Abstract
Background
The N-methyl-D-aspartate subtype of glutamate receptor plays a critical role in morphine tolerance. D-serine, a co-agonist of N-methyl-D-aspartate receptor, participates in many physiological and pathophysiological processes via regulating N-methyl-D-aspartate receptor activation. The purinergic P2X7 receptor activation can induce the D-serine release in the central nervous system. This study aimed to investigate the role of the ventrolateral midbrain periaqueductal gray D-serine in the mechanism of morphine tolerance in rats. The development of morphine tolerance was induced in normal adult male Sprague–Dawley rats through subcutaneous injection of morphine (10 mg/kg). The analgesic effect of morphine (5 mg/kg, i.p.) was assessed by measuring mechanical withdrawal thresholds in rats with an electronic von Frey anesthesiometer. The D-serine concentration and serine racemase expression levels in the ventrolateral midbrain periaqueductal gray were evaluated through enzyme-linked immunosorbent assay and Western blot analysis, respectively. The effects of intra-ventrolateral midbrain periaqueductal gray injections of the D-serine degrading enzyme D-amino acid oxidase and antisense oligodeoxynucleotide targeting the P2X7 receptor on chronic morphine-treated rats were also explored.
Results
We found that repeated morphine administrations decreased the antinociceptive potency of morphine evidenced by the percent changes in mechanical pain threshold in rats. By contrast, the D-serine contents and the expression levels of the serine racemase protein were upregulated in the ventrolateral midbrain periaqueductal gray in morphine-tolerant rats. The development of morphine tolerance was markedly alleviated by intra-ventrolateral midbrain periaqueductal gray injections of D-amino acid oxidase or antisense oligodeoxynucleotide targeting the P2X7 receptor.
Conclusions
Our data indicate that the development of antinociceptive tolerance to morphine is partially mediated by ventrolateral midbrain periaqueductal gray D-serine content, and the activation of the ventrolateral midbrain periaqueductal gray P2X7 receptor is an essential prelude to D-serine release. These results suggest that a cascade involving P2X7 receptor–D-serine–N-methyl-D-aspartate receptor mediated signaling pathway in the supraspinal mechanism of morphine tolerance.
Keywords: Morphine tolerance, D-serine, P2X7 receptor, midbrain periaqueductal gray
Background
Morphine is an opiate drug that is widely used for the clinical management of moderate to severe pain, but morphine-induced side effects, especially hyperalgesia and tolerance, significantly limit its clinical use. The N-methyl-D-aspartate (NMDA) subtype of glutamate receptor performs critical functions in pain processing including central sensitization that eventually causes hyperalgesia.1,2 Additionally, the activation of NMDA receptor has been implicated as an anti-opioid system in the development of morphine analgesic tolerance and dependence in the central nervous system (CNS).3–6
Numerous studies have revealed that the opening of the NMDA receptor ion channel requires occupation of two distinct binding sites, the glutamate site and the glycine site.7,8 As an endogenous ligand for glycine-bind site in NR1 subunit of NMDA glutamate receptors, D-serine (D-ser) is more potent than glycine.9,10 Besides predominantly expressing in astrocytes, and acting as an important gliotransmitter in the CNS,11 D-ser is also produced and released by neurons12 and behaves as a neurotransmitter in the same area of the brain. Accordingly, by modulating neurotransmission through NMDA receptor, D-ser involves in many vital physiological and pathological progresses, such as synaptic plasticity,13 neural development,14 social interactions,15,16 pain,17 neurotoxicity18 and is supposed as a potential therapeutic target for the treatment of nervous system diseases such as Alzheimer disease, epileptic seizures, amyotrophic lateral sclerosis,19 and psychiatric disorders.20 The D-ser is synthesized from L-serine via racemization of serine racemase (SR), which is a pyridoxal-5′-phosphate-dependent enzyme and regulated by many factors, and, importantly, the regional distribution of SR parallels that of D-ser.21 In the brain, SR is the major enzyme for D-ser production. Wolosker and Mori 22 reported that SR knockout (SR−/−) mice displayed 90% lower D-ser in the brain and deficits in NMDA receptor activity.
The midbrain periaqueductal gray (PAG) is a strategic site in endogenous nociceptive modulatory system.23,24 It is also an essential neural circuit for opioid-mediated analgesia,25–27 especially its ventrolateral PAG (vlPAG) region.28,29 The upregulation of NMDA receptor expression in the PAG facilitates morphine tolerance development. In the chronic morphine-treated mice, the expression of the NMDA receptor in the PAG was upregulated.30 A similar study found that epsilon 1 subunit of the NMDA receptor expression dramatically increased in the PAG of morphine-tolerant mice.6
Adenosine 5′-triphosphate (ATP) is released by neuronal and nonneuronal cells.31 In addition to being an intracellular energy source, ATP is an important neurotransmitter or neuromodulator that activates cation-permeable ion channels (P2X receptors) and G-protein-coupled receptors (P2Y receptors) on the cell surface.32 The P2X7 receptor subtype is widely distributed in glia cells and neuron,33 and its expression is altered in many pathophysiological processes, such as inflammation, pain, and cancer.34–36 The P2X7 receptors are also involved in the morphine tolerance. A previous study found that microglial cells in the rat spinal cord expressed the P2X7 receptor, and the protein level of this receptor was upregulated after chronic exposure to morphine. The suppression of the P2X7 receptor activation significantly attenuated the loss of morphine analgesic potency.37 The activation of P2X7 receptor triggers the release of D-ser from cultured astrocytes, and a spinal mechanism underlying morphine tolerance has been proposed, in which chronic morphine triggered multiple dialogues between glial and neuronal cells in the spinal cord via a cascade involving a P2X7 receptor–interleukin-18–D-ser–NMDA receptor–protein kinase C gamma (PKCγ)-mediated signaling pathway.38,39
Our previous study indicated that the P2X7 receptor expression was more pronounced in the vlPAG in morphine-tolerant rats.40 The current study aimed to elucidate the role of vlPAG D-ser in morphine tolerance. We explored the levels of D-ser and SR protein expression in the vlPAG of morphine-tolerant and control animals and investigated the effect of the D-ser degrading enzyme D-amino acid oxidase (DAAO) on the behavioral tolerance to morphine in rats. Furthermore, we studied whether the activation of P2X7 receptors in the vlPAG was involved in the mechanisms of morphine tolerance through the secretion of D-ser in rats.
Methods
Animals and ethics
Male Sprague–Dawley rats weighing 220 ± 10 g were purchased from the Center of Laboratory Animals, Third Military Medical University (Chongqing City, China). In a temperature-controlled room (25 ± 1℃), the rats were housed in groups of four or five per cage with a 12 h light-dark cycle (7:00 a.m.–7:00 p.m.). Food pellets and water were given to the rats ad libitum throughout the experiments, except during the experimental periods. All studies were approved by the Institutional Animal Care and Use Committee of the Zunyi Medical University and performed in strict compliance with the Ethical Issues of the International Association for the Study of Pain. All efforts were exerted to minimize the number of animals used and their suffering.
Induction of morphine tolerance
The rat model of morphine tolerance was established as previously described.39,40 Briefly, morphine was given subcutaneously (s.c.) twice daily (with a 12-h dosing interval) from day 1 to day 9 at 10 mg/kg body weight to establish systemic analgesic tolerance. The drug dosage was based on our previous study.40 To evaluate the development of morphine tolerance, we assessed morphine antinociception to mechanical stimuli at 30 min after an acute test dose (5 mg/kg) of morphine was intraperitoneally (i.p.) given. Morphine analgesic effects before and after a defined period of tolerance induction were compared. Baseline nociceptive thresholds were measured 15 min before subcutaneous injection of morphine.
Behavioral studies
On each designated test day, the rats were tested for mechanical allodynia after acclimatization to the testing apparatus, and allodynia was tested under nonrestrained conditions. To determine mechanical withdrawal threshold (MWT), we placed each rat in an individual transparent plexiglass cage (18 cm × 12 cm × 12 cm) with a wire mesh floor in a quiet room. The rats were allowed to explore and groom until settling down. The test involved evoking a hind paw flexion reflex (paw withdrawal) with a handheld force transducer (IITC 2390 series electronic von Frey anesthesiometer, Life Science Instruments, Los Angeles, California, USA) equipped with a 0.5-mm2 contact area polypropylene tip. The investigator was trained to perpendicularly apply the tip to the central area of the hind paw with gradually increasing pressure. Endpoint was characterized by removal of the paw, in which the animal actively lifted the whole paw on the tip of the anesthesiometer. Positive responses included prolonged hind paw withdrawal, licking or biting of the hind paw, or shaking the paw with high amplitude movements in response to the stimulus. Each hind paw was measured five times in grams, and the average values of five measurements were regarded as the paw MWT.41 The development of morphine-induced tolerance was detected by measuring percent changes in MWT after a challenge injection of morphine (5 mg/kg, i.p.) and calculated as follows: MWT (%) = (tested threshold − basal threshold)/basal threshold × 100%. A higher MWT% represented a better analgesic effect.
Surgical and microinjection procedures
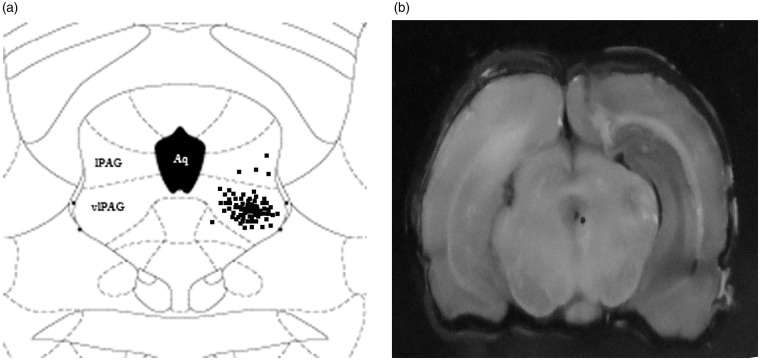
Anesthesia was induced through i.p. injection of 4% chloral hydrate (10 mL/kg body weight). The rats were mounted on a stereotaxic frame (Narishige SR-5R, Tokyo, Japan). The skull was exposed and the bregma was located. A stainless steel guide cannula (0.8 mm o.d.) was inserted unilaterally into the vlPAG and fixed to the skull by using dental zinc cement and jewelers’ screws. The stereotaxic coordinates for the vlPAG were 7.90 mm posterior to the bregma, 0.80 mm lateral to the midline, and 6.00 mm ventral to the skull surface. A dummy cannula was inserted into the guide cannula at the time of surgery to minimize occlusion. Skull screws and dental acrylic were used to hold the cannulae securely in place. After removal from the stereotaxic apparatus, the rats were (i.p.) administered with 1 mL of 0.9% sterile saline to prevent dehydration and then placed in a thermally controlled cage to avoid hypothermia until complete anesthetic recovery. Prior to any experiments, the animals were allowed to recover from the implantation surgery for five days and were monitored for signs of motor impairment. Rats with any neurological deficits caused by the surgical procedure were excluded from the experiments. On the day of intra-vlPAG injection, the rats were transferred from the main holding area to the laboratory and were left undisturbed for 1 h prior to drug administration. Each rat was lightly restrained, and a 32-gauge injection cannula (1.0 mm longer than the guide cannula) was inserted into the guide cannula. The injection cannula was connected to a 5 -µL Hamilton microsyringe. A total volume of 0.3 µL was injected over 3 min, and the injector was left in place for an additional 2 min before slow removal to ensure complete drug diffusion. Successful infusion was confirmed by monitoring the movement of a small air bubble in the microsyringe. After the experimental procedures, the animals were anesthetized through i.p. injection of 4% chloral hydrate (20 mL/kg body weight) and then intracardially perfused with physiological saline (0.9% NaCl) and 4% paraformaldehyde solution. The needle position of the cannula was visually confirmed with 0.2 µL of 2% Evans blue infusion through the microinjection cannula. Administration sites were verified through histological examination and plotted on coronal maps adapted from the atlas of Paxinos and Watson.42 Only rats with whole microinjection sites within the vlPAG were included in analysis. Animals with cannulae located outside the vlPAG (e.g., lateral PAG, dorsal raphe nucleus) were considered “cannulae misses” and discarded (Figure 1a and b).
Figure 1.
(a) Schematic diagram of the microinjection sites for saline, DAAO, and ODN. Dots represent the corresponding sites identified histologically through Evans blue dye microinjection. (b) Photograph of an injection site in the vlPAG. Microinjection sites were mostly distributed within the vlPAG region. Data from rats with an injection site outside the vlPAG region were discarded.
ODNs: oligodeoxynucleotides; DAAO: D-amino acid oxidase; vlPAG: ventrolateral midbrain periaqueductal gray.
Enzyme-linked immunosorbent assay
The vlPAG tissue (100 mg) was homogenized in 1 mL lysis buffer containing 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 0.5% Triton X-100, and Complete Protease Inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) and centrifuged at 3,000 r/min for 20 min at 4℃. The protein concentration of the supernatant (500 µL) was quantified by bicinchoninic acid (BCA) assay kit (Beyotime Institute of Biotechnology, Jiangsu, China). D-ser was measured using commercially available enzyme immunoassays (rat D-serine enzyme-linked immunosorbent assay (ELISA) set, Shanghai Biological Technology Co., Ltd. enzyme research, China) according to the manufacturer’s instructions. The standard curve was included in each experiment, and the protein expression was normalized to the total protein amount per vlPAG tissue and reported as ng/mg wet weight.
Western blot
Animals were anesthetized by an over dose of chloral hydrate (20 mL/kg body weight, i.p.), and the vlPAG tissue was rapidly removed. The collected tissue samples were homogenized in a lysis buffer containing a mixture of protease inhibitors (Roche Diagnostics) and phenylmethylsulfonyl fluoride (Sigma, St Louis, Missouri). Protein samples (20 µg/lane) were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis 5–12% gels, Bio-Rad, Canada) and transferred onto polyvinylidene fluoride membranes (Sigma Aldrich, St Louis, Missouri). The membranes were blocked with 5% nonfat milk and then incubated overnight at 4℃ with a primary antibody (rabbit anti-rat SR receptor, 1:400; rabbit anti-rat P2X7 receptor, 1:600; Abcam Corporation, Shanghai, China). Mouse anti-rat β-actin primary antibody (1:2,000; Sigma-Aldrich, , St Louis, Missouri) was included as a control for protein loading. The membranes were then incubated in goat anti-rabbit horseradish peroxidase (HRP)- or goat anti-mouse HRP-conjugated secondary antibody (1:5000; Santa Cruz Biotechnology, Paso Robles, CA) for 2 h at room temperature before the blots were visualized in enhanced chemiluminescence solution (Amersham Pharmacia Biotech, UK) and exposed to X-ray films. The developed X-ray films were scanned for data analysis. Protein levels were normalized to β-actin as the loading control. Relative optical density (ROD) of the protein bands was measured after subtracting the film background. Data are expressed as mean ratio ± S.E.M of the SR/β-actin protein.
Experimental design and drugs
The experiments consisted of four series. In series 1, changes in MWT values were determined after repeated administrations of morphine. The rats were randomly and equally divided into three groups according to a random number table: normal group, saline group, and morphine group (n = 12 per group). Morphine (10 mg/kg, s.c.) was administered to rats in the morphine group twice a day for nine days. In the saline group, the rats were injected with 1 mL/kg physiological saline solution (0.9% normal saline [NS]), instead of morphine, with the same schedule. The normal group served as control subjects. All experimental rats were subjected to MWT test daily for nine days.
In series 2, alterations in D-ser concentration and SR expression level in the vlPAG were observed. Six rats from each group in the first series were used for ELISA analysis of D-ser concentrations and the remaining six rats for Western blot analysis of SR expression on day 9 post morphine injection.
In series 3, we observed the effects of intra-vlPAG microinjection of different doses of DAAO. The rats were randomly and equally divided into six groups according to a random number table: normal group, normal + 0.1U DAAO group, morphine group, morphine + 0.001U DAAO group, morphine + 0.01U DAAO group, and morphine + 0.1U DAAO group (n = 8 per group). Morphine (10 mg/kg, s.c.) was administered twice a day for six days in all groups, except the normal and normal + 0.1U DAAO groups. DAAO at doses of 0.001U, 0.01U, and 0.1U in 0.3 µL sterile physiological saline (0.9% NS) were intra-vlPAG given on day 1 after morphine injection (s.c.) and once daily for five days. The experimental rats were subjected to MWT test on day 0 and day 6 after morphine injection (s.c.). The corresponding control (DAAO) was intra-vlPAG given with the same schedule.
In series 4, changes in contents of D-ser in the vlPAG in response to oligodeoxynucleotides (ODNs) targeted against P2X7 receptor were determined. A total of 36 normal rats were divided into three groups: saline + morphine group, mismatch ODN + morphine group (MM ODN + morphine), and antisense ODN + morphine group (AS ODN + morphine; n = 12 per group). The MM ODN + morphine and AS ODN + morphine groups were given ODN (15 nmol/0.3 µL) through vlPAG microinjection (from day 5 after morphine injection (s.c.), once daily for 5 days) and morphine (10 mg/kg, s.c.) twice daily for nine consecutive days. In the saline + morphine group, the rats were injected with 0.3 µL of 0.9% NS, instead of ODN, with the same schedule. At the end of the experiment (on day 9 after chronic morphine treatment), six rats from each group were used for Western blot analysis for P2X7 receptor expression and the remaining six rats for ELISA analysis for D-ser levels.
Morphine hydrochloride was purchased from Shenyang First Pharmaceutical Factory (Shenyang City, China). DAAO (Sigma-Aldrich, France) was used at a dose of 0.001U, 0.01U, and 0.1U in 0.3 µL sterilized normal saline. Phosphorothioate-modified oligonucleotides of the rat P2X7 receptor were synthesized and purified by Sangon Biological Engineering Technology Co. (Shanghai City, China). According to our previous report 40, the sequences were designed as follows: P2X7 receptor antisense ODN: 5′-TTG ATG GTG CCG TAA TTC ACG CTC T-3′ targeted to the nucleotide sequence 186 through 210 that directly follows the initiation codon of the rat P2X7 receptor; and mismatch ODN: 5′-AAT TAC ACA GTA AGC GAA CTT AGC C-3′. A database search using the BLAST program indicated that the antisense sequence was specific for the rodent P2X7 receptor. Nevertheless, the search did not identify the corresponding rodent sequence for the mismatch sequence. Antisense and mismatch ODNs for the P2X7 gene were dissolved in double distilled water to a concentration of 50 nmol/µL. ODNs were aliquoted and stored at −20℃, and oligonucleotide treatments were performed as previously described.43
Statistical analysis
Experimental data were processed using GraphPad Prism (version 6.01; GraphPad Software, Inc., San Diego, CA) and SPSS 17.0 (SPSS Inc., Chicago, IL). All data were presented as mean ± standard deviation. Results from the Western blot work and ELISA test were tested using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. Two-way ANOVA followed by Tukey’s post hoc test were used to analyze the data from the time course of morphine tolerance and the effects of drugs on the development of morphine tolerance. A p value < 0.05 was considered to be statistically significant in this study.
Results
Development of tolerance to analgesia produced by repeated morphine injections in rats
Before subcutaneous injection of morphine (day 0), the percentage change in MWT was not significantly different among the normal, saline, and morphine groups (p > 0.05, n = 12 in each group). In the morphine group, the analgesic effect induced by a test dose (5 mg/kg, i.p.) of morphine gradually decreased after the rats received multiple subcutaneous injections of 10 mg/kg morphine twice daily and was nearly abrogated on day 9. This phenomenon was considered as morphine tolerance. In contrast, the percentage changes in MWT in the saline group did not significantly change compared with that in the normal group at all observation time points (all p > 0.05, n = 12 in each group). Moreover, basal MWT did not significantly change during this time period (data not shown; Figure 2).
Figure 2.
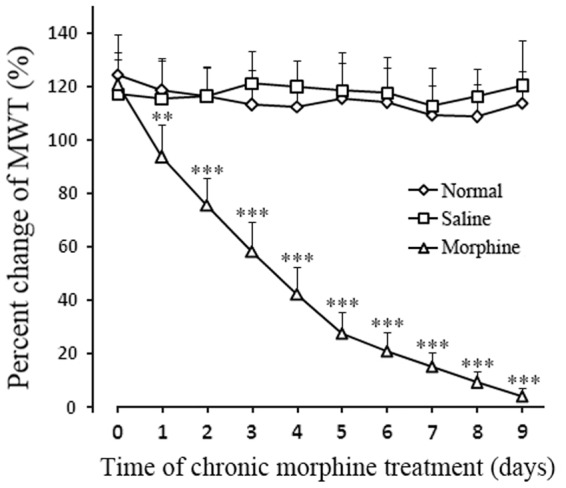
Percentage changes in MWT of the experimental rats subjected to repeated morphine/saline injections. Rats were administered with morphine (10 mg/kg, s.c.) twice daily for nine days. The antinociceptive effect of morphine was measured through the paw withdrawal test after treatment with a test dose of morphine treatment (5 mg/kg, i.p.). The analgesic efficacy of the test dose of morphine significantly decreased after repeated morphine administration. Results are means ± SEM (n = 12). **p < 0.01 and ***p < 0.001 versus data from the normal group.
MWT: mechanical withdrawal threshold.
Increased release of D-ser in the vlPAG induced by chronic administrations of morphine
By using the ELISA method, we detected D-ser release levels in the vlPAG in three experimental groups (normal, saline, and morphine group) of rats. As shown in Figure 3, there was a low level of D-ser in the vlPAG of normal rats and no detectable change in D-ser level following saline injection on day 9 time point (p > 0.05, n = 6, Figure 3). In contrast, D-ser content was significantly elevated on day 9 of chronic morphine treatment (10 mg/kg, s.c., twice daily for nine days consecutively; p < 0.001, n = 6, Figure 3) along with the development of tolerance to morphine analgesia, suggesting an involvement of D-ser release in the vlPAG in the development of morphine tolerance.
Figure 3.
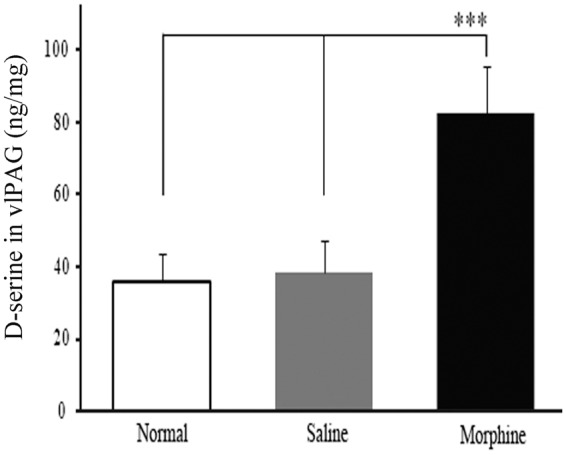
Alterations in D-ser levels in the vlPAG in experimental rats. The multiple morphine treatments induced an increased D-ser level in the vlPAG in morphine-tolerant rats. (n = 6). ***p < 0.001versus normal or saline group at the same time point.
vlPAG: ventrolateral midbrain periaqueductal gray.
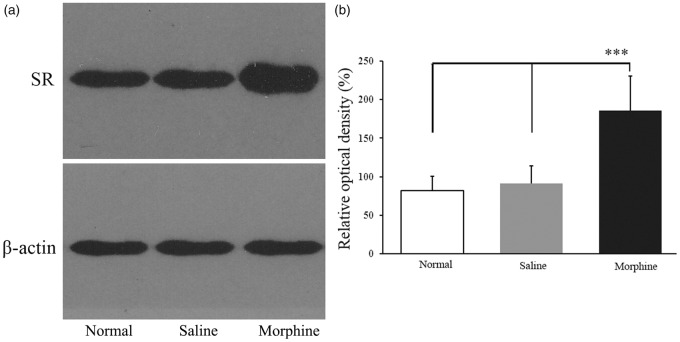
Upregulation of SR expression in the vlPAG in morphine-tolerant rats
Immunoblots from vlPAG homogenates revealed the presence of an immunopositive band of the SR in the normal, saline, and morphine groups. The results were quantified based on the ROD of the immunoblot bands compared with β-actin calculated from the densitometric quantification of the bands. No significant difference was detected between the normal and saline groups (p > 0.05, n = 6). The protein level of the SR was significantly higher in the morphine group than those in the normal and saline groups on nine days after chronic morphine treatment (all p < 0.001, n = 6; Figure 4a and b).
Figure 4.
(a) Upregulation of SR protein level in the vlPAG induced by chronic morphine treatment. Western blot analysis detected a protein band of approximately 40 kDa, which coincides with the known molecular weight of the SR. β-actin was used as the loading control. (b) The protein levels of the SR in different groups were expressed as ROD. (n = 6 in each group). ***p < 0.001 versus normal or saline group.
SR: serine racemase; vlPAG: ventrolateral midbrain periaqueductal gray; ROD: relative optical density.
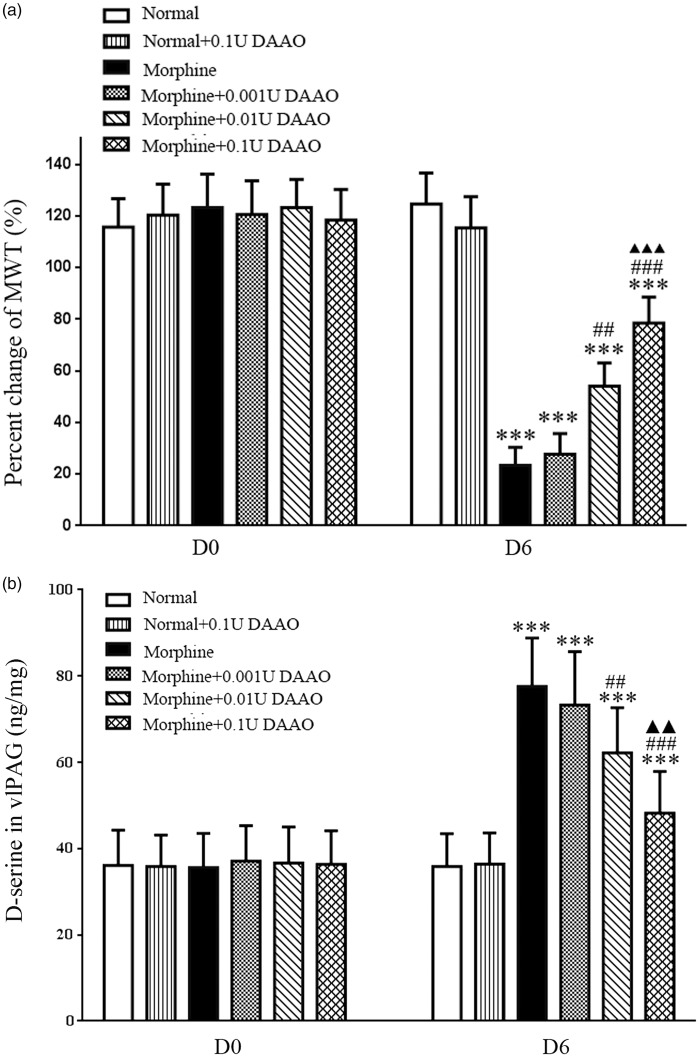
Effect of microinjection of DAAO on MWT in morphine-tolerant rats
To address the importance of vlPAG D-ser content in morphine tolerance, we undertook intra-vlPAG administration of DAAO, an enzyme that selectively degrading D-ser, on morphine-tolerant rats for six days. The administration of DAAO resulted in a markedly decrease in D-ser levels in the vlPAG and alleviated the morphine-tolerant behaviors (p < 0.001, n = 8). Moreover, DAAO acted in a dose-dependent manner, as the highest dose (0.1U) induced a more significant diminution in D-ser levels in the vlPAG and morphine-tolerant behaviors than a moderate dose (0.01U; p < 0.001, n = 8).The use of low dose (0.001 U) DAAO revealed no significant changes in D-ser level in the vlPAG and morphine-tolerant behaviors compared with morphine group (p > 0 .05, n = 8).The percentage changes in MWT in the normal + 0.1U DAAO group did not significantly change in D-ser levels in the vlPAG and morphine-tolerant behaviors compared with that in normal group (p > 0 .05, n = 8; Figure 5a and (b)).
Figure 5.
Attenuation of the development of morphine tolerance by intra-vlPAG injection of DAAO. The D-ser levels in the vlPAG and chronic morphine-induced analgesic tolerance were attenuated in mechanic withdrawal tests by the intra-vlPAG pretreatment of the DAAO. ***p < 0.001, versus normal group; ##p < 0.01, ###p < 0.001 versus morphine group; ▴▴p < 0.01, ▴▴▴p < 0.001 versus morphine + 0.01U DAAO group.
vlPAG: ventrolateral midbrain periaqueductal gray; DAAO: D-amino acid oxidase; D0: day 0; D6: day 6.
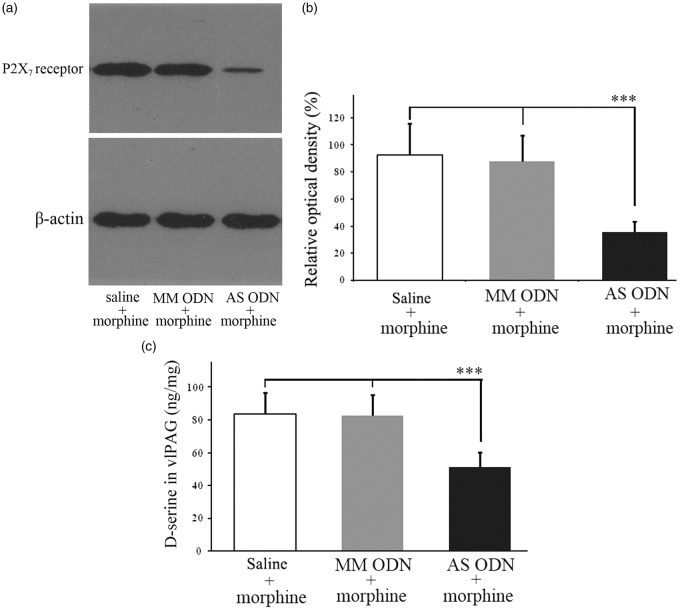
Effect of ODN against P2X7 receptor on D-ser level in the vlPAG
In this experimental procedure, a reversal effect of intra-vlPAG injection of AS ODN targeting the P2X7 receptor on D-ser level was observed. Western blot analysis indicated that delivery of AS ODN (15 nmol/0.3 µL) for five consecutive days in AS ODN + morphine group significantly downregulated the expression of the P2X7 receptor in the vlPAG compared with saline + morphine group or MM ODN + morphine group (all p < 0.001, n = 12). The expression levels of P2X7 receptor in the vlPAG were not significantly different between the saline + morphine and MM ODN + morphine group (p > 0.05, n = 12; Figure 6a and b). Using the ELISA method, we found that there was a lower level of D-ser in the vlPAG in AS ODN + morphine group compared with saline + morphine and MM ODN + morphine group (p < 0.001, n = 12). The difference in the level of D-ser in the vlPAG in MM ODN + morphine group, when compared with the saline + morphine group, was insignificant (p > 0.05, n = 12; Figure 6c).
Figure 6.
(a) and (b) P2X7 receptor antisense ODN, but not mismatch ODN, at 15 nmol/0.3 µL intra-vlPAG injection once daily for five days reduced P2X7 receptor expression in the vlPAG in rats. ***p < 0.001 versus data from the saline + morphine group or MM ODN + morphine group (n = 12 in each group). (c) ELISA showed the D-ser level in the AS ODN + morphine group was reduced compared with saline + morphine group (***p < 0.001). Conversely, the D-ser levels in the MM ODN + morphine group were not significantly different compared with that in the saline + morphine group (p > 0.05).
vlPAG: ventrolateral midbrain periaqueductal gray; MM ODN: mismatch oligodeoxynucleotide; AS ODN: antisense oligodeoxynucleotide
Discussion
The present set of experiments attempted to testify the hypothesis that D-ser in the vlPAG contributes to the development of chronic morphine tolerance in rats. The major findings in the current study are as follows: (a) repetitive applications of morphine could gradually induce morphine tolerance in rats; (b) the chronic morphine administration dramatically elevated the D-ser concentration and SR expression levels in the vlPAG in morphine-tolerant rats; (c) the analgesic effect of morphine was partly but significantly maintained by the vlPAG microinjection of DAAO; (d) intra-vlPAG injection of the ODN against P2X7 receptor markedly decreased D-ser level in the vlPAG and inhibited the development of morphine tolerance. These results demonstrated the important role of vlPAG D-ser in morphine-tolerant rats and suggested a direct implication of P2X7 receptor-D-ser pathway in the formation of morphine tolerance in rats.
D-amino acids are stereoisomers of naturally occurring L-amino acids. They are initially considered as unnatural amino acids and were thought to be only existed in bacteria and invertebrate species. However, the development and improvement of analytical methods and instruments have revealed the presence of D-amino acids such as D-ser and D-aspartate even in mammalian brains.44 It has been reported that D-ser immunoreactivity is abundant in the cerebral cortex, hippocampus, anterior olfactory nucleus, and amygdala of the rat brain.45 In the current study, we also found that a relative low level of D-ser content presented in the vlPAG in normal or saline-treated rats. Contrarily, after nine days of morphine treatment, the D-ser content in the vlPAG was obviously elevated, and the rats developed substantial tolerance to morphine analgesia, implicating an involvement of D-ser release in the vlPAG in the development of morphine tolerance.
D-ser has been initially reported to be released from astrocytes via large vesicles46 or exocytosis.47–49 It must be noted that several recent reports have described that neurons may also release D-ser and glycine.50,51 By using a conditional cell-specific SR-knockout mice, a lower forebrain D-ser level along with long-term potentiation (LTP) deficits were observed when SR gene was deleted in neurons while deletion in astrocytes only leads to a minimal decrease in forebrain SR expression and no significant change in D-ser level and NMDA receptor activity.52 Based on these findings, an elegant hypothesis of a serine shuttle between neurons and astrocytes was established.53 Namely, neuronal D-ser depends on the production of L-serine by astrocytes because the 3-phosphoglycerate dehydrogenase which catalyzes the production of L-serine from glucose is exclusively located in astrocytes.51,54,55 L-serine is then exported and shuttles to neurons to fuel the synthesis of D-ser by SR. Finally, D-ser is released by neurons and accumulates back in astrocytes.53 As a result, neurons and glial cells all play an important role in the metabolism of D-ser.
The glutamatergic NMDA receptor signaling pathways have been studied as targets for intervention in a variety of neuropathological conditions like neurodegenerations, epilepsy, neuropathic pain, drug addiction, and schizophrenia. In morphine tolerance, activation of the NMDA receptor has been implicated as an anti-opioid system in the development of morphine analgesic tolerance and dependence. At the supraspinal sites, both the NMDA receptor and μ-opioid receptor (MOR) are present in the PAG neurons, and the activation of NMDA receptor restricts the activity of the MOR. The cross talk between these receptors is sustained by the MOR-associated histidine triad nucleotide binding protein 1.56 In general, the activation of NMDA receptor can be an important factor for promoting the morphine tolerance.
As the activation of NMDA receptor is involved in above-mentioned disorders, high activity NMDA-blocking agents have been designed to treat some of these disorders; however, their effect is often compromised by undesirable side effects. Therefore, alternative ways of modulating NMDA receptor function need to be sought after. Numerous studies have revealed that D-ser is more effective than glycine as an NMDA receptor co-agonist9,10,57 and the distribution pattern of D-ser closely resembles that of NMDA receptors, therefore D-ser and its metabolism-related enzymes became the potential candidates for this purpose.
It has been reported that chronic administration of morphine produced a significant elevation of both the mRNA and protein expressions of SR in all the brain regions, whereas no significant change in the protein expression of DAAO was observed in all the brain regions.58 But SR is a very difficult target, with only few compounds so far identified exhibiting weak inhibitory activity,59 therefore we used DAAO in the current study and found that the vlPAG microinjection of DAAO was effective in restoring morphine analgesic effect, indicating the involvement of D-ser and glycine site in NMDA receptor in morphine tolerance. Together, our findings support the idea that endogenous D-ser in the vlPAG is a key co-agonist of NMDA receptor overactivation in the induction of morphine tolerance.
On the basis of the signal transduction mechanisms and characteristic molecular structures, the P2 purinoceptor can be divided into the P2X receptors (P2X1–7) and P2Y receptors (P2Y1,2,4,6,11,12,13,14).60–62 P2X receptors are cationic-selective ion channels gated by extracellular ATP. With techniques, results confirmed that the P2X7 receptors are localized in the rodent CNS neurons.63–65 Previous works have also suggested the expression of P2X7 receptor on CNS glial cells including astrocytes and microglial cells.66–68 Compared with other P2X receptors, P2X7 receptor have a lower affinity for ATP,69 indicating that their activation mostly occurs in pathological conditions associated with enhanced extracellular ATP levels. In peripheral tissues, P2X7 receptor mediates inflammation, cancer, cell proliferation, and apoptosis.70 In the nervous system, it modulates neurotransmitter release, as well as microglial and astroglial activation.33 The activation of P2X7 receptor on neuronal or nonneuronal cells is related to many brain disorders such as trauma,71,72 Alzheimer’s disease,73,74 Parkinson’s disease,75,76 and multiple sclerosis.77 In morphine tolerance, after chronic exposure to morphine, the protein expression of the P2X7 receptor in spinal microglia was upregulated and morphine tolerance was developed. Intrathecal administration of Brilliant Blue G, a potent P2X7 receptor inhibitor, or RNA interference targeting the spinal P2X7 receptor significantly attenuated the loss of morphine analgesic potency, upregulated P2X7 receptor expression, and activated microglia.37 So far, two mechanisms have been proposed to explain the P2X7 receptor activation-induced D-ser release. One is the pannexin-1 hemichannel, the P2X7 receptor-mediated D-ser release by the P2X7 receptor–pannexin-1 complex formation; the other one is the activated P2X7 receptor per se is also functioned as a permeation channel to release D-ser in part.38 In this study, we found that the use of AS ODN against P2X7 receptor reduced the level of D-ser in the vlPAG, so we speculated that under the circumstance of morphine tolerance, P2X7 receptor can promote the release of serine. As P2X7 receptor formed a channel or a P2X7 receptor–pannexin-1 complex in the D-ser-releasing mechanism, further investigations are demanded.
The racemase activity was independently stimulated by both Mg2+ and ATP.78 In our previous report, we found the expression of P2X7 receptor in the vlPAG was increased in morphine-tolerant rats. Combined with the findings from the current study that the expression of SR protein in the vlPAG was upregulated, we believed that an increased extracellular ATP level was presented in morphine-tolerant rat which is sufficient for the activation of both the P2X7 receptor and SR.
In our previous study, we found that the expression of the vlPAG P2X7 receptor significantly increased in morphine-tolerant rats, but P2X7 receptor antagonist A-740003 reduced the development of chronic morphine tolerance in rats.40 In the current study, we further revealed that intra-vlPAG injection of AS ODN against P2X7 receptor prominently decreased the P2X7 protein expression and D-ser concentration in the vlPAG in morphine-tolerant rats. Herein, the activation of P2X7 receptor leaded to an increased D-ser release in the vlPAG and thus at least in part contributed to the NMDA receptor activation-induced morphine tolerance in rats. The D-ser in the vlPAG is a potential molecule for the treatment of morphine tolerance.
Author contributions
SC, ZX, YL, and MS performed the experiments. ZX analyzed the data and drafted the manuscript. All authors read and approved the final version of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the National Natural Science Foundation of China (grant number 31160207 to Zhi Xiao).
References
- 1.Ma W, Quirion R. Targeting cell surface trafficking of pain-facilitating receptors to treat chronic pain conditions. Expert Opin Ther Targets 2014; 18: 459–472. [DOI] [PubMed] [Google Scholar]
- 2.Zhou HY, Chen SR, Pan HL. Targeting N-methyl-D-aspartate receptors for treatment of neuropathic pain. Expert Rev Clin Pharmacol 2011; 4: 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim G, Wang S, Zeng Q, et al. Expression of spinal NMDA receptor and PKC gamma after chronic morphine is regulated by spinal glucocorticoid receptor. J Neurosci 2005; 25: 11145–11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci 1994; 14: 2301–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martini L, Whistler JL. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol 2007; 17: 556–564. [DOI] [PubMed] [Google Scholar]
- 6.Inoue M, Mishina M, Ueda H. Locus-specific rescue of GluRepsilon1 NMDA receptors in mutant mice identifies the brain regions important for morphine tolerance and dependence. J Neurosci 2003; 23: 6529–6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schell MJ. The N-methyl D-aspartate receptor glycine site and D-serine metabolism: an evolutionary perspective. Philos Trans R Soc Lond B Biol Sci 2004; 359: 943–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henneberger C, Bard L, King C, et al. NMDA receptor activation: two targets for two co-agonists. Neurochem Res 2013; 38: 1156–1162. [DOI] [PubMed] [Google Scholar]
- 9.Shleper M, Kartvelishvily E, Wolosker H. D-serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J Neurosci 2005; 25: 9413–9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsui T, Sekiguchi M, Hashimoto A, et al. Functional comparison of D-serine and glycine in rodents: the effect on cloned NMDA receptors and the extracellular concentration. J Neurochem 1995; 65: 454–458. [DOI] [PubMed] [Google Scholar]
- 11.Martineau M. Gliotransmission: focus on exocytotic release of L-glutamate and D-serine from astrocytes. Biochem Soc Trans 2013; 41: 1557–1561. [DOI] [PubMed] [Google Scholar]
- 12.Wolosker H, Dumin E, Balan L, et al. D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration. FEBS J 2008; 275: 3514–3526. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg D, Artoul S, Segal AC, et al. Neuronal D-serine and glycine release via the Asc-1 transporter regulates NMDA receptor-dependent synaptic activity. J Neurosci 2013; 33: 3533–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim PM, Aizawa H, Kim PS, et al. Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc Natl Acad Sci U S A 2005; 102: 2105–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labrie V, Duffy S, Wang W, et al. Genetic inactivation of D-amino acid oxidase enhances extinction and reversal learning in mice. Learn Mem 2009; 16: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeVito LM, Balu DT, Kanter BR, et al. Serine racemase deletion disrupts memory for order and alters cortical dendritic morphology. Genes Brain Behav 2011; 10: 210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dieb W, Hafidi A. Astrocytes are involved in trigeminal dynamic mechanical allodynia: potential role of D-serine. J Dent Res 2013; 92: 808–813. [DOI] [PubMed] [Google Scholar]
- 18.Inoue R, Hashimoto K, Harai T, et al. NMDA- and beta-amyloid1-42-induced neurotoxicity is attenuated in serine racemase knock-out mice. J Neurosci 2008; 28: 14486–14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasabe J, Miyoshi Y, Suzuki M, et al. D-amino acid oxidase controls motoneuron degeneration through D-serine. Proc Natl Acad Sci U S A 2012; 109: 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacchi S, Caldinelli L, Cappelletti P, et al. Structure-function relationships in human D-amino acid oxidase. Amino Acids 2012; 43: 1833–1850. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto A, Oka T. Free D-aspartate and D-serine in the mammalian brain and periphery. Prog Neurobiol 1997; 52: 325–353. [DOI] [PubMed] [Google Scholar]
- 22.Wolosker H, Mori H. Serine racemase: an unconventional enzyme for an unconventional transmitter. Amino Acids 2012; 43: 1895–1904. [DOI] [PubMed] [Google Scholar]
- 23.Millan MJ. Descending control of pain. Prog Neurobiol 2002; 66: 355–474. [DOI] [PubMed] [Google Scholar]
- 24.Tavares I, Lima D. From neuroanatomy to gene therapy: searching for new ways to manipulate the supraspinal endogenous pain modulatory system. J Anat 2007; 211: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basbaum AI, Clanton CH, Fields HL. Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. J Comp Neurol 1978; 178: 209–224. [DOI] [PubMed] [Google Scholar]
- 26.Fyfe LW, Cleary DR, Macey TA, et al. Tolerance to the antinociceptive effect of morphine in the absence of short-term presynaptic desensitization in rat periaqueductal gray neurons. J Pharmacol Exp Ther 2010; 335: 674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tortorici V, Nogueira L, Salas R, et al. Involvement of local cholecystokinin in the tolerance induced by morphine microinjections into the periaqueductal gray of rats. Pain 2003; 102: 9–16. [DOI] [PubMed] [Google Scholar]
- 28.Mehalick ML, Ingram SL, Aicher SA, et al. Chronic inflammatory pain prevents tolerance to the antinociceptive effect of morphine microinjected into the ventrolateral periaqueductal gray of the rat. J Pain 2013; 14: 1601–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macey TA, Bobeck EN, Suchland KL, et al. Change in functional selectivity of morphine with the development of antinociceptive tolerance. Br J Pharmacol 2015; 172: 549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsushita Y, Omotuyi IO, Mukae T, et al. Microglia activation precedes the anti-opioid BDNF and NMDA receptor mechanisms underlying morphine analgesic tolerance. Curr Pharm Des 2013; 19: 7355–7361. [DOI] [PubMed] [Google Scholar]
- 31.Koles L, Furst S, Illes P. Purine ionotropic (P2X) receptors. Curr Pharm Des 2007; 13: 2368–2384. [DOI] [PubMed] [Google Scholar]
- 32.Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci 2006; 27: 166–176. [DOI] [PubMed] [Google Scholar]
- 33.Sperlagh B, Vizi ES, Wirkner K, et al. P2X7 receptors in the nervous system. Prog Neurobiol 2006; 78: 327–346. [DOI] [PubMed] [Google Scholar]
- 34.Alves LA, Bezerra RJ, Faria RX, et al. Physiological roles and potential therapeutic applications of the P2X7 receptor in inflammation and pain. Molecules 2013; 18: 10953–10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di, Virgilio F. Purines, purinergic receptors, and cancer. Cancer Res 2012; 72: 5441–5447. [DOI] [PubMed] [Google Scholar]
- 36.Franceschini A, Adinolfi E. P2X receptors: new players in cancer pain. World J Biol Chem 2014; 5: 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou D, Chen ML, Zhang YQ, et al. Involvement of spinal microglial P2X7 receptor in generation of tolerance to morphine analgesia in rats. J Neurosci 2010; 30: 8042–8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan HC, Chou YC, Sun SH. P2X7 R-mediated Ca(2+)-independent d-serine release via pannexin-1 of the P2X7 R-pannexin-1 complex in astrocytes. Glia 2015; 63: 877–893. [DOI] [PubMed] [Google Scholar]
- 39.Chen ML, Cao H, Chu YX, et al. Role of P2X7 receptor-mediated IL-18/IL-18R signaling in morphine tolerance: multiple glial-neuronal dialogues in the rat spinal cord. J Pain 2012; 13: 945–958. [DOI] [PubMed] [Google Scholar]
- 40.Xiao Z, Li YY, Sun MJ. Activation of P2X receptors in the midbrain periaqueductal gray of rats facilitates morphine tolerance. Pharmacol Biochem Behav 2015; 135: 145–153. [DOI] [PubMed] [Google Scholar]
- 41.Cunha TM, Verri WA, Jr, Vivancos GG, et al. An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res 2004; 37: 401–407. [DOI] [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 6th ed Amsterdam: Academic Press, 2007. [Google Scholar]
- 43.Xiao Z, Ou S, He WJ, et al. Role of midbrain periaqueductal gray P2X3 receptors in electroacupuncture-mediated endogenous pain modulatory systems. Brain Res 2010; 1330: 31–44. [DOI] [PubMed] [Google Scholar]
- 44.Hamase K, Morikawa A, Etoh S, et al. Analysis of small amounts of D-amino acids and the study of their physiological functions in mammals. Anal Sci 2009; 25: 961–968. [DOI] [PubMed] [Google Scholar]
- 45.Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A 1995; 92: 3948–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang N, Peng H, Yu Y, et al. Astrocytes release D-serine by a large vesicle. Neuroscience 2013; 240: 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coco S, Calegari F, Pravettoni E, et al. Storage and release of ATP from astrocytes in culture. J Biol Chem 2003; 278: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 48.Bezzi P, Gundersen V, Galbete JL, et al. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci 2004; 7: 613–620. [DOI] [PubMed] [Google Scholar]
- 49.Mothet JP, Pollegioni L, Ouanounou G, et al. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci U S A 2005; 102: 5606–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balu DT, Takagi S, Puhl MD, et al. D-serine and serine racemase are localized to neurons in the adult mouse and human forebrain. Cell Mol Neurobiol 2014; 34: 419–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ehmsen JT, Ma TM, Sason H, et al. D-serine in glia and neurons derives from 3-phosphoglycerate dehydrogenase. J Neurosci 2013; 33: 12464–12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benneyworth MA, Li Y, Basu AC, et al. Cell selective conditional null mutations of serine racemase demonstrate a predominate localization in cortical glutamatergic neurons. Cell Mol Neurobiol 2012; 32: 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolosker H. Serine racemase and the serine shuttle between neurons and astrocytes. Biochim Biophys Acta 2011; 1814: 1558–1566. [DOI] [PubMed] [Google Scholar]
- 54.Yamasaki M, Yamada K, Furuya S, et al. 3-Phosphoglycerate dehydrogenase, a key enzyme for l-serine biosynthesis, is preferentially expressed in the radial glia/astrocyte lineage and olfactory ensheathing glia in the mouse brain. J Neurosci 2001; 21: 7691–7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang JH, Wada A, Yoshida K, et al. Brain-specific Phgdh deletion reveals a pivotal role for L-serine biosynthesis in controlling the level of D-serine, an N-methyl-D-aspartate receptor co-agonist, in adult brain. J Biol Chem 2010; 285: 41380–41390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garzon J, Herrero-Labrador R, Rodriguez-Munoz M, et al. HINT1 protein: a new therapeutic target to enhance opioid antinociception and block mechanical allodynia. Neuropharmacology 2015; 89: 412–423. [DOI] [PubMed] [Google Scholar]
- 57.Fadda E, Danysz W, Wroblewski JT, et al. Glycine and D-serine increase the affinity of N-methyl-D-aspartate sensitive glutamate binding sites in rat brain synaptic membranes. Neuropharmacology 1988; 27: 1183–1185. [DOI] [PubMed] [Google Scholar]
- 58.Yoshikawa M, Shinomiya T, Takayasu N, et al. Long-term treatment with morphine increases the D-serine content in the rat brain by regulating the mRNA and protein expressions of serine racemase and D-amino acid oxidase. J Pharmacol Sci 2008; 107: 270–276. [DOI] [PubMed] [Google Scholar]
- 59.Beato C, Pecchini C, Cocconcelli C, et al. Cyclopropane derivatives as potential human serine racemase inhibitors: unveiling novel insights into a difficult target. J Enzyme Inhib Med Chem 2015.. DOI: 10.3109/14756366.2015.1057720. [DOI] [PubMed] [Google Scholar]
- 60.Chen ZP, Levy A, Lightman SL. Nucleotides as extracellular signalling molecules. J Neuroendocrinol 1995; 7: 83–96. [DOI] [PubMed] [Google Scholar]
- 61.Fredholm BB. Purinoceptors in the nervous system. Pharmacol Toxicol 1995; 76: 228–239. [DOI] [PubMed] [Google Scholar]
- 62.Illes P, Ribeiro JA. Neuronal P2 receptors of the central nervous system. Curr Top Med Chem 2004; 4: 831–838. [DOI] [PubMed] [Google Scholar]
- 63.Diaz-Hernandez M, Diez-Zaera M, Sanchez-Nogueiro J, et al. Altered P2X7-receptor level and function in mouse models of Huntington’s disease and therapeutic efficacy of antagonist administration. FASEB J 2009; 23: 1893–1906. [DOI] [PubMed] [Google Scholar]
- 64.Yu Y, Ugawa S, Ueda T, et al. Cellular localization of P2X7 receptor mRNA in the rat brain. Brain Res 2008; 1194: 45–55. [DOI] [PubMed] [Google Scholar]
- 65.Sanchez-Nogueiro J, Marin-Garcia P, Bustillo D, et al. Subcellular distribution and early signalling events of P2X7 receptors from mouse cerebellar granule neurons. Eur J Pharmacol 2014; 744: 190–202. [DOI] [PubMed] [Google Scholar]
- 66.Trang T, Beggs S, Salter MW. ATP receptors gate microglia signaling in neuropathic pain. Exp Neurol 2012; 234: 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ying YL, Wei XH, Xu XB, et al. Over-expression of P2X7 receptors in spinal glial cells contributes to the development of chronic postsurgical pain induced by skin/muscle incision and retraction (SMIR) in rats. Exp Neurol 2014; 261: 836–843. [DOI] [PubMed] [Google Scholar]
- 68.Hashioka S, Wang YF, Little JP, et al. Purinergic responses of calcium-dependent signaling pathways in cultured adult human astrocytes. BMC Neurosci 2014; 15: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol 2009; 71: 333–359. [DOI] [PubMed] [Google Scholar]
- 70.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 2007; 87: 659–797. [DOI] [PubMed] [Google Scholar]
- 71.Kimbler DE, Shields J, Yanasak N, et al. Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS One 2012; 7: e41229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Hare, Doig RL, Fitzgerald M. Novel combinations of ion channel inhibitors for treatment of neurotrauma. Discov Med 2015; 19: 41–47. [PubMed] [Google Scholar]
- 73.Sanz JM, Falzoni S, Rizzo R, et al. Possible protective role of the 489C>T P2X7R polymorphism in Alzheimer’s disease. Exp Gerontol 2014; 60: 117–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diaz-Hernandez JI, Gomez-Villafuertes R, Leon-Otegui M, et al. In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer’s disease through GSK3beta and secretases. Neurobiol Aging 2012; 33: 1816–1828. [DOI] [PubMed] [Google Scholar]
- 75.Liu H, Han X, Li Y, et al. Association of P2X7 receptor gene polymorphisms with sporadic Parkinson’s disease in a Han Chinese population. Neurosci Lett 2013; 546: 42–45. [DOI] [PubMed] [Google Scholar]
- 76.Carmo MR, Menezes AP, Nunes AC, et al. The P2X7 receptor antagonist Brilliant Blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology 2014; 81: 142–152. [DOI] [PubMed] [Google Scholar]
- 77.Grygorowicz T, Sulejczak D, Struzynska L. Expression of purinergic P2X7 receptor in rat brain during the symptomatic phase of experimental autoimmune encephalomyelitis and after recovery of neurological deficits. Acta Neurobiol Exp (Wars) 2011; 71: 65–73. [DOI] [PubMed] [Google Scholar]
- 78.De, Miranda J, Panizzutti R, Foltyn VN, et al. Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-D-aspartate (NMDA) receptor coagonist D-serine. Proc Natl Acad Sci U S A 2002; 99: 14542–14547. [DOI] [PMC free article] [PubMed] [Google Scholar]