Abstract
β-Cell proliferation and expansion during pregnancy are crucial for maintaining euglycemia in response to increased metabolic demands placed on the mother. Prolactin and placental lactogen signal through the prolactin receptor (PRLR) and contribute to adaptive β-cell responses in pregnancy; however, the in vivo requirement for PRLR signaling specifically in maternal β-cell adaptations remains unknown. We generated a floxed allele of Prlr, allowing conditional loss of PRLR in β-cells. In this study, we show that loss of PRLR signaling in β-cells results in gestational diabetes mellitus (GDM), reduced β-cell proliferation, and failure to expand β-cell mass during pregnancy. Targeted PRLR loss in maternal β-cells in vivo impaired expression of the transcription factor Foxm1, both G1/S and G2/M cyclins, tryptophan hydroxylase 1 (Tph1), and islet serotonin production, for which synthesis requires Tph1. This conditional system also revealed that PRLR signaling is required for the transient gestational expression of the transcription factor MafB within a subset of β-cells during pregnancy. MafB deletion in maternal β-cells also produced GDM, with inadequate β-cell expansion accompanied by failure to induce PRLR-dependent target genes regulating β-cell proliferation. These results unveil molecular roles for PRLR signaling in orchestrating the physiologic expansion of maternal β-cells during pregnancy.
Introduction
Pregnancy is a unique acquired physiologic state of increased metabolic demand, requiring increased output of insulin by islet β-cells. This adaptive response is accomplished both through enhanced insulin secretion and by β-cell proliferation and expansion in mice (1). Failure of adaptive β-cell expansion underlies dysregulated glucose homeostasis and progression to diabetes mellitus (2). Thus, the mechanisms underlying gestational β-cell proliferation are a focus of intensive ongoing investigation.
Pregnancy hormones are known regulators of β-cell growth and function (3). The lactogenic hormones prolactin and placental lactogen signal through the prolactin receptor (PRLR) and are crucial regulators of pregnancy adaptation in many maternal tissues (4). PRLR is expressed in both rodent and human pancreatic β-cells (5), and in vitro treatment of islets with prolactin has established it as a potent β-cell mitogen in both species (1). Although gene expression studies of islets during pregnancy identified strong induction of some prolactin signaling targets (6–8), the mechanisms underlying lactogen-stimulated changes in β-cells during pregnancy are incompletely understood. During transient β-cell proliferation and expansion during gestation, prior studies have reported increased expression of nuclear factors like FoxM1, the cyclin-dependent kinases cyclin A2 and cyclin B1, and MafB (6,9,10). However, the requirement for PRLR signaling to induce expression of these factors and the physiologic significance of the gestational MafB+ β-cell subpopulation are unknown.
Studies of β-cells during pregnancy in humans are confounded by practical and ethical challenges. Thus, animal studies remain critical for understanding β-cell biology during pregnancy (11). Mouse genetic studies of PRLR support a role in β-cell development and function; the global PRLR knockout has glucose intolerance and reduced β-cell mass (12). Unfortunately, the global Prlr knockout mouse is sterile, precluding pregnancy studies (13). Prlr+/− mice studied during pregnancy develop glucose intolerance and reduction in β-cell proliferation and mass expansion (14). Nevertheless, as loss of PRLR results in multiple abnormalities in other metabolic tissues that could indirectly influence β-cell function (15), it is essential to assess the consequences of targeted PRLR inactivation specifically in β-cells. In this study, we generated a conditional Prlr allele allowing Cre recombinase–mediated genetic ablation of PRLR signaling and identified a requirement for PRLR in molecular, hormonal, and proliferative adaptations by maternal β-cells in pregnancy. Collectively, our results suggest PRLR signaling is a master regulator of adaptive β-cell responses during pregnancy.
Research Design and Methods
Creation of the Floxed Prlr Allele, βPRLRKO, and βMafBKO Mice
A targeting vector containing Prlr genomic DNA encompassing exons 4 through 9 were subcloned into plasmid PL253 containing a thymidine kinase cassette. Using recombineering, loxP and FRT-neo-FRT-loxP cassettes were placed flanking exon 5. The targeting vector was electroporated into C57BL/6J embryonic stem cells and clones selected using G418; validated clones were injected into 129 blastocysts generating chimeric males (Stanford Transgenic Core). Germline transmission of Prlrf allele was identified by brown fur pups after crossing male chimeras with C57BL/6J females. Mouse tail genomic DNA was digested with NheI and Southern blotting performed with a 5′ external probe to confirm a correctly targeted allele. The FRT-flanked neo cassette was removed by crosses with FLPeR mice (The Jackson Laboratory, Bar Harbor, ME), generating floxed PRLR mice (Prlrf/+), which were backcrossed with C57BL/6J mice (The Jackson Laboratory) for more than eight generations. Prlrf/+ mice were crossed with mice with a transgene encoding Cre recombinase from rat insulin promoter elements (RIP-Cre) (16). Subsequently, RIP-Cre;Prlrf/+ males were crossed with Prlrf/+ females, generating βPRLRKO (PRLRf/f;RIP-Cre) and littermate Prlrf/+, Prlrf/f, RIP-Cre;Prlrf/+, and RIP-Cre;Prlr+/+ controls. MafBf/f mice (17) were crossed with RIP-CreM mice (18) and backcrossed to B6J mice at least six generations (see Supplementary Table 1: genotyping primers).
Mouse Husbandry, Breeding, and Experimentation
Mice were weaned 21–25 days after parturition. To avoid potential effects of altered maternal metabolism on offspring during in utero exposure, βPRLRKO females were solely used as experimental mice. Beginning at 8 weeks of age, βPRLRKO and control females were mated with wild-type FVB males. Vaginal plugs were scored at gestational day (GD) 0.5 and males removed. All experiments were repeated in at least two independent cohorts of mice. All procedures involving mice were approved and conducted in accordance with the Stanford Administrative Panel on Laboratory Animal Care or Vanderbilt Animal Care and Use Program.
Tolerance Testing and Serum Metabolite Analysis
Intraperitoneal glucose and insulin tolerance testing were performed as previously described (19). An oral glucose tolerance test (GTT) was performed using a 22-gauge rigid gavage needle to deliver an intragastric glucose bolus of 2 g/kg body weight. Blood was collected by tail vein bleeding. Blood glucose levels were determined by glucometer (Bayer Contour; Bayer). Ad libitum fed or overnight (16 h)–fasted blood glucose levels were measured at 8:30 a.m. Serum insulin levels were performed by ELISA (Crystal Chem) following the manufacturer’s directions.
Islet Isolation and Culture
Islets were isolated using retrograde perfusion of the pancreatic duct with collagenase, purified using density centrifugation, and cultured as previously described (20). Recombinant mouse prolactin (R&D Systems) was diluted in culture media to a final concentration of 500 ng/ml. Culture media was changed daily.
Imaging
Microscopy was performed on a Zeiss AxioM1 Fluorescence microscope with AxioVision software (Carl Zeiss). Confocal images were obtained using a Leica Sp2 microscope (Beckman Cell Sciences Imaging Facility; Leica Microsystems).
Quantifying β-Cell Mass and Proliferation
After weighing, the pancreas was fixed, embedded in optimal cutting temperature compound, and then frozen. Tissues were sectioned at 10-µm intervals using a Leica 3050S Cryostat (Leica Microsystems). We immunostained tissue with anti-insulin antibody and DAPI and then analyzed sections separated by 200 µm. β-Cell mass was measured using ImageJ (National Institutes of Health) to quantify insulin+ and total pancreas area in each section; β-cell area was the average of nine measured sections. The calculated β-cell mass = (β-cell area/total pancreas area) × (total pancreas weight). To measure proliferation, we immunostained tissues using antibodies against insulin and Ki-67 and the proportion of Ki-67+ β-cells calculated as a percentage. At least 1,500 β-cells were counted for each animal; only clusters containing at least 20 β-cells were counted. The total number of islets per mouse was the sum of clusters counted in nine analyzed sections. Antibodies used are listed in Supplementary Table 2.
Quantitative PCR
Total RNA was isolated using TRIzol (Invitrogen) and reverse transcribed using the Ambion Retroscript Kit (Ambion) according to the manufacturer’s instructions. Quantitative RT-PCR (qPCRs) were performed with FastStart Universal Probe Master (Roche) and TaqMan assays (see Supplementary Table 3) on an ABI 7500 RealTime PCR System (Applied Biosystems). Assays were performed in technical replicates and normalized to mouse actin as a reference standard.
Western Blotting
Islets were lysed in 2× Bio-Rad loading buffer (Bio-Rad) containing 10% BME and then boiled for 5 min. Following electrophoresis on Bio-Rad TGX 4–20% gels (Bio-Rad), proteins were transferred onto nitrocellulose using the tank apparatus (Bio-Rad). Chemiluminescent detection was performed with SuperSignal West Pico (Thermo) and Carestream Kodak BioMax XAR film (Sigma-Aldrich).
Statistical Analysis
Data are presented as means ± SEM. Statistical significance was determined by unpaired two-tailed Student t test, repeated-measures ANOVA (RM-ANOVA), or χ2 test as indicated using GraphPad Prism 6 (GraphPad Software, LaJolla, CA) or Microsoft Excel (Microsoft, Redmond, WA).
Results
Conditional Prlr Inactivation in Islet β-Cells
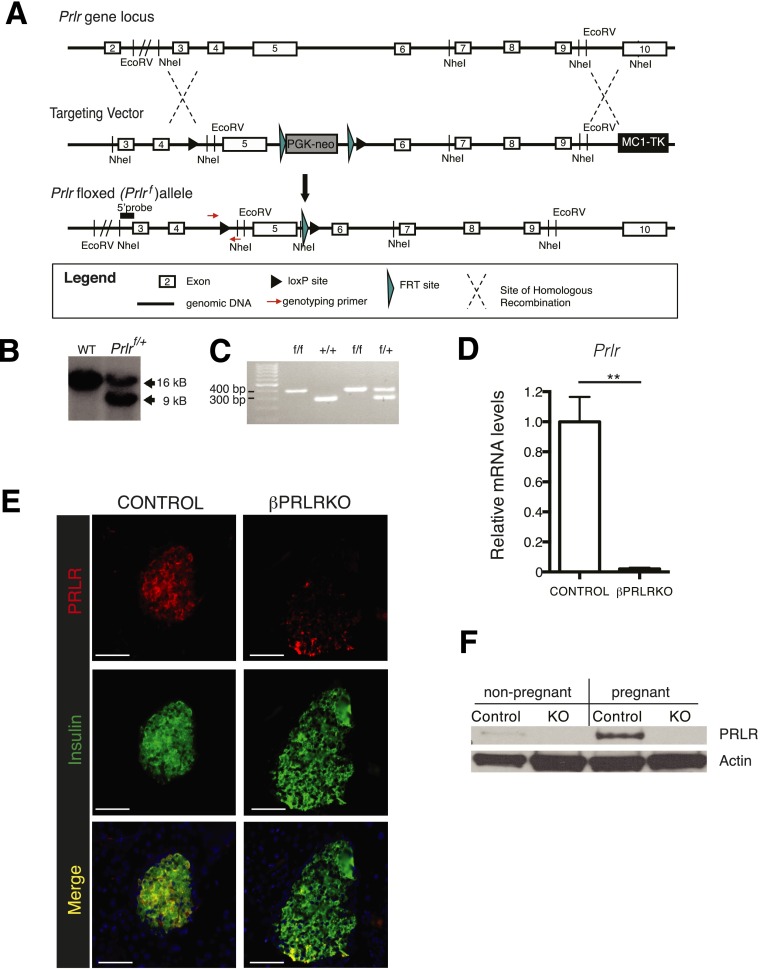
To permit targeted PRLR signaling disruption, we generated mice harboring a Cre recombinase-sensitive Prlrf allele (Fig. 1A and B) (see research design and methods). Intercrosses of Prlrf/+ mice generated Prlrf/f mice and siblings with expected genotypes in Mendelian ratio (Fig. 1C and data not shown). To generate mice lacking PRLR in pancreatic β-cells (abbreviated βPRLRKO mice), we bred RIP-Cre mice to Prlrf mice (see research design and methods). qPCR revealed that Prlr mRNA levels were reduced >95% in islets from βPRLRKO females as compared with littermate Prlrf/f controls (Fig. 1D). Immunohistology and Western blotting demonstrated that β-cell PRLR protein was reduced or almost undetectable in virgin or pregnant female βPRLRKO mice (Fig. 1E and F). Conception, nursing, and rearing of pups by βPRLRKO mothers was similar to that of controls (see gestational diabetes mellitus in βprlrko mice). Thus, the Prlrf/f mice permitted conditional genetic inactivation of Prlr in vivo.
Figure 1.
Conditional inactivation of PRLR signaling in pancreatic β-cells (βPRLRKO). A: Schematic diagram (not to scale) depicting the Prlr gene locus, targeting vector sequence, and Prlrf allele structure (see research design and methods). B: Southern blot of genomic DNA from mice demonstrating germline transmission of Prlrf. Introduction of a NheI site downstream of the loxP cassette resulted in a 9-kb (targeted allele) rather than a 16-kb fragment (wild-type [WT] allele). C: Genotyping PCR of a litter depicting representative progeny of all potential Prlr genotypes following a cross of Prlrf/+ mice. D: mRNA levels of Prlr in βPRLRKO females (RIP-Cre;Prlrf/f) and control littermates (Prlrf/f). n = 6 mice/group. E: Immunofluorescence images of islets from βPRLRKO female and control mice stained for PRLR and insulin (scale bar, 100 μm). F: Western blot of PRLR expression from islets isolated from virgin βPRLRKO female and controls. Actin was used as a loading control. **P ≤ 0.01 by t test.
Gestational Diabetes Mellitus in βPRLRKO Mice
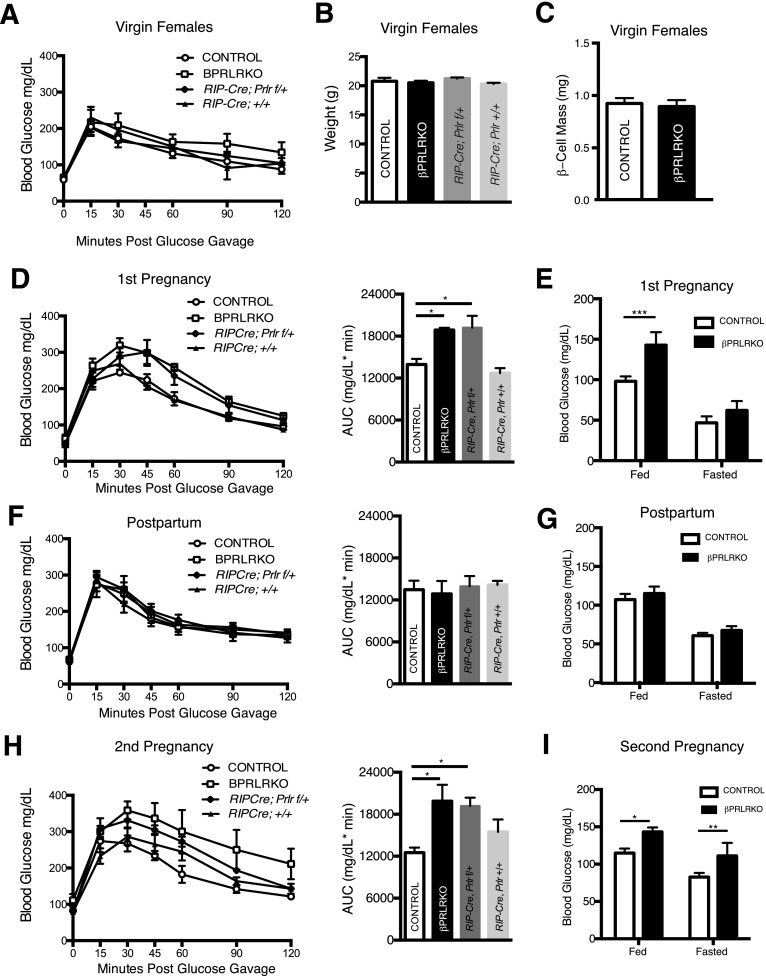
We next investigated the consequences of disrupting β-cell PRLR signaling on glucose homeostasis. Adult virgin female and male βPRLRKO mice did not show alterations in glucose tolerance, weight, or β-cell mass, in contrast to defects observed in global Prlr knockout mice (Fig. 2A–C) (data not shown). Ad libitum fed glucose and insulin levels, insulin tolerance, and total pancreas mass also did not differ in 2- to 3-month-old virgin βPRLRKO females compared with age-matched controls (Supplementary Fig. 1A–D), the age when all subsequent pregnancy studies were performed.
Figure 2.
βPRLRKO females have GDM. Virgin βPRLRKO females and control littermates were followed through pregnancy, postpartum, and a second pregnancy. A: GTT. B: Body weights. C: β-Cell mass in virgin females. D–I: Glucose tolerance with corresponding area under the curve (AUC) for each genotype and ad libitum fed and overnight fasted glucose for βPRLRKO females and controls. D and E: First pregnancy. F and G: Postpartum. H and I: Second pregnancy. n = 5–8 mice/group. *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001 for RM-ANOVA (GTT) or t test (blood glucose).
By contrast, we observed pronounced defects of glucose regulation in pregnant βPRLRKO females. Maternal insulin demand peaks late in pregnancy, ∼GD16.5 (10). On GD16.5, we observed marked glucose intolerance in βPRLRKO females (Fig. 2D) compared with control females (Prlrf/+ and Prlrf/f). We also observed glucose intolerance in RIP-Cre;Prlrf/+ mice (Fig. 2D). Blood glucose levels during ad libitum feeding were also elevated in GD16.5 βPRLRKO mice (Fig. 2E). Expression of Prlr mRNA in RIP-Cre;Prlrf/+ mice was intermediate between βPRLRKO and control females (Supplementary Fig. 2A). However, weight gain in pregnancy and litter sizes of βPRLRKO mothers were indistinguishable from that of controls (Supplementary Fig. 2B and C). Following parturition, βPRLRKO mothers lactated normally and nursed pups to weaning without impact on offspring survival rates (data not shown). Subsequent postpartum analyses showed that glucose intolerance and ad libitum glucose abnormalities in pregnant βPRLRKO mice resolved (Fig. 2F and G), a characteristic feature of gestational diabetes mellitus (GDM). Because some mouse strains harboring a RIP-Cre transgene develop glucose homeostasis abnormalities (21), we also evaluated RIP-Cre littermate controls but did not observe any differences in glucose tolerance in virgin, pregnant, or postpartum mice compared with controls (Fig. 2D, F, and H). Thus, βPRLRKO mice developed GDM.
Because GDM increases the risk of dysglycemia in future pregnancies, we investigated the effects of additional pregnancy on glucose regulation in βPRLRKO multigravida. During a second gestation in βPRLRKO females, glucose intolerance (Fig. 2H) and hyperglycemia in ad libitum feeding recurred (Fig. 2I). βPRLRKO females also developed evidence of worsened glucose control, including glycemia >200 mg/dL upon completion of the GTT (Fig. 2H) and fasting hyperglycemia (Fig. 2I), features not observed in the first pregnancy (Fig. 2D). Thus, we observed that glucose control worsened with additional pregnancies in βPRLRKO mice, similar to humans with multigravidity or GDM (22).
Failure to Proliferate and Expand β-Cells in Pregnant βPRLRKO Mice
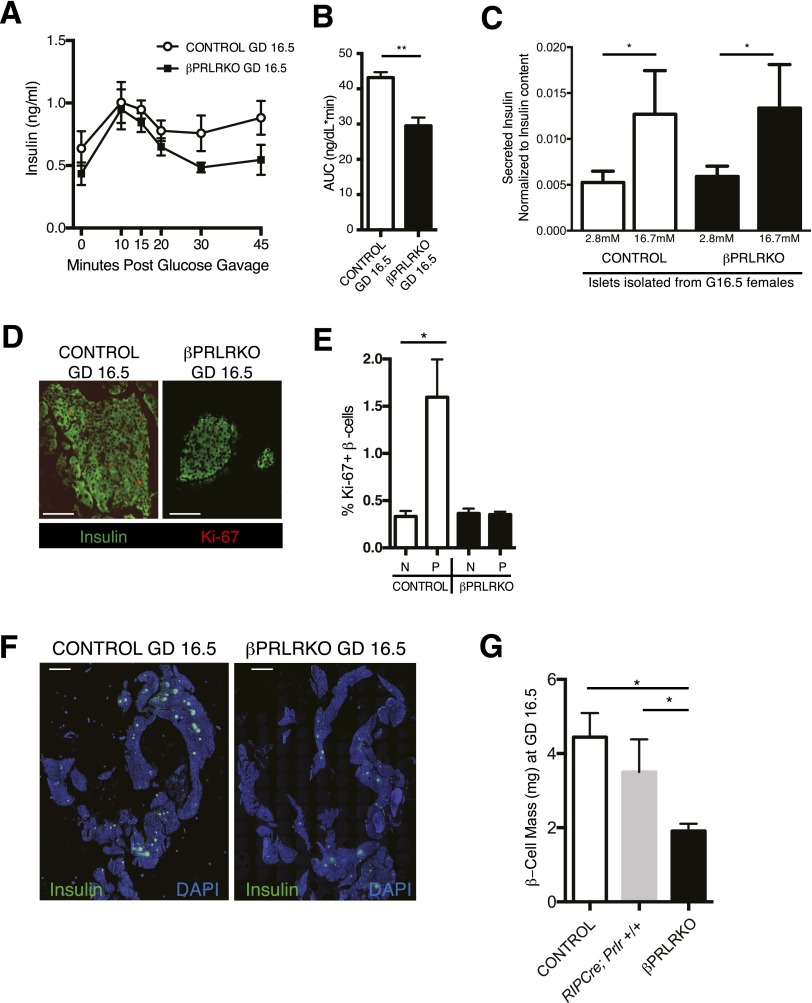
To identify the basis for GDM in βPRLRKO mothers, we assessed insulin output and β-cell growth, features known to undergo adaptive enhancement in pregnancy (1). We found reduced serum insulin levels in βPRLRKO mice during GTT in late gestation (GD16.5), consistent with relative insulin deficiency (Fig. 3A and B). We sought to understand the basis for this relative insulin deficiency by examining insulin secretion in islets isolated from mice at GD16.5. Islets from βPRLRKO or control mice showed similar basal and glucose-stimulated insulin secretion (GSIS), normalized to insulin content (Fig. 3C), indicating the insulin deficiency observed in vivo was not a consequence of reduced GSIS. Insulin tolerance was also unchanged in βPRLRKO mothers at this gestational stage, excluding altered peripheral insulin sensitivity (Supplementary Fig. 3A). Next, we used the cellular marker Ki-67 to quantify β-cell proliferation and observed a threefold reduction of β-cell labeling in islets of GD16.5 βPRLRKO mice compared with controls (Fig. 3D and E). No change in total islet number was observed (Supplementary Fig. 3C).
Figure 3.
βPRLRKO females have reduced β-cell proliferation and mass during pregnancy. Serum insulin levels during GTT (A) and corresponding area under the curve (AUC) measurements (B) in GD16.5 pregnant βPRLRKO females and controls (Prlrf/f). n = 4 mice/group. **P ≤ 0.01 by t test. C: GSIS on islets isolated from GD16.5 βPRLRKO females (n = 4) and controls (n = 6). D: Representative islet images of GD16.5 pancreas sections from βPRLRKO females and controls with Ki-67 immunofluorescence, insulin, and DAPI (scale bar, 100 μm). E: The percentage of β-cells with Ki-67+ nuclei staining in nonpregnant (N) and pregnant (P) (GD16.5) βPRLRKO females and controls. F: Representative images of pancreas sections from GD16.5 βPRLRKO females and controls used for β-cell area. Insulin immunofluorescence (green) and DAPI (blue) (scale bar, 2 mm). G: Calculated β-cell mass in GD16.5 βPRLRKO, RIP-Cre, Prlr+/+, and control (Prlrf/f) females. n = 3 or 4 mice/group. *P < 0.05 by t test.
Consistent with the observed reduction of Ki-67+ β-cells in pregnant βPRLRKO mice, β-cell area and mass were reduced approximately twofold compared with pregnant Prlrf/f and RIP-Cre controls (Fig. 3F and G and Supplementary Fig. 3C and D). Compared with nonpregnant βPRLRKO females (Fig. 2C) (0.89 ± 0.06 mg), pregnant βPRLRKO females (Fig. 3G) (1.91 ± 0.16 mg) exhibited a significant increase of β-cell mass during pregnancy (P = 0.004), suggesting PRLR-independent mechanisms of gestational mass expansion are active yet insufficient to compensate for the loss of PRLR signaling. Thus, loss of PRLR led to reduced β-cell proliferation and mass expansion in pregnant βPRLRKO.
Disrupted β-Cell Cycle Gene Expression and Serotonin Signaling in βPRLRKO Mice
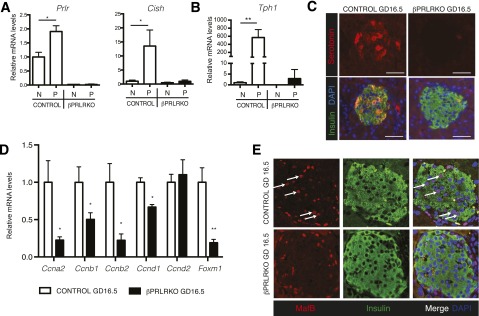
To investigate the molecular basis of reduced gestational β-cell proliferation in βPRLRKO mice, we measured gene expression. Gestational induction of known PRLR signaling targets like Prlr itself and cytokine-inducible Src homology 2–containing protein (Cish), a negative regulator of PRLR signaling (8), was markedly reduced as expected (Fig. 4A). Tryptophan hydroxylase 1 (Tph1) is highly induced by pregnancy in β-cells (6,23) and catalyzes the rate-limiting step of islet serotonin synthesis. In turn, serotonin contributes to β-cell proliferation through autocrine and paracrine effects (6). In βPRLRKO pregnant mice, islet Tph1 induction was eliminated (Fig. 4B), and immunoreactive serotonin in islets was absent during gestation (Fig. 4C). Thus, β-cell PRLR is required for islet serotonin production during pregnancy. Prior work suggests that lactogens alter expression of genes encoding β-cell cyclins, cyclin-dependent kinase inhibitors, and transcription factors Foxm1 and Foxd3 (4,10,24–26). In βPRLRKO pregnant females, qPCR with isolated islets at GD16.5 revealed reductions in Ccna2, Ccnb1, Ccnb2, and Ccnd1 (which, respectively, encode cyclins A2, B1, B2, and D1), and Foxm1, all previously implicated as regulators of gestational β-cell proliferation (6,8,10) (Fig. 4D). By contrast, we found that expression of Ccnd2 (Fig. 4D), Men1, Cdkn2c (p18), Cdkn1b (p27), and Foxd3 (Supplementary Fig. 4A), which prior studies indicate are regulated by lactogen signaling in pregnancy (24–27), were unaffected in βPRLRKO islets at GD16.5. Moreover, reduced Foxd3 expression during gestation (24) still occurred in βPRLRKO females, indicating PRLR signaling is not required for Foxd3 downregulation in pregnancy (Supplementary Fig. 4A). Collectively, our in vivo targeted genetic studies demonstrate that PRLR signaling is required for serotonin signaling and gene expression changes controlling adaptive maternal β-cell expansion.
Figure 4.
Altered cell cycle regulators and serotonin signaling in βPRLRKO islets during pregnancy. mRNA levels of Prlr and Cish (A) and Tph1 (B) in nonpregnant (N) or pregnant (P) βPRLRKO females (black bars) and controls (open bars). C: Immunofluorescence of islet serotonin, insulin, and DAPI (scale bar, 50 μm). D: Relative mRNA levels of Ccna2, Ccnb1, Ccnb2, Ccnd1, Ccnd2, and Foxm1 in βPRLRKO females and controls at GD16.5. E: Confocal immunofluorescence images of islets from GD16.5 βPRLRKO females and controls (original magnification ×40). MafB (red), insulin (green), and DAPI (blue) with MafB+Ins+ cells highlighted (white arrows). For mRNA studies, pregnant samples were from GD16.5 and expression levels normalized to nonpregnant controls = 1. n = 3–8 mice/group. *P < 0.05, **P ≤ 0.01 by t test.
To assess the formal possibility that GDM might indirectly alter gene expression in pregnant βPRLRKO mice, we measured the response of isolated βPRLRKO islets to lactogen treatment in vitro. PRLR activates several intracellular pathways, including Jak2/Stat5, a major mediator of its signal transduction (28). As expected, purified mouse prolactin robustly induced phosphorylation of Stat5 in control islets (Supplementary Fig. 4B). By contrast, phospho-Stat5 was reduced in islets from RIP-Cre;Prlrf/+ mice and absent in βPRLRKO islets treated with prolactin (Supplementary Fig. 4B). We next examined gene expression in cultured islets treated with prolactin for 24 h in concentrations comparable to peak lactogen levels in pregnancy (29). In controls, PRLR target genes, including Prlr, Cish, and Tph1, were induced to levels similar to those at GD16.5; this induction was eliminated in βPRLRKO islets (Supplementary Fig. 4C). FoxM1 expression was reported to increase in whole islets exposed in vitro to placental lactogen (10), but we did not detect consistent FoxM1 induction after treatment of islets with lactogens up to 4 days in culture (data not shown). These in vitro data are consistent with gene expression changes of βPRLRKO islets observed in vivo and suggest that altered expression of PRLR target genes reflects disrupted intrinsic islet signaling, not secondary effects of altered metabolism in GDM.
MafB Expression in β-Cells During Pregnancy Requires PRLR Signaling
In pancreatic islets from adult mice, the transcription factor MafB is exclusively expressed in α-cells (30), except during pregnancy when we detected transient expression in a subset of β-cells (9). This contrasts with MafB expression within both α-cells and β-cells during development (30,31). The physiologic significance of MafB expression in β-cells during pregnancy was unknown. We observed that up to 25% of maternal insulin+ (abbreviated Ins+) cells produced MafB by GD16.5 (Supplementary Fig. 5A). MafB+ β-cells diminished by postpartum day 7 and were undetectable by postpartum day 28 (Supplementary Fig. 5B). The maternal MafB+Ins+ cells at GC16.5 also produced Pdx1, a key transcription factor and regulator of β-cells (Supplementary Fig. 5C). MafB expression in adult α-cells promotes glucagon production, but we did not observe bihormonal Ins+Glucagon+ cells in maternal islets (data not shown). Strikingly, we found that MafB+ β-cells were absent from maternal βPRLRKO islets at GD16.5 or other gestational stages (Fig. 4E and data not shown). Thus, β-cell MafB expression in pregnancy requires PRLR signaling.
MafB Loss Impairs Gestational β-Cell Expansion and Promotes GDM
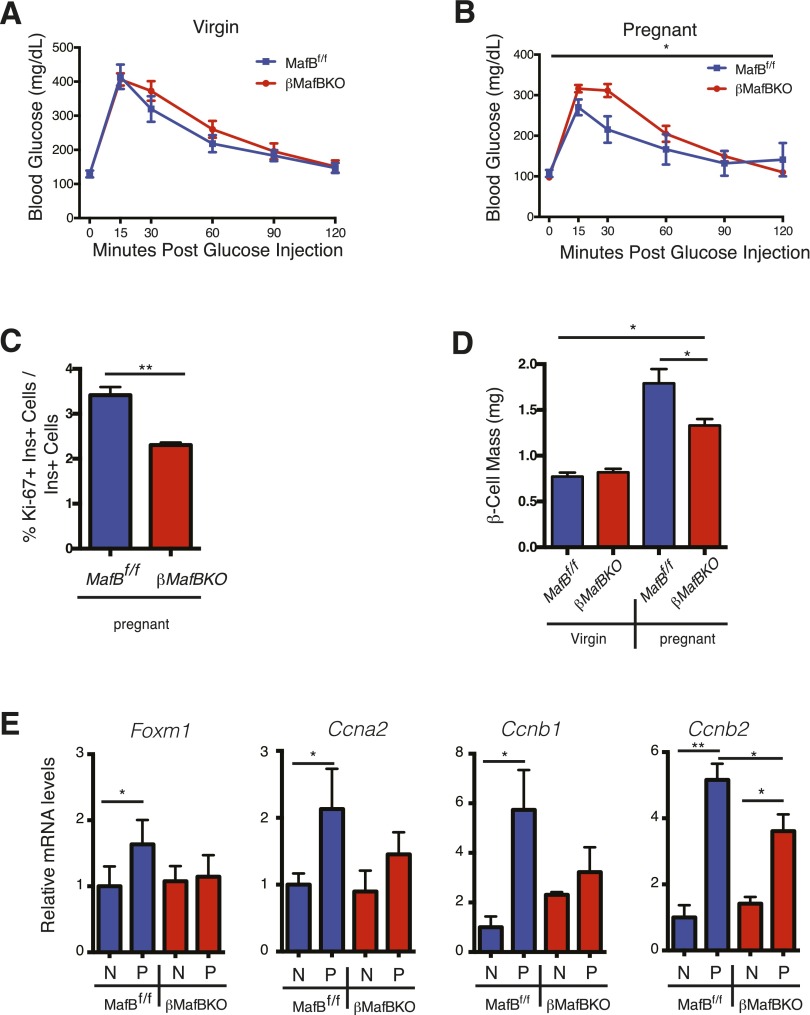
To identify physiologic roles for MafB in maternal β-cells, we generated a β-cell–specific deletion of MafB (βMafBKO) (see research design and methods). Nonpregnant βMafBKO mice had normal glucose tolerance (Fig. 5A), but showed glucose intolerance during pregnancy compared with control MafBf/f mice (Fig. 5B). This glucose intolerance was similar to that in βPRLRKO mice (Fig. 2). Additionally, both β-cell proliferation (Fig. 5C) and mass (Fig. 5D) were reduced in βMafBKO mice during pregnancy but not in virgin females.
Figure 5.
βMafBKO mice have GDM, reduced β-cell proliferation, and gene expression changes that overlap with βPRLRKO mice. GTT in βMafBKO females (MafBf/f;RIP-CreM) and controls (MafBf/f) as virgins (A) and during pregnancy (B) at GD15.5. The percentage of Ki-67+ nuclei within β-cells (Ki-67+Ins+/total Ins+ cells) (C) and β-cell mass (mg) in virgin and GD15.5 βMafBKO and controls (D). E: Relative mRNA levels of FoxM1, Ccna2, Ccnb1, and Ccnb2 in nonpregnant (N) or GD15.5 (P) βMafBKO females and controls. Expression levels were normalized to N controls = 1. n = 3–8 mice/group. *P < 0.05, **P ≤ 0.01 for RM-ANOVA (GTT) or t test.
Like βPRLRKO mice, pregnant βMafBKO mice failed to induce Foxm1, Ccna2, or Ccnb1 in pregnancy (Fig. 5E). Although levels of Ccnb2 mRNA increased with pregnancy in βMafBKO females, this induction was blunted compared with pregnant controls (Fig. 5E). The RIP-CreM allele used to generate βMafBKO mice also encodes human growth hormone that can activate PRLR signaling (32). Consistent with these prior findings, we observed significant elevations of PRLR signaling targets Cish and Tph1 in nonpregnant female RIP-CreM mice (Supplementary Fig. 5D). By contrast, Prlr expression in nonpregnant βMafBKO was similar to controls and was significantly induced during pregnancy. However, the induction was blunted compared with pregnant controls, including RIP-CreM pregnant females (Supplementary Fig. 5D). Despite this apparent partial activation of PRLR signaling, female βMafBKO mice did not show elevated Foxm1 or Ccna2 levels at baseline, and induction of both genes during pregnancy was blunted compared with pregnant RIP-CreM controls (Fig. 5E and Supplementary Fig. 5E). Moreover, despite elevated Tph1 expression, βMafBKO islets showed clear reduction of serotonin compared with control mice at GD16.5 (Supplementary Fig. 5F). Thus, maternal islet serotonin production requires MafB expression in gestation. Together with our finding that MafB induction fails in βPRLRKO mothers, these data indicate that MafB is regulated by PRLR signaling and required for β-cell adaptations during pregnancy.
Discussion
Genetic and cell biology studies have suggested that PRLR signaling is a crucial regulator of β-cell expansion (12,14,29,33), but prior loss-of-function studies were performed in global PRLR knockouts rather than examining PRLR loss specifically in β-cells. Our studies reveal a clear requirement for PRLR in β-cell function in vivo. A prior study of conventional PRLR knockout mice identified glucose intolerance, reduced body weight, and reduced β-cell mass in adult mice of both sexes (12), but it was unclear if these reflected islet or nonislet effects. By contrast, we observed normal glucose homeostasis and β-cell mass in adult prepartum βPRLRKO mice and no alteration in adult body weight. Our results suggest that defective PRLR signaling in nonislet tissues likely contributes to body weight and glucose homeostasis abnormalities previously observed in nonpregnant mice with global PRLR knockout. Some of these differences may also be related to genetic background differences between 129Sv and B6 mouse strains, known to affect PRLR-dependent phenotypes (34). Additional studies have indicated a role for PRLR signaling in fetal and early postnatal islet development (35). RIP-Cre expression commences in the fetal pancreas and has been used by us and others to inactivate β-cell genes in the fetal and neonatal period (20). Thus, βPRLRKO mice might be predicted to display defective developmental expansion of β-cells; however, virgin adult βPRLRKO mice displayed no alteration in β-cell mass. Further studies are required to assess the role of PRLR in embryonic or perinatal β-cell development.
PRLR signaling is thought to involve transduction through multiple effectors, including signal transducer and activator of transcription 5 (Stat5). However, mice carrying a β-cell–specific deletion of Stat5 did not develop GDM (36). Thus, although Stat5 is considered a principal mediator of PRLR signaling, our results suggest that other signal transduction pathways such as mitogen-activated protein kinase/extracellular signal-regulated kinase or phosphatidylinositol 3-kinase/Akt (4) are critical for gestational β-cell adaptation. Supporting this model, recent studies have demonstrated that prolactin induction of Tph1 is coordinated by activation of all three pathways (37). β-Cell–specific deletion of Tph1 has been reported, but gestational studies were not performed (38). Mice with pancreas-specific deletion of Foxm1 display profound GDM, but also exhibit prepregnancy defects in β-cell mass that likely reflect Foxm1 roles in physiological β-cell proliferation (10,39). Collectively, these prior studies support the view that targets of PRLR signaling regulate gestational β-cell expansion.
The significance of MafB+ β-cells during pregnancy was previously unknown. Our studies demonstrate intact PRLR signaling is required for the induction of MafB in a subset of β-cells during pregnancy. In this study, we report that MafB expression is necessary for proliferation of β-cells in pregnancy. Moreover, MafB was required for pregnancy-induced islet serotonin production and Foxm1 upregulation. Prior studies identified roles for MafB in islet development and postnatal expansion, in which it regulates terminal differentiation and maturation (17,31); a role for MafB in proliferation has also been established in pathological settings (40). Our studies reveal a role in physiologic proliferation of gestation, in which MafB+ β-cells maintain their hallmark features, including production of insulin and Pdx1 and suppression of glucagon. This contrasts with re-expression of MafB in α-like β-cells observed after Pdx1 or Foxo1 loss, in which β-cell dedifferentiation contributes to diabetes pathogenesis (41). Thus, in normal pregnancy, transient expression of MafB by Ins+ cells does not appear to reflect dedifferentiation. Future studies will be necessary to identify the specific mechanisms by which MafB, Foxm1, and Tph1 coordinate and control proliferation and mass expansion during gestation.
We and others have previously described additional putative PRLR signaling targets, including Men1, p18, p27, and Foxd3 (14,25,26,42). For example, we found prolactin was sufficient to induce Men1 gene expression changes in islets and that Men1 induction was sufficient to impair maternal β-cell expansion (25). However, in βPRLRKO pregnant mice, we did not detect altered expression of Men1, p18, or p27, suggesting PRLR signaling is not necessary for regulating these factors. These findings may reflect differences intrinsic to gain- and loss-of-function models, in vitro and in vivo experimentation, mouse strain differences, or yet-unidentified modifying factors that contribute to the regulation of these genes during pregnancy. Pregnancy is a complex, transient, polyhormonal state, and our findings highlight the importance of in vivo loss-of-function models for genetic analysis of putative target genes.
Brouwers et al. (32) report of human growth hormone minigene effects on islets in mice harboring transgenes like RIP-CreM highlights the importance of using RIP-CreM control littermates in physiologic studies. The βPRLRKO mice lack the very receptor believed to mediate the augmentation of β-cell mass by human growth hormone (32). Thus, the βPRLRKO model should be resistant to possible confounding changes caused by the RIP-Cre transgene.
Prior studies suggest that human β-cells expand in pregnancy, but the molecular and cellular basis of this is not clear (43,44). For example, Butler et al. (43) have suggested that this expansion may reflect β-cell neogenesis rather than proliferation, a possibility consistent with the recent finding of maternal β-cell neogenesis in rodents (10,45). A recent study identified single nucleotide polymorphisms in the 5′ untranslated region of PRLR associated with GDM risk in humans (46). Intriguingly, induction of TPH1 and islet serotonin also occurs in human pregnancy (6), but it is not known whether this reflects increased β-cell PRLR signaling. However, PRLR expression is much lower in human than rodent β-cells (47), and MafB expression, although restricted to α-cells in adult rodents, is also present in β-cells from a nonpregnant human (48). Other correlative studies have implicated a more general role for PRLR signaling in human glucose control and metabolism. Higher prolactin levels are associated with a reduced risk of glucose intolerance and diabetes in both men and women (49). A human PRLR disease-associated mutation causing familial hyperprolactinemia (50) was recently described, although information on diabetes or glucose homeostasis in this family was not reported. Thus, further studies are needed to assess the role of PRLR signaling in human β-cell biology in both gestational and nongestational settings.
In summary, we produced and characterized mice lacking PRLR in β-cells during gestation and found these mice developed hallmark features of GDM. These studies establish the in vivo requirement of PRLR in β-cells for serotonin production and for modulating expression of genes critical for β-cell proliferation during pregnancy and identify a novel role for MafB in gestational proliferation. PRLR signaling is thought to modulate the development and function of many tissues (5), including bone, immunity, adipose metabolism, and breast and prostate cancer pathogenesis (15). Thus, the conditional Prlrf allele developed in this study will be useful to examine PRLR signaling in other settings relevant to human health.
Article Information
Acknowledgments. The authors thank members of the Kim laboratory and Drs. Hail Kim (Korea Advanced Institute of Science and Technology), Justin Annes (Stanford University School of Medicine), and Mitchell Lazar (Perelman School of Medicine, University of Pennsylvania) for helpful discussions and advice. Kartik Viswanathan helped with initial stages of the project, and Dr. Pei Wang (School of Medicine, UT Health Sciences Center, San Antonio, TX) provided help with design and construction of the Prlrf allele.
Funding. R.R.B. is supported by a National Institute of Diabetes and Digestive and Kidney Diseases Mentored Clinical Scientist Research Career Development Award (K-08 DK091359). H.A.C. is supported by National Institutes of Health (NIH) National Research Service Award 1F-32 DK-102283. H.C. is supported by a JDRF postdoctoral award. H.P. is supported by American Diabetes Association grant 1-16-PDF-086. Work in the laboratory of R.W.S. is supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grants DK-090570, DK-089572, and DK-050203. The laboratory of S.K.K. is supported by the Snyder Foundation and grants from the NIH, JDRF, and Helmsley Trust. S.K.K. is a Howard Hughes Medical Institute Investigator.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.R.B., R.W.S., and S.K.K. conceived and designed the experiments and wrote the manuscript. R.R.B., H.A.C., E.M.W., H.C., H.P., X.G., Y.L., and E.C. performed experiments and analyzed data. L.G. provided MafB floxed mice. All authors reviewed and edited the manuscript. S.K.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1527/-/DC1.
References
- 1.Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res 1997;29:301–307 [DOI] [PubMed] [Google Scholar]
- 2.Halban PA, Polonsky KS, Bowden DW, et al. β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. J Clin Endocrinol Metab 2014;99:1983–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ernst S, Demirci C, Valle S, et al. Mechanisms in the adaptation of maternal beta-cells during pregnancy. Diabetes Manag (Lond) 2011;1:239–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goffin V, Binart N, Touraine P, Kelly PA. Prolactin: the new biology of an old hormone. Annu Rev Physiol 2002;64:47–67 [DOI] [PubMed] [Google Scholar]
- 5.Freemark M, Driscoll P, Maaskant R, Petryk A, Kelly PA. Ontogenesis of prolactin receptors in the human fetus in early gestation. Implications for tissue differentiation and development. J Clin Invest 1997;99:1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim H, Toyofuku Y, Lynn FC, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med 2010;16:804–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Layden BT, Durai V, Newman MV, et al. Regulation of pancreatic islet gene expression in mouse islets by pregnancy. J Endocrinol 2010;207:265–279 [DOI] [PubMed] [Google Scholar]
- 8.Rieck S, White P, Schug J, et al. The transcriptional response of the islet to pregnancy in mice. Mol Endocrinol 2009;23:1702–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pechhold S, Stouffer M, Walker G, et al. Transcriptional analysis of intracytoplasmically stained, FACS-purified cells by high-throughput, quantitative nuclease protection. Nat Biotechnol 2009;27:1038–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Zhang J, Pope CF, et al. Gestational diabetes mellitus resulting from impaired beta-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen. Diabetes 2010;59:143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasek RC, Gannon M. Advancements and challenges in generating accurate animal models of gestational diabetes mellitus. Am J Physiol Endocrinol Metab 2013;305:E1327–E1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freemark M, Avril I, Fleenor D, et al. Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology 2002;143:1378–1385 [DOI] [PubMed] [Google Scholar]
- 13.Ormandy CJ, Camus A, Barra J, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev 1997;11:167–178 [DOI] [PubMed] [Google Scholar]
- 14.Huang C, Snider F, Cross JC. Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology 2009;150:1618–1626 [DOI] [PubMed] [Google Scholar]
- 15.Ben-Jonathan N, Hugo ER, Brandebourg TD, LaPensee CR. Focus on prolactin as a metabolic hormone. Trends Endocrinol Metab 2006;17:110–116 [DOI] [PubMed] [Google Scholar]
- 16.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 2000;127:2317–2322 [DOI] [PubMed] [Google Scholar]
- 17.Yu WM, Appler JM, Kim YH, Nishitani AM, Holt JR, Goodrich LV. A Gata3-Mafb transcriptional network directs post-synaptic differentiation in synapses specialized for hearing. eLife 2013;2:e01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Postic C, Shiota M, Niswender KD, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem 1999;274:305–315 [DOI] [PubMed] [Google Scholar]
- 19.Banerjee RR, Rangwala SM, Shapiro JS, et al. Regulation of fasted blood glucose by resistin. Science 2004;303:1195–1198 [DOI] [PubMed] [Google Scholar]
- 20.Goodyer WR, Gu X, Liu Y, Bottino R, Crabtree GR, Kim SK. Neonatal β cell development in mice and humans is regulated by calcineurin/NFAT. Dev Cell 2012;23:21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JY, Ristow M, Lin X, White MF, Magnuson MA, Hennighausen L. RIP-Cre revisited, evidence for impairments of pancreatic beta-cell function. J Biol Chem 2006;281:2649–2653 [DOI] [PubMed] [Google Scholar]
- 22.Solomon CG, Willett WC, Carey VJ, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 1997;278:1078–1083 [PubMed] [Google Scholar]
- 23.Schraenen A, Lemaire K, de Faudeur G, et al. Placental lactogens induce serotonin biosynthesis in a subset of mouse beta cells during pregnancy. Diabetologia 2010;53:2589–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plank JL, Frist AY, LeGrone AW, Magnuson MA, Labosky PA. Loss of Foxd3 results in decreased β-cell proliferation and glucose intolerance during pregnancy. Endocrinology 2011;152:4589–4600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karnik SK, Chen H, McLean GW, et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science 2007;318:806–809 [DOI] [PubMed] [Google Scholar]
- 26.Arumugam R, Fleenor D, Lu D, Freemark M. Differential and complementary effects of glucose and prolactin on islet DNA synthesis and gene expression. Endocrinology 2011;152:856–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C. Wild-type offspring of heterozygous prolactin receptor-null female mice have maladaptive β-cell responses during pregnancy. J Physiol 2013;591:1325–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rieck S, Kaestner KH. Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol Metab 2010;21:151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brelje TC, Allaire P, Hegre O, Sorenson RL. Effect of prolactin versus growth hormone on islet function and the importance of using homologous mammosomatotropic hormones. Endocrinology 1989;125:2392–2399 [DOI] [PubMed] [Google Scholar]
- 30.Artner I, Le Lay J, Hang Y, et al. MafB: an activator of the glucagon gene expressed in developing islet alpha- and beta-cells. Diabetes 2006;55:297–304 [DOI] [PubMed] [Google Scholar]
- 31.Artner I, Blanchi B, Raum JC, et al. MafB is required for islet beta cell maturation. Proc Natl Acad Sci U S A 2007;104:3853–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brouwers B, de Faudeur G, Osipovich AB, et al. Impaired islet function in commonly used transgenic mouse lines due to human growth hormone minigene expression. Cell Metab 2014;20:979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vasavada RC, Garcia-Ocaña A, Zawalich WS, et al. Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem 2000;275:15399–15406 [DOI] [PubMed] [Google Scholar]
- 34.Binart N, Melaine N, Pineau C, et al. Male reproductive function is not affected in prolactin receptor-deficient mice. Endocrinology 2003;144:3779–3782 [DOI] [PubMed] [Google Scholar]
- 35.Auffret J, Freemark M, Carré N, et al. Defective prolactin signaling impairs pancreatic β-cell development during the perinatal period. Am J Physiol Endocrinol Metab 2013;305:E1309–E1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JY, Gavrilova O, Davani B, et al. The transcription factors Stat5a/b are not required for islet development but modulate pancreatic beta-cell physiology upon aging. Biochim Biophys Acta 2007;1773:1455–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iida H, Ogihara T, Min MK, et al. Expression mechanism of tryptophan hydroxylase 1 in mouse islets during pregnancy. J Mol Endocrinol 2015;55:41–53 [DOI] [PubMed] [Google Scholar]
- 38.Kim K, Oh CM, Ohara-Imaizumi M, et al. Functional role of serotonin in insulin secretion in a diet-induced insulin-resistant state. Endocrinology 2015;156:444–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Ackermann AM, Gusarova GA, et al. The FoxM1 transcription factor is required to maintain pancreatic beta-cell mass. Mol Endocrinol 2006;20:1853–1866 [DOI] [PubMed] [Google Scholar]
- 40.Lu J, Hamze Z, Bonnavion R, et al. Reexpression of oncoprotein MafB in proliferative β-cells and Men1 insulinomas in mouse. Oncogene 2012;31:3647–3654 [DOI] [PubMed] [Google Scholar]
- 41.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 2012;150:1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes E, Huang C. Participation of Akt, menin, and p21 in pregnancy-induced beta-cell proliferation. Endocrinology 2011;152:847–855 [DOI] [PubMed] [Google Scholar]
- 43.Butler AE, Cao-Minh L, Galasso R, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia 2010;53:2167–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Assche FA, Aerts L, De Prins F. A morphological study of the endocrine pancreas in human pregnancy. Br J Obstet Gynaecol 1978;85:818–820 [DOI] [PubMed] [Google Scholar]
- 45.Hakonen E, Ustinov J, Palgi J, Miettinen PJ, Otonkoski T. EGFR signaling promotes β-cell proliferation and survivin expression during pregnancy. PLoS One 2014;9:e93651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le TN, Elsea SH, Romero R, Chaiworapongsa T, Francis GL. Prolactin receptor gene polymorphisms are associated with gestational diabetes. Genet Test Mol Biomarkers 2013;17:567–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H, Kleinberger JW, Takane KK, et al. Augmented Stat5 signaling bypasses multiple impediments to lactogen-mediated proliferation in human β-cells. Diabetes 2015;64:3784–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai C, Brissova M, Hang Y, et al. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia 2012;55:707–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang T, Lu J, Xu Y, et al. Circulating prolactin associates with diabetes and impaired glucose regulation: a population-based study. Diabetes Care 2013;36:1974–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newey PJ, Gorvin CM, Cleland SJ, et al. Mutant prolactin receptor and familial hyperprolactinemia. N Engl J Med 2013;369:2012–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]