Abstract
Background
Visceral hypersensitivity is a complex pathophysiological paradigm with unclear mechanisms. Primary afferent neuronal plasticity marked by alterations in neuroactive compounds such as calcitonin gene-related peptide is suggested to underlie the heightened sensory responses. Signal transduction that leads to calcitonin gene-related peptide expression thereby sensory neuroplasticity during colitis remains to be elucidated.
Results
In a rat model with colitis induced by 2,4,6-trinitrobenzene sulfonic acid, we found that endogenously elevated brain-derived neurotrophic factor elicited an up-regulation of calcitonin gene-related peptide in the lumbar L1 dorsal root ganglia. At seven days of colitis, neutralization of brain-derived neurotrophic factor with a specific brain-derived neurotrophic factor antibody reversed calcitonin gene-related peptide up-regulation in the dorsal root ganglia. Colitis-induced calcitonin gene-related peptide transcription was also inhibited by brain-derived neurotrophic factor antibody treatment. Signal transduction studies with dorsal root ganglia explants showed that brain-derived neurotrophic factor-induced calcitonin gene-related peptide expression was mediated by the phospholipase C gamma, but not the phosphatidylinositol 3-kinase/Akt or the mitogen-activated protein kinase/extracellular signal-regulated protein kinase pathway. Application of PLC inhibitor U73122 in vivo confirmed that colitis-induced and brain-derived neurotrophic factor-mediated calcitonin gene-related peptide up-regulation in the dorsal root ganglia was regulated by the phospholipase C gamma pathway. In contrast, suppression of the phosphatidylinositol 3-kinase activity in vivo had no effect on colitis-induced calcitonin gene-related peptide expression. During colitis, calcitonin gene-related peptide also co-expressed with phospholipase C gamma but not with p-Akt. Calcitonin gene-related peptide up-regulation during colitis correlated to the activation of cAMP-responsive element binding protein in the same neurons. Consistently, colitis-induced cAMP-responsive element binding protein activation in the dorsal root ganglia was attenuated by brain-derived neurotrophic factor antibody treatment.
Conclusion
These results suggest that colitis-induced and brain-derived neurotrophic factor-mediated calcitonin gene-related peptide expression in sensory activation is regulated by a unique pathway involving brain-derived neurotrophic factor-phospholipase C gamma-cAMP-responsive element binding protein axis.
Keywords: brain-derived neurotrophic factor, calcitonin gene-related peptide, primary afferents, signal transduction, rat
Background
Visceral hypersensitivity is manifested by heightened sensory responses to visceral organ stimulation.1 Improper signaling in the viscera modulates sensory activity by regulating neurotransmitter production and release, resulting in chronic neuroplasticity of the primary sensory afferents in the dorsal root ganglia (DRG) and spinal cord.2,3 The increased sensory activity and altered neurotransmission is often accompanied by changes in the levels of neuropeptides and ion channels in the sensory reflex pathway which in turn play crucial roles in the development and maintenance of visceral pain.4,5 Calcitonin-gene related peptide (CGRP) is one of the most important nociceptive markers in the control of pain and inflammation2,6,7 and is most extensively studied in the trigeminal ganglia and DRG in migraine pathophysiology and peripheral and visceral pain.8,9 In adult rat DRG, about half of the primary sensory populations are peptidergic that are marked by CGRP;10,11 69% of CGRP visceral afferent neurons express nerve growth factor (NGF) high affinity receptor TrkA.12 CGRP expression in the DRG can be regulated by retrograde NGF signaling emanating from the inflamed visceral organ.13 CGRP is also colocalized with brain-derived neurotrophic factor (BDNF) high affinity receptor TrkB in the lumbar and sacral (L1 and S1) DRG, and the number of DRG neurons coexpressing these two molecules is increased in colitis.7 These reports indicate a positive correlation of BDNF action and CGRP expression. Indeed, exogenous BDNF treatment increases the number and density of CGRP fibers in the spinal cord.14 A significant interaction of BDNF and CGRP is also implicated in migraine susceptibility in patients.15
BDNF is an important neuromodulator in pain transduction by strengthening excitatory (glutamatergic) synapses.16,17 BDNF protein is localized mostly in the primary sensory nociceptors and is stored in dense core vesicles, possibly released in response to nociceptor activity.18–20 It has been demonstrated that acute intrathecal injection of BDNF could decrease the nociceptive threshold (i.e. hyperalgesia) in the rat,21 implicating an essential role of BDNF in the initiation of pain process. By binding to TrkB, BDNF initiates signal cascades including activation of three major signaling pathways: the extracellular signal-regulated protein kinase (ERK) pathway, the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, and the phospholipase C gamma (PLCγ) pathway. Activation of these pathways ultimately regulates gene transcription and protein production thereby modulating cellular physiology. The active form of ERK (i.e. ERK1/2, ERK5) and PI3K/Akt can directly phosphorylate transcription factor cAMP-responsive element binding protein (CREB) at the serine 133 site and enhance CREB transcriptional activity.22,23 Activation of the PLCγ pathway leads to Ca2+ and Na+ influx through the activation of ion channels, Ca2+ release from stores, and further leads to Ca2+-dependent kinase activity and CREB phosphorylation and activation.24
CREB is a transcription factor that regulates an estimated 4000 genes in humans and has been found to be involved in the perpetuation of neuropathic, inflammatory, and muscle pain.25–28 This transcription factor is demonstrated to be extensively involved in synaptic plasticity and long-term potentiation and is commonly used as a marker for pain-related neuronal changes.29 The CGRP promoter contains a cAMP-responsive element, and CGRP expression is regulated by CREB-mediated transcription.30,31 Taken together, it is likely that CGRP expression level is modulated by CREB activity level in the primary afferent neurons.
Our previous study demonstrates that BDNF application in DRG mass culture elicits an increase in CGRP expression which is mediated by TrkB.7 To further examine the interplay of BDNF and CGRP, and their explicit roles in sensory activation during visceral inflammation, the present study is undertaken to investigate the intracellular signaling pathways initiated by BDNF in vivo in colitis and examine which pathway(s) are responsible for BDNF-initiated CGRP expression. Using an experimental colitis model induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS), we show that endogenously elevated BDNF contributes to the induction of CGRP expression. CGRP production in vivo and in vitro is attenuated by suppression of the PLCγ pathway but not the PI3K/Akt pathway, revealing a unique signaling cascade in the regulation of CGRP expression. Our findings will increase the understanding of the molecular mechanisms of visceral hypersensitivity as a result of visceral inflammation.
Materials and methods
Experimental animals
Adult male Sprague-Dawley rats (150–200 g) (Harlan Sprague Dawley, Inc.) were used in the current studies. All experimental protocols involving animal use were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University (IACUC#: AM10315). Animal care was in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and National Institutes of Health guidelines. All efforts were made to minimize the potential for animal pain, stress, or distress.
Induction of colonic inflammation
To induce inflammation in the distal colon, fasted rats were anesthetized and TNBS (Sigma–Aldrich Co. LLC) was instilled into the lumen of the colon 6 cm proximal to the anus at a single dose of 90 mg/kg (60 mg/mL solution in 50% EtOH) through a syringe-attached polyethylene catheter. Animals that received similar volume of 50% EtOH enema or saline served as control. All colonic instillations were performed under isoflurane anesthesia (2.5%, SurgiVet, Smiths Medical PM, Inc. Waukesha, WI). Euthanasia of animals was performed on either day 3 (termed as three days of colitis) or day 7 (termed as seven days of colitis) after a single dose of intracolonic instillation of TNBS. To ensure exposure of the distal colon to TNBS, rats were held head-down by lifting up the tail for 1 min.
Tissue harvesting
For immunostaining, intracardiac perfusion was performed for euthanasia of animals. Under anesthesia (3%–4% isoflurane), animals were euthanized via perfusion first with oxygenated Krebs buffer (pH 7.4) (95% O2, 5% CO2) followed by 4% paraformaldehyde. After perfusion, the DRGs were quickly removed and postfixed for 6 h. Tissue was then rinsed in phosphate buffered saline (0.1 M PBS, pH 7.4) and placed in ascending concentrations of sucrose (20 %) for cryoprotection. For Western blot and culture, DRGs were freshly dissected out and either homogenized or processed to extract DRG explants.
DRG explants culture
The segment-matched DRG pairs from L1 level were freshly isolated from naïve animals. After peeling off the surrounding membrane that wrapped the ganglion, these DRGs were acutely cultured in Dulbecco’s Modified Eagle Medium for 2–4 h before treatment. To study the effect of BDNF, one DRG explant was treated with BDNF (50 ng/mL, EMD Millipore Corporation) for designated time point. The contralateral DRG served as control. To study the effect of a specific inhibitor, one DRG was treated with BDNF plus inhibitor and the contralateral served as control by receiving treatment of BDNF and vehicle (DMSO). The inhibitor and vehicle were added to the culture 1 h prior to BDNF treatment.
Immunostaining
DRGs were sectioned at a 20 µm thickness and processed for on-slide immunostaining. Primary antibodies used were mouse anti-CGRP (1:2000, Abcam), rabbit anti-phospho-CREB (1:1000, Cell Signaling), rabbit anti-phospho-Akt (1:500, Cell Signaling), and rabbit anti-PLC-γ (1:1500, Santa Cruz Biotechnology). The specificity of these antibodies had been carefully characterized in our previous studies.2,13 The secondary antibodies used were Cy3- or Alexa 488-conjugated species-specific antibody. The final product of the slides was coverslipped with Citifluor (Citifluor Ltd., London) and viewed under a Zeiss fluorescent photomicroscope. Immunoreactive positive cells were counted in 6 to 10 sections randomly chosen from each ganglion and averaged as one sample. The area of section containing cells (excluding the area containing fibers) was selected using free-line tools integrated in the AxioVision measurement software (Carl Zeiss, Inc.) and was measured as mm2. The number of positively stained cells was normalized against the measured area and expressed as number cells per mm2. This method of quantification has been validated in our previous studies.13 To avoid double counting, we chose every third section for one specific antibody stained.
Western blot
Ganglia were homogenized in T-Per solution (Pierce Biotechnology, Rockford, IL) with addition of protease and phosphatase inhibitor cocktails (Sigma). Proteins were denatured in an equivalent amount of Laemmli Sample Buffer (Bio-Rad) and were separated on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane using Trans-Blot Turbo (Bio-Rad). The membrane was blocked using 5% milk in Tris-buffered saline, and incubated at 4℃ with primary antibody against phospho-CREB (1:1000, Cell Signaling). A horseradish peroxidase-conjugated secondary antibody (1:2000, Cell Signaling) and enhanced chemiluminescence system was used to visualize immunoreactive bands. For endogenous control, the same membrane was stripped and re-incubated with antibody against total CREB (1:1000, Cell Signaling), or β-actin (1:5000, Sigma). The densitometric quantification of immunoreactive bands was performed using the software FluorChem 8800 (Alpha Innotech, San Leabdro, CA).
RNA extraction and quantitative real-time PCR
Total RNA was extracted using a RNA extraction kit RNAqueous (Ambion, TX). RNA concentration was determined spectrophotometrically. cDNA was synthesized using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, ABI) under manufacturer’s instruction. Specific Taqman probes were used in real-time PCR quantification. β-actin was used as an endogenous control. The changes in the target genes were normalized against the endogenous control and were calculated to express fold changes using 2−ΔΔCt method.
Drug treatment
Animals received antagonists via intravenous (i.v.) injection. Antagonists used in this study were BDNF neutralizing antibody (36 µg/kg body weight, Santa Cruz Biotechnology, Inc.),17 PLC inhibitor U73122 (1 mg/kg body weight, Calbiochem-EMD Millipore Corporation),32 and PI3K inhibitor LY 294002 (50 µg/kg body weight, Calbiochem-EMD Millipore Corporation).17 A single dose of antagonist was injected. When animals were examined on day 3 post colitis induction, the antagonist was injected on the same day and post TNBS treatment. When animals were examined at day 7 post colitis induction, the antagonist was injected on day 3 after colitis induction. Both U73122 and LY294002 solution were prepared by dissolving in DMSO as stock and then diluting in saline for injection. Animals control to drug treatment received either the same amount of control IgG (to anti-BDNF) or DMSO (to pharmacological inhibitors). The treatment designs for each drug were customized by us through preliminary studies.
Data analysis
The results from each study were presented as mean ± SD. Comparison between control and experimental groups was made by using Kruskal-Wallis nonparametric one-way ANOVA, or student t test. Differences between means at a level of p ≤ 0.05 were considered to be significant.
Results
Inhibition of BDNF in vivo attenuated CGRP expression in DRG during colitis
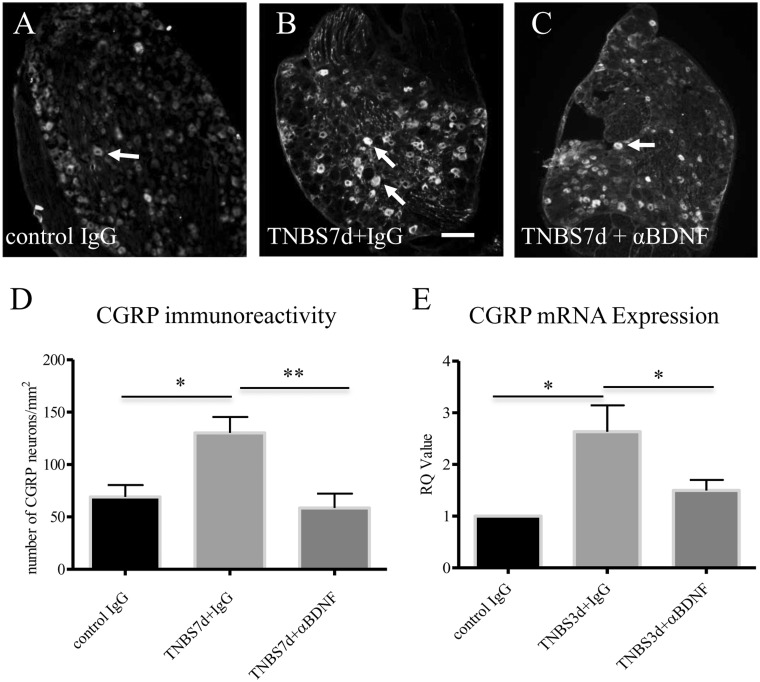
The excitatory neurotransmitter CGRP immunoreactivity is up-regulated in TrkB-expressing DRG neurons at seven days of colitis;7 this suggests an association of the BDNF/TrkB system and CGRP expression in the DRG. We have shown that the endogenous BDNF levels are increased in DRG neurons during colitis,19 and BDNF has a paracrine role in regulating DRG neuronal activity.5,33 To examine whether BDNF regulates CGRP expression in the DRG in vivo, we injected BDNF neutralizing antibody to animals with colitis to block the endogenous BDNF action. We chose the L1 DRG segment to study because both CGRP mRNA and protein levels were up-regulated in this segment during colitis, in a time-dependent manner, i.e., CGRP mRNA levels were the highest at three days of colitis and CGRP protein levels were peaked at seven days of colitis,2 thus these time points were examined (Figure 1). Consistently in the current study, colitis also increased the level of CGRP protein in L1 DRG at day 7 following TNBS treatment; this increase was not affected by normal IgG intervention (Figure 1: ompare 1(b) to 1(a); summary data shown in Figure 1(d)), however, was attenuated by anti-BDNF treatment (Figure 1: compare 1(c) to 1(b); summary data shown in Figure 1(d)). To examine whether endogenous BDNF had a role in regulating CGRP transcription, we performed qPCR of CGRP.2 Our results showed that BDNF also had a role in regulating CGRP mRNA levels in the DRG during colitis. The relative levels of CGRP mRNA were increased in TNBS-treated animals that received control IgG when compared to IgG-treated control animals (Figure 1(e)). BDNF neutralization blocked colitis-induced CGRP transcriptional up-regulation (Figure 1(e)).
Figure 1.
Colitis-increased CGRP expression in L1 DRG was blocked by BDNF neutralization. TNBS treatment increased CGRP immunoreactivity in L1 DRG in the presence of normal IgG (A, B, and D). BDNF antibody treatment of colitis animals reduced CGRP immunoreactivity when compared to colitis (B, C, and D). TNBS treatment also increased CGRP mRNA levels examined by real-time PCR in L1 DRG in the presence of normal IgG (E). BDNF antibody treatment reduced CGRP mRNA level in colitis (E). Bar = 60 µm. *p < 0.05; **p < 0.01. n = 4–6 animals for each group.
BDNF increased CGRP expression through the PLCγ pathway
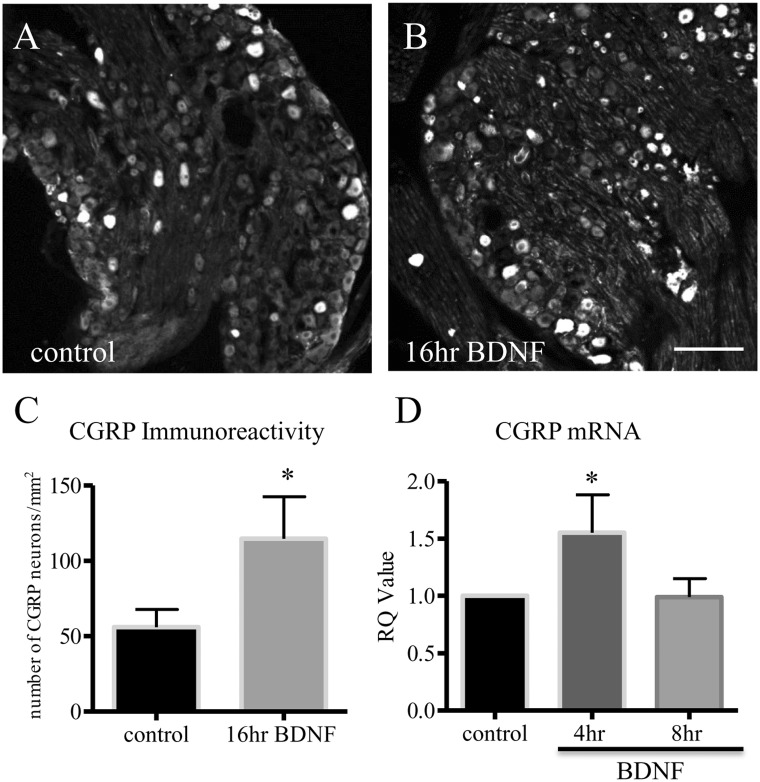
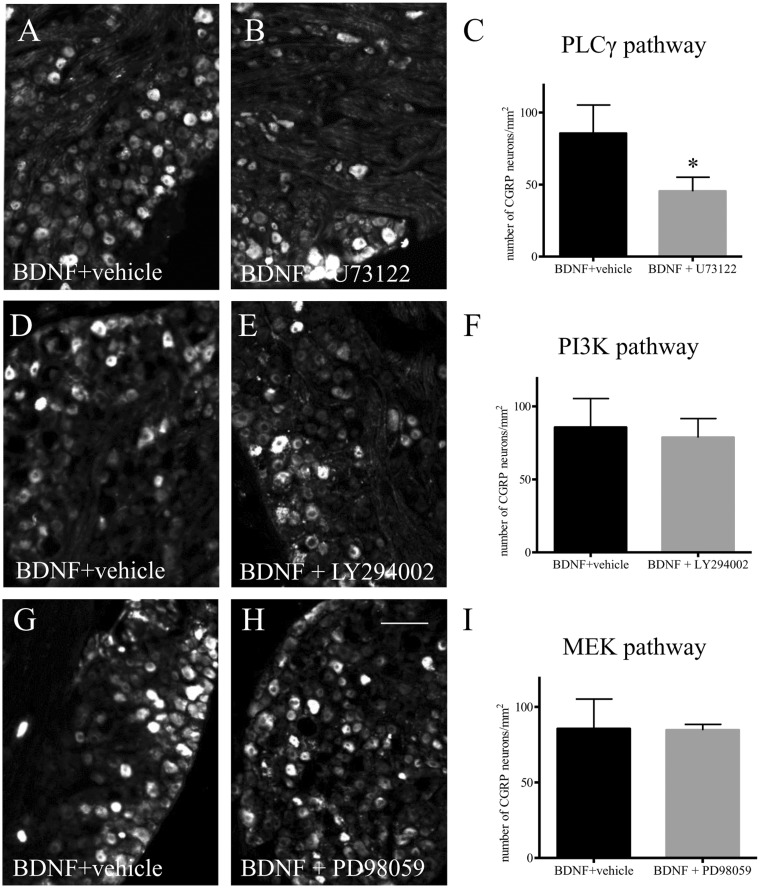
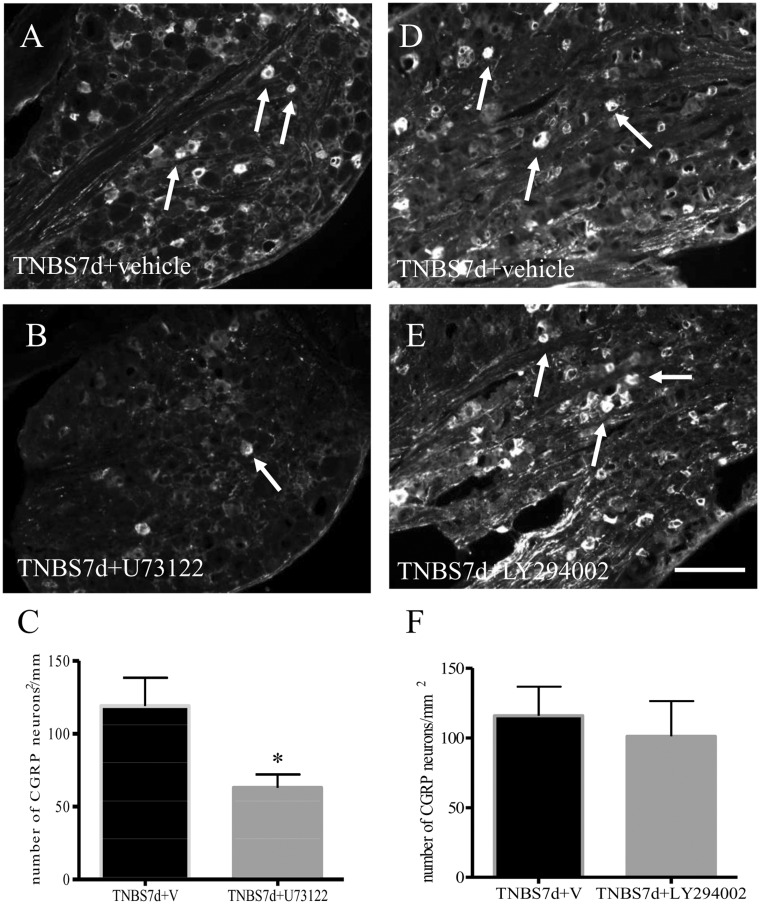
To examine the mechanism of action of BDNF in CGRP expression in the DRG, we used an ex vivo approach by incubating segment-matched DRG explants pair with or without BDNF (Figure 2(a): without BDNF; Figure 2(b): with BDNF). Our results showed that exogenous BDNF (50 ng/mL) elicited a two-fold increase in CGRP immunoreactivity in the DRG after 16 h incubation (Figure 2(c)). To examine whether BDNF also regulated CGRP mRNA levels, we performed a time-course study in order to catch the window of the CGRP transcriptional activity prior to mRNA translation. We showed that BDNF indeed increased CGRP transcription, but in a time-dependent manner (Figure 2(d)). We then examined the signal transduction pathways that could mediate BDNF-regulated CGRP up-regulation. Specific inhibitors were used, including PD98059 (5 μM) to block the mitogen-activated protein kinase/extracellular signal-regulated protein kinase (MEK/ERK) pathway, LY294002 (5 μM) to block the PI3K/Akt pathway, and U73122 (5 μM) to block the PLC/Ca2+ pathway. Pair-matched DRG explants were pre-treated with either vehicle or the specific inhibitor for 1 h, followed by BDNF treatment of both DRGs for additional 16 h. Our results showed that pre-treatment of the DRG with PLC inhibitor U73122 reduced CGRP immunoreactivity caused by BDNF (Figure 3(a)–(c)). In contrast, both LY294002 (Figure 3(d)–(f)) and PD98059 (Sigma) (Figure 3(g)–(i)) were unable to block BDNF-induced CGRP expression in the DRG which resulted in a similar amount of CGRP immunoreactivity in the DRG pairs treated by BDNF (plus vehicle) (Figure 3(d) and (g)) or BDNF plus inhibitors (Figure 3(e) and (h)), respectively.
Figure 2.
Exogenous BDNF increased CGRP expression in DRG explants. One ganglion of the ganglia pair was treated with BDNF (50 ng/mL), and the contralateral was used as control. CGRP immunoreactivity was examined and compared within the pairs (A, B). BDNF treatment elicited an up-regulation of CGRP immunoreactivity in the DRG after treatment for 16 h (C). Bar = 60 µm. Time course studies showed that BDNF treatment also increased the levels of CGRP mRNA in the DRG (D). *p < 0.05. n = 3 independent experiments.
Figure 3.
BDNF-induced CGRP expression was mediated by the PLCγ pathway but not by the PI3K/Akt or the MEK/ERK pathway. The ganglia pairs were pre-treated with vehicle plus BDNF (A, D, G), or one of the inhibitors against the PLCγ pathway (B, C: U73122), the PI3K/Akt pathway (E, F: LY294002), or the MEK/ERK pathway (H, I: PD98059) followed by BDNF treatment for 16 h (B, E, H). CGRP immunoreactivity was then examined and compared in these ganglia pairs (C, F, I). U73122 (C) pre-treatment blocked BDNF-facilitated CGRP expression in DRG. LY294002 (F) and PD98059 (I) pre-treatment did not affect the level of CGRP in the ganglia induced by BDNF. Bar = 80 µm. *p < 0.05. Results were from four independent experiments for each treatment.
Inhibition of PLC but not PI3K in vivo attenuated colitis-induced CGRP expression in DRG
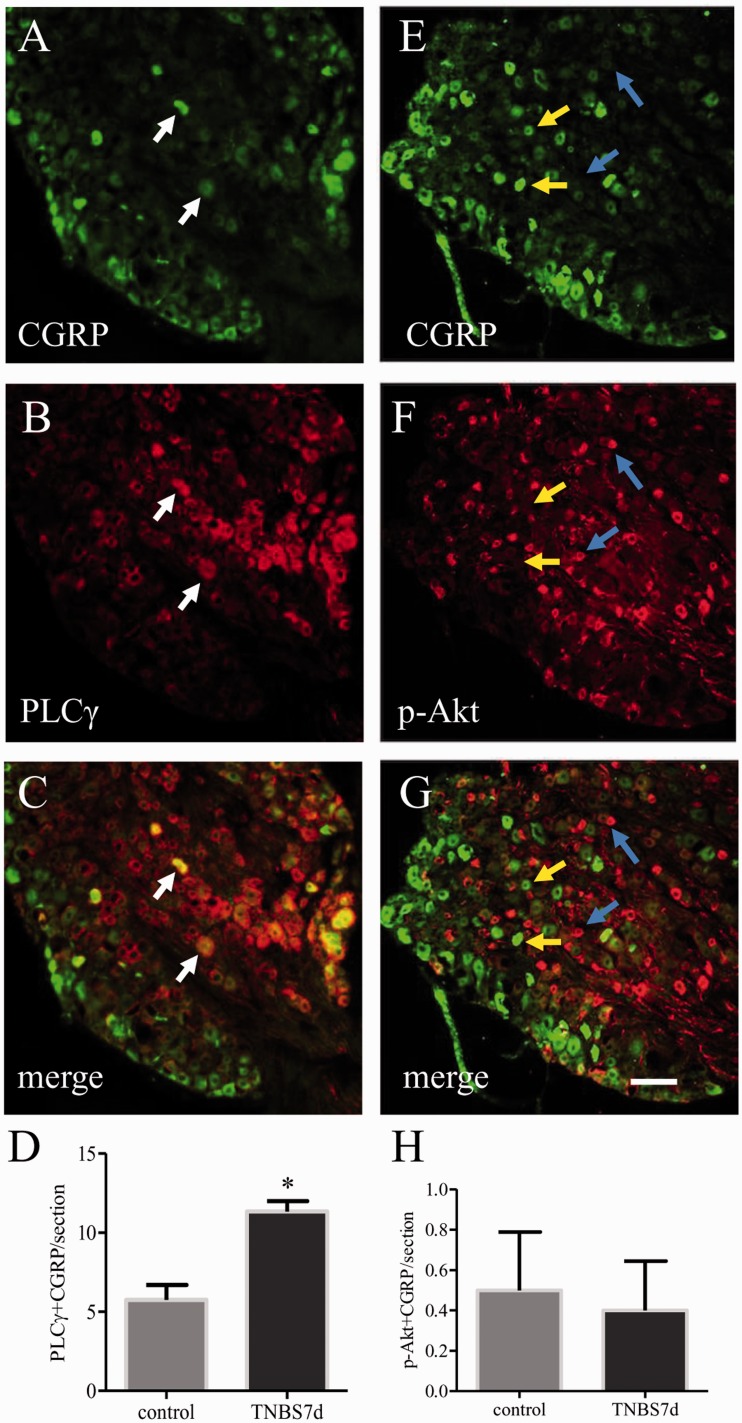
We next examined whether the PLCγ pathway was also involved in CGRP up-regulation in the L1 DRG in the in vivo model of colitis. Our published results showed that PLCγ expression and Akt phosphorylation were up-regulated in the DRG at seven days of colitis.34 Here we showed that on day 7 post colitis induction, coexpression of CGRP (Figure 4(a), green cells) and PLCγ (Figure 4(b), red cells) was increased in the L1 DRG (Figure 4(d)), however, CGRP was scarcely colocalized with p-Akt (Figure 4(e)–(h)). Administration of the PLC specific inhibitor U73122 to animals with colitis significantly reduced the number of L1 DRG neurons expressing CGRP immunoreactivity (Figure 5(a)–(c)). In contrast, administration of the PI3K inhibitor LY294002 had no effect on CGRP expression level in the DRG when compared to colitis (Figure 5(d)–(f)). As for the ERK pathway, our earlier studies showed that the ERK1/2 activity was not affected in the DRG by colitis.5 Figure 3 also demonstrated that the MEK/ERK pathway was not involved in BDNF-induced CGRP expression in culture. Thus, we did not perform the in vivo test of PD98059 on colitis-induced CGRP expression.
Figure 4.
Increases in the co-localization of CGRP with PLCγ but not phospho-Akt in L1 DRG during colitis. Double immunostaining showed that a subpopulation of CGRP cells (A, green cells indicated by white arrows) also expressed PLCγ (B, red cells). In contrast, very few CGRP cells expressed phospho (p)-Akt (E–G, yellow arrows indicated CGRP cells, blue arrows indicated p-Akt cells). TNBS treatment of animals increased the number of DRG neurons co-expressing CGRP and PLCγ (D) but had no effect on the number of cells co-expressing CGRP and p-Akt (H). Bar = 60 µm. *p < 0.05. n = 4 animals in each group.
Figure 5.
Inhibition of PLCγ but not PI3K/Akt attenuated colitis-induced CGRP expression in L1 DRG. Animals were induced for colitis followed by treatment with either vehicle (A, D), or specific inhibitors against PLC (B: U73122) or PI3K (E: LY294002). CGRP immunoreactivity was examined in L1 DRG from all animals. Colitis-induced CGRP expression was attenuated by inhibition of the PLCγ pathway (C). However, suppression of the endogenous PI3K activity with LY294002 had no effect on CGRP expression level evoked by colitis (F). Bar = 80 µm. *p < 0.05. n = 3 animals for each group.
Up-regulation of CREB activity was associated with BDNF-induced CGRP up-regulation in DRG during colitis
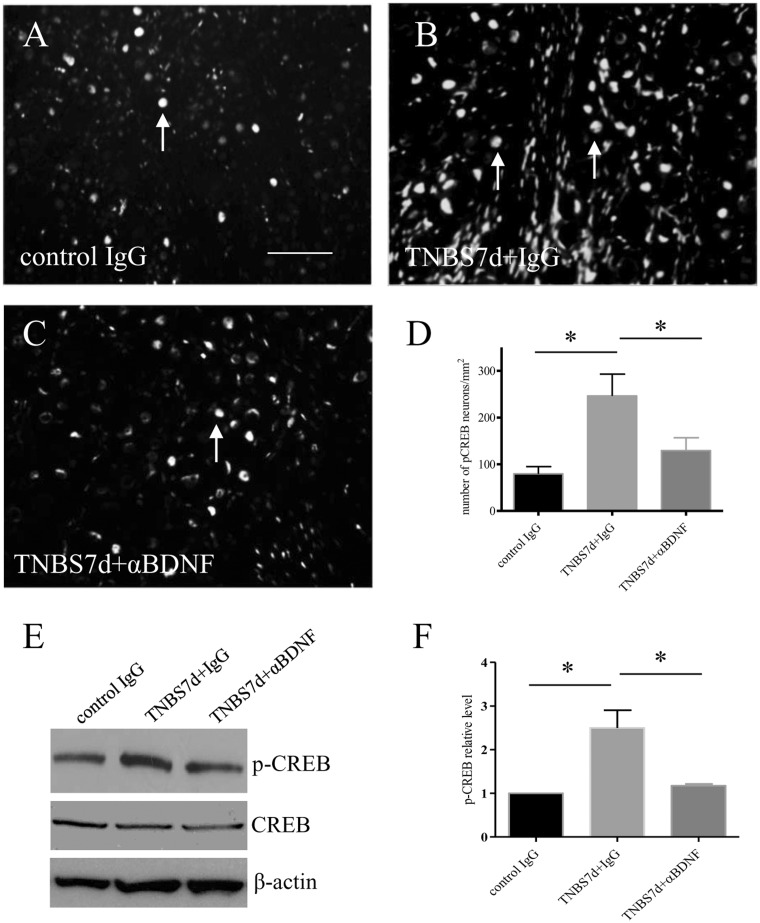
The transcription factor CREB acts as a molecular switch in neuronal plasticity and is activated dependent of Ca2+. To examine whether BDNF has a role in CREB activation in DRG during colitis, we examined the phosphorylation (activation) level of CREB (p-CREB Ser133) in the DRG during colitis and post anti-BDNF treatment (Figure 6). At seven days of colitis, the level of p-CREB was increased in the L1 DRG examined by immunohistochemistry (compare Figure 6(b)–(a), arrows show nuclear staining), and Western blot when both total CREB and β-actin were used as internal controls (Figure 6(e); summary data Figure 6(f)). Neutralization of BDNF action in vivo attenuated colitis-induced CREB activation in the DRG (compare Figure 6(c)–(b), Figure 6(d)–(f)), suggesting that CREB served as a downstream mediator in BDNF signaling cascade in DRG during colitis.
Figure 6.
BDNF immuno-neutralization reduced the level of CREB phosphorylation in L1 DRG during colitis. The number of phospho (p)-CREB immunoreactive neurons was significantly higher in L1 DRG from animals treated with TNBS in the presence of control IgG (compare A and B). BDNF neutralization reduced colitis-induced increases in p-CREB expression (C and D). Western blot confirmed the effects of BDNF antibody in reducing CREB phosphorylation in colitis when both total CREB, and β-actin were used as internal control (E and F). Bar = 60 µm. *p < 0.05. n = 4–5 animals for each group.
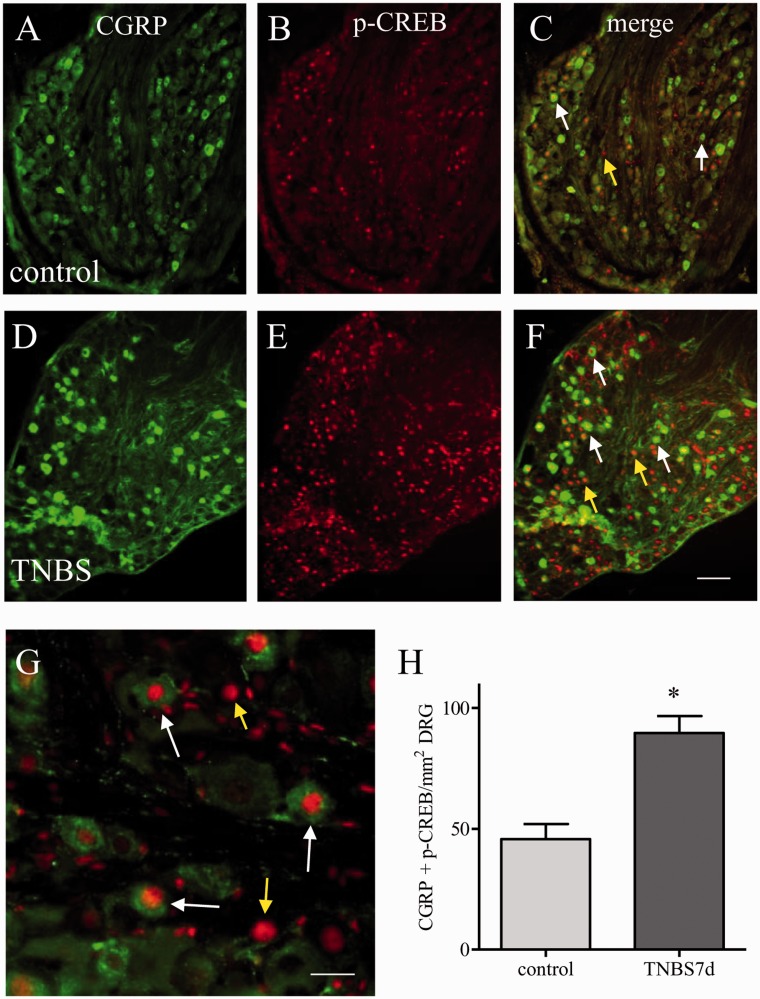
Since both CGRP and p-CREB were up-regulated during colitis and both CGRP and p-CREB were regulated by BDNF (compare Figure 6(a)–(d) to Figure 1(a)–(d)), we next examined the association of CREB activation and CGRP expression in DRG during colitis. Colocalization study showed that a subpopulation of DRG neurons coexpressed both CGRP (Figure 7(a), (d), green cells) and phospho-form of CREB (Figure 7(b), (e), red nuclear staining). The majority of CGRP neurons contained p-CREB (Figure 7(c), (f), (g) white arrows); however, not all p-CREB positive cells had CGRP (Figure 7(c), (f), (g), yellow arrows) in both control (Figure 7(a)–(c)) and TNBS-treated groups (Figure 7(d)–(e)). At seven days of colitis, the number of DRG neurons expressing both CGRP and p-CREB was increased in the L1 DRG (Figure 7(h)), suggesting a parallel up-regulation of these two molecules in DRG during colitis.
Figure 7.
Correlation of CREB phosphorylation and CGRP expression in L1 DRG during colitis. Double immunostaining showed that CGRP expression (A, D: green cells) was largely colocalized with phospho (p)-CREB (B, E: red nuclear staining) in L1 DRG in both control (A–C) and seven days of colitis animals (D–F). The merged microphotograph (C, F) showed green CGRP cells having red p-CREB nuclear stain (white arrows). Colitis increased the number of DRG cells expressing both CGRP and p-CREB (H). Bar = 60 µm in A–F; 20 µm in G. *p < 0.05. n = 5 for each control and TNBS treatment.
Discussion
The present study demonstrates that the PLCγ pathway is a unique pathway downstream of BDNF in colitis-induced CGRP expression in DRG. CGRP is an excitatory neurotransmitter that marks the nociceptive activity of the primary sensory neurons. During colitis, CGRP transcripts and protein levels alter dynamically in a time-dependent manner in the lumbar and sacral DRG due to their production and release.2,35 It is important but not yet understood how CGRP production in DRG is regulated. Extended from our previous study showing that TNBS colitis caused an up-regulation of CGRP transcripts and protein levels in L1 DRG, and CGRP co-expressed with BDNF high affinity receptor TrkB,2,7 we examined the role of endogenous BDNF in CGRP expression during colitis. The hypothesis of BDNF regulation of CGRP during colitis is based on the following observations: (1) BDNF protein up-regulation in the DRG is at the same time block (i.e. three days of colitis) with CGRP mRNA up-regulation and is prior to CGRP protein up-regulation (i.e. seven days of colitis),2,19 and (2) BDNF increases CGRP expression in DRG neuronal culture which is blocked by inhibition of high affinity BDNF receptor TrkB.7 Expanding on these in vitro studies,7,14 the present study demonstrated that BDNF action in vivo also regulated CGRP expression in DRG examined in the colitis model. Among the three major signaling pathways downstream of BDNF/TrkB, the PLCγ pathway was found to be involved in CGRP up-regulation, while the MEK/ERK and PI3K/Akt pathway were not. This conclusion was confirmed in culture and in vivo, suggesting a unique role of PLCγ in mediating the activity of CGRP-containing nociceptors during colitis. Additional study showed that Ca2+-sensitive transcription factor CREB activity was also regulated by BDNF and was associated with CGRP up-regulation in the DRG during colitis. These findings further strengthen the importance of PLCγ-Ca2+ axis in BDNF action and colitis-associated nociceptive transmission.
PLC is a class of enzymes that cleave phospholipids such as PtdIns(4,5)P2 to produce the secondary messenger inositol trisphosphate (IP3). Six isotypes of PLC: β, γ, δ, ɛ, ζ, η are identified according to their structures and functions in signal transduction. PLCγ is downstream of receptor tyrosine kinase and is responsive to neurotrophin/Trk action. In the neuronal system, PLCγ is well characterized for its role in BDNF-initiated long-term potentiation, synaptic plasticity, and remodeling.36 The function of PLCγ is carried on by IP3-facilitated Ca2+ release from the intracellular store, and/or diacylglycerol/protein kinase C-modulated ion channel activity leading to Ca2+ influx, thereby increasing the intracellular Ca2+ ([Ca2+]i) level and the activity of Ca2+-dependent pathways.37,38 Subsequently, the transcription factor CREB, which activity is dependent on Ca2+ levels, will undergo trafficking and phosphorylation, and promote gene transcription.39 One of the genes targeted by CREB is CGRP.30 Indeed, CREB activation and CGRP up-regulation in DRG during colitis are parallel and coexisting in the same neurons, and both are regulated by the BDNF-PLC cascade.
CREB can also be activated by a number of other kinases including the Ca2+/CaM-dependent kinase II, PKA, MAPK, and Akt40 and is involved in other gene expression in addition to CGRP.41 Although Akt and CREB activation are correlated in cancer cells42 and can be activated by growth factors,43 in the present study, we show that phospho-Akt does not colocalize with CGRP in DRG neurons even though the level of phospho-Akt was also increased in the DRG during colitis,34 thus it is unlikely that the Akt pathway directly regulates CGRP expression in the DRG neurons. One explanation is that the PI3K/Akt pathway may activate CREB in non-CGRP neurons. This is true that a large number of p-CREB positive cells do not express CGRP (Figure 7) while most of the CGRP neurons have p-CREB. This suggests that p-CREB could have multiple roles in the DRG by not only regulating CGRP but also regulating other molecules.
In addition to the BDNF-PLCγ pathway, CGRP expression can also be regulated by other signaling in the DRG. ERK5 is a novel member of the ERK family that is sensitive to cytokine, stress, and mitogenic factors. The phosphorylation level of ERK5, not the ERK1/2, is reported to increase in DRG during colitis.5 Activation of ERK5 is a key pathway in retrograde NGF-induced sensory neuronal survival response.22 In culture, prevention of ERK5 activity attenuates retrograde NGF-induced CGRP up-regulation in DRG neuronal soma.13 Up-regulation of NGF in the inflamed distal colon and retrograde transport of TrkA to DRG neuronal cell body may facilitate this interaction and also contribute to CGRP up-regulation in DRG.13,44 The interplay between NGF and CGRP pathways has long been suggested. The peptidergic DRG neurons contain both CGRP and TrkA. CGRP mRNA in DRG was also decreased in TrkA−/− mice as well as in NGF-deprived DRG explants.45 Some of the DRG neurons that contain CGRP also contain TrkB thus are also responsive to BDNF. These results suggest that CGRP as a nociceptive marker can be regulated by a number of modulators synergistically to fine-tune the activity of the sensory neurons. Other factors such as cytokines, purinergic system, and glutamate and receptors may also have roles in CGRP expression due to their ability in regulating the Ca2+ pathway. The cytokine Activin is able to increase CGRP expression in sensory neurons in culture and in vivo after peripheral inflammation.46,47
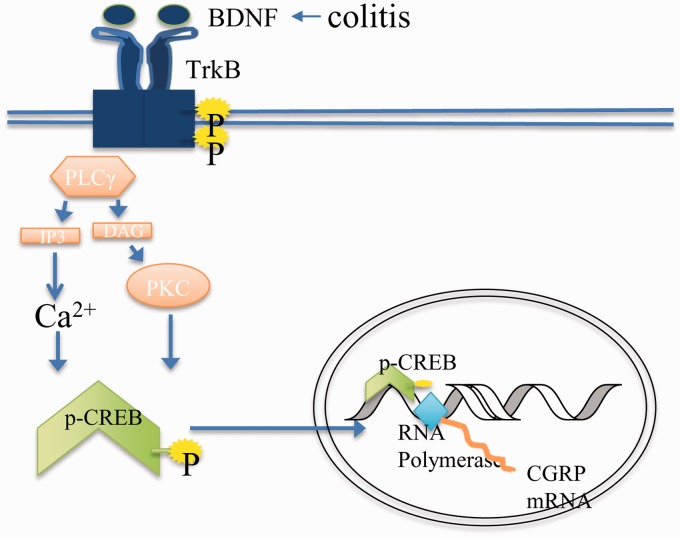
Visceral hypersensitivity is a highly complex entity that can occur due to hyperexcitability of the primary sensory afferents in DRG. Aberrant levels of neurochemical compounds within DRG neurons are critical components in modulating neuronal excitability. CGRP is highly recognized for its neurotransmitter nature in mediating sensory activity and central sensitization in the spinal cord27 and is widely considered as a nociceptive marker.6,48 Mice lacking CGRP or receiving pharmacological inhibition of CGRP activity do not develop hyperalgesia or central neuropathic pain after inflammation.49,50 Conversely, mice receiving intrathecal CGRP peptide exhibit nociceptive behavior.51,52 In regard to visceral hypersensitivity induced by TNBS colitis, CGRP expression is increased in the lumbar L1 DRG,2 and inhibition of endogenous CGRP activity reduces visceral hypersensitivity.53 As of today, limited studies have been implemented to investigate the mechanism of CGRP generation in sensory neurons. The present study combined in vivo and in vitro techniques and systemically explored the signal transduction that mediates CGRP expression in primary sensory neurons. Through these studies, we conclude that in TrkB-expressing cells, CGRP is regulated by a paracrine action of endogenous BDNF through a unique pathway involving PLCγ and CREB (Figure 8). CGRP expression in other cell populations can be modulated by different factors such as NGF/TrkA and cytokines. These results suggest that blockade of a single pathway may be necessary but may not be sufficient to decrease nociception during visceral inflammation.
Figure 8.
Schematic diagram illustrates the putative mechanism for BDNF signaling in mediating CGRP expression in DRG. Endogenously elevated BDNF in sensory neurons upon visceral inflammation (colitis in the present study) binds to TrkB and activates the PLCγ pathway which leads to Ca2+ mobilization and Ca2+ dependent CREB phosphorylation. Activity of CREB further contributes to CGRP up-regulation in primary afferent neurons.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grant NIH DK077917 to LYQ.
References
- 1.Qiao LY. Neurotrophin signaling and visceral hypersensitivity. Front Biol (Beijing) 2014; 9: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiao LY, Grider JR. Colitis induces calcitonin gene-related peptide expression and Akt activation in rat primary afferent pathways. Exp Neurol 2009; 219: 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nazif O, Teichman JM, Gebhart GF. Neural upregulation in interstitial cystitis. Urology 2007; 69: 24–33. [DOI] [PubMed] [Google Scholar]
- 4.Gottmann K, Mittmann T, Lessmann V. BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp Brain Res 2009; 199: 203–234. [DOI] [PubMed] [Google Scholar]
- 5.Yu SJ, Grider JR, Gulick MA, et al. Up-regulation of brain-derived neurotrophic factor is regulated by extracellular signal-regulated protein kinase 5 and by nerve growth factor retrograde signaling in colonic afferent neurons in colitis. Exp Neurol 2012; 238: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benemei S, Nicoletti P, Capone JG, et al. CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol 2009; 9: 9–14. [DOI] [PubMed] [Google Scholar]
- 7.Qiao LY, Grider JR. Up-regulation of calcitonin gene-related peptide and receptor tyrosine kinase TrkB in rat bladder afferent neurons following TNBS colitis. Exp Neurol 2007; 204: 667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eftekhari S, Edvinsson L. Calcitonin gene-related peptide (CGRP) and its receptor components in human and rat spinal trigeminal nucleus and spinal cord at C1-level. BMC Neurosci 2011; 12: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benemei S, Nicoletti P, Capone JG, et al. CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol 2009; 9: 9–14. [DOI] [PubMed] [Google Scholar]
- 10.Ju G, Hokfelt T, Brodin E, et al. Primary sensory neurons of the rat showing calcitonin gene-related peptide immunoreactivity and their relation to substance p-immunoreactive, somatostatin-immunoreactive, galanin-immunoreactive, vasoactive intestinal polypeptide-immunoreactive and cholecystokinin-immunoreactive ganglion-cellse. Cell Tissue Res 1987; 247: 417–431. [DOI] [PubMed] [Google Scholar]
- 11.Mccarthy PW, Lawson SN. Cell type and conduction-velocity of rat primary sensory neurons with calcitonin gene-related peptide-like immunoreactivity. Neuroscience 1990; 34: 623–632. [DOI] [PubMed] [Google Scholar]
- 12.Bennett DL, Dmietrieva N, Priestley JV, et al. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett 1996; 206: 33–36. [DOI] [PubMed] [Google Scholar]
- 13.Yu SJ, Xia CM, Kay JC, et al. Activation of extracellular signal-regulated protein kinase 5 is essential for cystitis- and nerve growth factor-induced calcitonin gene-related peptide expression in sensory neurons. Mol Pain 2012; 8: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song XY, Li F, Zhang FH, et al. Peripherally-derived BDNF promotes regeneration of ascending sensory neurons after spinal cord injury. PLoS One 2008; 3: e1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemos C, Mendonca D, Pereira-Monteiro J, et al. BDNF and CGRP interaction: implications in migraine susceptibility. Cephalalgia 2010; 30: 1375–1382. [DOI] [PubMed] [Google Scholar]
- 16.Geng SJ, Liao FF, Dang WH, et al. Contribution of the spinal cord BDNF to the development of neuropathic pain by activation of the NR2B-containing NMDA receptors in rats with spinal nerve ligation. Exp Neurol 2010; 222: 256–266. [DOI] [PubMed] [Google Scholar]
- 17.Liu M, Kay JC, Shen S, et al. Endogenous BDNF augments NMDA receptor phosphorylation in the spinal cord via PLCgamma, PKC, and PI3K/Akt pathways during colitis. J Neuroinflammation 2015; 12: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lever IJ, Bradbury EJ, Cunningham JR, et al. Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. J Neurosci 2001; 21: 4469–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiao LY, Gulick MA, Bowers J, et al. Differential changes in brain-derived neurotrophic factor and extracellular signal-regulated kinase in rat primary afferent pathways with colitis. Neurogastroenterol Motil 2008; 20: 928–938. [DOI] [PubMed] [Google Scholar]
- 20.Conner JM, Lauterborn JC, Yan Q, et al. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci 1997; 17: 2295–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Constandil L, Aguilera R, Goich M, et al. Involvement of spinal cord BDNF in the generation and maintenance of chronic neuropathic pain in rats. Brain Res Bull 2011; 86: 454–459. [DOI] [PubMed] [Google Scholar]
- 22.Watson FL, Heerssen HM, Bhattacharyya A, et al. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response (vol 4, pg 981, 2001). Nat Neurosci 2002; 5: 1017. [DOI] [PubMed] [Google Scholar]
- 23.Perkinton MS, Ip JK, Wood GL, et al. Phosphatidylinositol 3-kinase is a central mediator of NMDA receptor signalling to MAP kinase (Erk1/2), Akt/PKB and CREB in striatal neurones. J Neurochem 2002; 80: 239–254. [DOI] [PubMed] [Google Scholar]
- 24.Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiology 2005; 20: 70–78. [DOI] [PubMed] [Google Scholar]
- 25.Wang YY, Wu SX, Zhou L, et al. Dose-related antiallodynic effects of cyclic AMP response element-binding protein-antisense oligonucleotide in the spared nerve injury model of neuropathic pain. Neuroscience 2006; 139: 1083–1093. [DOI] [PubMed] [Google Scholar]
- 26.Descalzi G, Fukushima H, Suzuki A, et al. Genetic enhancement of neuropathic and inflammatory pain by forebrain upregulation of CREB-mediated transcription. Mol Pain 2012; 8: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kay JC, Xia CM, Liu M, et al. Endogenous PI3K/Akt and NMDAR act independently in the regulation of CREB activity in lumbosacral spinal cord in cystitis. Exp Neurol 2013; 250: 366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bement MK, Sluka KA. Co-localization of p-CREB and p-NR1 in spinothalamic neurons in a chronic muscle pain model. Neurosci Lett 2007; 418: 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Todorovski Z, Asrar S, Liu J, et al. LIMK1 regulates long-term memory and synaptic plasticity via the transcriptional factor CREB. Mol Cell Biol 2015; 35: 1316–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanigan TM, Russo AF. Binding of upstream stimulatory factor and a cell-specific activator to the calcitonin/calcitonin gene-related peptide enhancer. J Biol Chem 1997; 272: 18316–18324. [DOI] [PubMed] [Google Scholar]
- 31.Freeland K, Liu YZ, Latchman DS. Distinct signalling pathways mediate the cAMP response element (CRE)-dependent activation of the calcitonin gene-related peptide gene promoter by cAMP and nerve growth factor. Biochem J 2000; 345: 233–238. [PMC free article] [PubMed] [Google Scholar]
- 32.Hou C, Kirchner T, Singer M, et al. In vivo activity of a phospholipase C inhibitor, 1-(6-((17beta-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole-2,5-di one (U73122), in acute and chronic inflammatory reactions. J Pharmacol Exp Ther 2004; 309: 697–704. [DOI] [PubMed] [Google Scholar]
- 33.Xia CM, Gulick MA, Yu SJ, et al. Up-regulation of brain-derived neurotrophic factor in primary afferent pathway regulates colon-to-bladder cross-sensitization in rat. J Neuroinflammation 2012; 9: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia C, Shen S, Hashmi F, et al. Colitis-induced bladder afferent neuronal activation is regulated by BDNF through PLCgamma pathway. Exp Neurol 2015. DOI: 10.1016/j.expneurol.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Traub RJ, Hutchcroft K, Gebhart GF. The peptide content of colonic afferents decreases following colonic inflammation. Peptides 1999; 20: 267–273. [DOI] [PubMed] [Google Scholar]
- 36.Gartner A, Polnau DG, Staiger V, et al. Hippocampal long-term potentiation is supported by presynaptic and postsynaptic tyrosine receptor kinase B-mediated phospholipase C gamma signaling. J Neurosci 2006; 26: 3496–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizoguchi Y, Monji A, Nabekura J. Brain-derived neurotrophic factor induces long-lasting Ca2+-activated K+ currents in rat visual cortex neurons. Eur J Neurosci 2002; 16: 1417–1424. [DOI] [PubMed] [Google Scholar]
- 38.Numakawa T, Matsumoto T, Adachi N, et al. Brain-derived neurotrophic factor triggers a rapid glutamate release through increase of intracellular Ca2+ and Na+ in cultured cerebellar neurons. J Neurosci Res 2001; 66: 96–108. [DOI] [PubMed] [Google Scholar]
- 39.Sheng M, McFadden G, Greenberg ME. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron 1990; 4: 571–582. [DOI] [PubMed] [Google Scholar]
- 40.Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cell Signal 2004; 16: 1211–1227. [DOI] [PubMed] [Google Scholar]
- 41.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2001; 2: 599–609. [DOI] [PubMed] [Google Scholar]
- 42.Garcia GE, Nicole A, Bhaskaran S, et al. Akt-and CREB-mediated prostate cancer cell proliferation inhibition by Nexrutine, a Phellodendron amurense extract. Neoplasia 2006; 8: 523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peltier J, O’Neill A, Schaffer DV. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol 2007; 67: 1348–1361. [DOI] [PubMed] [Google Scholar]
- 44.Qiao LY, Grider JR. Colitis elicits differential changes in the expression levels of receptor tyrosine kinase TrkA and TrkB in colonic afferent neurons: a possible involvement of axonal transport. Pain 2010; 151: 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel TD, Jackman A, Rice FL, et al. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo (vol 25, pg 345, 2000). Neuron 2003; 37: 183. [DOI] [PubMed] [Google Scholar]
- 46.Ai XB, Cappuzzello J, Hall AK. Activin and bone morphogenetic proteins induce calcitonin gene-related peptide in embryonic sensory neurons in vitro. Mol Cell Neurosci 1999; 14: 506–518. [DOI] [PubMed] [Google Scholar]
- 47.Xu P, Van Slambrouck C, Berti-Mattera L, et al. Activin induces tactile allodynia and increases calcitonin gene-related peptide after peripheral inflammation. J Neurosci 2005; 25: 9227–9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han JS, Adwanikar H, Li Z, et al. Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats. Mol Pain 2010; 6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang LP, Hoff AO, Wimalawansa SJ, et al. Arthritic calcitonin/alpha calcitonin gene-related peptide knockout mice have reduced nociceptive hypersensitivity. Pain 2001; 89: 265–273. [DOI] [PubMed] [Google Scholar]
- 50.Bennett AD, Chastain KM, Hulsebosch CE. Alleviation of mechanical and thermal allodynia by CGRP(8-37) in a rodent model of chronic central pain. Pain 2000; 86: 163–175. [DOI] [PubMed] [Google Scholar]
- 51.Oku R, Satoh M, Fujii N, et al. Calcitonin gene-related peptide promotes mechanical nociception by potentiating release of substance P from the spinal dorsal horn in rats. Brain Res 1987; 403: 350–354. [DOI] [PubMed] [Google Scholar]
- 52.Sun RQ, Lawand NB, Willis WD. The role of calcitonin gene-related peptide (CGRP) in the generation and maintenance of mechanical allodynia and hyperalgesia in rats after intradermal injection of capsaicin. Pain 2003; 104: 201–208. [DOI] [PubMed] [Google Scholar]
- 53.Delafoy L, Gelot A, Ardid D, et al. Interactive involvement of brain derived neurotrophic factor, nerve growth factor, and calcitonin gene related peptide in colonic hypersensitivity in the rat. Gut 2006; 55: 940–945. [DOI] [PMC free article] [PubMed] [Google Scholar]