Abstract
Dopamine (DA) possesses potent neuromodulatory properties in the central nervous system. In the anterior cingulate cortex, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPAR) are key ion channels in mediating nerve injury induced long-term potentiation (LTP) and chronic pain phenotype. In the present study, we reported the effects of DA on glutamate mediated excitatory post-synaptic currents (EPSCs) in pyramidal neurons of layer II/III of the ACC in adult mice. Bath application of DA (50 μM) caused a significant, rapid and reversible inhibition of evoked EPSCs (eEPSC). This inhibitory effect is dose-related and was absent in lower concentration of DA (5 μM). Furthermore, selective postsynaptic application of GDP-β-S (1.6 mM) in the internal solution completely abolished the inhibitory effects of DA (50 μM). We also investigated modulation of spontaneous EPSCs (sEPSCs) and TTX sensitive, miniature EPSCs (mEPSCs) by DA. Our results indicated mixed effects of potentiation and inhibition of frequency and amplitude for sEPSCs and mEPSCs. Furthermore, high doses of SCH23390 (100 μM) and sulpiride (100 μM) revealed that, inhibition of eEPSCs is mediated by postsynaptic D2-receptors (D2R). Our finding posits a pre- and postsynaptic mode of pyramidal neuron EPSC modulation in mice ACC by DA.
Keywords: Anterior cingulate cortex, AMPA/KA receptors, dopamine, D2 receptors, evoked excitatory postsynaptic currents, miniature excitatory postsynaptic currents, spontaneous excitatory postsynaptic currents
Introduction
Dopamine (DA) as a catecholamine is best known as the “reward” neurotransmitter in the general terms.1 However, DA is extremely potent and imperative in modulating many aspects of the central nervous system. Its absence can manifest as Parkinsonism2,3 and excessive and peculiar receptor signaling is observed in Schizophrenia.4,5 DA modulates synaptic transmission through interaction with five G protein-coupled receptor subtypes (GPCRs; D1–D5), divided in to two classes of D1- (D1–D5) and D2-like (D2–D3–D4) receptors (DAR).6 Previously, in vitro application of DA at a high dose has been shown to have presynaptic inhibitory effect on mediated excitatory postsynaptic currents (EPSCs) in rat brain slices (Table 1). Thus far, physiological profiling of DA’s activity has been well characterized in the prefrontal cortex (PFC), a critical area for cognition and working memory.7
Table 1.
Summary of whole-cell intracellular studies on the effects of DA (≥20 µM) bath application and AMPA/KAR-mediated EPSC modulation.
| Region | Species | DA dose | eEPSC | mEPSC | sEPSC | Locus | References |
|---|---|---|---|---|---|---|---|
| PFC | Rat | 20 µM | Increase | N/A | N/A | Post | Gonzalez-Islas and Hablitz45 |
| Rat | 100 µM | Inhibit | N/A | N/A | N/A | Law-Tho et al.35 | |
| Rat | 100 µM | Inhibit | N/A | N/A | N/A | Otani et al.36 | |
| Rat | 100 µM | N/A | ↑ Hz | N/A | N/A | Marek and Aghajanian53 | |
| Ferret | 10 mM local | Inhibit | N/A | N/A | Pre | Gao et al.51 | |
| NAc | Rat | 50 µM | Inhibit | N/A | N/A | Pre | Zhang et al.39 |
| Rat | 30 µM 30 µM | Inhibit Inhibit | N/A N/A | N/A N/A | Pre | Harvey and Lacey37 Harvey and Lacey41 | |
| Rat | 100 µM | Inhibit | N/A | N.A | Pre | Pennartz et al.44 | |
| Rat | 75 µM | Inhibit | ↓ Hz | N/A | Pre | Nicola et al.40 | |
| Prelimbic Cortex | Rat | 100 µM | N/A | No effect | ↑ Hz | Pre | Wang et al., 2002 |
| VTA | Rat | 30 µM | Inhibit | ↓ Hz | N/A | Pre | Koga and Momiyama, 2000 |
| NTS | Rat | 100 µM | Inhibit | N/A | ↓ Hz | Pre | Kline et al. 2002 |
| LEC | Rat | 50 µM | Inhibit | N/A | N/A | Pre | Caruana and Chapman43 |
| PBN | Rat | 50 µM | Inhibit | ↓ Hz | N/A | Pre | Chen et al.42 |
| ACC | Mice | 50 µM | Present | Present | Present | Present | Present |
AMPA: α-amino-3-hydroxy-5-methyl-4isoxazolepropionic acid; KAR: kainite receptors; EPSCs: excitatory postsynaptic currents; DA: Dopamine; eEPSCs: evoked excitatory postsynaptic currents; mEPSCs: miniature excitatory postsynaptic currents; sEPSCs: spontaneous excitatory postsynaptic currents; PFC: prefrontal cortex; NAc: nucleus accumbens; VTA: ventral tegmental area; LEC: lateral entorhinal cortex; PBN: parabrachial nucleus; ACC: anterior cingulate cortex; N/A: authors did not perform the experiments; local, pressure application of DA at the local neuron.
The anterior cingulate cortex (ACC) is a subregion of the cingulate cortex, selective for integrating the sensation of pain.8–10 Human imaging studies have revealed that ACC neurons are activated by noxious stimuli11,12 and elevated activity of the region in individuals with anxiety disorders.13 Furthermore, central plasticity in the ACC is affective in manifesting chronic pain.9 In rodents, peripheral nerve injury and visceral pain induces both presynaptic enchantment of glutamate release and postsynaptic up-regulation of α-amino-3-hydroxy-5-methyl-4isoxazolepropionic acid receptors (AMPAR’s; α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor) in the ACC.14–17 This enhanced glutamate mediate synaptic transmission in the ACC is the proposed mechanism behind chronic pain and anxiety.10,18 The presence of dopaminergic fibers in the ACC has been documented,19 and the presence of DA’s receptor proteins20 and mRNA21 provides the basis for DA to have neuromodulatory effects on synaptic transmission of neurons in the ACC. Indeed, Wang et al.22 (neuron) have shown that DA signaling through D1R protein kinase A (PKA) signaling is necessary for surface expression of AMPA glutamate receptor subtype 1 (GluR1) and associated plasticity in a subregion of the cingulate cortex.22 Given the necessity of synaptic transmission and excitatory-mediated activity of the ACC in sensation of pain and pathological neuropathic pain, and strong indications for dopaminergic abnormalities in chronic pain,23,24 we asked the question of how DA modulates AMPA/kainite receptors (KAR)-mediated EPSCs in pyramidal neurons of the ACC.
In the present study, we used electrophysiological approach to investigate the effects of DA for the first time on pyramidal neurons of layers II/III in mice ACC. Our results established an inhibitory role for DA on AMPA/KA evoked EPSCs (eEPSCs) in pyramidal neurons of the superficial layers. This inhibition was observed as a reduction in the amplitude of currents that were evoked by stimulation of neurons in layer V/IV. To address the locus of action for this inhibition, we used pharmacological tools, paired pulse facilitation (PPF) protocol, and analysis of spontaneous EPSC (sEPSC) or miniature EPSC (mEPSC) frequency and amplitude. We conclude DA to module both presynaptic and postsynaptic properties, and the inhibition of eEPSCs is mediated by postsynaptic D2Rs.
Materials and methods
Animals
Adult (six to eight weeks) male C57/Bl6 mice were used for in vitro electrophysiological experiments. All mice were kept in cages holding maximum of four mice. Handling and housing were done in accordance with the University of Toronto DCM standards. Each cage contained a red plastic house with chew toy and material for nest formation as two enrichment items. Animals were subjected to 12 h on and off light cycle.
Slice preparation
Mice were anesthetized with 5% isoflurane and sacrificed by decapitation. The brains were quickly removed and placed in cold (4℃) oxygenated (95% O2 and 5% CO2) artificial cerebrospinal fluid (ACSF:124 mM NaCl, 4.4 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 25 mM NaHCO3, 1 mM NaH2PO4, and 10 mM glucose). For making of coronal brain slices (300 µm), the brains were glued to the cutting stage of the tissue slicer (Leica VT1200S) and slices were made while the brains were submerged in cold oxygenated ACSF. Prior to recording, coronal slices were submerged in a recovery chamber filled with oxygenated ACSF at room temperature (25℃) for at least 1 h.
Whole-cell patch-clamp recording
Whole-cell recordings were done on a recording chamber placed on an Olympus BX51WI microscope. We measured glutamate-mediated AMPAR/KA eEPSCs from layer II/III pyramidal neurons of the ACC, using Axon 200B amplifier (Axon Instruments, CA). For eEPSCs, neurons were voltage clamped at –60 mV and stimulations were performed on layer V/IV using tungsten bipolar stimulating electrodes (Microprobes). The recording electrodes (4–6 MΩ) were filled with internal solution containing 145 mM K-gluconate, 5 mM NaCl, 1 mM MgCl2, 0.2 mM EGTA, 10 mM HEPES, 2 mM Mg-ATP, and 0.1 mM Na3-GTP (adjusted to pH 7.2 with KOH). For PPF protocol, we applied dual stimulation, 50 ms apart, following previous literature.17 Stable baseline recordings were obtained for 5 min and DA at different concentrations were perfused to the recording chamber for 10 min and subsequently washed out for 15 min while eEPSCs were recorded (total of 30 min of recording). Recordings of sEPSCs were performed in voltage clamp mode (–60 mV). For mEPSCs, we included tetrodotoxin (TTX; 1 µM) during the entire duration of the experiments. For the spontaneous currents, we obtained baseline recordings of 5 min and added DA (50 µM) and recorded sEPSCs and mEPSCs currents for a total of 25 min. For exclusive postsynaptic GPCR inhibition, guanosine 5′-[beta-thio] diphosphate (GDP-β-S; 1.6 mM) was added to the recording pipette filled with internal solution. For investigating the role of D1 and D2 receptors, we repeated the evoked experiments with the addition of sulpiride and (R)-(+)-7-Chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390) as specific and potent D2 and D1R antagonists. All of the recordings were done in presence of picrotoxin (PTX; 100 µM) to block γ-aminobutyric acid type A (GABAA) receptor-mediated inhibitory synaptic currents.
Pharmacological agents
The drugs used in the experiments include Dopamine hydrochloride, (±) Sulpiride, R (+)-SCH-23390 hydrochloride, GDP-β-S, tetrodotoxin (with citrate acid), and PTX. All drugs, except tetrodotoxin (Cedarlane), were purchased from Sigma Aldrich, CA.
Data and statistical analysis
Data were collected and analyzed by pClamp 9.2 software (Axon Instruments). Access resistance was monitored throughout the experiment, and data were discarded if access resistance changed >15% during the experiment. For experiments that pertained a washout phase after drug application, one-way analysis of variance (ANOVA) was used to compare the EPSCs during course of drug action versus the baseline and the washout phase. T-test was used for comparison of drug effect and baseline. *P < 0.05 was considered statistically significant.
Results
DA inhibition of AMPA/KAR eEPSCs
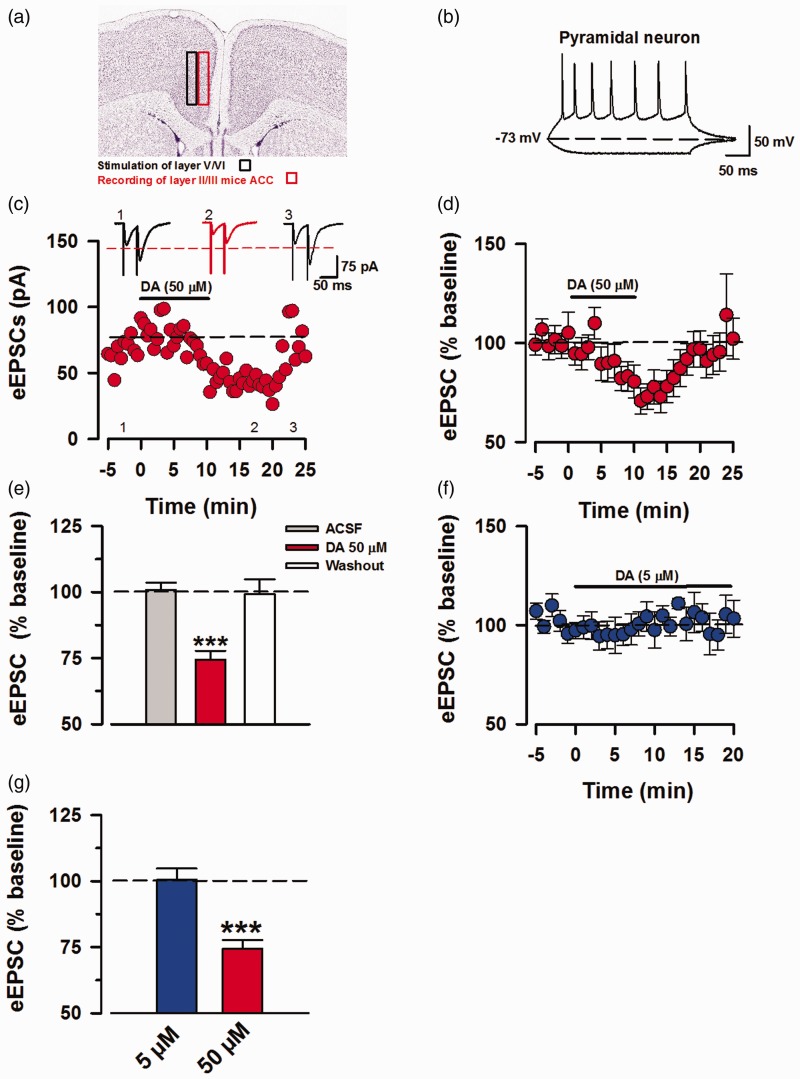
For our experiments, we performed whole-cell patch-clamp recordings of pyramidal neurons in layer II/III of mice ACC and stimulated layer V/IV (Figure 1(a); image courtesy of Allen Mouse Brain Atlas, version 1 (2008), coronal level 43).25 We characterized pyramidal neurons based on their pyramidal shape and action potential (AP) firing that is characteristic of ACC pyramids with firing frequency adaptation and slow hyperpolarization (Figure 1(b)).26 DA (50 µM) was applied for 10 min after obtaining 5 min of baseline recording, followed by 15 min of washing out DA (50 µM) with fresh ACSF (Figure 1(c)). Based on our protocol, the eEPSCs recorded are CNQX (6-cyano-7-nitroquinoxaline-2, 3-Dione) sensitive; hence, these depolarizing eEPSCs depicted as inward currents are primarily mediated by AMPA/KARs.27 Perfusion of DA (50 µM) rapidly inhibited the eEPSCs amplitude (74.5% ± 3.28% of baseline; Figure 1(d)) and washout with fresh ACSF restored the current amplitude similar to the baseline levels (99.3% ± 5.5% of baseline; Figure 1(d)). This inhibition was statistically significant in comparison to the baseline and washout phase (DA 50 µM: 74.5% ±3.28%, baseline: 100.88 ± 2.69%, washout: 99.3 ± 5.53. DA vs. baseline, P < 0.001; DA vs. washout, P < 0.001, One-way ANOVA, n = 11/8 mice; Figure 1(e)). Furthermore, application of DA at a lower concentration (5 µM) did not inhibit eEPSC amplitudes (100.6% ± 4.2% of baseline, P = 0.654, t-test, n = 5/4 mice; Figure 1(f) and (g)). Our results indicate that simultaneous net activation of D1- and D-2-like receptors by high concentration of DA produces inhibition of AMPAR/KA-mediated eEPSCs in mice ACC brain slices. This high-dose inhibition is consistent with the previous reports from other regions of rat brain slices (Table 1).
Figure 1.
Perfusion of high dose DA inhibits ACC pyramidal eEPSCs. Voltage-clamp whole-cell recordings of eEPSCs in pyramidal neurons of layer II/III of mice ACC. (a) Representative diagram of recording location at layer II/III and stimulation of layer V/IV in mice ACC, image credit: Allen Institute, mouse coronal brain atlas, version 1:2008, coronal level 43. (b) Sample action potential firing pattern of pyramidal neuron in current-clamp mode by injection of 15 mV currents for 400 ms, every 15 s. (c) Sample EPSC trace of AMPA/KA currents in layers II/III, depicting the time course for reduction of EPSC amplitude due to DA interaction with DARs and recovery after washing out DA. The AMPA/KAR currents were recorded in presence of PTX (100 μM). (d) Averaged and normalized (%) of eEPSCs (n = 11/8 mice). (e) Inhibition induced by DA (50 μM) was statistically significant in comparison to the baseline and washout (one-way ANOVA, ***P < 0.001). (f) Averaged and normalized (%) of eEPSCs showing no inhibition in response to DA (5 μM; blue, n = 5/4 mice). (g) Results for statistical analysis of DA (5 μM) against baseline (t-test, P = 0.65), data shown in comparison to DA (50 μM). Statistical analyses were performed for 5 min bins. Error Bars represent SEM. eEPSCs: evoked excitatory postsynaptic currents.
DA postsynaptic mode of action
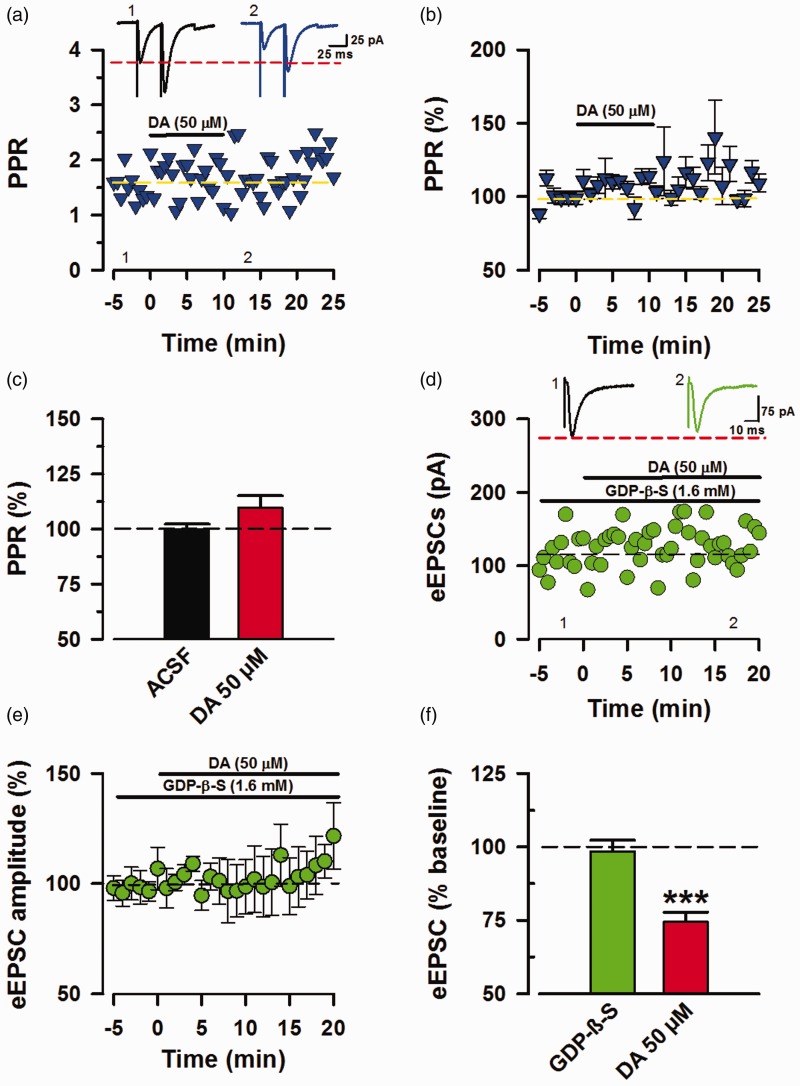
Thus far, the modulatory effects of DA have been accounted mostly by presynaptic mechanisms. In order to understand the locus of action for the observed inhibition, we analyzed paired-pulse ratio (PPR) during the frame of DA inhibition (11–16 min post application). PPF is a form of short-term facilitation that is indicative of presynaptic function and glutamate release.28 Upon dual stimulation, the second pulse would normally have higher amplitude than the first due to residual Ca2 + from the first pulse (Figure 2(a)). A decrease in PPR is a characteristic of a presynaptic specific form of mood related long-term potentiation (LTP) observed in the ACC.17 In our experiments, application of DA (50 µM) produced negligible (109.7% ± 5.46% of baseline; Figure 2(b)) and insignificant changes (P = 0.158, t-test, n = 11/8 mice; Figure 2(c)) in the PPR. Given that DARs are classified as GPCRs, we were interested to see the effects of exclusive postsynaptic G-protein inhibition on the inhibitory effects of DA. Hence, we applied GDP-β-S (1.6 mM), a broad inhibitor of GPCRs29 in the recording pipette, and tested the inhibitory modulation of eEPSCs by DA (Figure 2(d)). DA (50 µM) in the presence of GDP-β-S (1.6 mM) at the postsynaptic site had insignificant effects on eEPSCs (98.5% ± 3.762% of baseline; Figure 2(e), P = 0.631, t-test, n = 6/5 animals; Figure 2(f)). Together, the absence of a significant change in the PPR during DA course of action and the near blockage of DA-induced inhibition indicate that postsynaptic DAR activation is sufficient for inhibiting eEPSCs.
Figure 2.
DA inhibition requires postsynaptic GPCR activation. Determination of DA synaptic locus of action from PPR analysis and postsynaptic GPCR inhibition. (a) Sample trace and raw values of PPR during baseline and DA inhibitory time course (11–16 min post DA application). (b) Summarized (%) PPR values (n = 11/8 mice). (c) Statistical analysis of PPR raw values presented as % change (P = 0.158). (d) Sample traces and raw values of eEPSCs recording with GDP-β-S (1.6 mM) in the recording pipette (n = 1). (e) Averaged data showing GDP-β-S (1.6 mM) in the recording pipette blocked the inhibition by DA (n = 6/5 mice). (f) Green: Postsynaptic GPCR inhibition by GDP-β-S prevented DA to have any significant effect on the eEPSCs (P = 0.631). Red: DA (50 μM) induced inhibition from Figure 1(e) for comparison. Statistical analyses were performed 11 to 16 min after DA application relative to 5 min of baseline. Error bars represent SEM. PPR: paired-pulse ratio; ACSF: artificial cerebrospinal fluid; eEPSCs: evoked excitatory postsynaptic currents; DA: Dopamine; GDP-β-S: guanosine 5′-[beta-thio] diphosphate.
DA modulates sEPSC amplitude and frequency
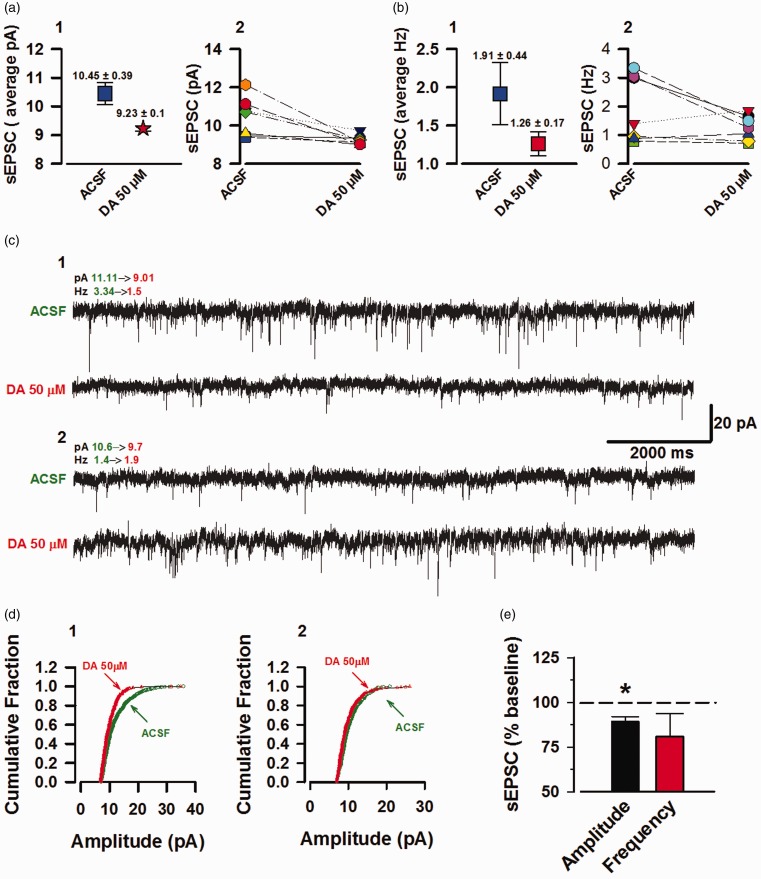
Under basal conditions, presynaptic neurotransmitter containing vesicles are randomly docked and fused to produce spontaneous currents EPSCs (sEPSCs & mEPSCs). Currently, the exact functional role of these spontaneously transmitted currents remains elusive.30,31 Here, we recorded such sEPSC currents and analyzed the frequency and amplitude before and during the course of DA action. The recorded neurons had average amplitude of 10.45 ± 0.39 pA at baseline and 9.23 ± 0.1 pA after DA application (Figure 3(a)-1). Analysis at the individual neuron level revealed that DA signaling inhibited the sEPSC amplitude of 3/7 neurons and had negligible effect on the remaining 4/7 neurons (Figure 3(a)-2). Furthermore, the neurons had a baseline frequency of 1.91 ± 0.44 Hz and 1.26 ± 0.17 Hz after DA application (Figure 3(b)-1). At the individual level, DA inhibited the frequency of 4/7 neurons, increased the frequency of 2/7 neurons, and 1/7 neurons had negligible changes (Figure 3(b)-2). Figure 3(c)-1 is an example trace of a neuron with decreased amplitude (19% decrease from baseline) and frequency (55% decrease from baseline), that is accompanied by a left shift in the amplitude cumulative fraction (Figure 3(d)-1). In addition, Figure 3(c)-2 is a sample trace of a neuron with increased frequency (36% from baseline) upon DA signaling and negligible changes in the amplitude (8.5% decrease from baseline); the amplitude cumulative fraction of this neuron did not show a shift from baseline (Figure 3(d)-2). The averaged data show a decrease in both the frequency and amplitude of sEPSCs from DA application. However, only the inhibition of amplitude was statistically significant (Amplitude: 89 ± 3.03 from baseline, P = 0.010; frequency: 80% ± 12.7%, P = 0.186, n = 7/4 mice, t-test, Figure 3(e)). Therefore, we conclude mixed effect by DA on both the amplitude and frequency of these spontaneously firing currents.
Figure 3.
DA inhibits the amplitude of sEPSCs with mixed effects on the frequency. sEPSCs in the ACC, layer II/III pyramidal neurons. (a) 1: Blue box: mean raw amplitude (pA) values at baseline (5 min). Red star: mean sEPSC amplitudes during DA (15–20 min post DA application) course of action. 2: Individual neuron amplitudes at baseline and DA (n = 7/4 mice). (b) 1: Average of raw frequencies (Hz) at baseline (blue box) and DA (red box). 2: Individual neuron frequencies at baseline and DA. (c) 1: Example sEPSC trace of a neuron with inhibition in frequency and amplitude by DA application. 2: Example sEPSC trace of a neuron with increased frequency in response to DA application. (d) 1: Amplitude cumulative fraction for the neuron with inhibition of frequency and amplitude (trace c, 1). 2: Amplitude cumulative fraction for neuron with increased frequency and no substantial change in the amplitude (trace c, 2). (e) Only effects of DA on sEPSC amplitude were statistically significance (*P = 0.010) and not the frequency (P = 0.186). Error bars represent SEM. ACSF: artificial cerebrospinal fluid; sEPSCs: spontaneous excitatory postsynaptic currents; DA: Dopamine.
DA can modulate both frequency and amplitude of TTX sensitive mEPSCs
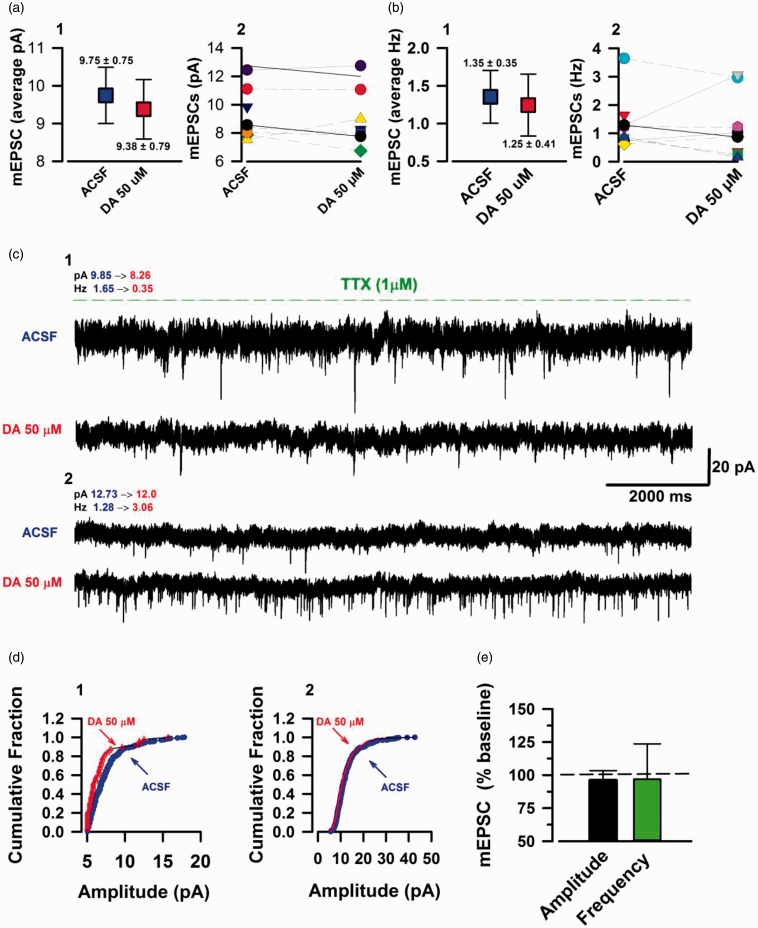
In this part of our investigation, we examined the TTX sensitive component of sPESCs. The previous sEPSCs that we analyzed have spontaneous AP component from peripheral neurons,32 hence we recorded spontaneous currents in the presence of TTX (1 µM) to block any AP firing. In the absence of potential spontaneous APs, the mEPSCs observed are exclusively from the presynaptic terminal forming synapses with the neuron that is being recorded. This allows for an additional way to examine presynaptic changes,33 in addition to PPR analysis. The mEPSCs had average amplitude of 9.75 ± 0.75 pA at baseline and 9.38 ± 0.70 pA upon DA course of action (Figure 4(a)-1). Furthermore, mEPSCs displayed an average frequency of 1.35 ± 0.35 Hz at the baseline and 1.25 ± 0.41 Hz post DA application (Figure 4(b)-1). Looking at the individual data, we observed a consistent lack of substantial change in the mEPSC amplitudes upon DA modulation of all the neurons (n = 9/9 neurons; Figure 4(a)-2). Interestingly, DA perfusion resulted in differential modulation of mEPSC frequencies in a similar fashion to its TTX sensitive counterpart. Application of DA (50 µM) potentiated the frequency of 3/8 neurons, inhibited 5/9 neurons, and did not change the frequency of 1/9 neurons (Figure 4(b)-2). Figure 4(c)-1 represents the trace of a neuron with a large decline in frequency (78%) from the baseline with a marginal shift in amplitude cumulative fraction (Figure 4(d)-1). Also, Figure 4(c)-2 is a sample of a neuron with an even greater increase (138.6%) in frequency from the baseline; this neuron did not have a shift in the amplitude cumulative fraction (Figure 4(d)-2). Statistical analysis revealed no significance changes of mEPSCs amplitudes upon DAR signaling (Amplitude: 96.02% ± 7.22% of baseline, P = 0.738, n = 9/6 mice, t-test; Figure 4(e)), and despite the clear individual changes in frequency, the summed data did not produce a significant statistical value (Frequency; 96.83% ± 26.8% of baseline, P = 0.842, n = 9/6 mice, t-test; Figure 4(e)). Based on these results, we cannot decisively conclude a role for DA in modulating mEPSCs in pyramidal neurons of ACC. However, modulation of mEPSC frequency is evident.
Figure 4.
DA can potentiate and inhibit mEPSC frequency. TTX sensitive mEPSC currents in the ACC layer II/III pyramidal neurons. (a) 1: Blue box indicating the mean value of raw amplitudes (pA) data at baseline (5 min). Red box: mean sEPSC amplitudes during DA (15–20 min post DA (50 μM) application) course of action. 2: Individual neuron amplitudes at baseline and DA (n = 9/6 mice). (b) 1: Average raw frequencies (Hz) at baseline (blue box) and DA (blue box). 2: Individual neuron frequencies at baseline and DA. (c) 1: Example mEPSC trace of a neuron with inhibited frequency by DA application. 2: Example mEPSC trace of a neuron with a very large increase in frequency in response to DA. (d) 1: Cumulative fraction of amplitudes for the neuron with inhibition of frequency (trace c, 1). 2: Cumulative fraction of amplitude for neuron with increase in frequency (trace c, 2). (e) DA had no significant influence on mEPSC amplitude (P = 0.738) and the mixture of potentiation and inhibition of frequency at the individual neuronal level yielded an insignificant statistical value (P = 0.842). Error bars represent SEM. ACSF: artificial cerebrospinal fluid; mEPSCs: miniature excitatory postsynaptic currents; DA: Dopamine.
SCH 23390 at high concentration potentiate the inhibitory effects of DA
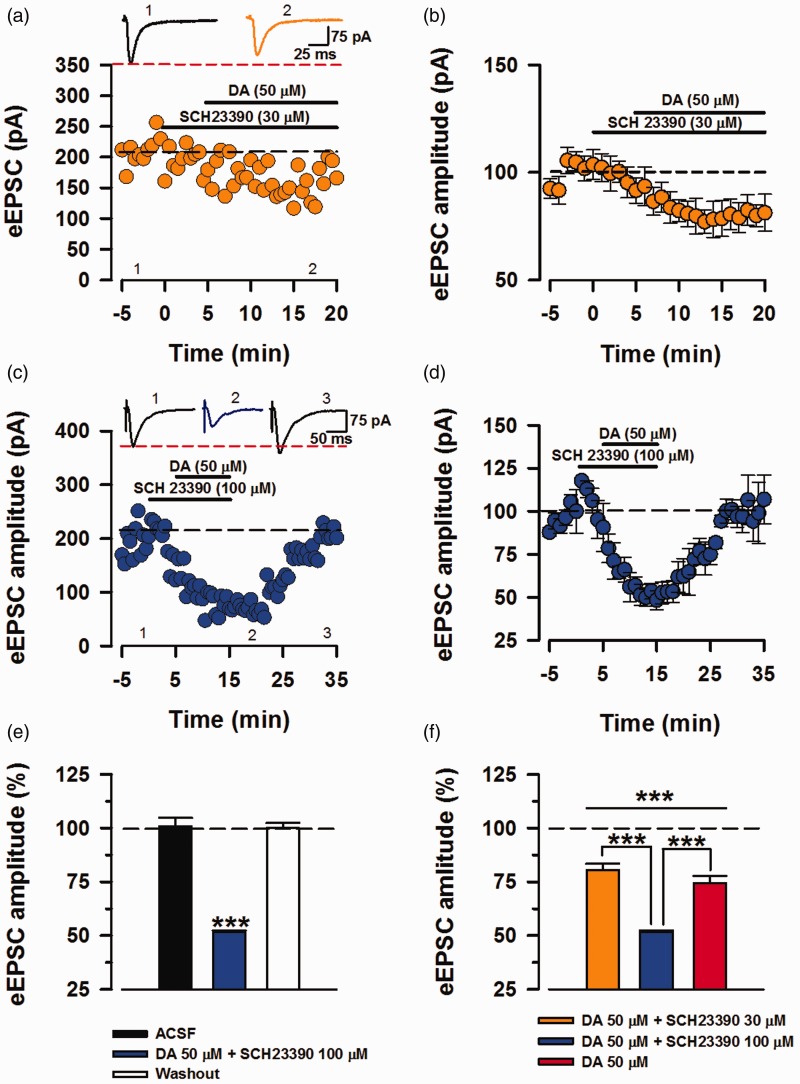
For the next part of the experiments, we investigated the role of D1Rs and D2Rs in the inhibition eEPSC, as all of the DARs are activated in the initial experiments (Figure 1(d)). D1Rs activate adenylyl cyclase (AC) and PKA by coupling to either Go or Gs,22,34 and Wang et al.35 showed this pathway to be imperative in LTP and the behavioral deficits of fragile X knock out mice. We recorded eEPSCs and applied D1-Like receptor antagonist SCH23390 (30 µM) after 5 min of baseline recording and subsequently applied DA (50 µM) after 10 min of baseline recording (Figure 5(a)). Application of DA (50 µM) in the presence of SCH 23390 (30 µM) was able to significantly inhibit the eEPSC amplitude (80.6% ± 2.89% of baseline, P < 0.001, n = 9/7 mice, t-test; Figure 5(b) and (f)). Given that D1Rs activate AC-PKA pathway that is a strong positive modulator of AMPA/KA activity, we increased the concentration of SCH23390 (100 µM) to double the concentration of DA (Figure 5(c)). Application of DA (50 µM) in presence of SCH (100 µM) inhibited the eEPSCs two-folds higher than DA alone and washing out with fresh ACSF returned the eEPSC amplitudes towards the baseline. This strong inhibition of transmission was significant compare to the baseline and washout phase (Baseline: 100.1 + 2.18 %, DA + SCH23390 (100 µM): 51.53% ±0.8% of baseline, washout: 101.26% + 2.8% of baseline; Figure 5(d). DA + SCH23390 (100 µM) vs. baseline, P < 0.001; DA + SCH23390 (100 µM) vs. washout, P < 0.001, washout vs. baseline, P = 0.118, One-way ANOVA, n = 4/4 mice; Figure 5(e)). Furthermore, this exacerbated inhibition by addition of SCH23390 (100 µM) is significantly greater than DA and DA +SCH23390 (30 µM; P < 0.001, t-test; Figure 5(f)). Together, these observations conclude that SCH23390 as a potent D1 antagonist had dose-related inhibitory effects when combined with DA.
Figure 5.
Antagonism of D1 receptors potentiates the inhibitory effects of DA. Investigating the role of D1 receptors by co-application of potent D1R antagonist (SCH23390) and DA (50 μM). (a) Sample trace and raw eEPSC values. SCH23390 (30 μM) was applied 5 min prior to the application of DA. (b) Averaged and normalized (%) eEPSC values displaying inhibition by DA in presence of SCH23390 (30 μM; n = 9/7 mice). (c) Sample trace and raw eEPSC values for DA in presence of SCH23390 (100 μM), exhibiting a strong inhibition. (d) Averaged and normalized (%) data for SCH23390 (100 μM) + DA (n = 4/4 mice). (e) In comparison to the baseline and washout phase, co-application of SCH23390 (100 μM) and DA significantly reduced synaptic transmission that was rapidly recovered upon washing out the drugs (one-way ANOVA, ***P < 0.001). (f) Application of DA alone and with both concentrations of SCH23390 significantly lowered the eEPSC amplitudes from their respective baselines (***P < 0.001). In comparison, inhibition by SCH23390 (100 μM) + DA was significantly greater than inhibition by DA (red bar) and SCH23390 (30 μM) + DA (***P < 0.001). Errors bars represent SEM. eEPSCs: evoked excitatory postsynaptic currents; DA: Dopamine; ACSF: artificial cerebrospinal fluid.
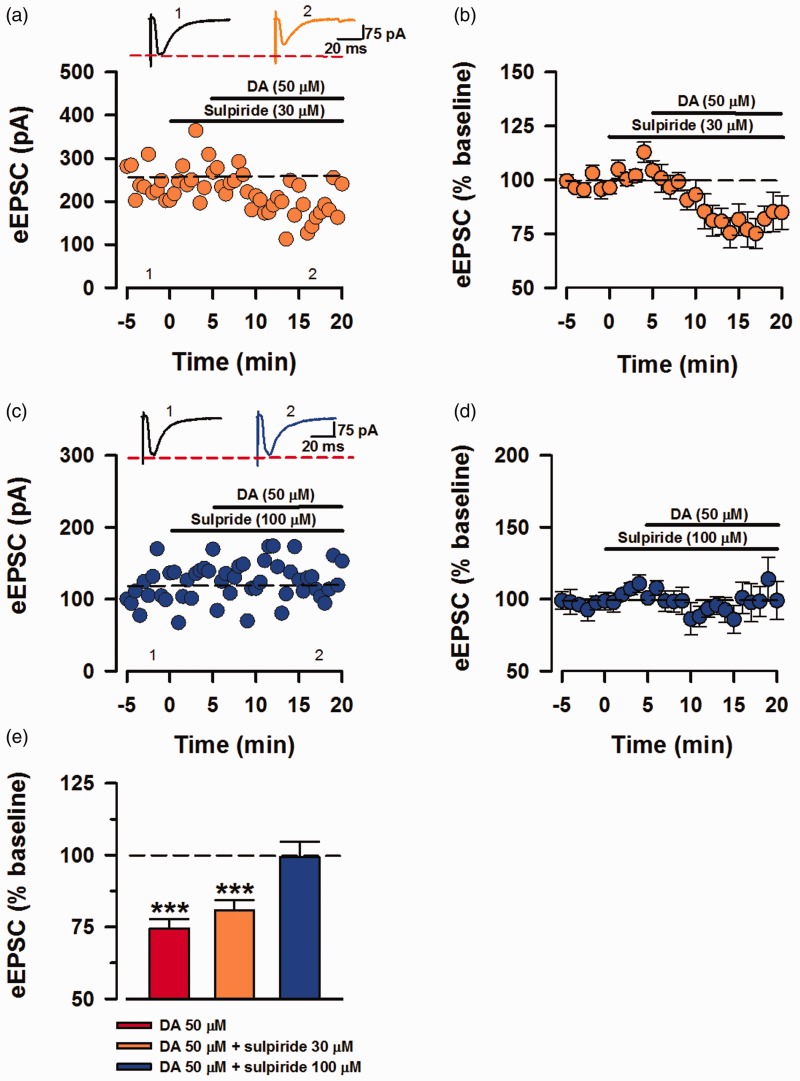
Sulpiride at high dosage blocks the inhibitory effects of DA
To further elucidate the inhibitory modulation by DA, we perfused sulpiride as a selective D2 antagonist, prior to application of DA (50 µM). In the same order of application as before (Figure 5(a)), sulpiride (30 µM) was applied after 5 min of baseline recording, followed by application of DA, 5 min after the addition of sulpiride (Figure 6(a)). The mixture of sulpiride (30 µM) and DA inhibited eEPSC currents (80.79% ± 3.57% of baseline; Figure 6(b)). However, increase the dose of sulpiride to 100 µM prevented the inhibition of eEPSCs by DA (99.36% ± 5.29% of baseline; Figure 6(d)). Similar to the inhibition in presence of low concentration of SCH23390 (30 µM), DA and sulpiride (30 µM) significantly inhibited eEPSC transmission, and DA in presence of 100 µM has no significant influence on baseline transmission (sulpiride (30 µM) + DA (50 µM), 80.79% ± 3.57% of baseline, P < 0.001, n = 7/5 mice, t-test, sulpiride (100 µM) + DA, 99.36% ± 5.29% of baseline, P = 0.649, n = 6/4 mice, t-test; Figure 6(e)). Here we are able to show that inhibiting the activity of D2Rs by high concentration of sulpiride is sufficient to block the DA-induced inhibition.
Figure 6.
DA mediates inhibition by D2. Inhibition of D2Rs by sulpiride blocked the inhibitory effects of DA. (a) Sample trace of a neuron recorded with sulpiride (30 μM) applied 5 min after baseline and DA (50 μM), 10 minu after the baseline. (b) Averaged and normalized (%) eEPSCs recorded in presence of sulpiride (30 μM) and DA (n = 7/5 mice). (c) Sample eEPSC trace for sulpiride (100 μM) and DA. (d) Averaged data (%) showing no inhibition by DA in presence of sulpiride (100 μM; n = 6/4 mice). (e) Statistical analysis of mean eEPSCs for DA alone and DA in presence of both concentrations of sulpiride. Combination of sulpiride (30 μM) and DA significantly lowered eEPSC amplitude (***P < 0.001) and sulpiride (100 μM) + DA did not have significant influence on the baseline transmission (t-test, P = 0.649). Error bars represent SEM. eEPSCs: evoked excitatory postsynaptic currents; DA: Dopamine.
Discussion
In the present study, we show that DA can reversibly inhibit excitatory transmission of ACC pyramidal neurons. Given the lack of change in PPR, complete abolishment of this inhibition by selectively blocking postsynaptic GCRPs or D2Rs, we conclude that DA inhibits eEPSCs through postsynaptic D2R signaling. Furthermore, we found that DA was able to inhibit or potentiate sEPSC & mEPSC release probability in the ACC, suggesting that DA may modulate spontaneous release and evoked synaptic transmission through different mechanisms. Our results provide the novel evidence for inhibitory modulation of ACC synaptic transmission, and this inhibitory modulation is in consistent with previously reported inhibitory function of DA in rat PFC36,37 and nucleus accumbens (NAc).38–42
Postsynaptic effects of DA on evoked mediated EPSCs
PPR is often used and as a mean of determining presynaptic changes. Previous work has found PPR to have either decreased40,43 or increased41,44 in response to bath application of DA. In the ACC, we did not observe significant changes in the PPR during DA’s inhibitory course of action. In light of this apparent difference from previous studies, blockade of DA-induced inhibition by exclusive postsynaptic GCRPs inactivation validated the inhibition to be mediated by postsynaptic DARs. Although in NAc, presynaptic mode of action is accounted by a PKA independent D1 pathway,38,45 our results are consistent with DA postsynaptic mode of action observed in PFC and the cingulate.22,46,47 Application of DA at a low concentration (5 µM) did not influence the eEPSC amplitudes. In the PFC, DA is proven as inhibitory at high concentration (100 µM)36 and enchanting at a lower concentration (20 µM).46 Given the approbation of DA dose-dependent effects; lower doses of DA can induce D1-AC-PKA-mediated pathway, and higher doses activate the D2 pathway48 that is inhibitory to the AC-PKA pathway.47,49 Strong evidence suggests that D1R-PKA pathway potentiates AMPAR transmission22,46,49 by promoting AMPAR surface expression50–52 and conductance through phosphorylation of GluR1 subunit at ser845.22,53 Therefore enchantment of AMPA/KA EPSCs observed by Gonzalez-Islas & Hablitz46 is most likely a dose-dependent observation. In the present study, DA at a concentration of 5 µM did not cause any facilitation, and 50 µM as a high concentration is mediating a D2 response as observed in subsequent DA and DAR antagonist co-application experiments.
DA produces mixed modulation of spontaneous neurotransmission
Based on the averaged data analysis, DA had no significant effect on frequency of mEPSCs and sEPSCs. At the first glance, this lack of change is in line with DA postsynaptic locus of action, albeit previous reports noting DA to decrease mEPSC frequency in NAc39 and increase it in mPFC.54 Regardless of statistically insignificant changes, we do observe clear inhibition, or potentiation of frequencies at the individual neuronal level for both sEPSC and mEPSCs. In terms of the amplitude changes, the significant reduction in sEPSC amplitude was due to reduction of amplitudes that were over 10 pA, with no substantial changes in amplitudes with baseline amplitude of < 10 pA. Furthermore, mEPSC amplitudes displayed very marginal changes regardless of the initial value, which is contrary to the postsynaptic mode of inhibition for eEPSCs, as one would expect a reduction in mEPSC amplitude.33 One possible explanation for this phenomenon is the heterogeneity of presynaptic vesicle pools and their relative postsynaptic channels,30,31,55 and future studies are clearly needed to investigate basic mechanism for these mixed effects.
Antagonism of D2-like receptors blocks AMPA/KA-R eEPSCs inhibition by DA
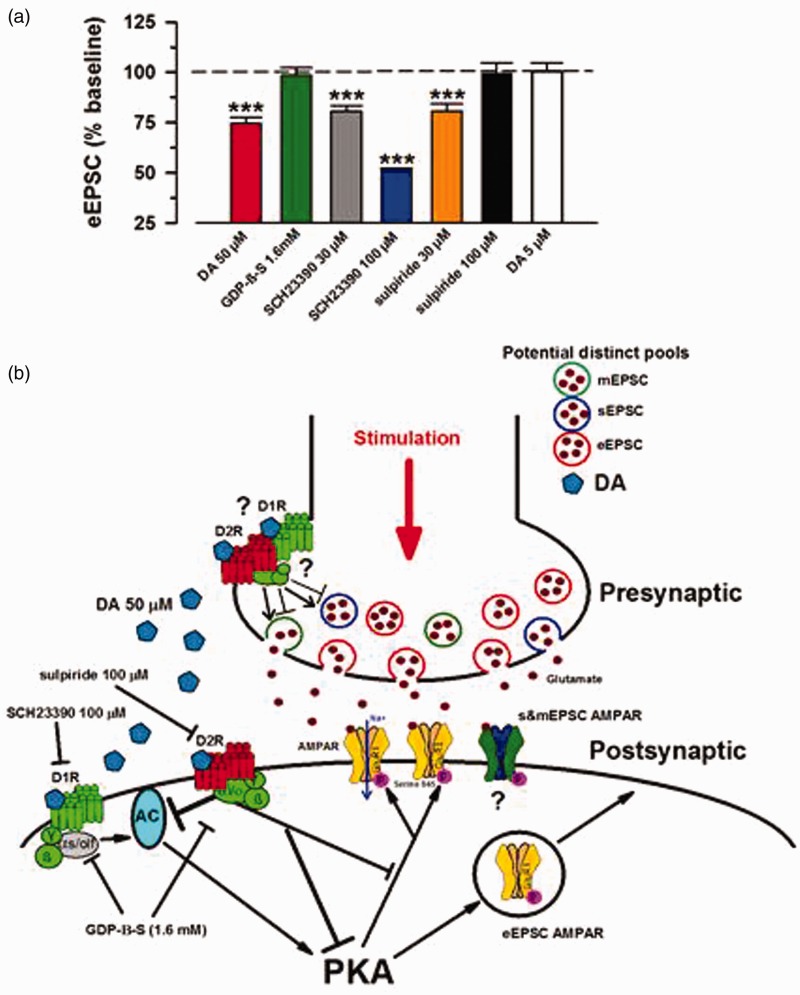
In the present study, we applied D1- and D2-like receptor antagonists along with DA (50 µM) and characterized the inhibition as D2R-mediated. Initially, co-application of neither SCH23990 (30 µM) or sulpiride (30 µM) with DA (50 µM) had any significant influence on inhibitory effects of DA. However, once the dosages of antagonists were increased to 100 µM, we observed a robust inhibition by DA in presence of potent D1 antagonist, SCH23390 that was significantly greater than inhibition by DA alone. Sulpiride at 100 µM blocked the inhibition of eEPSCs by DA. Possible postsynaptic interaction of DA with both D1 and D2 components yields a D2-mediated inhibition of AMPA/KA eEPSCs that is exacerbated by inhibition of the D1 system. This is in accordance with a D2 inhibitory tone by inhibiting AC-PKA pathway6 and de-phosphorylation of AMPAR GluR1 ser845.56 Figure 7 is a representative diagram of potential mechanism based on our observations and previous findings. In relation to our work, microinjection of DA itself to the ACC has been shown to prolong the induction and lessen the intensity of chronic pain phenotype,57 thus providing a possible connection at the physiological level.
Figure 7.
Schematic of DA postsynaptic signaling events. DA mediated inhibition of AMPA/KA eEPSCs by postsynaptic D2R signaling. (a) Compiled summarized (%) data for all of the eEPSC experiments (***P < 0.001). (b) Schematic overview of the potential mechanism for eEPSC inhibition based on the provided data and previous literature. Bath application of high dose DA (50 μM) yields a D2-dominated pathway that is inhibitory to AMPA/KAR-mediated eEPSCs. D2-mediated inhibition of AMPAR EPSCs involves inactivation of AC-PKA pathway that is activated by D1 signaling to promote upregulation of AMPAR surface expression and conductance via phosphorylation. Interaction of DA with its presynaptic receptors can both increase and decrease mEPSC/sEPSC frequency of occurrence, as well as inhibit sEPSC amplitudes in certain neurons. This mixed response is indicative of a physiologically distinct mechanism from that of evoked transmission. Question marks indicate lack of direct explanation. Arrow represents potentiation and blunt-end lines depict inhibition. eEPSCs: evoked excitatory postsynaptic currents; DA: Dopamine; mEPSCs: miniature excitatory postsynaptic currents; GDP-β-S: guanosine 5′-[beta-thio] diphosphate.
Authors’ Contributions
SDG performed electrophysiological experiments and drafted the manuscript. SDG and MZ designed the project. SDG, MY, and MZ finished the final version of the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the EJLB-CIHR Michael Smith Chair in Neurosciences and Mental Health, Canada Research Chair, Canadian Institute for Health Research operating Grants (MOP-124807), NSERC Discovery Grant (RGPIN 402555), and the Azrieli Neurodevelopmental Research Program and Brain Canada.
References
- 1.Pignatelli M, Bonci A. Role of dopamine neurons in reward and aversion: a synaptic plasticity perspective. Neuron 2015; 86: 1145–1157. [DOI] [PubMed] [Google Scholar]
- 2.Remy P, et al. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 2005; 128: 1314–1322. [DOI] [PubMed] [Google Scholar]
- 3.Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci 2002; 3: 932–942. [DOI] [PubMed] [Google Scholar]
- 4.Seeman P, Kapur S. Schizophrenia: more dopamine, more D2 receptors. Proc Natl Acad Sci U S A 2000; 97(14): 7673–7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brisch R, et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psychiatry 2014; 5: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 2011; 63: 182–217. [DOI] [PubMed] [Google Scholar]
- 7.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 2004; 74: 1–58. [DOI] [PubMed] [Google Scholar]
- 8.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 2005; 6: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhuo M. Cortical excitation and chronic pain. Trends Neurosci 2008; 31: 199–207. [DOI] [PubMed] [Google Scholar]
- 10.Zhuo M. Long-term potentiation in the anterior cingulate cortex and chronic pain. Philos Trans R Soc Lond B Biol Sci 2014; 369: 20130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013; 14: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apkarian AV, et al. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005; 9: 463–484. [DOI] [PubMed] [Google Scholar]
- 13.Osuch EA, et al. Regional cerebral metabolism associated with anxiety symptoms in affective disorder patients. Biol Psychiatry 2000; 48: 1020–1023. [DOI] [PubMed] [Google Scholar]
- 14.Chen T, et al. Postsynaptic insertion of AMPA receptor onto cortical pyramidal neurons in the anterior cingulate cortex after peripheral nerve injury. Mol Brain 2014; 7: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu SB, et al. Long-term upregulation of cortical glutamatergic AMPA receptors in a mouse model of chronic visceral pain. Mol Brain 2015; 8: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, et al. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci 2008; 28: 7445–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koga K, et al. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron 2015; 85: 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhuo M. Neural mechanisms underlying anxiety-chronic pain interactions. Trends Neurosci 2016; 39: 136–145. [DOI] [PubMed]
- 18.Walton ME, et al. The mesocortical dopamine projection to anterior cingulate cortex plays no role in guiding effort-related decisions. Behav Neurosci 2005; 119: 323–328. [DOI] [PubMed] [Google Scholar]
- 19.Xu L, Zhang XH. Distribution of D1 and D2-dopamine receptors in calcium-binding-protein expressing interneurons in rat anterior cingulate cortex. Sheng Li Xue Bao 2015; 67: 163–172. [PubMed] [Google Scholar]
- 20.Ortega-Legaspi JM, et al. Expression of the dopaminergic D1 and D2 receptors in the anterior cingulate cortex in a model of neuropathic pain. Mol Pain 2011; 7: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, et al. FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron 2008; 59: 634–647. [DOI] [PubMed] [Google Scholar]
- 22.Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron 2016; 89: 11–36. [DOI] [PubMed] [Google Scholar]
- 23.Navratilova E, et al. Positive emotions and brain reward circuits in chronic pain. J Comp Neurol 2016; 524: 1646–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007; 445: 168–176. Atlas: Website: © 2015 Allen Institute for Brain Science. Allen Mouse Brain Atlas [Internet]. Available from: (http://mouse.brain-map.org/static/atlas. [DOI] [PubMed] [Google Scholar]
- 25.Li XY, et al. Characterization of neuronal intrinsic properties and synaptic transmission in layer I of anterior cingulate cortex from adult mice. Mol Pain 2012; 8: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koga K, et al. Kainate receptor-mediated synaptic transmissions in the adult rodent insular cortex. J Neurophysiol 2012; 108: 1988–1998. [DOI] [PubMed] [Google Scholar]
- 27.Schulz PE, Cook EP, Johnston D. Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J Neurosci 1994; 14: 5325–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holz GGT, Rane SG, Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature 1986; 319: 670–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kavalali ET. The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci 2015; 16: 5–16. [DOI] [PubMed] [Google Scholar]
- 30.Zucker RS. Minis: whence and wherefore? Neuron 2005; 45: 482–484. [DOI] [PubMed] [Google Scholar]
- 31.Lee D, O’Dowd DK. Fast excitatory synaptic transmission mediated by nicotinic acetylcholine receptors in Drosophila neurons. J Neurosci 1999; 19: 5311–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malenka RC, Nicoll RA. Long-term potentiation–a decade of progress? Science 1999; 285: 1870–1874. [DOI] [PubMed] [Google Scholar]
- 33.Beaulieu JM, Espinoza S, Gainetdinov RR. Dopamine receptors - IUPHAR review 13. Br J Pharmacol 2015; 172: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang LY, Salter MW, MacDonald JF. Regulation of kainate receptors by cAMP-dependent protein kinase and phosphatases. Science 1991; 253: 1132–1135. [DOI] [PubMed] [Google Scholar]
- 35.Law-Tho D, Hirsch JC, Crepel F. Dopamine modulation of synaptic transmission in rat prefrontal cortex: an in vitro electrophysiological study. Neurosci Res 1994; 21: 151–160. [DOI] [PubMed] [Google Scholar]
- 36.Otani S, et al. Dopamine facilitates long-term depression of glutamatergic transmission in rat prefrontal cortex. Neuroscience 1998; 85: 669–676. [DOI] [PubMed] [Google Scholar]
- 37.Harvey J, Lacey MG. A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J Neurosci 1997; 17: 5271–5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicola SM, Kombian SB, Malenka RC. Psychostimulants depress excitatory synaptic transmission in the nucleus accumbens via presynaptic D1-like dopamine receptors. J Neurosci 1996; 16: 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Bose P, Warren RA. Dopamine preferentially inhibits NMDA receptor-mediated EPSCs by acting on presynaptic D1 receptors in nucleus accumbens during postnatal development. PLoS One 2014; 9: e86970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicola SM, Malenka RC. Dopamine depresses excitatory and inhibitory synaptic transmission by distinct mechanisms in the nucleus accumbens. J Neurosci 1997; 17: 5697–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harvey J, Lacey MG. Endogenous and exogenous dopamine depress EPSCs in rat nucleus accumbens in vitro via D1 receptors activation. J Physiol 1996; 492: 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, et al. Dopamine depresses glutamatergic synaptic transmission in the rat parabrachial nucleus in vitro. Neuroscience 1999; 90: 457–468. [DOI] [PubMed] [Google Scholar]
- 43.Caruana DA, Chapman CA. Dopaminergic suppression of synaptic transmission in the lateral entorhinal cortex. Neural Plast 2008; 2008: 203514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pennartz CM, et al. Presynaptic dopamine D1 receptors attenuate excitatory and inhibitory limbic inputs to the shell region of the rat nucleus accumbens studied in vitro. J Neurophysiol 1992; 67: 1325–1334. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Islas C, Hablitz JJ. Dopamine enhances EPSCs in layer II-III pyramidal neurons in rat prefrontal cortex. J Neurosci 2003; 23: 867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tseng KY, O’Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci 2004; 24: 5131–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trantham-Davidson H, et al. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J Neurosci 2004; 24: 10652–10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glovaci I, Caruana DA, Chapman CA. Dopaminergic enhancement of excitatory synaptic transmission in layer II entorhinal neurons is dependent on D(1)-like receptor-mediated signaling. Neuroscience 2014; 258: 74–83. [DOI] [PubMed] [Google Scholar]
- 49.Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci 2005; 25: 7342–7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mangiavacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J Neurochem 2004; 88: 1261–1271. [DOI] [PubMed] [Google Scholar]
- 51.Gao C, Sun X, Wolf ME. Activation of D1 dopamine receptors increases surface expression of AMPA receptors and facilitates their synaptic incorporation in cultured hippocampal neurons. J Neurochem 2006; 98: 1664–1677. [DOI] [PubMed] [Google Scholar]
- 52.Esteban JA, et al. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 2003; 6: 136–143. [DOI] [PubMed] [Google Scholar]
- 53.Marek GJ, Aghajanian GK. 5-HT2A receptor or alpha1-adrenoceptor activation induces excitatory postsynaptic currents in layer V pyramidal cells of the medial prefrontal cortex. Eur J Pharmacol 1999; 367: 197–206. [DOI] [PubMed] [Google Scholar]
- 54.Sara Y, et al. Use-dependent AMPA receptor block reveals segregation of spontaneous and evoked glutamatergic neurotransmission. J Neurosci 2011; 31: 5378–5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hakansson K, et al. Regulation of phosphorylation of the GluR1 AMPA receptor by dopamine D2 receptors. J Neurochem 2006; 96: 482–488. [DOI] [PubMed] [Google Scholar]
- 56.Lopez-Avila A, et al. Dopamine and NMDA systems modulate long-term nociception in the rat anterior cingulate cortex. Pain 2004; 111: 136–143. [DOI] [PubMed] [Google Scholar]