Abstract
In humans, the glucagon response to moderate-to-marked insulin-induced hypoglycemia (IIH) is largely mediated by the autonomic nervous system. Because this glucagon response is impaired early in type 1 diabetes, we sought to determine if these patients, like animal models of autoimmune diabetes, have an early and severe loss of islet sympathetic nerves. We also tested whether this nerve loss is a permanent feature of type 1 diabetes, is islet-selective, and is not seen in type 2 diabetes. To do so, we quantified pancreatic islet and exocrine sympathetic nerve fiber area from autopsy samples of patients with type 1 or 2 diabetes and control subjects without diabetes. Our central finding is that patients with either very recent onset (<2 weeks) or long duration (>10 years) of type 1 diabetes have a severe loss of islet sympathetic nerves (Δ = −88% and Δ = −79%, respectively). In contrast, patients with type 2 diabetes lose no islet sympathetic nerves. There is no loss of exocrine sympathetic nerves in either type 1 or type 2 diabetes. We conclude that patients with type 1, but not type 2, diabetes have an early, marked, sustained, and islet-selective loss of sympathetic nerves, one that may impair their glucagon response to IIH.
Introduction
Patients with type 1 diabetes have a marked impairment of their glucagon response to insulin-induced hypoglycemia (IIH) (1,2) even within the 1st year after diagnosis (3). This impaired glucagon response can increase their risk for severe and prolonged hypoglycemia (4), which is aversive and thereby decreases patient compliance (5) with the intensive glucose-lowering therapy that is designed to both delay the onset and slow the progression of the long-term complications of this disease (6–9). In humans without diabetes, there is a major contribution of the autonomic nervous system to the glucagon response to IIH (10), which is consistent with both the activation of the autonomic nervous system during hypoglycemia (11–14) and the ability of each autonomic branch to stimulate glucagon secretion (15–18). Therefore, a defect in the autonomic-islet pathway could contribute to the known impairment of their glucagon response to IIH (19,20). However, early in human type 1 diabetes, the IIH-induced activation of two of the three autonomic inputs to the islet, the parasympathetic nerves (21,22) and adrenal medullary epinephrine (3,23), appears normal. Therefore, if a defect in the autonomic-islet pathway exists, it is likely in the third autonomic input to the islet, its sympathetic nerves.
Indeed, a major defect in the islet sympathetic pathway, a marked loss of sympathetic nerves within the islet, has been demonstrated in three separate animal models of autoimmune diabetes: the biobreeder (BB) rat (24), the nonobese diabetic (NOD) mouse (25), and the rat insulin promoter-glycoprotein (RIP-GP) mouse (26). This novel neuropathy is different in several respects from classical diabetic autonomic neuropathy (27,28), including those that cause the loss of cardiac sympathetic nerves (29,30). First, this islet nerve loss is fully established in the first few weeks after (24,25), or even immediately before (26), the development of overt diabetes. In contrast, diabetic autonomic neuropathy takes months/years to develop (27,28). Second, the loss of sympathetic nerves is restricted to the pancreatic islet; the parent axons in the adjacent exocrine pancreas remain intact (24,26). In contrast, diabetic autonomic neuropathy causes nerve dysfunction in a variety of tissues (29,31,32). Third, in contrast to the association of classical diabetic autonomic neuropathy with chronic hyperglycemia (6,29,33) and damage mediated by reactive oxygen species (34), the loss of islet sympathetic nerves is associated with the immune attack of the islet (25,26) and activation of a neurotrophin receptor that causes rapid degeneration of sympathetic axons (26,35).
Because type 1 diabetes is widely accepted as due to a lymphocyte-mediated attack on the islet (36), we hypothesized that, like the animal models of autoimmune diabetes, patients with type 1 diabetes would have an early, marked, and islet-selective loss of sympathetic nerves. Conversely, because the islets of patients with traditional type 2 diabetes do not undergo severe autoimmune attack (37), we also hypothesized that subjects with type 2 diabetes would not exhibit this islet nerve loss. To test these hypotheses, we obtained autopsy specimens of pancreas from patients with diabetes and their age-matched control subjects without diabetes and used the combination of antigen retrieval and immunohistochemistry to detect and quantify islet and exocrine pancreatic sympathetic nerves. Specifically, to determine if there was marked loss of islet sympathetic nerves that was unlikely to be due to the chronic hyperglycemia of long-duration (ld) diabetes, we compared islet sympathetic nerve area in patients with very recent onset (<2 weeks) of type 1 diabetes to that of young control subjects without diabetes. Second, to determine if such nerve loss was selective for the islet, we compared the sympathetic nerve area in the exocrine pancreas of the same two groups. Third, to determine if this loss of islet sympathetic nerves is associated only with the autoimmune form of diabetes, we compared the islet sympathetic nerve area of patients with type 2 diabetes to an older, age-matched control group without diabetes. Last, to determine if the early loss of islet sympathetic nerves seen in patients with type 1 diabetes was sustained over time, we compared the islet sympathetic nerve area in patients with ld-type 1 diabetes (>10 years) to the same older, age-matched control subjects without diabetes.
Research Design and Methods
Subjects and Pancreatic Tissue
Autopsies of subjects with ld-type 1 diabetes and type 1 diabetes and both young and older subjects without diabetes were performed at the University of Washington and at Seattle Children’s, Seattle, WA. Autopsies of subjects with very short duration (sd) type 1 diabetes were performed throughout the U.K. and were collected by one of the authors (A.K.F.). Individuals with a history of pancreatic cancer, pancreatitis, end-stage liver disease, hepatitis, organ transplantation, or chronic glucocorticoid treatment were excluded. The study was approved by institutional review boards at the University of Washington and the VA Puget Sound Health Care System.
Prior consent to use these pancreata for research purposes was obtained from either the individuals themselves or family members. Pancreatic tissue was usually sampled from the body of the pancreas, fixed in formalin for variable periods of time, and then paraffin embedded. All samples were received as 4-μm sections of pancreas mounted on microscope slides, but only those that showed no or minimal autolysis, as judged by clear and distinct morphology, were analyzed further.
Immunohistochemistry
Antigen retrieval was performed by first immersing all slide-mounted sections in 10 mmol/L citrate buffer and then microwaving them at high power (1,750 W) for 35 min. This procedure dramatically improved the visualization of the tyrosine hydroxylase (TH) contained in the fine sympathetic nerve fibers and varicosities of the pancreas.
Selected sections processed for antigen retrieval were subsequently immunostained with a mouse monoclonal glucagon antibody at a dilution of 1:1,000 (G2654 clone K79; Sigma-Aldrich) to identify islets and, on an adjacent section, a mouse monoclonal antibody against TH at a dilution of 1:100 (catalog no. MAB318; Millipore, Billerica, MA) to identify sympathetic nerve fibers and varicosities. The second antibodies against glucagon and TH were an Alexa Fluor 488–conjugated goat anti-mouse antibody (1:200, A-11029; Molecular Probes, Invitrogen, Carlsbad, CA) and an Alexa Fluor 555–conjugated goat anti-mouse antibody used at a dilution of 1:200 (catalog no. A21424; Life Technologies, Grand Island, NY), respectively.
Since the goal of this study was to determine if patients with type 1 diabetes had a major loss of islet sympathetic nerves similar to that seen in animal models of autoimmune diabetes, it was critical to rule out false negatives that would bias the data in favor of this hypothesis. Therefore, sections were excluded from analysis if the large arterioles in the exocrine pancreas of that section failed to show the well-known sympathetic innervation of vascular smooth muscle, as identified by TH fiber staining in thick-walled vessels.
Neural Quantification
Islets in sections of pancreas from patients without and with diabetes were confirmed by glucagon staining (see Fig. 1A and B and Fig. 3A–C). Ten islets per subject were chosen for measurement of islet area and islet sympathetic nerve area. For this purpose, the perimeter of the islets was traced and total islet area calculated by a computer-assisted image analysis system (Nikon Elements, Melville, NY). Sympathetic nerve fibers within the islet were identified by TH immunoreactivity localized to fine varicosities and fibers. The nerve fiber area was quantified by counting all areas of fluorescence above a set threshold and below a size of 8 µm2, criteria that selects for nerve varicosities and small axon segments. Islet TH nerve fiber area was reported as an absolute value (µm2 TH/islet), as we have done previously in mouse models of autoimmune diabetes (25,26). Islet TH nerve fiber density was reported as nerve fiber area relative to islet size (percentage of islet area occupied by neural TH).
Figure 1.
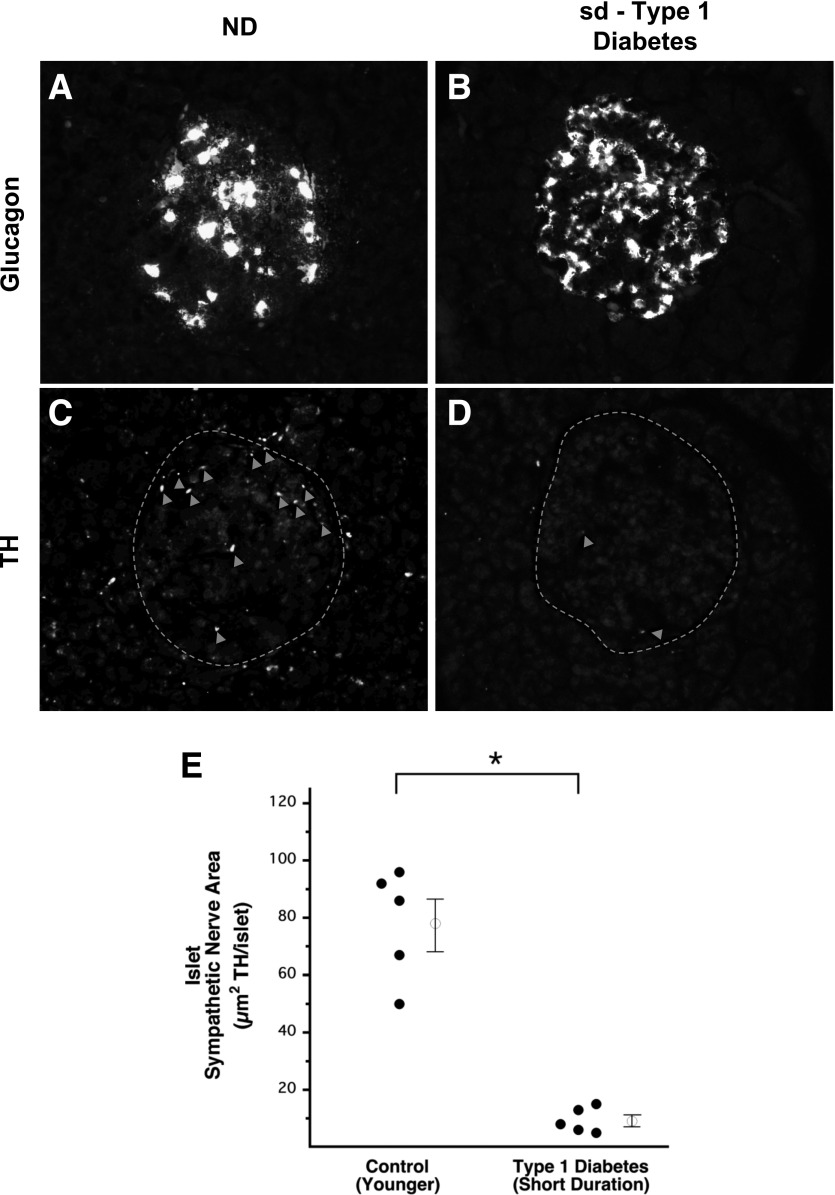
Marked loss of islet sympathetic nerves in sd-type 1 diabetes. Representative images of glucagon staining defining the boundary of an islet from a control subject without diabetes (A) and a patient with sd-type 1 diabetes (B). Representative images of TH staining defining sympathetic nerve varicosities (arrowheads) within those islets (dashed lines) (C and D). Sympathetic nerve fiber area within the islets of patients with sd-type 1 diabetes and of age-matched control subjects without diabetes (E). Open circles with bars represent group mean ± SEM. ND, control subjects without diabetes. *Significant difference between groups (P < 0.001).
Figure 3.
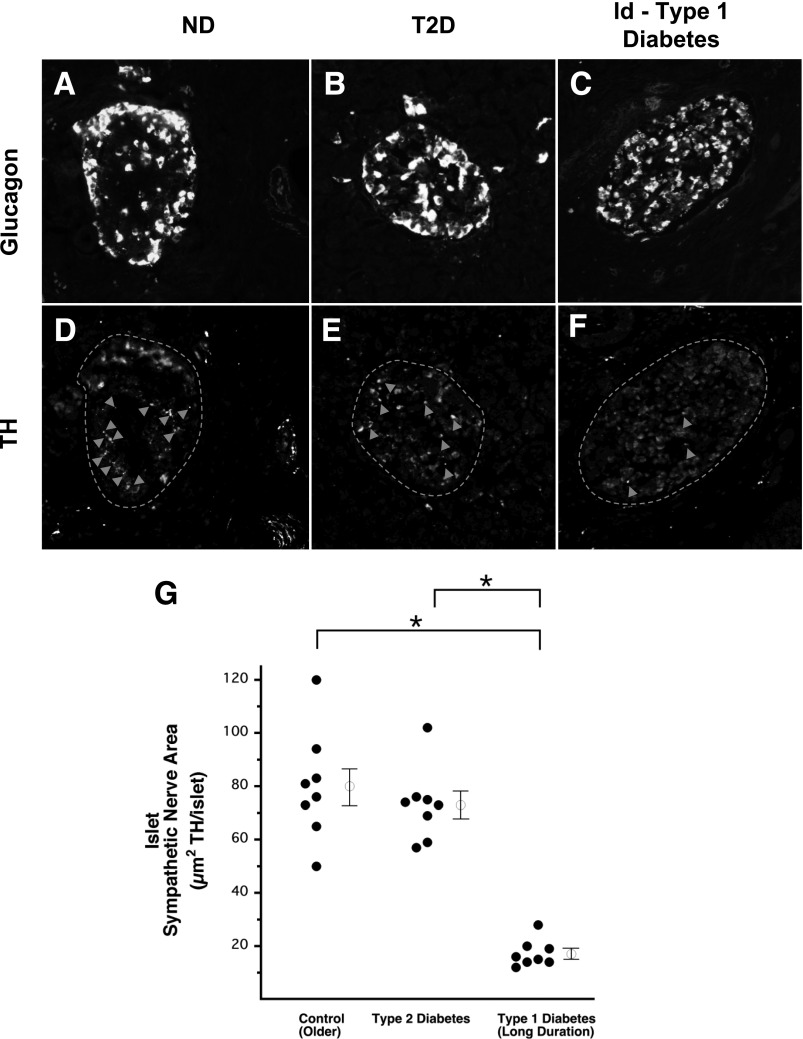
No loss of islet sympathetic nerves in type 2 diabetes, but marked loss in ld-type 1 diabetes. Representative images of glucagon staining defining the boundary of an islet from a control subject without diabetes (A), a patient with type 2 diabetes (T2D) (B), and a patient with ld-type 1 diabetes (C). Representative images of TH staining defining sympathetic nerve varicosities (arrowheads) within those islets (dashed lines) (D–F). Sympathetic nerve area within the islets of patients with T2D and ld-type 1 diabetes and of age-matched control subjects without diabetes (G). Open circles with bars represent group mean ± SEM. ND, control subjects without diabetes. *Significant difference between groups (P < 0.001).
TH fiber area in the exocrine pancreas was calculated after selecting large areas (∼135,000 µm2 each) devoid of, yet near, islets and is reported as both µm2 TH/mm2 exocrine pancreas and percentage of exocrine area occupied by neural TH. Although arterial structures were excluded from these exocrine fields, due to their high sympathetic nerve density, inclusions of veins, ducts, and thin interlobular spaces were unavoidable. These lightly innervated, nonacinar exocrine structures were equally distributed throughout the five groups and had minimal effect on the quantification of exocrine sympathetic nerve area.
Statistical Analysis
To compare continuous variables across the five groups, ANOVA was used followed by a post hoc Scheffe test. All data are expressed as mean ± SEM.
Results
Loss of Islet, but Not Exocrine, Sympathetic Nerves in Patients With Very Short Duration Type 1 Diabetes
The characteristics of the patients with sd-type 1 diabetes and their young, age-matched control subjects without diabetes are listed in Table 1. These two young groups were not significantly different in either age or sex.
Table 1.
Phenotypic characteristics of the study cohort
| Sd-type 1 diabetes | Younger control subjects | Ld-type 2 diabetes | Older control subjects | Ld-type 1 diabetes | |
|---|---|---|---|---|---|
| Sample size (n) | 5 | 5 | 8 | 8 | 8 |
| Age (years) | 13 ± 2* | 18 ± 2 | 65 ± 4 | 59 ± 3 | 56 ± 5 |
| Sex (% female) | 80 | 60 | 50 | 100 | 50 |
| BMI (kg/m2) | 30.5 ± 1.3 | 30.9 ± 4.3 | 32.7 ± 4.4 | ||
| Diabetes duration (years) | 0.02 ± 0.01* | NA | 11 ± 4 | NA | 14 ± 4 |
| (<2 weeks) | |||||
| Cause of death, n (%) | |||||
| Cardiovascular disease/stroke | 3 (37.5) | 1 (12.5) | 3 (37.5) | ||
| Cancer | 0 (0) | 3 (37.5) | 0 (0) | ||
| Pulmonary | 2 (25) | 2 (25) | 1 (12.5) | ||
| Surgical | 0 (0) | 1 (12.5) | 1 (12.5) | ||
| Infection | 1 (12.5) | 0 (0) | 2 (25) | ||
| Gastrointestinal | 2 (25) | 1 (12.5) | 0 (0) | ||
| Unknown | 5 (100) | 5 (100) | 0 (0) | 0 (0) | 1 (12.5) |
| Diabetes medication, n (%) | |||||
| None | 1 (12.5) | NA | 0 (0) | ||
| Oral | 3 (37.5) | NA | 0 (0) | ||
| Insulin | 3 (37.5) | NA | 6 (75) |
Data presented as mean ± SEM. NA, not applicable.
*Significantly different (P < 0.05) from ld-type 1 diabetes.
TH-positive nerve area within islets from patients with sd-type 1 diabetes (9 ± 2 µm2 TH/islet) (Fig. 1D and E) was reduced by 88% (P < 0.001) compared with that of their control subjects without diabetes (78 ± 9 µm2 TH/islet) (Fig. 1C and E). The cross-sectional area of islets from patients with sd-type 1 diabetes (16,055 ± 2,318 µm2) was decreased by 44% (P < 0.05) compared with that of their control subjects without diabetes (28,779 ± 5,142 µm2). Islet TH nerve density of patients with sd-type 1 diabetes (0.06 ± 0.01% of islet area occupied by neural TH) was decreased by 80% (P < 0.001) compared with that of their control group without diabetes (0.30 ± 0.05%).
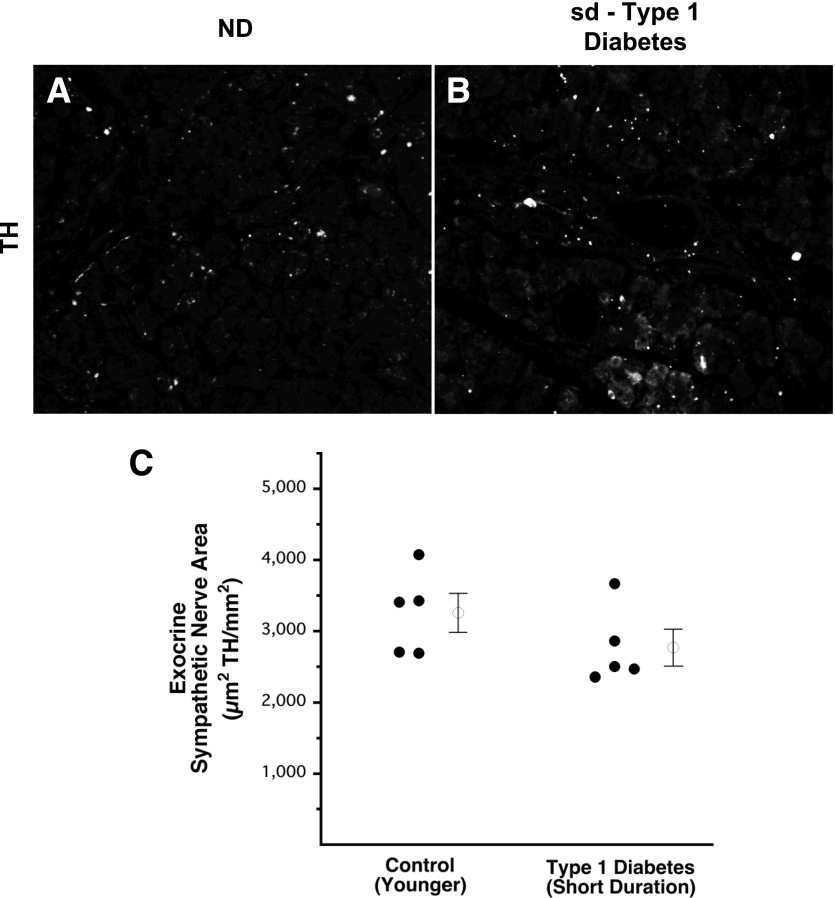
In contrast to the marked decrease of islet TH nerve area, TH-positive nerve area in the exocrine pancreas of patients with sd-type 1 diabetes (2,772 ± 240 µm2 TH/mm2 = 0.28 ± 0.02% exocrine area occupied by TH) (Fig. 2B and C) did not differ significantly from that of their control subjects without diabetes (3,261 ± 260 µm2 TH/mm2 = 0.33 ± 0.03%) (Fig. 2A and C). A comparison of exocrine to islet nerve density for the subjects with sd-type 1 diabetes and their age-matched control subjects is provided by Supplementary Table 1.
Figure 2.
No loss of exocrine sympathetic nerves in sd-type 1 diabetes. Representative images of TH staining in the exocrine pancreas of a control subject without diabetes (A) and a patient with sd-type 1 diabetes (B). Sympathetic nerve area in the exocrine pancreas, including veins, ducts, and thin interlobular spaces, of patients with sd-type 1 diabetes and of age-matched control subjects without diabetes (C). Open circles with bars represent group mean ± SEM. ND, control subjects without diabetes.
No Loss of Either Islet or Exocrine Sympathetic Nerves in Patients with Type 2 Diabetes
The characteristics of patients with type 2 diabetes and their older age-matched control subjects without diabetes are listed in Table 1. The two groups were well matched for age and BMI, but not sex.
In contrast to the marked decrease of islet TH nerve area seen in patients with sd-type 1 diabetes, no islet nerve loss was observed in patients with type 2 diabetes. In patients with type 2 diabetes, islet TH nerve area (73 ± 5 µm2 TH/islet) (Fig. 3E and G) was not different from that of their age-matched control subjects without diabetes (80 ± 7 µm2 TH/islet) (Fig. 3D and G). Similarly, neither the islet size (21,921 ± 1,177 µm2) nor the islet nerve density (0.35 ± 0.03% of islet area occupied by neural TH) of patients with type 2 diabetes differed from that of their control subjects without diabetes (24,751 ± 1,411 µm2 and 0.32 ± 0.02% of islet area occupied by neural TH, respectively).
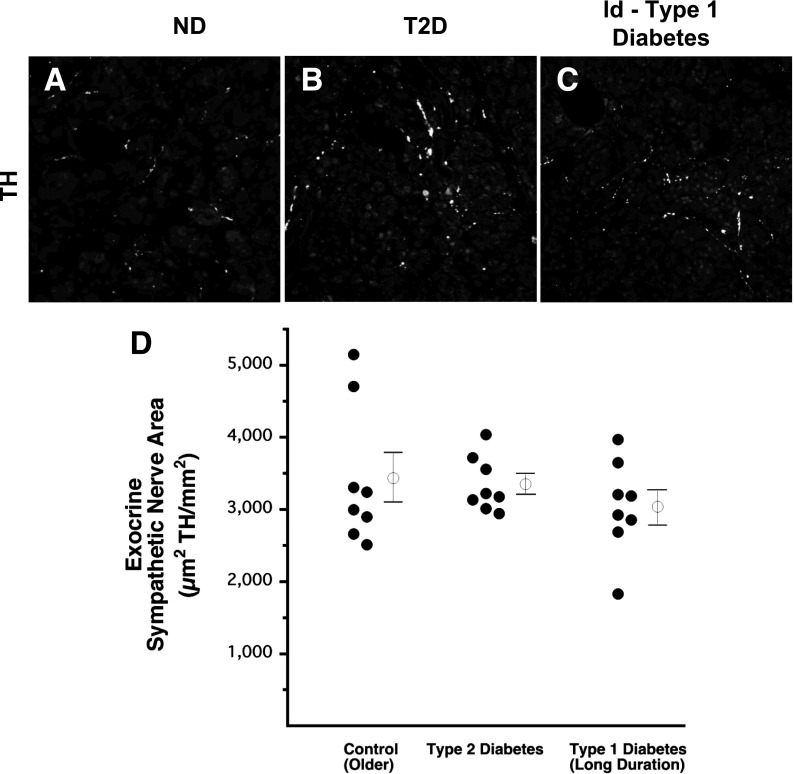
Likewise, exocrine TH nerve area in subjects with type 2 diabetes (3,350 ± 136 µm2 TH/mm2 = 0.34 ± 0.01% of exocrine area occupied by TH) (Fig. 4B and D) was not different from that of their control subjects without diabetes (3,433 ± 342 µm2 TH/mm2 = 0.34 ± 0.03% of exocrine area occupied by TH) (Fig. 4A and D). A comparison of exocrine to islet nerve density for subjects with type 2 diabetes and their age-matched control subjects is provided by Supplementary Table 1.
Figure 4.
No loss of exocrine sympathetic nerves in either type 2 diabetes or ld-type 1 diabetes. Representative images of TH staining in the exocrine pancreas of a control subject without diabetes (A), a patient with type 2 diabetes (T2D) (B), and a patient with ld-type 1 diabetes (C). Sympathetic nerve area in the exocrine pancreas, including veins, ducts, and thin interlobular spaces, of patients with T2D and ld-type 1 diabetes and of age-matched control subjects without diabetes (D). Open circles with bars represent group mean ± SEM. ND, control subjects without diabetes.
Loss of Islet, but Not Exocrine, Sympathetic Nerves in Patients With Longer-Duration Type 1 Diabetes
The characteristics of patients with ld-type 1 diabetes (>10 years) and the same older control subjects without diabetes are listed in Table 1. The two groups of subjects were well matched for age and BMI, but not for sex.
Similar to the findings in sd-type 1 diabetes, the TH-positive nerve fiber area within islets from subjects with ld-type 1 diabetes (17 ± 2 µm2 TH/islet) (Fig. 3F and G) was decreased by 79% (P < 0.001) compared with that of their age-matched control subjects without diabetes (80 ± 7 µm2 TH/islet) (Fig. 3D and G). Cross-sectional islet area in the subjects with ld-type 1 diabetes (15,517 ± 1,363 µm2) was reduced by 37% (P < 0.05) compared with that of their control subjects without diabetes (24,751 ± 1,411 µm2). Islet TH nerve density of subjects with ld-type 1 diabetes (0.11 ± 0.01% of islet area occupied by neural TH) was 66% lower (P < 0.001) than that of their control subjects without diabetes (0.32 ± 0.02% of islet area occupied by TH).
In contrast to the marked loss of islet TH in ld-type 1 diabetes, TH-positive nerve area in the exocrine pancreas of subjects with ld-type 1 diabetes (3,038 ± 228 µm2 TH/mm2 = 0.30 ± 0.02% of exocrine area occupied by TH) (Fig. 4C and D) did not differ from that of their control subjects without diabetes (3,433 ± 342 µm2 TH/mm2 = 0.34 ± 0.03% of exocrine area occupied by TH) (Fig. 4A and D). A comparison of exocrine to islet nerve density for subjects with ld-type 1 diabetes and their age-matched control subjects is provided in Supplementary Table 1.
The duration of diabetes for the patients with ld-type 1 diabetes was significantly greater than that of the patients with sd-type 1 diabetes (P < 0.05). However, islet TH nerve area, islet cross-sectional area, islet TH nerve density, and exocrine TH nerve area were all similar between the groups with ld-type 1 diabetes and sd-type 1 diabetes, despite the difference in their age and diabetes duration. Similarly, these measures did not differ between the young and old control groups without diabetes, despite the difference in their ages.
Islet TH nerve area and islet nerve density of subjects with ld-type 1 diabetes were less than that of subjects with type 2 diabetes (both P < 0.001); the duration of diabetes was not significantly different between the groups with ld-type 1 diabetes and type 2 diabetes (see Table 1). In contrast, exocrine TH nerve areas were similar between these two groups with longstanding diabetes. A larger proportion of patients with ld-type 1 diabetes were treated with insulin, compared with the group with type 2 diabetes (see Table 1).
Discussion
We describe for the first time a marked loss of islet sympathetic nerves in human type 1 diabetes. The fact that this islet nerve loss occurs very early in type 1 diabetes and that it is not present in type 2 diabetes strongly suggests that it is not caused by the chronic diabetic hyperglycemia usually invoked as a cause of diabetic autonomic neuropathy (6,29,33). Rather we conclude that the loss of islet sympathetic nerves in human type 1 diabetes is triggered by the autoimmune attack of the islet. This conclusion is in keeping with those based on the study of animal models of both nonautoimmune and autoimmune diabetes. First, noninsulitis-mediated chemical destruction of islet β-cells does not result in a loss of islet sympathetic nerves (24,25). Second, in the NOD mouse, there is a positive correlation between the amount of immune cells in the islet and the loss of islet sympathetic nerves (25), and blocking islet infiltration prevents the loss of islet sympathetic nerves (25). Third, in the virally treated RIP-GP mouse, a marked loss of islet sympathetic nerves begins when the islet is first infiltrated, even before diabetic hyperglycemia develops (26). Thus, lymphocytic infiltration of the islet is a strong candidate for the specific autoimmune component responsible for triggering islet sympathetic nerve loss in human type 1 diabetes.
Although islet infiltration is prominent in the mouse models of autoimmune diabetes (25,26), it is very sparse in pancreata from patients with type 1 diabetes; a recent, comprehensive study reports that >95% of islets are insulitis free (38). Nonetheless, type 1 diabetes in humans is accepted as an autoimmune disease in which T lymphocytes invade the islet (36) over time, presumably in successive, acute phases characteristic of a relapsing-remitting disease (39). If so, insulitis at the time of tissue harvest provides only a snapshot of the most recent relapse phase. Therefore, directly demonstrating the relation between the loss of islet sympathetic nerves, which is permanent and therefore cumulative, and insulitis in humans may require an index of cumulative insulitis. The degree of residual β-cell area, used by others to “stage” the autoimmune attack of the islet (40), is one possible, although indirect, index of cumulative insulitis. In the absence of such data, we cannot yet directly implicate the lymphocytic attack of the islet as the trigger for the loss of islet sympathetic nerves in human type 1 diabetes, as we have in animal models of autoimmune diabetes (25,26). However, our current findings that patients with nonautoimmune type 2 diabetes do not lose their islet sympathetic nerves and that patients with type 1 diabetes do not lose their exocrine sympathetic nerves both support this hypothesis.
Because patients with type 1 diabetes with longer diabetes duration had an islet nerve loss similar to those with very short diabetes duration, there appears to be no major regrowth of islet sympathetic nerves over time in type 1 diabetes. This absence of islet reinnervation is, at first glance, surprising because their unmyelinated parent axons in the adjacent exocrine pancreas appear intact. Therefore, the reinnervation distance, a major barrier for reinnervation of human tissue (41), is very short. On the other hand, the lack of significant reinnervation of ld-type 1 diabetes islets is consistent with the known effect of diabetes to retard nerve regrowth after crush injury or axotomy (42).
Although the sympathetic nerves of the human islet do not directly contact islet endocrine cells (43,44), as they do in mice (44), activation of sympathetic nerves still influences islet hormone secretion in human subjects (45). Presumably sympathetic neurotransmitters are released into the intraislet arterioles that directly perfuse the endocrine cells of the islet (46). Thus, although there seems to be little direct nerve–α cell contact in human islets, the loss of sympathetic nerves from the islets of patients with type 1 diabetes would be expected to impair their glucagon response to sympathetic activation, as has been demonstrated directly (25,47) and indirectly (26) in rodent models of autoimmune diabetes. Because pancreatic sympathetic nerves are activated by IIH (48,49), and because activation of these nerves stimulates glucagon secretion (15,50–52), the loss of islet sympathetic nerves may also contribute to the known impairment of the glucagon response to IIH in human type 1 diabetes (1–3). In contrast, the retention of islet sympathetic nerves in type 2 diabetes may explain why there is no impairment of the glucagon response to IIH in patients with type 2 diabetes of <10 years duration (53).
With regard to the function of the exocrine pancreas, the finding of normal sympathetic innervation of the exocrine pancreas of subjects with ld-type 1 diabetes and type 2 diabetes could lead one to conclude, perhaps inappropriately, that classical diabetic autonomic neuropathy (27,28,54) is absent in these two groups with longstanding diabetes. However, the diagnosis of diabetic autonomic neuropathy is usually based on functional tests, such as decreased nerve conduction velocity (55,56). There may well be such functional impairment in longstanding diabetes even when gross nerve structure appears normal, as suggested by a recent review of diabetic autonomic neuropathy (54). Thus, although we cannot rule out possible impairments of exocrine sympathetic function in ld-type 1 diabetes and type 2 diabetes, the major loss of islet sympathetic nerves in both sd- and ld-type 1 diabetes almost certainly implies a serious impairment of islet function.
In summary, we report for the first time that humans with <2 weeks of type 1 diabetes have a marked loss of islet, but not pancreatic exocrine, sympathetic nerves. We also show for the first time that subjects with type 2 diabetes do not lose islet sympathetic nerves, thereby restricting this islet-selective nerve loss to the autoimmune form of diabetes. Importantly, because subjects with sd- and ld-type 1 diabetes both have a major loss of islet sympathetic nerves, this nerve loss appears to be both an early and a permanent feature of type 1 diabetes. As such, we suggest that this novel neural defect contributes to the impairment of the glucagon responses to IIH, seen in both patients with sd-type 1 diabetes and patients with ld-type 1 diabetes, thereby increasing their risk for episodes of severe hypoglycemia.
Article Information
Acknowledgments. The authors thank Dr. Steven Kahn (VA Puget Sound Health Care System and University of Washington) for critical review of both the project and the manuscript. The authors thank Daryl Hackney (VA Puget Sound Health Care System and University of Washington) and Pam Henderson (VA Puget Sound Health Care System) for assistance with the preparation of the figures and the manuscript, respectively.
Funding. This work was supported by U.S. National Institutes of Health grants R01-DK-088082 (R.L.H.) and R01-DK-050154 (G.J.T.), the Biomedical Laboratory Research and Development Service of the U.S. Department of Veterans Affairs (G.J.T.), and University of Washington Diabetes Research Center grant P30-DK-017047.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. T.O.M. and G.J.T. researched the data and wrote the manuscript. Q.M. performed the immunohistochemistry and researched the data. A.K.F., C.L.F., and R.L.H. provided the tissue. T.O.M. and G.J.T. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-0284/-/DC1.
References
- 1.Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science 1973;182:171–173 [DOI] [PubMed] [Google Scholar]
- 2.Benson JW Jr, Johnson DG, Palmer JP, Werner PL, Ensinck JW. Glucagon and catecholamine secretion during hypoglycemia in normal and diabetic man. J Clin Endocrinol Metab 1977;44:459–464 [DOI] [PubMed] [Google Scholar]
- 3.Arbelaez AM, Xing D, Cryer PE, et al.; Diabetes Research in Children Network (DirecNet) Study Group . Blunted glucagon but not epinephrine responses to hypoglycemia occurs in youth with less than 1 yr duration of type 1 diabetes mellitus. Pediatr Diabetes 2014;15:127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cryer PE, Gerich JE. Relevance of glucose counterregulatory systems to patients with diabetes: critical roles of glucagon and epinephrine. Diabetes Care 1983;6:95–99 [DOI] [PubMed] [Google Scholar]
- 5.Böhme P, Bertin E, Cosson E, Chevalier N; GEODE group . Fear of hypoglycaemia in patients with type 1 diabetes: do patients and diabetologists feel the same way? Diabetes Metab 2013;39:63–70 [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 7.Aiello LP, Sun W, Das A, et al.; DCCT/EDIC Research Group . Intensive diabetes therapy and ocular surgery in type 1 diabetes. N Engl J Med 2015;372:1722–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Boer IH, Sun W, Cleary PA, et al.; DCCT/EDIC Research Group . Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 2011;365:2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havel PJ, Ahren B. Activation of autonomic nerves and the adrenal medulla contributes to increased glucagon secretion during moderate insulin-induced hypoglycemia in women. Diabetes 1997;46:801–807 [DOI] [PubMed] [Google Scholar]
- 11.Schwartz TW, Holst JJ, Fahrenkrug J, et al. Vagal, cholinergic regulation of pancreatic polypeptide secretion. J Clin Invest 1978;61:781–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Havel PJ, Mundinger TO, Taborsky GJ Jr. Pancreatic sympathetic nerves contribute to increased glucagon secretion during severe hypoglycemia in dogs. Am J Physiol 1996;270:E20–E26 [DOI] [PubMed] [Google Scholar]
- 13.Garber AJ, Cryer PE, Santiago JV, Haymond MW, Pagliara AS, Kipnis DM. The role of adrenergic mechanisms in the substrate and hormonal response to insulin-induced hypoglycemia in man. J Clin Invest 1976;58:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havel PJ, Taborsky GJ Jr. The contribution of the autonomic nervous system to changes of glucagon and insulin secretion during hypoglycemic stress. Endocr Rev 1989;10:332–350 [DOI] [PubMed] [Google Scholar]
- 15.Marliss EB, Girardier L, Seydoux J, et al. Glucagon release induced by pancreatic nerve stimulation in the dog. J Clin Invest 1973;52:1246–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloom SR, Edwards AV. Pancreatic endocrine responses to stimulation of the peripheral ends of the vagus nerves in conscious calves. J Physiol 1981;315:31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerich JE, Karam JH, Forsham PH. Stimulation of glucagon secretion by epinephrine in man. J Clin Endocrinol Metab 1973;37:479–481 [DOI] [PubMed] [Google Scholar]
- 18.Ahrén B, Taborsky GJ Jr, Porte D Jr. Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia 1986;29:827–836 [DOI] [PubMed] [Google Scholar]
- 19.Taborsky GJ Jr, Ahrén B, Havel PJ. Autonomic mediation of glucagon secretion during hypoglycemia: implications for impaired alpha-cell responses in type 1 diabetes. Diabetes 1998;47:995–1005 [DOI] [PubMed] [Google Scholar]
- 20.Taborsky GJ Jr, Mundinger TO. Minireview: the role of the autonomic nervous system in mediating the glucagon response to hypoglycemia. Endocrinology 2012;153:1055–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Järhult J, Farnebo LO, Hamberger B, Holst J, Schwartz TW. The relation between catecholamines, glucagon and pancreatic polypeptide during hypoglycaemia in man. Acta Endocrinol (Copenh) 1981;98:402–406 [DOI] [PubMed] [Google Scholar]
- 22.White NH, Gingerich RL, Levandoski LA, Cryer PE, Santiago JV. Plasma pancreatic polypeptide response to insulin-induced hypoglycemia as a marker for defective glucose counterregulation in insulin-dependent diabetes mellitus. Diabetes 1985;34:870–875 [DOI] [PubMed] [Google Scholar]
- 23.Bolli G, de Feo P, Compagnucci P, et al. Abnormal glucose counterregulation in insulin-dependent diabetes mellitus. Interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes 1983;32:134–141 [DOI] [PubMed] [Google Scholar]
- 24.Mei Q, Mundinger TO, Lernmark A, Taborsky GJ Jr. Early, selective, and marked loss of sympathetic nerves from the islets of BioBreeder diabetic rats. Diabetes 2002;51:2997–3002 [DOI] [PubMed] [Google Scholar]
- 25.Taborsky GJ Jr, Mei Q, Hackney DJ, Figlewicz DP, LeBoeuf R, Mundinger TO. Loss of islet sympathetic nerves and impairment of glucagon secretion in the NOD mouse: relationship to invasive insulitis. Diabetologia 2009;52:2602–2611 [DOI] [PubMed] [Google Scholar]
- 26.Taborsky GJ Jr, Mei Q, Bornfeldt KE, Hackney DJ, Mundinger TO. The p75 neurotrophin receptor is required for the major loss of sympathetic nerves from islets under autoimmune attack. Diabetes 2014;63:2369–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tesfaye S, Boulton AJ, Dyck PJ, et al.; Toronto Diabetic Neuropathy Expert Group . Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boulton AJ, Vinik AI, Arezzo JC, et al.; American Diabetes Association . Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956–962 [DOI] [PubMed] [Google Scholar]
- 29.Stevens MJ. New imaging techniques for cardiovascular autonomic neuropathy: a window on the heart. Diabetes Technol Ther 2001;3:9–22 [DOI] [PubMed] [Google Scholar]
- 30.Langer A, Freeman MR, Josse RG, Armstrong PW. Metaiodobenzylguanidine imaging in diabetes mellitus: assessment of cardiac sympathetic denervation and its relation to autonomic dysfunction and silent myocardial ischemia. J Am Coll Cardiol 1995;25:610–618 [DOI] [PubMed] [Google Scholar]
- 31.Schmidt RE, Plurad SB, Modert CW. Experimental diabetic autonomic neuropathy characterization in streptozotocin-diabetic Sprague-Dawley rats. Lab Invest 1983;49:538–552 [PubMed] [Google Scholar]
- 32.The DCCT Research Group . Factors in development of diabetic neuropathy. Baseline analysis of neuropathy in feasibility phase of Diabetes Control and Complications Trial (DCCT). Diabetes 1988;37:476–481 [PubMed] [Google Scholar]
- 33.UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 34.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813–820 [DOI] [PubMed] [Google Scholar]
- 35.Singh KK, Park KJ, Hong EJ, et al. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci 2008;11:649–658 [DOI] [PubMed] [Google Scholar]
- 36.Gale EA. The discovery of type 1 diabetes. Diabetes 2001;50:217–226 [DOI] [PubMed] [Google Scholar]
- 37.Velloso LA, Eizirik DL, Cnop M. Type 2 diabetes mellitus--an autoimmune disease? Nat Rev Endocrinol 2013;9:750–755 [DOI] [PubMed] [Google Scholar]
- 38.Campbell-Thompson M, Fu A, Kaddis JS, et al. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes 2016;65:719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol 2007;7:988–994 [DOI] [PubMed] [Google Scholar]
- 40.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 2009;155:173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheib J, Höke A. Advances in peripheral nerve regeneration. Nat Rev Neurol 2013;9:668–676 [DOI] [PubMed] [Google Scholar]
- 42.Kennedy JM, Zochodne DW. Impaired peripheral nerve regeneration in diabetes mellitus. J Peripher Nerv Syst 2005;10:144–157 [DOI] [PubMed] [Google Scholar]
- 43.Pettersson M, Ahrén B, Lundquist I, Böttcher G, Sundler F. Neuropeptide Y: intrapancreatic neuronal localization and effects on insulin secretion in the mouse. Cell Tissue Res 1987;248:43–48 [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Diaz R, Abdulred MH, Formoso A, et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 2011;14:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilliam LK, Palmer JP, Taborsky GJ Jr. Tyramine-mediated activation of sympathetic nerves inhibits insulin secretion in humans. J Clin Endocrinol Metab 2007;92:4035–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taborsky GJ, Jr. Islets have a lot of nerve! Or do they? Cell Metab 2011;14:5–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mundinger TO, Mei Q, Figlewicz DP, Lernmark A, Taborsky GJ Jr. Impaired glucagon response to sympathetic nerve stimulation in the BB diabetic rat: effect of early sympathetic islet neuropathy. Am J Physiol Endocrinol Metab 2003;285:E1047–E1054 [DOI] [PubMed] [Google Scholar]
- 48.Havel PJ, Veith RC, Dunning BE, Taborsky GJ Jr. Pancreatic noradrenergic nerves are activated by neuroglucopenia but not by hypotension or hypoxia in the dog. Evidence for stress-specific and regionally selective activation of the sympathetic nervous system. J Clin Invest 1988;82:1538–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunning BE, Scott MF, Neal DW, Cherrington AD. Direct quantification of norepinephrine spillover and hormone output from the pancreas of the conscious dog. Am J Physiol 1997;272:E746–E755 [DOI] [PubMed] [Google Scholar]
- 50.Bloom SR, Edwards AV. Certain pharmacological characteristics of the release of pancreatic glucagon in response to stimulation of the splanchnic nerves. J Physiol 1978;280:25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holst JJ, Gronholt R, Schaffalitzky de Muckadell OB, Fahrenkrug J. Nervous control of pancreatic endocrine secretion in pigs. II. The effect of pharmacological blocking agents on the response to vagal stimulation. Acta Physiol Scand 1981;111:9–14 [DOI] [PubMed] [Google Scholar]
- 52.Ahrén B, Veith RC, Taborsky GJ Jr. Sympathetic nerve stimulation versus pancreatic norepinephrine infusion in the dog: 1). Effects on basal release of insulin and glucagon. Endocrinology 1987;121:323–331 [DOI] [PubMed] [Google Scholar]
- 53.Heller SR, Macdonald IA, Tattersall RB. Counterregulation in type 2 (non-insulin-dependent) diabetes mellitus. Normal endocrine and glycaemic responses, up to ten years after diagnosis. Diabetologia 1987;30:924–929 [DOI] [PubMed] [Google Scholar]
- 54.Schmidt RE. Autonomic neuropathy in experimental models of diabetes mellitus. Handb Clin Neurol 2014;126:579–602 [DOI] [PubMed] [Google Scholar]
- 55.Pfeifer MA, Schumer MP. Clinical trials of diabetic neuropathy: past, present, and future. Diabetes 1995;44:1355–1361 [DOI] [PubMed] [Google Scholar]
- 56.Quasthoff S. The role of axonal ion conductances in diabetic neuropathy: a review. Muscle Nerve 1998;21:1246–1255 [DOI] [PubMed] [Google Scholar]