Abstract
Histone deacetylase inhibitors (HDACIs) have been shown to have antiproliferative activity through cell-cycle arrest, differentiation, and apoptosis in colorectal cancer (CRC) cells. Our present study revealed that one HDAC inhibitor, valproic acid (VPA), can obviously promote in vitro motility of HCT-116 and SW480 cells. VPA treatment significantly down regulates the expression of epithelial markers E-Cadherin (E-Cad) and Zona occludin-1(ZO-1) while up regulates the mesenchymal markers Vimentin (Vim) and N-cadherin (N-Cad), suggesting that VPA can trigger the epithelial–mesenchymal transition (EMT) of CRC cells. VPA treatment significantly increases the expression and nuclear localization of Snail, the key transcription factors of EMT. Snail knockdown by siRNAs obviously reverses VPA induced EMT of HCT-116 and SW480 cells. Further, VPA can decrease the ubiquitination, increase the acetylation, and then elevate the stabilization of Snail. VPA also increases the phosphorylation of Akt/GSK-3β. The inhibitor of PI3K/Akt, LY2994002, significantly attenuates VPA induced phosphorylation of Akt and GSK-3β and up regulation of Snail and Vim. Collectively, our data reveal that VPA can trigger the EMT of CRC cells via up regulation of Snail through AKT/GSK-3β signals and post-transcriptional modification. It suggests that more attention should be paid when VPA used as a new anticancer drug for CRC patients.
Keywords: Akt/GSK-3β, colorectal cancer, EMT, Snail, VPA
Introduction
Human colorectal cancer (CRC) is a major worldwide health concern, with around 1.2 million new cancer cases each year.1 Most deaths from CRC are due to metastases that are resistant to conventional therapies. It is estimated that about 20% of CRC patients experienced tumor metastasis at diagnosis and lost the best treatment opportunity.2 Surgery, accompanied with chemotherapy, radiotherapy and biological target therapy, have greatly improved the prognosis of CRC patients. However, there are still 50% of patients died of tumor recurrence or metastasis in the second year of operation.3 Therefore the investigation of underlying pathological mechanisms of cancer genesis, development, invasion and metastasis is important to improve the cure rate and life quality of CRC patients.
Emerging evidences suggested that epithelial–mesenchymal transition (EMT), which refers to the epithelial cells' transfer to the mesenchymal cells, acts as an integral component of CRC.4 It is the first step in metastatic dissemination of cancer cells and characterized by loss of cell-cell adhesion and gain of migratory and invasive traits. The down-regulation of E-cadherin (E-Cad) and up-regulation of Vimentin (Vim) have been regarded as the markers of EMT.5,6 A number of key transcription factors, such as Snail, Slug, Zeb, and Twist, were identified to potentiate EMT progression.7 They can bind to E-cad promoter and inhibit its transcription activity and expression.8 Therefore, the loss or decrease of E-Cad is considered to be the primary and most important step of EMT.9
Valproic acid (VPA, 2-propylpentanoic acid) has been widely used as an anti-convulsant for more than 40 y.10 As a histone deacetylase inhibitor (HDACI), VPA has been suggested to induce the differentiation of many kinds of cancer cells in vitro and suppress tumor growth in vivo.11 It shows significantly anti-cancer effects in various cancer models including lung, renal, bladder, and cervical cancer.12–15 Recently, numerous studies suggested that HDACIs including VPA can modulate the EMT of cancer cells. However, existing data about the effects of HDACIs on EMT were contradictory.16–19 HDACIs can inhibit EMT by up regulation of E-Cad.20,21 While other studies suggested that HDACIs can promote the EMT in various cancer cells.16-19 As to CRC, one recent study indicated that HDACIs can induce the EMT.16 While the roles and mechanisms of VPA on the EMT of CRC cells remain to be further illustrated.
Considering that HDACs might be considered as anticancer therapeutic targets for CRC treatment,22 we therefore evaluate the effects and related mechanism of VPA on the progression and development of CRC cancers. Our results revealed that VPA significantly promotes the in vitro migration and invasion and then promotes the EMT of CRC cells. Further, our studies reveal the stabilization of Snail via acetylation and repression of GSK-3β mediated VPA induced EMT of CRC.
Materials and Methods
Chemicals and reagents
VPA and other chemicals were of reagent grade or better and purchased from Sigma Chemical Co. (St. Louis, MO, USA) unless otherwise noted. The monoclonal antibodies and the secondary anti-mouse antibody conjugated to horseradish peroxidase (HRP) are products of Cell Signaling Technology (MA, USA). Alexa Fluor 488/594 conjugated secondary antibody, DAPI and Lipofectamine 2000 were purchased from Invitrogen (Carlsbad, CA, USA). SYBR Premix Ex Taq II is a product of TaKaRa BIO Inc.. (TBI, Japan). All compounds were solubilized in dimethyl sulfoxide (DMSO). Steroid-free medium containing DMSO (0.5% v/v) was used as the control.
Cell culture, treatment, and transfection
Human CRC cell lines HCT-116 and SW480 were obtained from the American Type Culture Collection (ATCC) and cultured in RPMI-1640 medium containing 10% fetal bovine serum (FBS) (Hyclone, UT, USA) and antibiotics in a humidified incubator with 5% CO2 at 37 °C. VPA was dissolved in DMSO with the final concentration of DMSO in the medium less than 0.5%. For transfection, HCT-116 or SW480 cells were seeded into plates and transfected with siRNA negative control or si-Snail1˜3 by use of Lipofectamine 2000 reagent according to the manufacture's instructions.
Cell viability assay
Methyl-thiazol tetrazolium (MTT) assay was use to evaluate the effects of VPA on the in vitro proliferation of CRC cells. Briefly, HCT-116 or SW480 cells were inoculated into 96-well plates with 5 × 103 cells per well for 24 h, and then treated with increasing concentrations of VPA for 48 h. Then, 50 μl of sterile MTT (5 mg/ml, Sigma) was added to each well and incubated for 4 h. The reaction was stopped by adding 150 μl DMSO. After mixed for 10 min, the absorbance was measured at 450 nm using a microplate reader. At least 6 independent experiments were performed for each concentration.
Cell migration and invasion assay
The effects of VPA on the migration and invasion of CRC cells were evaluated in triplicate by modified Boyden chambers and wound healing assay according to previous study.23 For wound healing assay, HCT-116 or SW480 cells were cultured on 6-well plates for 24 h and then treated with or without VPA. Then a defined scratch was applied on the well bottom, which detached cells within a definite corridor. The recovering of scratch wound was monitored 48 h later by use of a microscope. Image J software (National Institutes of Health, Bethesda, MD) was then used to quantify the initial scratch area and the final area of the scratch 24 h later. The distance of cell migration was calculated and compared with the control group. For in vitro invasion assay, the polycarbonate filters (8 μm pore size) were coated with Matrigel TM until dried. Then cells in suspension (1 × 105) treated with or without VPA were loaded into the upper chamber in medium without FBS. After treatment, cells that had spread through the pores of the filter and into the lower chamber were fixed in 70% methanol at −20°C and counted under a phase contrastmicroscope (five fields per chamber).
Western blot analysis
After treatment with or without VPA, cells were harvested lysed and prepared for western blot analysis as described previously.24 Briefly, the protein concentrations were measured by use of Bradford method. Then 30 μg total protein were loaded onto 8% SDS-PAGE for electrophoresis and then transferred onto a PVDF membrane (Millipore) by electroblotting. Then membrane was then incubated with primary antibodies at 4 °C overnight against human Snail, E-cad, FN, Vim, ZO-1, and GAPDH. Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 2 h. Blots were developed using an ECL kit (Amersham, Arlington Heights, IL, USA).
Quantitative real-time PCR
After transfected with siRNAs, cells were washed twice with PBS. Total RNA from each group of cells was extracted with Trizol (Invitrogen Life Technologies, CA, USA). The cDNA was synthesized using Takara reverse transcriptase and oligo(dT) primer from 2 μg of total RNA. Reverse transcription was performed at 37°C for 50 min followed by 15 min at 70°C for inactivation. The primers used in each reaction were as follows: Snail, forward 5′- GAC CAC TAT GCC GCG CTC TT -3′ and reverse 5′- TCG CTG TAG TTA GGC TTC CGA TT -3′; GAPDH, forward 5′-GCA CCG TCA AGG CTG AGA AC-3′ and reverse 5′-TGG TGA AGA CGC CAG TGG A-3′. The PCR amplification were performed for 35 cycles of 94°C for 30 s, 60°C for 30 s, and 72 °C for 30 s. After normalized to GAPDH gene, expression levels for each target gene were calculated using the comparative threshold cycle (CT) method. Each experiment was performed in triplicates and repeated 3 times.
Immunofluorescence
HCT-116 cells were grown on chamber slides. After 12 h of cultivation, cells were stimulated with VPA for 24 h. Cells were fixed in 4% paraformaldehyde for 10 minutes and blocked with goat serum over night at 4°C, then incubated with anti-Snail antibody at 1:100 for 1 h at 37 °C. Cells were washed three times with PBS and then incubated with a secondary anti-mouse antibody conjugated to FITC at 1:1000 for 1 h at 37°C. Finally, cells were washed, incubated with DAPI (10 μg/ml) for 10 min to visualize cell nuclei, and examined with Confocal Laser Scanning Microscopy (Zeiss, Germany) to analyze nuclear translocation of Snail.
Immunoprecipitation
To assess the ubiquitination and acetylation of Snail, treated cells were washed twice with ice-cold PBS and harvested at 4°C in immunoprecipitation lysis buffer. The lysates were prepared with 20 strokes, and then supernatants collected after centrifugation at 14,000 rpm for 10 min at 4°C. Equal amounts of protein were immunoprecipitated using anti-Snail antibody or negative control IgG, and the immune complexes were bound to 20 μl of Protein A/G beads (Pierce Classic IP kit, Thermo Scientific, IL, USA) for 2 h at 4°C. The beads were washed with lysis buffer and subjected to protein gel blotting with anti-ubiquitin or anti-acetylate antibody.
Statistical analysis
The statistical analyses were performed using SPSS 17.0 (Aspire Software International, Leesburg, VA, USA) for Windows. Statistical analysis of the average value between 2 groups was done by Student's t-test, and differences with P value less than 0.05 were considered statistical significance.
Results
VPA promotes the in vitro motility of CRC cells
Firstly, we investigated the effects of VPA on the cell viability of HCT-116 and SW480 cells for 48 h. As shown in Figure 1 A, VPA greater than 5 mM can significantly inhibit the in vitro proliferation of CRC cells. The IC50 of VPA on the proliferation of HCT116 and SW480 was 11.2 and 16.4 mM, respectively. Therefore, 1 mM VPA, which has no significant effect on the cell proliferation, was used in the next study to investigate the effect of VPA on motility of CRC cells. The results of wound healing assay revealed that 1 mM VPA significantly increased the wound closure of both HCT-116 and SW480 cells (Fig. 1B-C). Transwell analysis confirmed that 1 mM VPA significantly (p < 0 .01) promoted the in vitro invasion of both HCT-116 and SW480 cells(Fig. 1D). Collectively, our results suggested that VPA can significantly promote the in vitro migration and invasion of CRC cells.
Figure 1.
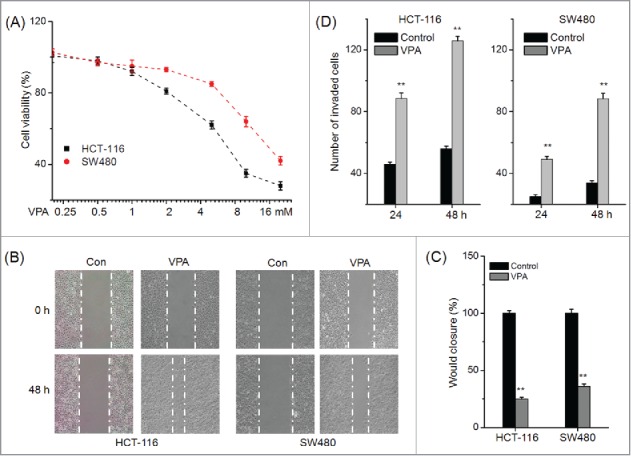
VPA promotes the in vitro motility of CRC cells. (A) HCT-116 and SW480 cells were treated with various concentrations of VPA for 48 h, and then the cell viability was assessed by use of MTT assay; (B) Representative images of wounds at 0 and 48 h in the presence or absence of 1 mM VPA; (C) Quantitative analysis of wound healing assay for CRC cells treated with 1 mM VPA for 48 h; (D) HCT-116 or SW480 cells were allowed to invade spread through the matrix gel and into the under-side of the filter for 24 h and 48 h in the presence or absence of 1 mM VPA. The number of invaded cells were fixed, stained, photographed, and compared with the control group. **p < 0.01 compared with control.
VPA induces the epithelial-mesenchymal transition (EMT) of CRC cells
EMT has been considered as the first and key step for the metastasis of cancer cells.25 Therefore whether VPA can induce the EMT phenotype of CRC cells was further investigated. Western blot analysis showed that VPA can significantly decrease the epithelial makers E-cad and ZO-1, whereas increase the mesenchymal markers N-cad and FN, in both HCT-116 and SW480 cells (Fig. 2A). It suggested that CRC cells have undergone an EMT phenotype after treatment with VPA. Further, immunofluorescence staining showed that VPA treated HCT-116 cells had decreased E-Cad while increased Vim and FN as compared with the control cells (Fig. 2B). All these data suggested that VPA can induce the EMT of CRC cells.
Figure 2.
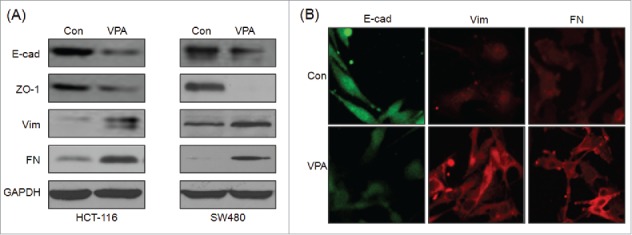
VPA induces the EMT of CRC cells. (A) HCT-116 or SW480 cells were treated with 1 mM VPA for 48 h, and then the expression of E-cad, ZO-1, Vim, and FN were analyzed by Western blot analysis; (B) HCT-116 cells were treated with 1 mM VPA for 48 h, and then the expression of E-cad, Vim, and FN were analyzed by immunofluorescence.
VPA significantly increases the expression of Snail
Transcription factors including Snail, Slug, ZEB1, and Twist have been reported to play essential roles in regulating EMT.26 Therefore the effects of VPA on the expression of transcription factors were measured by use of Western blot analysis. The result showed that VPA treatment for 24 h significantly elevated the expression of Snail, while not others, in both HCT-116 and SW480 cells (Fig. 3A). Further the sub cellular localization of Snail in VPA treated HCT-116 cells was checked by use of immunofluorescence staining. The result showed that VPA treatment obviously triggered the nuclear translocation of Snail in HCT-116 cells (Fig. 3B). These results revealed that VPA can significantly increase the expression and nuclear translocation of Snail in CRC cells.
Figure 3.
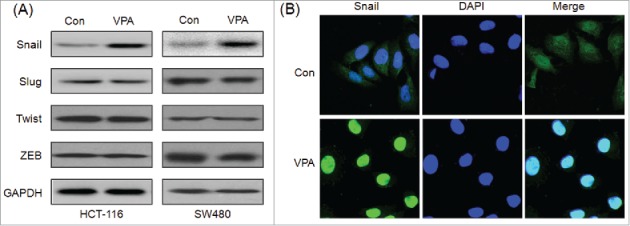
VPA significantly increases the expression of Snail. (A) HCT-116 or SW480 cells were treated with 1 mM VPA for 24 h, and then the expression of Snail, Slug, Zeb, Twist were measured by use of Western blot analysis; (B) HCT-116 cells were treated with 1 mM VPA for 24 h, the cellular location of Snail (green) were examined by immunofluorescence staining and nuclei were stained with DAPI (blue).
Snail plays an essential role in VPA induced EMT
To verify the roles of Snail in VPA induced EMT of CRC cells, HCT-116 and SW480 cells were transfected with non-targeting control si-RNA (siNC) or si-Snail1∼3 for 24 h, and then treated with VPA for 48 h. The silence of Snail was confirmed by the results of real time PCR and Western blot analysis in HCT-116 cells (Fig. 4A), which showed that si-Snail1 had the most great efficiency for Snail knockdown. Then si-Snail1 was chose for the next studies. The EMT markers were measured by Western blot analysis in CRC cells transfected with siNC or si-Snail1. The results revealed that silence of Snail significantly attenuated VPA induced down regulation of E-cad and up regulation of Vim and FN in both HCT-116 (Fig. 4B) and SW480 (Fig. 4C) cells. It suggested that Snail is essential for VPA induced EMT in CRC cells.
Figure 4.
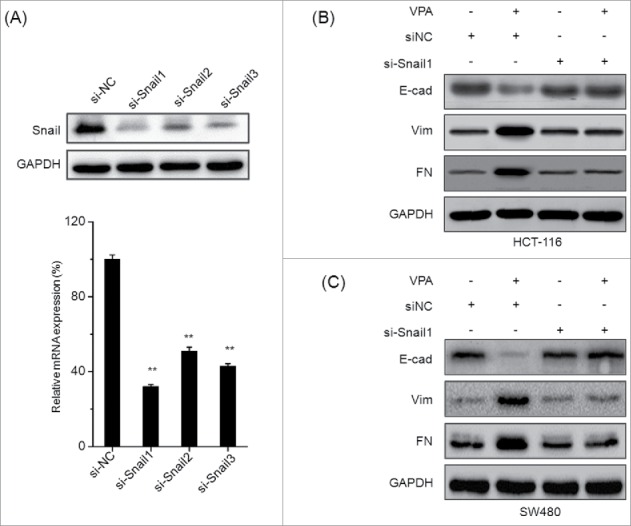
Snail plays an essential role in VPA induced EMT. (A) HCT-116 cells were transfected with Snail specific si-RNA1˜3 (si-Snail1˜3) or negative control si-RNA (si-NC) for 24 h, and then the mRNA and protein expression of Snail were analyzed by qRT-PCR and Western-blot, respectively. HCT-116 (B) or SW480 (C) cells were transfected with si-Snail1 or negative control si-RNA (si-NC) for 24 h and then exposed to 1 mM VPA for another 48 h, the expression of EMT related markers were measured by use of Western blot analysis. **p < 0.01 compared with control.
VPA increases the acetylation while decreases the ubiquitination of Snail
To investigate the mechanisms responsible for VPA induced expression of Snail, we checked the shorter time for upregulation of Snail protein by VPA. The results revealed that the expression of Snail protein was significantly upregulated by VPA within 1 h in both HCT-116 and SW480 cells (Fig. 5A), suggesting that up-regulation of Snail quantity partially may be via post-transcriptional processes. Then the acetylation of Snail was further checked due to VPA can increase the acetylation of both histone and non-histone proteins. HCT-116 cells were treated with 2 μM Bortezomib to inhibit degradation of ubiquitinated proteins and then treated with or without 1 mM VPA for another 2 h. The acetylation and ubiquitination of Snail levels in immune complex were detected by western blotting with an anti-acetyalte and an anti-ubiquitin antibody. The results revealed that VPA can significantly increased the acetylation (Fig. 5B) while decreased the ubiquitination (Fig. 5C) of Snail. These results suggested that VPA can increase the acetylation and decrease the ubiquitination of Snail and then elevate its stabilization.
Figure 5.
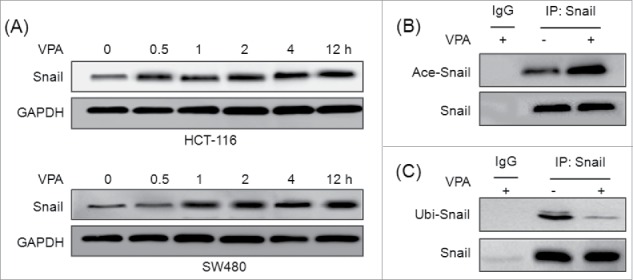
VPA increases the acetylation while decreases the ubiquitination of Snail. (A) HCT-116 or SW480 cells were treated with 1 mM VPA for the indicated times, the expression of Snail were measured by use of Western blot analysis; HCT-116 cells were treated with 2 μM Bortezomib and then exposed to VPA for 2 h. The total proteins in the cell lysate were subjected to immunoprecipitation with an anti-Snail antibody or negative control IgG. The acetylation or ubiquitination of Snail levels in immune complex was detected by protein gel blotting with an anti-acetyalte (B) or an anti-ubiqutin (C) antibody (upper panel).
Phosphorylation of Akt/GSK-3β is involved in VPA induced Snail expression and EMT
Recent studies indicated that AKT/GSK-3β mediated stabilization of Snail participates the EMT of CRC cells.27 Therefore the phosphorylation and protein levels of GSK-3β were also measured in VPA treated HCT-116 and SW480 cells. The results revealed that VPA treatment significantly increased the phosphorylation levels of Akt and GSK-3β in both HCT-116 and SW480 cells (Fig. 6A). To verify whether Akt/GSK-3β was involved in VPA induced EMT of CRC cells. HCT-116 cells were treated with the inhibitor of PI3K/Akt, LY294002 (20 μM), prior to exposure to VPA, expression of p-AKT, p-GSK-3β, Snail, and markers of EMT were determined by Western blot analysis. The results revealed that LY294002 significantly attenuated VPA induced phosphorylation of Akt and GSK-3β and up regulation of Snail and Vim (Fig. 6B). Further, LY294002 also abolished the VPA induced EMT-like morphological changes of HCT-116 cells (Fig. 6C). Collectively, these observations suggested that phosphorylation of Akt/GSK-3β mediates, at least partially, VPA induced up regulation of Snail and EMT of CRC cells.
Figure 6.
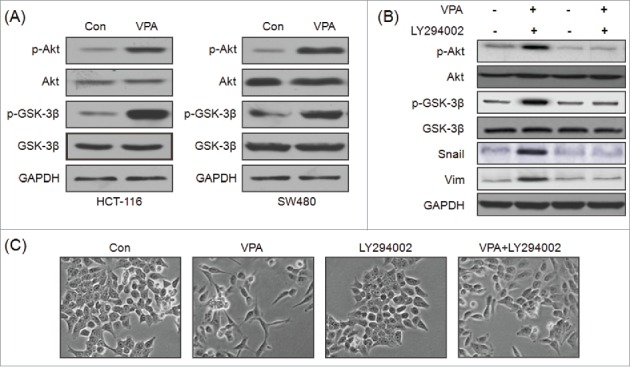
Phosphorylation of Akt/GSK-3β is involved in VPA induced Snail expression and EMT. (A) HCT-116 or SW480 cells were treated with 1 mM VPA for 2 h, and then the phosphorylation and protein levels of Akt, GSK-3βwere measured by use of Western blot analysis; (B) HCT-116 cells were pretreated with or without LY294002 (20 μM) for 1 h, followed by stimulation with or without 1 mM VPA for 48 h. The expression of Snail, E-Cad and the activation of AKT and GSK-3β were examined by Western blot analysis; (C) Changes in morphology of HCT-116 cells were analyzed by a phase-contrast microscopy.
Discussion
Several HDAC inhibitors are in clinical trials for the treatment of solid and hematological malignancies (Khan and 28). VPA has several advantages including low cost and favorable safety profile, therefore it has been considered as candidate drugs for various cancer cell types.29-31 However, recent studies revealed that HDACIs such as SAHA and NaB can promote the metastasis of cancer cells.16–19 In the present study, we revealed that VPA can significantly promote the in vitro migration and invasion of CRC cells via induction of EMT. The key transcription factor Snail, which was up regulated and accumulated in nuclear in VPA treated CRC cells, mediated VPA induced EMT. Further, VPA can increase the expression of Snail via elevating its stabilization and activating Akt/GSK-3β signals.
Nowadays, the limited data concerning the effects of HDACIs on the motility of cancer cells are contradictory. Several studies reported that SAHA can suppress the migration and invasion of cancer cells via inhibition of EMT.20,32,33 VPA also can significantly inhibit high-invasive renal cancer ACHN cell15 and breast cancer MDA-MB-231 cell.34 It has been reported to upregulate the expression of E-Cad, inhibit the EMT, and then suppress the migration of prostate cancer cells.35 However, recent studies indicated that HDACIs including VPA and SAHA can promote the metastasis of cancer cells.16–19 Trichostatin A (TSA) and VPA can induced mesenchymal features in the colon carcinoma cells by a decrease in E-cad and an increase in Vim expression in colon carcinoma cells.16 VPA can increase the expression of MMP-2 and then induced the migration of cord blood mesenchymal stromal cells.36 The expression of MMP-2 and MMP-9 was also up regulated in VPA treated human glioma cells.37 The contradictory data may be due to the different of cell lines, HDACIs, and treatment approaches.38 Our present study revealed that 1 mM VPA can significantly promote the in vitro motility of CRC cells via induction of EMT. It hence suggested that VPA requires careful caution before their application as new anticancer drugs for colon cancers.
The alteration of transcription is one of the main mechanisms for EMT regulation in tumor cells. Transcription factors such as Snail, Slug, Twist1 and Zeb act as an key modulator on the initiation and progression of EMT.26 Snail and Slug can bind to the E-box site in the promoter of E-cad and trigger the EMT of many types of cancer.39 Whereas factors such as Twist, Zeb, E2.2 and FoxC2 repress E-cadherin transcription indirectly.26 In the present study, our data revealed that VPA can trigger the expression and nuclear localization of Snail while not others. Further, silence of Snail can attenuate the VPA induced EMT of CRC cells. The up regulation and nuclear translocation of Snail can promote the EMT of cancer cells via directly suppressing the expression of E-cad in various cancers.39 Although our results did not exclude the possibility that VPA could influence other regulation approaches to trigger the EMT, our data revealed that up regulation of Snail participated VPA induced EMT of CRC cells.
Our results indicated that Snail protein was significantly upregulated by VPA within 1 h in both HCT-116 and SW480 cells, which suggested that upregulation of Snail quantity partially may be via post-transcriptional processes. Snail is a highly unstable protein, our data revealed that VPA can significantly increased its acetylation while decreased its ubiquitination. It was well known that ubiquitination of proteins will lead to proteasomal degradation. Further, acetylation of lysines can equally block ubiquitination of the same residue of protein and then inhibit its degradation.40,41 Further, our results found that VPA can up regulate the expression of Snail via Akt/GSK-3β signal pathway. The activation of Akt/GSK-3β signal has been reported to be associated with a loss of cell adhesion, increased of cell motility, and poor prognosis of CRC.42 VPA can activation of Akt in pancreatic cancer cells.43 That stabilization of Snail mediated by AKT/GSK-3β mediates EMT of colorectal cancer induced by TNFα.27 In our study, the phosphorylation of GSK-3β, up regulation of Snail and Vim was blocked by treatment with the PI3K inhibitor, suggesting that AKT/GSK-3β signaling pathway plays an essential role for VPA induced EMT of CRC cells.
In summary, we here demonstrated more molecular evidences of VPA on the migration and invasion of CRC cells. The results showed that VPA can trigger the in vitro motility of CRC cells via induction of EMT. In addition, the up regulation of Snail via AKT/GSK-3β and post-transcriptional modification mediates VPA induced EMT. Although our data were only based on just 2 cell lines, in addition, VPA is mostly used in combination with other agents/irradiation for clinical therapy, our findings will help to better understand the roles of VPA on the progression of CRC and suggest that more attention should be paid when VPA used as new anticancer drugs for CRC patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (2013) and the Science and Technology Planning Project of Guangdong Province, China (2012B031800494).
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2012; 65:87-108; http://dx.doi.org/ 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res 2015a; 27:2-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schule S, Dittmar Y, Knosel T, Krieg P, Albrecht R, Settmacher U, Altendorf-Hofmann A. Long-term results and prognostic factors after resection of hepatic and pulmonary metastases of colorectal cancer. Int J Colorectal Dis 2013; 28:537-45; PMID:22885838; http://dx.doi.org/ 10.1007/s00384-012-1553-0 [DOI] [PubMed] [Google Scholar]
- 4.Bates RC, Mercurio AM. The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol Ther 2005; 4:365-70; PMID:15846061; http://dx.doi.org/ 10.4161/cbt.4.4.1655 [DOI] [PubMed] [Google Scholar]
- 5.Zavadil J, Bottinger EP. TGF-β and epithelial-to-mesenchymal transitions. Oncogene 2005; 24:5764-74; PMID:16123809; http://dx.doi.org/ 10.1038/sj.onc.1208927 [DOI] [PubMed] [Google Scholar]
- 6.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 2009; 119:1429-37; PMID:19487819; http://dx.doi.org/ 10.1172/JCI36183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014; 15:178-96; PMID:24556840; http://dx.doi.org/ 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haley JD, Thomson S, Petti F, Sujka-Kwok I, Mercado P, Bean J, Monaghan M, Seymour SL, Argast GM, Epstein DM. A systems view of epithelial-mesenchymal transition signaling states. Clin Exp Metastas 2011; 28:137-55; http://dx.doi.org/ 10.1007/s10585-010-9367-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Bras GF, Taubenslag KJ, Andl CD. The regulation of cell-cell adhesion during epithelial-mesenchymal transition, motility and tumor progression. Cell Adh Migr 2012; 6:365-73; PMID:22796940; http://dx.doi.org/ 10.4161/cam.21326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Breemen MS, Rijsman RM, Taphoorn MJ, Walchenbach R, Zwinkels H, Vecht CJ. Efficacy of anti-epileptic drugs in patients with gliomas and seizures. J Neurol 2009; 256:1519-26; PMID:19434440; http://dx.doi.org/ 10.1007/s00415-009-5156-9 [DOI] [PubMed] [Google Scholar]
- 11.Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, et al.. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J 2001; 20:6969-78; PMID:11742974; http://dx.doi.org/ 10.1093/emboj/20.24.6969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Chen L, Sun F, Zhang G. Enhanced suppression of proliferation and migration in highly-metastatic lung cancer cells by combination of valproic acid and coumarin-3-carboxylic acid and its molecular mechanisms of action. Cytotechnology 2013; 65:597-608; PMID:23161221; http://dx.doi.org/ 10.1007/s10616-012-9513-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreaux J, Reme T, Leonard W, Veyrune JL, Requirand G, Goldschmidt H, Hose D, Klein B. Gene expression-based prediction of myeloma cell sensitivity to histone deacetylase inhibitors. Br J Cancer 2013; 109:676-85; PMID:23868005; http://dx.doi.org/ 10.1038/bjc.2013.392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Jing Y, Ouyang S, Liu B, Zhu T, Niu H, Tian Y. Inhibitory effect of valproic acid on bladder cancer in combination with chemotherapeutic agents and. Oncol Lett 2013a; 6:1492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang FQ, Liu M, Yang FP, Che J, Li W, Zhai W, Wang GC, Zheng JH, Li X. VPA inhibits renal cancer cell migration by targeting HDAC2 and down-regulating HIF-1alpha. Mol Biol Rep 2014; 41:1511-8; PMID:24390319; http://dx.doi.org/ 10.1007/s11033-013-2996-2 [DOI] [PubMed] [Google Scholar]
- 16.Ji M, Lee EJ, Kim KB, Kim Y, Sung R, Lee SJ, Kim DS, Park SM. HDAC inhibitors induce epithelial-mesenchymal transition in colon carcinoma cells. Oncol Rep 2015; 33:2299-308; PMID:25813246 [DOI] [PubMed] [Google Scholar]
- 17.Jiang GM, Wang HS, Zhang F, Zhang KS, Liu ZC, Fang R, Wang H, Cai SH, Du J. Histone deacetylase inhibitor induction of epithelial-mesenchymal transitions via up-regulation of Snail facilitates cancer progression. BBA-Mol Cell Res 2013; 1833:663-71 [DOI] [PubMed] [Google Scholar]
- 18.Kong D, Ahmad A, Bao B, Li Y, Banerjee S, Sarkar FH. Histone deacetylase inhibitors induce epithelial-to-mesenchymal transition in prostate cancer cells. Plos One 2013; 7:e45045; http://dx.doi.org/ 10.1371/journal.pone.0045045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchida H, Maruyama T, Nishikawa-Uchida S, Oda H, Miyazaki K, Yamasaki A, Yoshimura Y. Studies using an in vitro model show evidence of involvement of epithelial-mesenchymal transition of human endometrial epithelial cells in human embryo implantation. J Biol Chem 2012; 287:4441-50; PMID:22174415; http://dx.doi.org/ 10.1074/jbc.M111.286138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei WW, Zhang KH, Pan XC, Hu Y, Wang DM, Yuan XW, Shu GW, Song JG. Histone deacetylase 1 is required for transforming growth factor-β 1-induced epithelial-mesenchymal transition. Int J Biochem Cell Biol 2010; 42:1489-97; PMID:20580679; http://dx.doi.org/ 10.1016/j.biocel.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 21.Srivastava RK, Kurzrock R, Shankar S. MS-275 sensitizes TRAIL-resistant breast cancer cells, inhibits angiogenesis and metastasis, and reverses epithelial-mesenchymal transition in vivo. Mol Cancer Ther 2010; 9:3254-66; PMID:21041383; http://dx.doi.org/ 10.1158/1535-7163.MCT-10-0582 [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Zhong Q. Histone deacetylase inhibitors and cell death. Cell Mol Life Sci 2014; 71:3885-901; PMID:24898083; http://dx.doi.org/ 10.1007/s00018-014-1656-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen ZJ, Yang XL, Liu H, Wei W, Zhang KS, Huang HB, Giesy JP, Liu HL, Du J, Wang HS. Bisphenol A modulates colorectal cancer protein profile and promotes the metastasis via induction of epithelial to mesenchymal transitions. Arch Toxicol 2015b; 89:1371-81; http://dx.doi.org/ 10.1007/s00204-014-1301-z [DOI] [PubMed] [Google Scholar]
- 24.Wei W, Chen ZJ, Zhang KS, Yang XL, Wu YM, Chen XH, Huang HB, Liu HL, Cai SH, Du J, et al.. The activation of G protein-coupled receptor 30 (GPR30) inhibits proliferation of estrogen receptor-negative breast cancer cells in vitro and in vivo. Cell Death Dis 2014; 5:e1428; PMID:25275589; http://dx.doi.org/ 10.1038/cddis.2014.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev 2009; 28:15-33; PMID:19169796; http://dx.doi.org/ 10.1007/s10555-008-9169-0 [DOI] [PubMed] [Google Scholar]
- 26.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139:871-90; PMID:19945376; http://dx.doi.org/ 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Wang HS, Zhou BH, Li CL, Zhang F, Wang XF, Zhang G, Bu XZ, Cai SH, Du J. Epithelial-mesenchymal transition (EMT) induced by TNF-α requires AKT/GSK-3beta-mediated stabilization of snail in colorectal cancer. Plos One 2013b; 8:e56664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan O, La Thangue NB. HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol 2012; 90:85-94; PMID:22124371; http://dx.doi.org/ 10.1038/icb.2011.100 [DOI] [PubMed] [Google Scholar]
- 29.Byler TK, Leocadio D, Shapiro O, Bratslavsky G, Stodgell CJ, Wood RW, Messing EM, Reeder JE. Valproic acid decreases urothelial cancer cell proliferation and induces thrombospondin-1 expression. BMC Urol 2012; 12:21; PMID:22898175; http://dx.doi.org/ 10.1186/1471-2490-12-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das CM, Aguilera D, Vasquez H, Prasad P, Zhang M, Wolff JE, Gopalakrishnan V. Valproic acid induces p21 and topoisomerase-II (α/β) expression and synergistically enhances etoposide cytotoxicity in human glioblastoma cell lines. J Neurooncol 2007; 85:159-70; PMID:17534580; http://dx.doi.org/ 10.1007/s11060-007-9402-7 [DOI] [PubMed] [Google Scholar]
- 31.Soriano AO, Yang H, Faderl S, Estrov Z, Giles F, Ravandi F, Cortes J, Wierda WG, Ouzounian S, Quezada A, et al.. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood 2007; 110:2302-8; PMID:17596541; http://dx.doi.org/ 10.1182/blood-2007-03-078576 [DOI] [PubMed] [Google Scholar]
- 32.Bruzzese F, Leone A, Rocco M, Carbone C, Piro G, Caraglia M, Di Gennaro E, Budillon A. HDAC inhibitor vorinostat enhances the antitumor effect of gefitinib in squamous cell carcinoma of head and neck by modulating ErbB receptor expression and reverting EMT. J Cell Physiol 2011; 226:2378-90; PMID:21660961; http://dx.doi.org/ 10.1002/jcp.22574 [DOI] [PubMed] [Google Scholar]
- 33.Nalls D, Tang SN, Rodova M, Srivastava RK, Shankar S. Targeting epigenetic regulation of miR-34a for treatment of pancreatic cancer by inhibition of pancreatic cancer stem cells. Plos One 2011; 6:e24099; PMID:21909380; http://dx.doi.org/ 10.1371/journal.pone.0024099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li GF, Qian TL, Li GS, Yang CX, Qin M, Huang J, Sun M, Han YQ. Sodium valproate inhibits MDA-MB-231 breast cancer cell migration by upregulating NM23H1 expression. Genet Mol Res 2012; 11:77-86; PMID:22290468; http://dx.doi.org/ 10.4238/2012.January.13.1 [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Wang G, Wang L, Song C, Wang X, Kang J. Valproic acid inhibits prostate cancer cell migration by up-regulating E-cadherin expression. Pharmazie 2011; 66:614-8; PMID:21901986 [PubMed] [Google Scholar]
- 36.Marquez-Curtis LA, Qiu Y, Xu A, Janowska-Wieczorek A. Migration, proliferation, and differentiation of cord blood mesenchymal stromal cells treated with histone deacetylase inhibitor valproic Acid. Stem Cells Int 2014; 2014:610495; PMID:24757448; http://dx.doi.org/ 10.1155/2014/610495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Tsai YH, Tseng SH. Valproic acid affected the survival and invasiveness of human glioma cells through diverse mechanisms. J Neurooncol 2012; 109:23-33; PMID:22528797; http://dx.doi.org/ 10.1007/s11060-012-0871-y [DOI] [PubMed] [Google Scholar]
- 38.Gotfryd K, Skladchikova G, Lepekhin EA, Berezin V, Bock E, Walmod PS. Cell type-specific anti-cancer properties of valproic acid: independent effects on HDAC activity and Erk1/2 phosphorylation. Bmc Cancer 2010; 10:383; PMID:20663132; http://dx.doi.org/ 10.1186/1471-2407-10-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell 2009; 15:195-206; PMID:19249678; http://dx.doi.org/ 10.1016/j.ccr.2009.01.023 [DOI] [PubMed] [Google Scholar]
- 40.Hernandez-Hernandez A, Ray P, Litos G, Ciro M, Ottolenghi S, Beug H, Boyes J. Acetylation and MAPK phosphorylation cooperate to regulate the degradation of active GATA-1. EMBO J 2006; 25:3264-74; PMID:16858405; http://dx.doi.org/ 10.1038/sj.emboj.7601228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeong JW, Bae MK, Ahn MY, Kim SH, Sohn TK, Bae MH, Yoo MA, Song EJ, Lee KJ, Kim KW. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell 2002; 111:709-20; PMID:12464182; http://dx.doi.org/ 10.1016/S0092-8674(02)01085-1 [DOI] [PubMed] [Google Scholar]
- 42.Pal I, Mandal M.. PI3K and Akt as molecular targets for cancer therapy: current clinical outcomes. Acta Pharmacol Sin 2012; 33:1441-58; PMID:22983389; http://dx.doi.org/ 10.1038/aps.2012.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi PF, Yin T, Zhou F, Cui PF, Gou SM, Wang CY. Valproic acid sensitizes pancreatic cancer cells to natural killer cell-mediated lysis by upregulating MICA and MICB via the PI3K/Akt signaling pathway. Bmc Cancer 2014; 14; PMID:24410891 [DOI] [PMC free article] [PubMed] [Google Scholar]


