Abstract
Background
Chronic idiopathic pain syndromes are major causes of personal suffering, disability, and societal expense. Dietary n-6 linoleic acid has increased markedly in modern industrialized populations over the past century. These high amounts of linoleic acid could hypothetically predispose to physical pain by increasing the production of pro-nociceptive linoleic acid-derived lipid autacoids and by interfering with the production of anti-nociceptive lipid autacoids derived from n-3 fatty acids. Here, we used a rat model to determine the effect of increasing dietary linoleic acid as a controlled variable for 15 weeks on nociceptive lipid autacoids and their precursor n-6 and n-3 fatty acids in tissues associated with idiopathic pain syndromes.
Results
Increasing dietary linoleic acid markedly increased the abundance of linoleic acid and its pro-nociceptive derivatives and reduced the abundance of n-3 eicosapentaenoic acid and docosahexaenoic acid and their anti-nociceptive monoepoxide derivatives. Diet-induced changes occurred in a tissue-specific manner, with marked alterations of nociceptive lipid autacoids in both peripheral and central tissues, and the most pronounced changes in their fatty acid precursors in peripheral tissues.
Conclusions
The present findings provide biochemical support for the hypothesis that the high linoleic acid content of modern industrialized diets may create a biochemical susceptibility to develop chronic pain. Dietary linoleic acid lowering should be further investigated as part of an integrative strategy for the prevention and management of idiopathic pain syndromes.
Keywords: Oxylipin, linoleic acid, omega-6, omega-3, idiopathic, pain
Introduction
Idiopathic and poorly understood persistent pain syndromes (Table 1) are major causes of personal suffering, disability, and societal expense.1,17 The majority of patients with idiopathic pain in one location report pain at other body regions.1,18 For example, patients with migraine headaches have high prevalence of bladder pain syndrome,19 irritable bowel syndrome,2 vulvodynia,20 fibromyalgia,3 and low back pain.21 This overlapping nature suggests that shared mechanisms may underlie initiation and perpetuation of pain at multiple sites. However, few biochemical or genetic susceptibility factors for idiopathic pain syndromes have been identified.
Table 1.
| Pain syndrome(s) | Associated tissue(s) | Estimated prevalence |
|---|---|---|
| Bladder pain syndrome/Interstitial cystitis | Bladder | 2–6.5% |
| Vulvodynia | Perineum | 7–8% of women |
| Severe headaches or migraine | Skeletal muscle, meninges, trigeminal nerve, and cranial vessels | 14–23% |
| Fibromyalgia syndrome/chronic widespread pain | Skeletal muscle | 2–8% |
| Regional myofascial pain | Skeletal muscle | Unknown, common |
| Chronic low back or neck pain | Skeletal muscle, fascia, intervertebral discs, and facet joints | 10.2%a (low back pain) |
| 2.2%a (neck pain) | ||
| Idiopathic orchialgia | Testis, epididymis, and perineum | 4.8% of men presenting to urology clinics |
| Irritable bowel syndrome | Small intestine | 4–6% |
| Gastroesophageal reflux disease | Esophagus | 17–18%b |
aPercentage of U.S. adults reporting chronic impairing low back or neck pain for >3 months in North Carolina, USA.
bPercentage of U.S. adults with painful reflux in Olmstead County, MN, USA.
Mechanisms linking n-6 and n-3 fatty acids to nociception
As major components of circulating lipoproteins, and of skin, muscle, immune, myelin, glial, and neuronal membranes,4,22 n-6 and n-3 fatty acids can be endogenously converted to autacoids with pro- or anti-nociceptive properties (e.g., oxidized linoleic acid (LA) metabolites, prostanoids, and monoepoxides).4,5–8,23–26 With notable exceptions,27,28 mediators derived from n-6 LA and arachidonic acid (AA) promote nociception,5–7,23 while mediators derived from n-3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) promote anti-nociception.24–26 Thus, an imbalance of mediators derived from n-6 and n-3 fatty acids is a plausible mechanism contributing to initiation and perpetuation of chronic pain. As mammals lack the capacity for de novo biosynthesis of n-6 and n-3 fatty acids, diet is the sole source in mammalian tissues.29,30 These observations led us to hypothesize that targeted dietary alterations may be able to reduce pain by favorably influencing the balance of pro- and anti-nociceptive mediators. 4,9,31
We recently demonstrated proof of principle for this hypothesis in a randomized trial among 67 patients with chronic headaches.4 The combination of increasing n-3 fatty acids with concurrent reduction in LA (the H3-L6 intervention) increased anti-nociceptive and reduced pro-nociceptive mediators in circulation and reduced headache frequency and severity.4,10 Reduction in circulating LA was closely associated with pain reduction, suggesting that LA lowering was a key component of the intervention.10
High intake of LA could increase the susceptibility to chronic pain via several mechanisms (Figure 1). Most directly, LA is the precursor to pro-nociceptive mediators which induce hyperalgesia and allodynia in rodents.5,6,23 A fraction of LA is enzymatically converted to AA,32 the precursor to pro-nociceptive prostanoids7 and endovanilloids,33 as well as several anti-nociceptive mediators (e.g., lipoxins27 and epoxyeicosatrienoic acid [EpETrEs]28). High LA intake may reduce the production of anti-nociceptive mediators derived from EPA and DHA.24,30 Thus, we hypothesize that high dietary LA could predispose to the development of chronic pain. However, the effects of alterations in dietary LA on nociceptive autacoids and their precursor fatty acids in tissues associated with pain conditions are largely unknown.
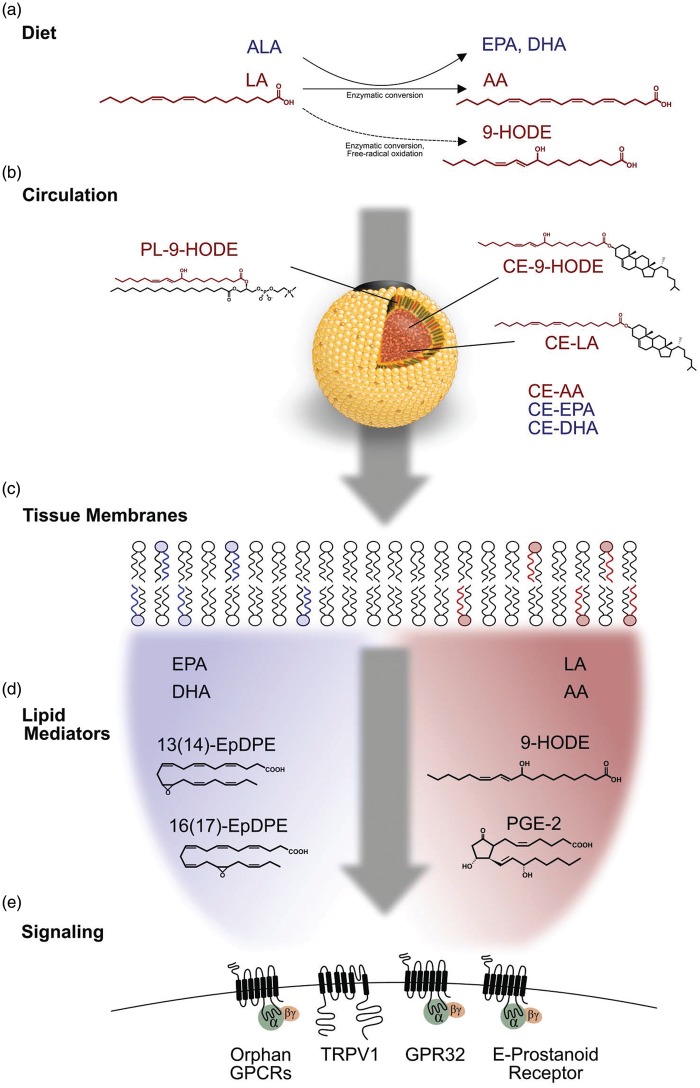
Figure 1.
Proposed mechanisms linking high intake of linoleic acid to chronic pain. (a) Dietary LA can be endogenously converted to pro-nociceptive mediators (e.g. 9-HODE). A small fraction of dietary LA is converted to n-6 AA, the precursor to pro- and anti-nociceptive mediators. High intakes of dietary LA competitively inhibit hepatic conversion of n-3 ALA into EPA and DHA. (b) In circulation, LA and HODE are predominantly esterified in cholesteryl esters, triacylglycerol, and phospholipid components of lipoproteins. LA and HODE in circulating LDL are delivered to peripheral tissues via LDL receptors and scavenger receptors. (c) High intakes of LA produce tissue-specific increases in LA and AA and reduction in EPA and DHA content of cellular membranes. (d) High intake of dietary LA increases the production of pro-nociceptive mediators (e.g. 9-HODE and PGE2) and reduces the production of anti-nociceptive lipid autacoids (e.g. EpDPEs and EpETEs). (e) These alterations in nociceptive lipid mediators modulate receptors (e.g. TRPV1, E-prostanoid) creating a biochemical susceptibility to develop chronic pain. LA: linoleic acid; ALA: α-linolenic acid; AA: arachidonic acid; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid; CE: cholesteryl ester; PL: phospholipid; HODE: hydroxyoctadecadienoic acid; EpDPE: Epoxy-docosapentaenoic acid GPCR, G-protein coupled receptor; TRPV1: transient receptor potential vanilloid, type 1.
As a first step in testing this hypothesis, we investigated the effects of controlled alterations in dietary LA on nociceptive autacoids and their precursor fatty acids in rat tissues associated with idiopathic pain syndromes and in tissues of the peripheral and central nervous system (CNS).
Methods
Animals
The animal protocol was approved by the Animal Care and Use Committee of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23). Male Fischer-344 (CDF) rat pups (18–21 days old) and their surrogate mothers were purchased from Charles River Laboratories (Portage, MI). Upon arrival, the pups were weaned from their surrogate mothers and randomly assigned to a very low (n = 8), moderate (n = 8), or high LA diet (n = 8) for 15 weeks. Animals were housed in an animal facility having regulated temperature, humidity, and a 12 h light/dark cycle. In order to maintain equivalency of calories and nutrients, dietary LA was altered in these three groups via isocaloric replacement with coconut oil, which is rich in saturated fatty acids. The three study diets were prepared by Dyets Inc. (Bethlehem, PA) based on the AIN-93 G formulation and contained 10% fat by weight.34,35 Dietary fatty acids were analyzed by gas chromatography (GC) as previously reported.36 Briefly, food pellets were crushed with pestle and mortar, weighed and extracted in chloroform/methanol (2:1 v/v) by the Folch method.37 A portion of the extracted lipids was methylated with 1% H2SO4 in methanol after adding 1,2-diheptadecanoyl-sn-glycero-3-phosphocholine as an internal standard. The fatty acid methyl esters were extracted in heptane, reconstituted in isooctane, and analyzed by gas chromatography as detailed elsewhere.36 Fatty acid composition of the three diets is shown in Table 2. Rats had free access to food and water throughout the study; their food was replaced every three or four days.
Table 2.
Fatty acid and macronutrient compositions of study diets.
| LA (%E) | ALA (%E) | MUFA (%E) | SFAa (%E) | Total fat (%E) | Protein (%E) | Carbohydrate (%E) | |
|---|---|---|---|---|---|---|---|
| Diet group | |||||||
| 0.4 %E LA | 0.4 | 0.9 | 1.1 | 12.1 | 22.0 | 19.5 | 58.5 |
| 5 %E LA | 5.2 | 0.9 | 1.5 | 9.8 | 22.0 | 19.5 | 58.5 |
| 10 %E LA | 10.5 | 0.9 | 2.8 | 6.0 | 22.0 | 19.5 | 58.5 |
aTotal SFA energy content listed here does not include the contribution from short chain fatty acids (8:0, 6:0, and 4:0) present in coconut oil. These fatty acids were not quantifiable with our extraction and analytical method. Diets did not contain AA, EPA, and DHA. LA: linoleic acid; ALA: alpha-linolenic acid; AA: arachidonic acid; MUFA: monounsaturated fatty acid; SFA: saturated fatty acid; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid.
Tissue sampling rationale
The tissue sampling strategy was designed to provide insight into the hypothesis that diet can alter local synthesis of nociceptive lipid autacoids in peripheral tissues associated with idiopathic pain syndromes. To limit lipid autacoid degradation, peripheral tissues that could be quickly collected and frozen were selected including: perineum (vulvodynia), bladder (bladder pain syndrome/interstitial cystitis), epididymis (idiopathic orchialgia), esophagus (gastroesophageal reflux disease), duodenum (irritable bowel syndrome), and trapezius muscle. Trapezius, which is commonly associated with regional myofascial pain38 and may be involved in fibromyalgia syndrome,39 was selected as representative and readily accessible skeletal muscle. Additional tissues—including blood and nervous system tissues—that could be quickly harvested were selected to provide an opportunity to broadly compare whether similar diet-induced changes occurred in peripheral tissues, nervous system tissues, and in circulation.
Rat tissue collection and fatty acid analyses
The rats were killed by CO2 overdose. Tissue samples were immediately collected, frozen on dry-ice chilled isobutane, and stored at −80℃. Tissue fatty acids were analyzed as previously described.40 Briefly, samples were thawed, weighed, and homogenized in butylated hydroxytoluene (BHT)/methanol for fatty acid extraction according to the method of Folch et al.37 BHT was added in the methanol to reduce lipid oxidation during the procedures. The internal standard methyl tricosanoate (23:0) was added to each sample. This was followed by methylation with 14% BF3/methanol. The hexane extracts were concentrated to a small volume with a stream of nitrogen and transferred to microvials for GC analysis. Fatty acid methyl esters were analyzed with an HP-7890 A gas chromatograph equipped with a flame ionization detector (Hewlett-Packard, Palo Alto, CA) and a fused silica capillary column (DB-FFAP, 15 m × 0.100 mm i.d. × 0.10 µm film thickness, J & W Scientific, Folsom, CA). The detector and injector temperatures were set to 250℃. The oven temperature program began at 150℃ for 0.25 min and increased to 200℃ at the rate of 10℃/min, then at the rate of 3.5℃/min to 225℃ for 0.5 min, and finally increased at the rate of 40℃/min to 245℃, with a final hold for 15 min. Hydrogen was used as carrier gas at a linear velocity of 50 cm/s. A custom mixed, 30-component, quantitative methyl ester standard containing 10–24 carbons and 0–6 double bonds was used for assignment of retention times and to ensure accurate quantification (Nu Chek Prep 462, Elysian, MN). Fatty acid data were expressed as % of total peak area, which corresponded to weight% to within 5%, as demonstrated by quantitative standard mixtures. Internal standards were used to calculate tissue fatty acid concentrations. Fatty acid data were expressed as a percentage of total fatty acids (%FA) and are also provided as a concentration in micrograms per gram of tissue (µg/g) in the Supplementary materials.
Lipid autacoid analyses
Lipid autacoids were analyzed as free oxylipins and after saponification to determine the sum of both free and esterified oxylipins as previously described.16 Ice-cold methanol (400–800 µl) containing 0.1% acetic acid and 0.1% BHT was added to approximately 0.1 g of frozen tissue, following the addition of 10 µl antioxidant mix and 20 µl surrogate standard. The antioxidant solution contained three antioxidants mixed at a 1:1:1 ratio (v/v/v) consisting of 6.9 mg/ml ethylenediaminetetraacetic acid in water, 1.12 mg/ml BHT in methanol, and 0.4 mg/ml triphenylphosphine in water. The antioxidant solution was passed through a Millipore filter to remove solid particles. The 20 µl surrogate standard contained 200 nmol of d11-11(12)-EpETrE, d11-14,15-DiHETrE, d4-6-keto-PGF1a, d4-9-hydroxyoctadecadienoic acid (HODE), d4-LTB4, d4-PGE2, d4-TXB2, d6-20-HETE, and d8-5-HETE dissolved in methanol (abbreviations for oxylipins are in Supplementary Materials Table S1). The sample containing the extraction solvent, antioxidant mix, and surrogate standards was cooled in −80℃ freezer for 30 min and then homogenized for 5 to 10 min at 30 vibrations per second using a bead homogenizer. A Polytron was used to further homogenize the tissue for approximately 30 s on dry ice if large tissue particles were observed. The homogenized samples were stored overnight at −80℃ freezer, following which they were centrifuged at high speed in a 5145 R microcentrifuge (Eppendorf) for 10 min. Approximately half of the supernatant was subjected to direct solid phase extraction to extract free oxylipins. The remaining supernatant was hydrolyzed in equal volumes of 0.5 M sodium carbonate solution (26.5 mg per ml of 1:1 v/v methanol/water) at 60℃ for 30 min under constant shaking. Following neutralization with 25 or 50 µl acetic acid and 1575 or 3150 µl water, depending on whether the volume of the supernatant extract was 200 or 400 µl, the hydrolyzed oxylipins were also subjected to solid phase extraction. Oxylipins were quantified by liquid chromatography-electrospray ionization tandem mass spectrometry as previously described.16
Data analysis and graphical representation
Nonparametric analyses were employed due to the presence of non-normal distributions. A Kruskal–Wallis test was used for between-group comparisons. Oxylipin values that were below the detection or quantitation limit were imputed as one half the limit of quantitation. Diet-induced changes in selected pro- and anti-nociceptive variables and their precursor n-3 and n-6 fatty acids were graphed using boxplots with medians and interquartile ranges.
Results
Body weights in the three diet groups did not differ (median weights in grams were 373, 390, and 350 for 0.4, 5, and 10%E diets, respectively; p = 0.53).
Dietary LA-induced alterations in tissue n-6 and n-3 fatty acids
Tissue-specific alterations in LA, 18:2n-6
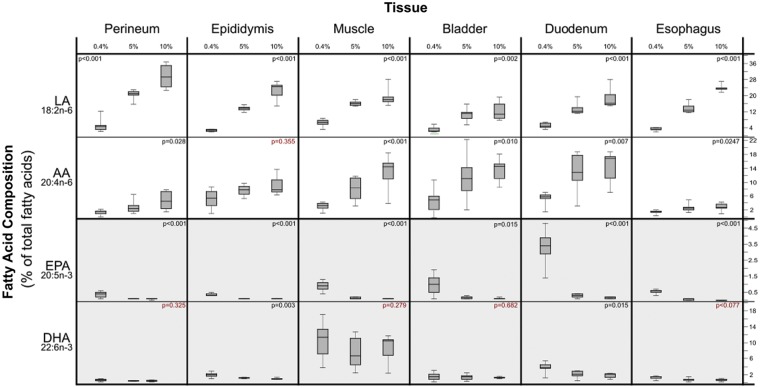
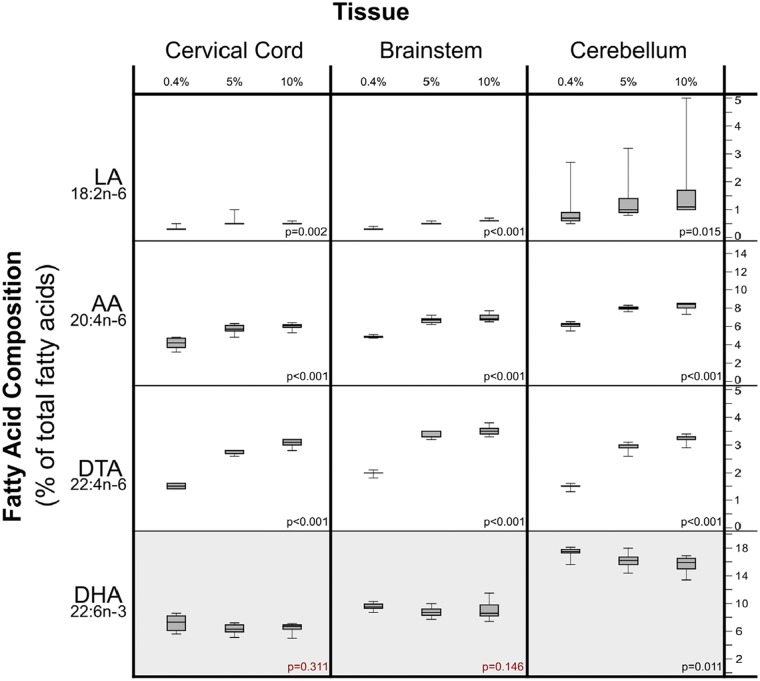
Increasing dietary LA produced marked increases in the LA content of several peripheral tissues associated with idiopathic pain syndromes (Tables S2 and S3). The most pronounced increases were observed in perineum, epididymis, esophagus, muscle, duodenum, and bladder (Figure 2). Increases in dietary LA also produced statistically significant, but comparatively modest increases in the LA content of CNS tissues including cervical cord, brainstem, and cerebellum (Figure 3).
Figure 2.
Dietary LA-induced changes in the fatty acid content of peripheral tissues associated with idiopathic pain syndromes. Note that the Y-axis scales differ in these graphs. X-axis % values refer to percentage of food energy. Box plots include medians and interquartile ranges with end whiskers set to minimum and maximum values. Number of samples for each tissue (perineum n = 21, epididymis n = 21, skeletal muscle n = 24, bladder n = 21, duodenum n = 21, and esophagus n = 20). LA: linoleic acid; AA: arachidonic acid; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid.
Figure 3.
Dietary LA-induced changes in fatty acids in central nervous system tissues. Note that the Y-axis scales differ in these graphs. X-axis % values refer to percentage of food energy. Box plots include medians and interquartile ranges with end whiskers set to minimum and maximum values. Number of samples for each tissue (cervical cord n = 23, brainstem n = 24, and cerebellum n = 24). LA: linoleic acid; AA: arachidonic acid; DTA: docosatetraenoic acid; DHA: docosahexaenoic acid.
Tissue-specific alterations in AA, 20:4n-6
Increasing dietary LA produced marked increases in the AA content of certain peripheral tissues associated with idiopathic pain syndromes (e.g., bladder, muscle, and duodenum) but had comparatively minor effects on several other peripheral tissues (e.g., perineum, esophagus, and epididymis; Figure 2 and Table S2). Increases in dietary LA also produced statistically significant and substantial increases in the AA content of nervous system tissues including sciatic nerve, cervical cord, brainstem, and cerebellum.
Tissue-specific alterations in docosatetraenoic acid (22:4n-6)
Docosatetraenoic acid (DTA) tended to be less abundant than LA and AA, accounting for 0.01 to 5.3% of total fatty acids. Increasing dietary LA produced substantial increases in the DTA content of several tissues associated with idiopathic pain syndromes (e.g., bladder and duodenum), as well as several CNS tissues (Figure 3).
Tissue-specific alterations in EPA, 20:5n-3
The 5 and 10%E groups both had very low EPA content (less than 0.3% of total fatty acids) in tissues associated with idiopathic pain syndromes and nervous system tissues. Lowering dietary LA from 5 to 0.4%E significantly increased the EPA content of several tissues (e.g., duodenum, bladder, and muscle); however, EPA remained less than 3.4% of total fatty acids in all tissues (Table S2).
Tissue-specific alterations in DHA, 22:6n-3
Increasing dietary LA significantly decreased the DHA content of cerebellum, duodenum, epididymis, and testis but did not alter the DHA content of bladder, muscle, or sciatic nerve (Table S2).
Tissue-specific alterations in oleic acid (18:1n-9) and palmitic acid (16:0)
Increasing dietary LA significantly decreased the oleic acid content of certain peripheral tissues including duodenum, epididymis, and muscle and modestly reduced the oleic acid content of cervical cord, brainstem, and cerebellum. Dietary LA had a comparatively minor impact on the palmitic acid content of most tissues. Notable exceptions include sciatic nerve and epididymis (Table S2).
Dietary LA-induced alterations in lipid autacoids derived from n-3 and n-6 fatty acids
Dose-dependent increases in oxidized LA metabolites
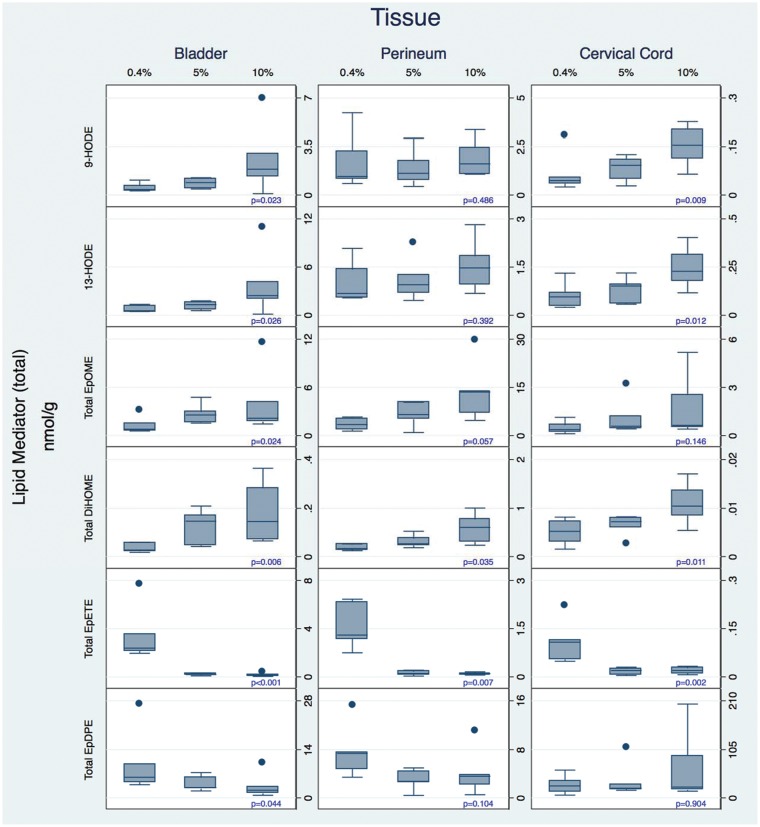
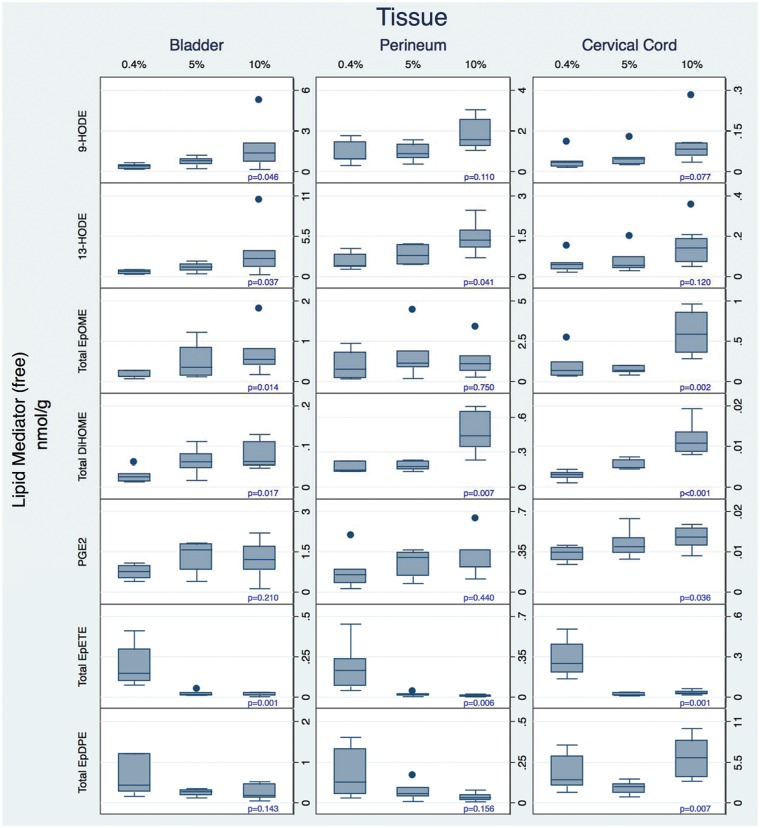
Increasing dietary LA as a controlled variable from 0.4 to 5 to 10%E markedly increased the concentrations of several oxidized LA metabolite (OXLAM) species with putative pro-nociceptive properties5,6,23 in perineum, bladder, and cervical cord (Figures 4 and 5 and Tables S4–S6). While both total and free OXLAMs tended to increase with higher LA intakes, changes in specific regioisomers (e.g., 9- and 13-HODE, 9(10)- and 12(13)-epoxyoctadecamonoenoic acid (EpOME), and 9(10)- and 12(13)-DiHOME) occurred in a tissue-specific manner.
Figure 4.
Diet-induced changes in total (sum of free and esterified) n-3 and n-6 derived oxylipins in tissues associated with idiopathic pain syndromes. Note that the Y-axis scales differ in these graphs. Note that the Y-axis scales differ in these graphs. Box plots include medians and interquartile ranges with end whiskers set to 1.5 times interquartile values. Number of samples for each tissue (bladder n = 20, perineum n = 18, and cervical cord n = 21). HODE: hydroxyoctadecadienoic acid; EpOME: epoxyoctadecamonoenoic acid; EpETrE: epoxyeicosateteaenoic acid; EpDPE: epoxydocosapentaenoic acid.
Figure 5.
Diet-induced changes in free (unesterified) n-3 and n-6 derived oxylipins in tissues associated with idiopathic pain syndromes. Note that the Y-axis scales differ in these graphs. Box plots include medians and interquartile ranges with end whiskers set to 1.5 times interquartile values. Number of samples for each tissue (bladder n = 20, perineum n = 18, and cervical cord n = 21). HODE: hydroxyoctadecadienoic acid; EpOME: epoxyoctadecamonoenoic acid; PG: prostaglandin; EpETE: epoxyeicosateteaenoic acid; EpDPE: epoxydocosapentaenoic acid.
Alterations in pro- and anti-nociceptive oxylipin derivatives of n-6 AA
Increasing dietary LA had comparatively minor effects on AA-derived oxylipins in perineum, bladder, and cervical cord. Exceptions include significant increases in TXB2 in perineum and free PGE2 and free 11,12,15-TriHETrE (hepoxilin inactivation product) in cervical cord, as well as a significant reduction in LXA4 in bladder.
Reductions in oxylipin derivatives of EPA and DHA
Increasing dietary LA reduced the concentrations of anti-nociceptive EPA-derived (e.g. epoxyeicosateteaenoic acids [EpETEs]) and DHA-derived (e.g. epoxydocosapentaenoic acids [EpDPEs]) n-3 monoepoxides in bladder, perineum, and cervical cord. Reductions in n-3 monoepoxides were most pronounced when increasing dietary LA from 0.4%E to 5%E, with only minor differences observed between 5 and 10%E.
Discussion
Here, we showed that increasing dietary n-6 LA as a controlled variable in rats markedly increased the LA content of several peripheral tissues associated with idiopathic pain syndromes, with the most pronounced increases in perineum (vulvodynia), epididymis (idiopathic orchialgia), skeletal muscle (fibromyalgia syndrome and regional myofascial pain syndrome), esophagus (gastroesophageal reflux disease), duodenum (irritable bowel syndrome), and bladder (bladder pain syndrome/interstitial cystitis). Moreover, these diet-induced increases in tissue LA were accompanied by increased concentrations of (total and free) OXLAMs in perineum, bladder, and cervical cord. Importantly, OXLAMs have been shown to have pro-nociceptive properties in rodent pain behavioral models.5,6,23 For example, HODE-mediated allodynia and peripheral inflammatory pain in rodents were attenuated by immunological neutralization of HODEs using antibodies23 or by indirect inhibition of HODE biosynthesis using the broad spectrum cytochrome P450 inhibitor ketoconazole.41 OXLAMs have been implicated in the hyperalgesia that accompanies thermal burn injury,5 Achilles tendinopathy-related pain,42 and the persistent nociception triggered by peripheral or central injection of nerve growth factor.11
Previous findings in rodents and humans indicate that dietary LA can alter the amounts of LA43 and its bioactive oxidation products in certain tissues.44 For example, we previously demonstrated in humans that dietary LA lowering from about 7 to 2.5%E for 12 weeks significantly reduced the abundance of LA in circulating phospholipids, triglycerides, and cholesteryl esters, as well as the concentrations of 9- and 13-HODEs and oxo-ODEs in plasma.44 Johnson et al. reported that a fourfold increase in dietary LA produced a fivefold increase in the 9- and 13-HODE content of mammary tissue in female mice.45 Importantly, however, the present study is the first to show that increasing dietary LA markedly increases the abundance of LA and OXLAMs in tissues associated with idiopathic pain syndromes. This finding supports the hypothesis that high n-6 LA intakes could contribute to the initiation and/or perpetuation of chronic pain via the straightforward mechanism of increasing pro-nociceptive lipid autacoids in peripheral tissues that supply such mediators to peripheral sensory nerve terminals (Figure 1).
Given the low abundance of LA in cervical cord (<1% of fatty acids), the findings that OXLAMs were relatively abundant (median ranges for total HODEs 146–399 and EpOMEs 377-642 pmol/g), and markedly increased with high LA intakes in cervical cord, were unexpected. While the reason for this discrepancy between LA and its bioactive products in CNS tissues is not entirely clear, it suggests that the mechanism(s) that limit accumulation of LA may not extend to LA oxidation products. Alternatively, the small amount of LA in CNS tissues may be preferentially metabolized to bioactive autacoids. Consistent with this suggestion, Demar et al.46 found that radiolabeled unesterified LA infused into rats readily crossed the blood–brain barrier and was metabolized into unidentified polar compounds. While it is possible that these unidentified compounds are OXLAMs (e.g. HODEs and EpOMEs), this requires confirmation.
Dietary LA-induced reductions in n-3 monoepoxides
Dietary LA decreased n-3 DHA and EPA, and their n-3 monoepoxide derivatives EpDPE and EpETrE, in peripheral tissues associated with idiopathic pain syndromes, and in cervical cord. Morriseau et al.24 demonstrated in rat carrageenan inflammatory pain model that injection of EpDPE or EpETrE peripherally (intraplantar) or centrally (intrathecal) resulted in significant antihyperalgesic activity. Notably, 13(14)-EpDPE and 16(17)-EpDPE are intermediates (pathway precursors) for the biosynthesis of maresin-126 and protectins,12 which have demonstrated anti-nociceptive properties in preclinical models.25,47 Hence, increased concentrations of EPA and DHA-derived autacoids in both peripheral and central tissues could contribute to the anti-nociceptive properties of low-LA diets.
Dietary LA had modest effects on AA-derived lipid autacoids
Increasing dietary LA increased the abundance of n-6 AA in several tissues associated with idiopathic pain syndromes, as well as CNS tissues (Figures 2 and 3). As certain AA-derived oxylipins (e.g. PGE2 and hepoxilins) have widely recognized pro-nociceptive properties,7 and others (e.g. EpETrEs and lipoxins) have anti-nociceptive properties,27,28 diet-induced increases in these AA-derivatives could have important implications for pain. Unexpectedly, however, despite producing major increases in tissue AA, increasing dietary LA in the present study translated to inconsistent and comparatively minor effects on AA-derived oxylipins. However, the few changes that did occur—increases in PGE2 and the hepoxilin pathway marker (11,12,15-TriHETrE) in cervical cord and the paradoxical reduction in the anti-nociceptive AA-derivative LXA4 in bladder—would be expected to promote nociception.7,33 Thus, it is possible that dietary LA-induced changes in AA-derived mediators could enhance nociception in certain tissues. However, as observed for LA and OXLAMs, the observed discrepancies between changes in tissue AA and AA-derived lipid autacoids indicate that measuring the fatty acid composition alone provides an incomplete representation of the biochemical milieu of tissues.
Collective findings from the present study suggest that low-LA diets could potentially have anti-nociceptive properties due to a combination of reductions in OXLAMs and increases in n-3 monoepoxides. Consistent with these rodent findings, diet-induced reductions in plasma LA and increases in EPA and DHA were closely correlated with decreased headache frequency and severity4 in 67 patients with chronic daily headaches; diet-induced reductions in n-6 AA were not associated with pain reduction.10
Potential implications of modern high-LA diets
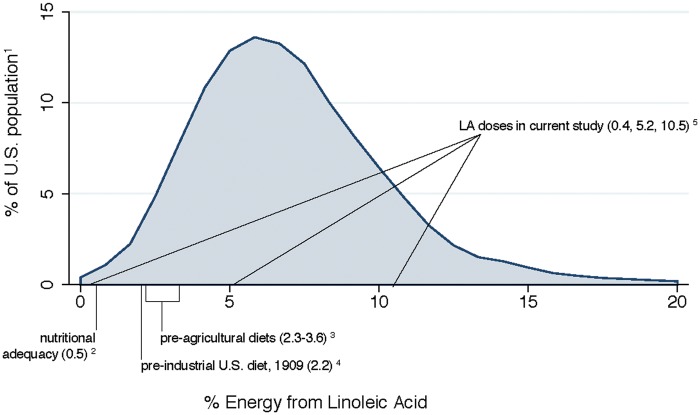
If the hypothesis that high intake of dietary LA produces a biochemical susceptibility to develop chronic pain is correct, the tripling of U.S. per capita average dietary LA from about 2%E to 7%E during the 20th century48 (Figure 6) may contribute to the pervasive nature of idiopathic pain syndromes in the U.S. population. Notably, approximately 80% of Americans currently consume ≥5%E as LA and 18% consume ≥10%E.49 Thus, doses selected for the present study fall within ranges commonly consumed by the U.S. population.
Figure 6.
Linoleic acid content of study diets compared to current and historical intakes. 1Distribution of LA intakes in U.S. adults.49
2Dose of LA needed to prevent deficiency symptoms.43
Strengths and limitations
The present study had several important strengths. The use of controlled alterations in dietary LA with otherwise equivalent micronutrient and macronutrient contents minimized potential confounding effects of other dietary constituents. Quantitation of both free and total (sum of free and esterified) oxylipins provided a more complete picture of lipid autacoid-related biochemical milieu of tissues. While free oxylipins represent the pool of bioactive autacoids at any given moment, a substantial portion of certain oxylipins (e.g. hydroxy fatty acids) are esterified in phospholipids and other complex lipids.13–16,51 Since this preformed pool of esterified oxylipins can be mobilized to influence pathophysiology, both measures provide useful information.16
This study also had several important limitations. The present findings are biochemical in nature only and do not demonstrate hyper-excitation of the peripheral or CNS tissues. Additional experiments are required to determine whether the observed diet-induced changes in lipid autacoids are accompanied by changes in pain phenotype in inflammatory, neuropathic, or widespread pain models. Present findings are limited to biochemical results using only three doses of dietary LA. We do not yet know whether doses higher than 10%E produce even more marked alterations in OXLAMs, or the effects of graded reduction in dietary LA (between 5%E and 0.4%E) on EPA and DHA monoepoxide concentrations. The oxylipins measured in the present study do not reflect all possibly formed lipid autacoids, because of limited coverage of the analytical method used. Because of the saponification used to assess total (sum of free and esterified) oxylipins, certain lipid mediators, e.g. prostanoids, were degraded making it impossible to compare the total levels of these oxylipins. In addition, rats were not subjected to microwave fixation,52 which has been shown to reduce postmortem release of several lipid mediators in brain.53 To our knowledge, the effects of microwave fixation have not been examined in the peripheral tissues studied here. An additional consideration is that the specific molecular mechanisms (e.g. biosynthetic enzymes and receptors) linking lipid autacoids to behavioral and clinical pain outcomes are incompletely understood.
Future research should be directed toward (a) assessing the behavioral and clinical effects of controlled alterations in dietary LA and other dietary constituents in rodent models of pain and human pain conditions, respectively; (b) delineating the biochemical effects of altering dietary LA above 10%E and at finer gradients between 0.4 and 5%E; (c) identifying and characterizing the specific autacoids, including regio- and stereoisomers, that are most responsible for pain reduction in each tissue; and (d) characterizing the specific molecular pathways utilized for biosynthesis, transport, signaling, and inactivation of each lipid mediator in peripheral and CNS tissues. Findings in rats are not necessarily generalizable to humans. However, the demonstration that dietary LA strongly influences circulating levels of nociceptive autacoids in patients with chronic headaches suggests that this finding may be relevant for humans.4,10
Randomized controlled trials altering n-6 LA and n-3 EPA and DHA as controlled variables are underway in several human populations with idiopathic chronic pain syndromes (e.g. Clinicaltrials.gov NCT02012790, NCT02272010). The clinical and biochemical findings from these human trials, combined with biochemical data reported here, are intended to provide key insights needed to move this line of inquiry forward. Ultimately, these efforts could lead to the development of complementary and integrative approaches for preventing and treating pain.
Conclusions
In summary, the present study demonstrates that dietary LA dose dependently increases the abundance of LA and its pro-nociceptive lipid derivatives, and reduces the abundance of EPA and DHA and several of their anti-nociceptive mediators, in tissues associated with common idiopathic pain syndromes. These findings provide biochemical support for the hypothesis that high-LA diets could contribute to the initiation and/or perpetuation of persistent pain. Dietary LA lowering should be further investigated as part of an integrative strategy for the prevention and management of idiopathic pain syndromes.
Acknowledgments
The authors thank Mark Horowitz for data management and oxylipin tables and graphs.
Author contributions
Experimental design: CER, AYT; data collection: CER, AT, HB, JDL, AYT; data analysis: SFM, JY, DZ, AYT; manuscript preparation: CER, AR, AYT; manuscript revision: SFM, JY, HB, DZ, JDL, SIR, JRH, JMD, BDH. All authors read and approved the final manuscript.
Authors’ Note
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Partial support was provided by the Mayday Fund, NIEHS RO1ES002710, NINDS U54NS079202, and NIDDK U24097154, and the Intramural Programs of the National Institute on Aging and the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
References
- 1.Johannes CB, Le TK, Zhou X, et al. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain 2010; 11: 1230–1239. DOI: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Lau CI, Lin CC, Chen WH, et al. Association between migraine and irritable bowel syndrome: a population-based retrospective cohort study. Eur J Neurol 2014; 21: 1198–1204. DOI: 10.1111/ene.12468. [DOI] [PubMed] [Google Scholar]
- 3.Liu HY, Fuh JL, Lin YY, et al. Suicide risk in patients with migraine and comorbid fibromyalgia. Neurology 2015; 85: 1017–1023. DOI: 10.1212/WNL.0000000000001943. [DOI] [PubMed] [Google Scholar]
- 4.Ramsden CE, Faurot KR, Zamora D, et al. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial. Pain 2013; 154: 2441–2451. DOI: 10.1016/j.pain.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patwardhan AM, Akopian AN, Ruparel NB, et al. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest 2010; 120: 1617–1626. DOI: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green DP, Ruparel S, Roman L, et al. Role of endogenous TRPV1 agonists in a postburn pain model of partial-thickness injury. Pain 2013; 154: 2512–2520. DOI: 10.1016/j.pain.2013.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonova M, Wienecke T, Olesen J, et al. Prostaglandin E(2) induces immediate migraine-like attack in migraine patients without aura. Cephalalgia 2012; 32: 822–833. DOI: 10.1177/0333102412451360. [DOI] [PubMed] [Google Scholar]
- 8.Ji RR, Xu ZZ, Strichartz G, et al. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci 2011; 34: 599–609. DOI: 10.1016/j.tins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsden CE, Mann JD, Faurot KR, et al. Low omega-6 vs. low omega-6 plus high omega-3 dietary intervention for chronic daily headache: protocol for a randomized clinical trial. Trials 2011; 12: 97 DOI: 10.1186/1745-6215-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsden CE, Faurot KR, Zamora D, et al. Targeted alterations in dietary n-3 and n-6 fatty acids improve life functioning and reduce psychological distress among patients with chronic headache: a secondary analysis of a randomized trial. Pain 2015; 156: 587–596. DOI: 10.1097/01.j.pain.0000460348.84965.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskander MA, Ruparel S, Green DP, et al. Persistent nociception triggered by nerve growth factor (NGF) is mediated by TRPV1 and oxidative mechanisms. J Neurosci 2015; 35: 8593–8603. DOI: 10.1523/JNEUROSCI.3993-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serhan CN, Dalli J, Colas RA, et al. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta 2015; 1851: 397–413. DOI: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gron B, Iversen L, Ziboh V, et al. Monohydroxy fatty acids esterified to phospholipids are decreased in lesional psoriatic skin. Arch Dermatol Res 1993; 285: 449–454. [DOI] [PubMed] [Google Scholar]
- 14.Bayer M, Mosandl A, Thaci D. Improved enantioselective analysis of polyunsaturated hydroxy fatty acids in psoriatic skin scales using high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 2005; 819: 323–328. DOI: 10.1016/j.jchromb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Shearer GC, Newman JW. Lipoprotein lipase releases esterified oxylipins from very low-density lipoproteins. Prostaglandins Leukot Essent Fatty Acids 2008; 79: 215–222. DOI: 10.1016/j.plefa.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schebb NH, Ostermann AI, Yang J, et al. Comparison of the effects of long-chain omega-3 fatty acid supplementation on plasma levels of free and esterified oxylipins. Prostaglandins Other Lipid Mediat 2014; 113–115: 21–29. DOI: 10.1016/j.prostaglandins.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.IOM Report. Relieving pain in America: A blueprint for transforming prevention, care, education, and research, www.iom.edu/relievingpain (2011, accessed 18 February 2016). [DOI] [PubMed]
- 18.Diatchenko L, Nackley AG, Slade GD, et al. Idiopathic pain disorders—pathways of vulnerability. Pain 2006; 123: 226–230. DOI: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Clemens JQ, Elliott MN, Suttorp M, et al. Temporal ordering of interstitial cystitis/bladder pain syndrome and non-bladder conditions. Urology 2012; 80: 1227–1231. DOI: 10.1016/j.urology.2012.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen RH, Veasley C, Smolenski D. Latent class analysis of comorbidity patterns among women with generalized and localized vulvodynia: preliminary findings. J Pain Res 2013; 6: 303–309. DOI: 10.2147/JPR.S42940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plesh O, Adams SH, Gansky SA. Self-reported comorbid pains in severe headaches or migraines in a US national sample. Headache 2012; 52: 946–956. DOI: 10.1111/j.1526-4610.2012.02155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sastry PS. Lipids of nervous tissue: composition and metabolism. Prog Lipid Res 1985; 24: 69–176. [DOI] [PubMed] [Google Scholar]
- 23.Patwardhan AM, Scotland PE, Akopian AN, et al. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci U S A 2009; 106: 18820–18824. DOI: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morisseau C, Inceoglu B, Schmelzer K, et al. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res 2010; 51: 3481–3490. DOI: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu ZZ, Liu XJ, Berta T, et al. Neuroprotectin/protectin D1 protects against neuropathic pain in mice after nerve trauma. Ann Neurol 2013; 74: 490–495. DOI: 10.1002/ana.23928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalli J, Zhu M, Vlasenko NA, et al. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J 2013; 27: 2573–2583. DOI: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun T, Yu E, Yu L, et al. LipoxinA(4) induced antinociception and decreased expression of NF-kappaB and pro-inflammatory cytokines after chronic dorsal root ganglia compression in rats. Eur J Pain 2012; 16: 18–27. DOI: 10.1016/j.ejpain.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Inceoglu B, Jinks SL, Ulu A, et al. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci U S A 2008; 105: 18901–18906. DOI: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holman RT. Metabolism of isomers of linoleic and linolenic acids. Proc Soc Exp Biol Med 1951; 76: 100–102. [DOI] [PubMed] [Google Scholar]
- 30.Taha AY, Cheon Y, Faurot KF, et al. Dietary omega-6 fatty acid lowering increases bioavailability of omega-3 polyunsaturated fatty acids in human plasma lipid pools. Prostaglandins Leukot Essent Fatty Acids 2014; 90: 151–157. DOI: 10.1016/j.plefa.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsden C, Gagnon C, Graciosa J, et al. Do omega-6 and trans fatty acids play a role in complex regional pain syndrome? A pilot study. Pain Med 2010; 11: 1115–1125. DOI: 10.1111/j.1526-4637.2010.00882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emken EA, Adlof RO, Duval SM, et al. Effect of dietary arachidonic acid on metabolism of deuterated linoleic acid by adult male subjects. Lipids 1998; 33: 471–480. [DOI] [PubMed] [Google Scholar]
- 33.Gregus AM, Doolen S, Dumlao DS, et al. Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors. Proc Natl Acad Sci U S A 2012; 109: 6721–6726. DOI: 10.1073/pnas.1110460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993; 123: 1939–1951. [DOI] [PubMed] [Google Scholar]
- 35.Igarashi M, Gao F, Kim HW, et al. Dietary n-6 PUFA deprivation for 15 weeks reduces arachidonic acid concentrations while increasing n-3 PUFA concentrations in organs of post-weaning male rats. Biochim Biophys Acta 2009; 1791: 132–139. DOI: 10.1016/j.bbalip.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeMar JC, Jr, Ma K, Bell JM, et al. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res 2006; 47: 172–180. DOI: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957; 226: 497–509. [PubMed] [Google Scholar]
- 38.Fernandez-de-Las-Penas C, Simons D, Cuadrado ML, et al. The role of myofascial trigger points in musculoskeletal pain syndromes of the head and neck. Curr Pain Headache Rep 2007; 11: 365–372. [DOI] [PubMed] [Google Scholar]
- 39.Westgaard RH, Mork PJ, Loras HW, et al. Trapezius activity of fibromyalgia patients is enhanced in stressful situations, but is similar to healthy controls in a quiet naturalistic setting: a case-control study. BMC Musculoskelet Disord 2013; 14: 97 DOI: 10.1186/1471-2474-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvheim AR, Torstensen BE, Lin YH, et al. Dietary linoleic acid elevates the endocannabinoids 2-AG and anandamide and promotes weight gain in mice fed a low fat diet. Lipids 2014; 49: 59–69. DOI: 10.1007/s11745-013-3842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruparel S, Green D, Chen P, et al. The cytochrome P450 inhibitor, ketoconazole, inhibits oxidized linoleic acid metabolite-mediated peripheral inflammatory pain. Mol Pain 2012; 8: 73 DOI: 10.1186/1744-8069-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gouveia-Figueira S, Nording ML, Gaida JE, et al. Serum levels of oxylipins in achilles tendinopathy: an exploratory study. PLoS One 2015; 10: e0123114 DOI: 10.1371/journal.pone.0123114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bourre JM, Piciotti M, Dumont O, et al. Dietary linoleic acid and polyunsaturated fatty acids in rat brain and other organs. Minimal requirements of linoleic acid. Lipids 1990; 25: 465–472. [DOI] [PubMed] [Google Scholar]
- 44.Ramsden CE, Ringel A, Feldstein AE, et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids 2012; 87: 135–141. DOI: 10.1016/j.plefa.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson JA, Blackburn ML, Bull AW, et al. Separation and quantitation of linoleic acid oxidation products in mammary gland tissue from mice fed low- and high-fat diets. Lipids 1997; 32: 369–375. [DOI] [PubMed] [Google Scholar]
- 46.DeMar JC, Jr, Lee HJ, Ma K, et al. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim Biophys Acta 2006; 1761: 1050–1059. DOI: 10.1016/j.bbalip.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Serhan CN, Dalli J, Karamnov S, et al. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J 2012; 26: 1755–1765. DOI: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blasbalg TL, Hibbeln JR, Ramsden CE, et al. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr 2011; 93: 950–962. DOI: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.U.S. Department of Agriculture, Agricultural Research Service. Nutrient Intakes from Food: Mean Amounts Consumed per Individual, by Gender and Age, What We Eat in America, National Health and Nutrition Examination Survey (NHANES 2011-2012). www.ars.usda.gov/Services/docs.htm?docid=18349 (accessed 1 August 2015).
- 50.Kuipers RS, Luxwolda MF, Dijck-Brouwer DA, et al. Estimated macronutrient and fatty acid intakes from an East African Paleolithic diet. Br J Nutr 2010; 104: 1666–1687. DOI: 10.1017/S0007114510002679. [DOI] [PubMed] [Google Scholar]
- 51.Camp RD, Mallet AI, Woollard PM, et al. The identification of hydroxy fatty acids in psoriatic skin. Prostaglandins 1983; 26: 431–447. [DOI] [PubMed] [Google Scholar]
- 52.Taha AY, Basselin M, Ramadan E, et al. Altered lipid concentrations of liver, heart and plasma but not brain in HIV-1 transgenic rats. Prostaglandins Leukot Essent Fatty Acids 2012; 87: 91–101. DOI: 10.1016/j.plefa.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farias SE, Basselin M, Chang L, et al. Formation of eicosanoids, E2/D2 isoprostanes, and docosanoids following decapitation-induced ischemia, measured in high-energy-microwaved rat brain. J Lipid Res 2008; 49: 1990–2000. DOI: 10.1194/jlr.M800200-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]