F, Sachs F, Rahimzadeh J, Hua SZ. Interplay between cytoskeletal stresses and cell adaptation under chronic flow. PloS One 2012; 7(9):e44167; PMID:23028495; http://dx.doi.org/ 10.1371/journal.pone.0044167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannone G, Dubin-Thaler BJ, Rossier O, Cai YF, Chaga O, Jiang GY, Beaver W, Döbereiner HG, Freund Y, Borisy G, et al.. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell 2007; 128:561-75; PMID:17289574; http://dx.doi.org/ 10.1016/j.cell.2006.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, et al.. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol 2001; 3:466-72; PMID:11331874; http://dx.doi.org/ 10.1038/35074532 [DOI] [PubMed] [Google Scholar]
- 18.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 1997; 275:1308-11; PMID:9036856; http://dx.doi.org/ 10.1126/science.275.5304.1308 [DOI] [PubMed] [Google Scholar]
- 19.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol 2010; 188:877-90; PMID:20308429; http://dx.doi.org/ 10.1083/jcb.200906012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oakes PW, Beckham Y, Stricker J, Gardel ML. Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J Cell Biol 2012; 196:363-74; PMID:22291038; http://dx.doi.org/ 10.1083/jcb.201107042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye N, Verma D, Meng F, Davidson MW, Suffoletto K, Hua SZ. Direct observation of alpha-actinin tension and recruitment at focal adhesions during contact growth. Exp Cell Res 2014; 327:57-67; PMID:25088253; http://dx.doi.org/ 10.1016/j.yexcr.2014.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res 2006; 98:176-85; PMID:16456110; http://dx.doi.org/ 10.1161/01.RES.0000200162.94463.d7 [DOI] [PubMed] [Google Scholar]
- 23.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 1999; 285:895-8; PMID:10436159; http://dx.doi.org/ 10.1126/science.285.5429.895 [DOI] [PubMed] [Google Scholar]
- 24.Meng F, Sachs F. Visualizing dynamic cytoplasmic forces with a compliance-matched FRET sensor. J Cell Sci 2011; 124:261-9; PMID:21172803; http://dx.doi.org/ 10.1242/jcs.071928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng CY, Wang A, Zocchi G, Rolih B, Levine AJ. Elastic energy of protein-DNA chimeras. Phys Rev E Stat Nonlin Soft Matter Phys 2009; 80:061912; PMID:20365195; http://dx.doi.org/ 10.1103/PhysRevE.80.061912 [DOI] [PubMed] [Google Scholar]
- 26.Meng F, Suchyna TM, Sachs F. A fluorescence energy transfer-based mechanical stress sensor for specific proteins in situ. FEBS J 2008; 275:3072-87; PMID:18479457; http://dx.doi.org/ 10.1111/j.1742-4658.2008.06461.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng F, Sachs F. Orientation-based FRET sensor for real-time imaging of cellular forces. J Cell Sci 2012; 125:743-50; PMID:22389408; http://dx.doi.org/ 10.1242/jcs.093104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaner NC, Lin MZ, McKeown MR, Steinbach PA, Hazelwood KL, Davidson MW, Tsien RY. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat Methods 2008; 5:545-51; PMID:18454154; http://dx.doi.org/ 10.1038/nmeth.1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat Rev 2001; 2:793-805; PMID:11715046; http://dx.doi.org/ 10.1038/35099066 [DOI] [PubMed] [Google Scholar]
- 30.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. Nanoscale architecture of integrin-based cell adhesions. Nature 2010; 468:580-4; PMID:21107430; http://dx.doi.org/ 10.1038/nature09621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J. Polycystins and primary cilia: primers for cell cycle progression. Ann Rev Physiol 2009; 71:83-113; PMID:19572811; http://dx.doi.org/ 10.1146/annurev.physiol.70.113006.100621 [DOI] [PubMed] [Google Scholar]
- 32.Prahalad P, Calvo I, Waechter H, Matthews JB, Zuk A, Matlin KS. Regulation of MDCK cell-substratum adhesion by RhoA and myosin light chain kinase after ATP depletion. Am J Physiol Cell Physiol 2004; 286:C693-C707; PMID:14644769; http://dx.doi.org/ 10.1152/ajpcell.00124.2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


