Abstract
Background
Anaphase-promoting complex/cyclosome (APC/C) and its co-activator Cdh1 are important ubiquitin-ligases in proliferating cells and terminally differentiated neurons. In recent years, APC/C-Cdh1 has been reported as an important complex contributing to synaptic development and transmission. Interestingly, cortical APC/C-Cdh1 is found to play a critical role in the maintenance of neuropathic pain, but it is not clear whether APC/C-Cdh1 in spinal dorsal cord is involved in molecular mechanisms of neuropathic pain conditions.
Results
Immunostaining showed that Cdh1 was mainly distributed in dorsal horn neurons of the spinal cord in rats. Its expression was downregulated in the ipsilateral dorsal horn at 14 days after spared nerve injury. Rescued expression of Cdh1 in spinal cord by intrathecal administration of recombinant lentivirus encoding Cdh1 (Lenti-Cdh1-GFP) significantly attenuated spared nerve injury-induced mechanical allodynia. Furthermore, rescued expression of spinal Cdh1 significantly reduced surface membrane expression of GluR1, but increased the expression of GluR1-related erythropoietin-producing human hepatocellular receptor A4 and its ligand EphrinA1 in dorsal horn of spared nerve injury-treated animals.
Conclusions
This study indicates that a downregulation of Cdh1 expression in spinal dorsal horn is involved in molecular mechanisms underlying the maintenance of neuropathic pain. Upregulation of spinal Cdh1 may be a promising approach to treat neuropathic pain.
Keywords: Anaphase-promoting complex, Cdh1, neuropathic pain, GluR1, spinal cord
Background
Neuropathic pain is an important public health problem caused by nerve damage or pathological adaptations in the somatosensory system.1 Understanding cellular and molecular mechanisms is helpful for clinical management of neuropathic pain.
The ubiquitin proteasome system (UPS), a major proteolytic system in eukaryotic cells,2 has been reported to be involved in mechanisms underlying the development and maintenance of chronic pain. The protein degradation mediated by the UPS involves two steps: the coordinated action of the E1 ubiquitin-activating enzyme, E2 ubiquitin-conjugating enzymes and E3 ubiquitin ligases that leads to the conjugation of ubiquitin to a given cellular protein; and the degradation of the ubiquitinated protein mediated by the 26 S proteasome complex.3 Levels of ubiquitin-activating enzyme E1A, 20 S proteasome unit and free ubiquitin are reduced in the spinal cord of neuropathic rats, which demonstrated the downregulation of the UPS activity under the condition of neuropathic pain.4 Inhibition of the UPS activity by proteasome inhibitors attenuates the initiation and maintenance of adjuvant arthritis5,6 and neuropathic pain.4,7
The first step of protein degradation mediated by the UPS is named as ubiquitination. Briefly, at first the ubiquitin is activated and transferred to the E1; the activated ubiquitin is subsequently conjugated to the E2; then the E2-loaded ubiquitin is transferred to a substrate, catalyzed by an E3.3 Although a large number of components in the UPS have been identified and characterised, the high efficiency and selectivity of ubiquitination reactions are based on the E3 ubiquitin ligases.3 Taken together, focus on E3 ubiquitin ligases as specific drug targets might be a promising approach to chronic pain administration. However, little is currently known about the role of E3 ligases in the development of persistent pain.8–10
Anaphase-promoting complex/cyclosome (APC/C), a cullin-RING-E3 ubiquitin ligase, plays an important role in the cell cycle transition in proliferating cells.11 APC/C is activated by interaction with either Cdc20 or Cdh1, co-activators that determine the substrate specificity of APC/C.12 Since the discovery of the APC/C-Cdh1 axis in postmitotic neurons,13 several studies have demonstrated the critical roles of APC/C-Cdh1 in the central nervous system, including control of axonal growth14–16 and regulation of neuronal survival and differentiation.17–19 Importantly, a series of experiments have shown that APC/C-Cdh1 is also required for synaptic development and activity-dependent synaptic plasticity.20–22 Additionally, long-lasting synaptic plasticity in the somatosensory system has been recognised as molecular mechanisms of persistent pain.23 These synaptic changes occur on both functional and structural levels.24 For example, studies have shown that the development of neuropathic pain is accompanied by activity-dependent synaptic plasticity in the spinal dorsal horn.25,26 Based on the role of Cdh1 in regulating synaptic plasticity and involvement of some neurological disorders,27 we hypothesise that APC/C-Cdh1 signalling pathway may contribute to molecular mechanisms of synaptic plasticity in spinal cord underlying the development of persistent pain.
In this study, we examined the distribution of Cdh1 and its changes in rat spinal cord after spared nerve injury (SNI). We also analysed the effects of rescued expression of Cdh1 in spinal cord by intrathecal administration of lentivirus encoding Cdh1 on SNI-induced mechanical hypersensitivity. Finally, a study showed that Cdh1 is involved in the downregulation of the surface expression of the calcium-permeable alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptor GluR1 subunit in mammalian neurons by interacting with erythropoietin-producing human hepatocellular receptor A4 (EphA4).21 It has been demonstrated that increased expression of GluR1 in spinal dorsal horn neurons is responsible to central sensitisation and behavioural hypersensitivity after tissue and nerve injury.23 Together, these prompted us to investigate whether the function of APC/C-Cdh1 signalling in neuropathic pain is linked to GluR1.
Methods
Animals
Male Sprague-Dawley rats (200–250 g, eight weeks old) were obtained from Tongji Medical College Experimental Animal Center (Huazhong University of Science and Technology, Wuhan, China). Rats were maintained under controlled laboratory conditions (temperature: 22 ± 0.5℃, humidity: 60% ± 15%, 12 h alternate light–dark cycles, free access to water and food). All animal experiments were approved by the Animal Care and Use Committee of Tongji Medical College. This study was conducted in accordance with the guidelines accepted by the International Association for the Study of Pain28 and in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health of the United States of America.
Peripheral nerve injury model
Peripheral neuropathic pain was induced by SNI, as previously described.29 Briefly, under anaesthesia with sodium pentobarbital (40–50 mg/kg, i.p.), the left sciatic nerve of rats was exposed through an incision at the mid-thigh level. The tibial and common peroneal nerves were ligated with 5.0 silk sutures and then sectioned distal to the ligation, removing 2–3 mm of the distal nerve stump while leaving the remaining sural nerve intact. In sham-operated control rats, the sciatic nerve and its branches were exposed but not ligated.
Behavioural testing
Animals were habituated to the environment in Plexiglass enclosures with a wire mesh floor on an elevated platform for three days before basal measurements. Mechanical withdrawal thresholds (MWTs) of both the surgical ipsilateral (left) and contralateral (right) hind paws were assessed by von Frey filaments (0.4–15.0 g; Stoelting, Wood Dale, IL, USA) and Dixon’s up-down method.30 Biting, sharp withdrawal and licking of the hind paw were considered as positive responses. For each test, five trials were performed at intervals of at least 5 min to obtain a mean value. Each test was performed by the same investigator who was blinded to the grouping method.
Intrathecal catheter implantation and lentivirus administration
Animals were implanted with a single intrathecal catheter for lentivirus injection as described previously.31 Briefly, rats were placed in a prone position after anaesthesia. After creating a small opening at the intervertebral space between the L3–L4 vertebrae, a sterile PE10 intrathecal catheter was inserted through the opening into the lumbar enlargement. The tail withdrawal reflex was used to determine the success of intrathecal catheter implantation. After catheterisation, all rats were allowed to recover for a minimum of three days before being used in other experiments.
Previously, we constructed a recombinant rat Lenti-Cdh1-GFP (pGC-FU-Cdh1-GFP).32 A lentivirus construct expressing GFP only (pGC-FU-GFP) was used as a control. The lentiviral package was supplied by Shanghai Gene Chem (Shanghai, China). The titre of Lenti-Cdh1-GFP was 2.0 × 109 transduction units (TU)/mL, while that of control lentivirus (Lenti-GFP) was 4.0 × 109 TU/mL. Lenti-Cdh1-GFP (4.0 × 108 TU/mL, 10 µL), Lenti-GFP (4.0 × 108 TU/mL, 10 µL), or vehicle (0.9% saline, 10 µL) was intrathecally injected through the catheter on day 7 after SNI when mechanical allodynia was fully developed (Figure 2(a)).
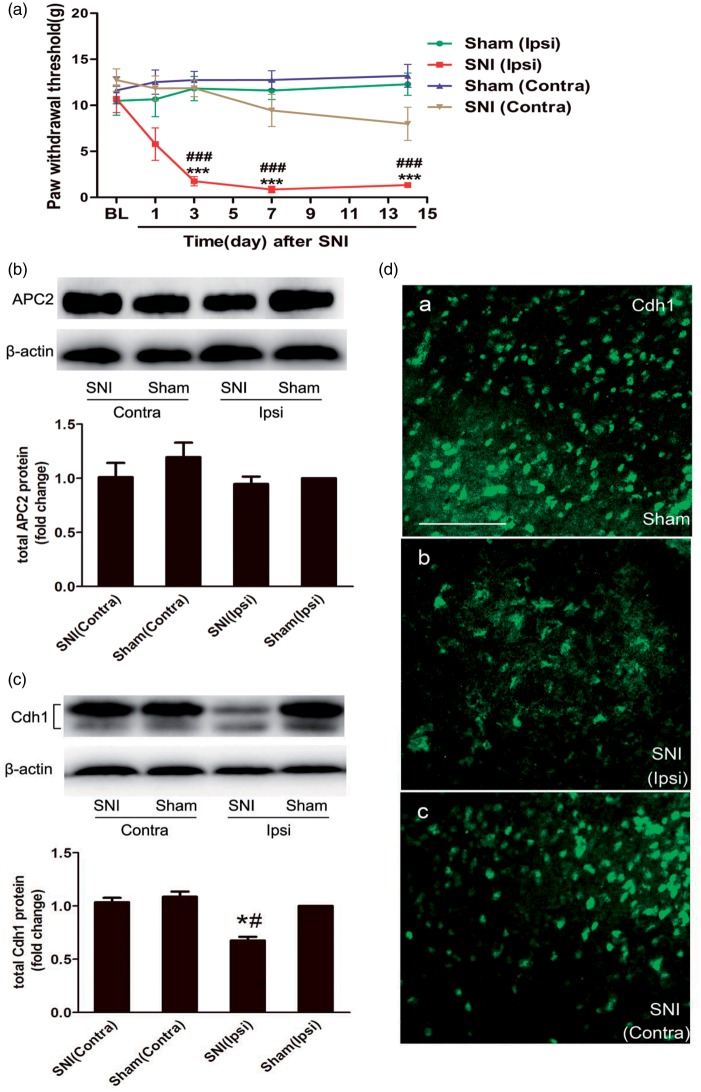
Figure 2.
Changes in APC/C-Cdh1 expression in the dorsal horn after nerve injury. (a) The MWTs of bilateral hind paws to von Frey filament probing were measured, and spared nerve injury (SNI) produced persistent mechanical allodynia in the ipsilateral hind paws starting at day 3 and continuing up to day 14 post-injury (n = 10 in each group). (b and c) Representative bands (top) for the expression of anaphase-promoting complex subunit 2 (APC2) and Cdh1 in the bilateral spinal cord at 14 d after SNI. Quantitative data (bottom) for Western blotting bands (n = 3 in each group) are shown. β-Actin was used as an internal reference, and the fold change for density in the sham (Ipsi) group was set at one for quantification. (d) Immunofluorescence shows Cdh1 expression in the superficial laminae of the dorsal horn at 14 days after SNI. Note that Cdh1 immunoreactivity in the superficial dorsal horn is less intense at the ipsilateral side. Data are expressed as the mean ± standard error of the mean (SEM). ***P < 0.001 versus sham (Ipsi); ###P < 0.001 versus baseline; two-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) post hoc test. *P < 0.05 versus sham (Ipsi); #P < 0.05 versus SNI (Contra); one-way ANOVA followed by Fisher’s LSD post hoc test. BL: baseline; Contra: contralateral; Ipsi: ipsilateral. Scale bars: 200 µm.
Immunofluorescence analysis
Under deep anaesthesia, rats were perfused with 0.1 M phosphate-buffered saline (PBS, pH 7.2–7.4), followed by ice-cold 4% paraformaldehyde in 0.1 M PBS at 14 days after SNI or 14 days after intrathecal injection of lentivirus. Lumbar spinal cords were removed, postfixed in 4% paraformaldehyde/PBS overnight and cryoprotected with 30% sucrose/PBS for 24 h at 4℃. For immunostaining assay, transverse lumbar spinal cord sections (20 µm) were serially cut on a cryostat. After permeabilization with 0.3% TritonX-100/PBS for 15 min, the sections were treated with 5% goat serum for 1 h at room temperature and then incubated with rabbit anti-Fzr1/Cdh1 (1:200 dilution; Beijing Aviva Systems Biology, Beijing, China) and rabbit anti-GFP antibodies (1: 100 dilution; Beyotime Institute of Biotechnology, Jiangsu, China) for 48 h at 4℃. The selectivity of the anti-Cdh1 antibody has been identified in our lab.32–34 After washing with PBS three times for 10 min each, the sections were incubated with Cy2-conjugated goat anti-rabbit secondary antibodies (1:200 dilution; Jackson Immunoresearch, West Grove, PA, USA) for 2 h at room temperature.
For double-labelling assays, the sections were incubated with a mixture of primary antibodies – rabbit anti-Fzr1/Cdh1 and mouse anti-glial fibrillary acidic protein (GFAP) (1:100 dilution; Merck Millipore, Billerica, MA, USA) or mouse anti-neuron-specific nuclear protein (anti-NeuN) antibodies (1:100 dilution; Merck Millipore) – for 48 h at 4℃. Sections were then incubated with Cy3-conjugated goat anti-rabbit (1:200 dilution; Jackson Immunoresearch) and Cy2-conjugated goat anti-mouse secondary antibodies (1:200 dilution; Jackson Immunoresearch) for 2 h at room temperature. The images were captured using a fluorescent microscope (DM2500; Leica Microsystems, Wetzlar, Germany).
Western blotting
Under deep anaesthesia, rats were sacrificed by decapitation at 14 days after SNI or 14 days after intrathecal injection of lentivirus. Hemisected spinal cords at the L3–L6 segments were rapidly removed and preserved in liquid nitrogen. To prepare tissue lysates, spinal cord tissues were homogenised in ice-cold histolysis buffer (Radio-Immunoprecipitation Assay [RIPA] lysis buffer; Beyotime Institute of Biotechnology) containing 0.1 mM phenylmethylsulphonyl fluoride (phenylmethylsulphonyl fluoride; Beyotime Institute of Biotechnology) protease inhibitor and then centrifuged at 12,000 × g for 15 min at 4℃.
The cytosolic and membrane fractions were obtained using a nucl-cyto-mem preparation kit (Applygen Technologies, Beijing, China). The protein concentrations were determined with Bradford reagent (Bio-Rad, Hercules, CA, USA) using bovine serum albumin as a standard. Equivalent amounts of extracts (30 µg per lane) were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% gels and subsequently transferred to polyvinylidene difluoride membranes (Merck Millipore). The blots were blocked with 5% non-fat milk in TBST (0.1% Tween-20, 25 mM Tris, 150 mM NaCl, pH 7.5) for 1 h at room temperature and then incubated with rabbit anti-Fzr1/Cdh1 (1:500 dilution), mouse anti-anaphase-promoting complex subunit 2 (APC2) (1:1000 dilution; Abcam, Cambridge, UK), rabbit anti-GluR1 (1:1000 dilution; Merck Millipore), rabbit anti-EphA4 (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-EphrinA1 (1:200 dilution; Santa Cruz Biotechnology), mouse anti-β-actin (1:500 dilution; Wuhan Boster Biological Technology, Hubei, China) and rabbit anti-N-cadherin antibodies (1:50000 dilution; Merck Millipore) overnight at 4℃. After washing with TBST three times for 10 min each, the membranes were incubated in horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG (1:5000, Abcam, Cambridge, UK) for 2 h at room temperature. Blots were treated with enhanced chemiluminescence reagents (Thermo Scientific, Kalamazoo, MI, USA), visualised using a Chemi-Doc XRS imaging system (Bio-Rad) and quantified using Image J 1.48 u software. N-Cadherin and β-actin were used as internal references for the membrane and cytosolic fractions, respectively. The loading control for total lysates was β-actin. The densities of bands on the blots were normalised to those of the control group.
Co-immunoprecipitation assay
At 14 days after SNI, tissue spanning from the ipsilateral spinal cord to the incision was homogenised and lysed in ice-cold immunoprecipitation buffer (1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, 0.57 mM PMSF, 1 mM leupeptin in PBS, pH 7.4) with various protease inhibitors. Next, 500 µg of the ipsilateral spinal cord lysate was incubated with 10 µg of rabbit anti-GluR1 antibody overnight at 4℃ and then incubated with protein A/G agarose (Beyotime Institute of Biotechnology) on a rotator for 3–4 h at 4℃. As a negative control, equivalent amounts of spinal cord lysates were incubated with 2 µg of normal rabbit IgG (Merck Millipore). After washing with immunoprecipitation buffer four times, the mixture was denatured with SDS-PAGE loading buffer at 95℃ for 5 min and stored at –80℃.
Equivalent amounts of samples (30 µg per lane) were detected by Western blotting. Additionally, 10 µg of total extract was also assessed by Western blotting as a positive control (Input). The antibodies used for the assay were as follows: rabbit anti-Fzr1/Cdh1, mouse anti-APC2, rabbit anti-GluR1, rabbit anti-EphA4 and rabbit anti-EphrinA1 antibodies.
Statistical analysis
All data were normally distributed and are shown as the mean ± standard error of the mean. Statistical analysis was performed with SPSS software (version 19.0; IBM, Ehningen, Germany). Densitometric quantification of protein band intensity was analysed using one-way analysis of variance (ANOVA), followed by Fisher’s least significant difference (LSD) post hoc test. Behavioural results were tested using two-way ANOVA (group × time), followed by Fisher’s LSD post hoc test. Differences with P values of less than 0.05 were considered significant.
Results
An enriched distribution of Cdh1 in neurons of the superficial dorsal horn
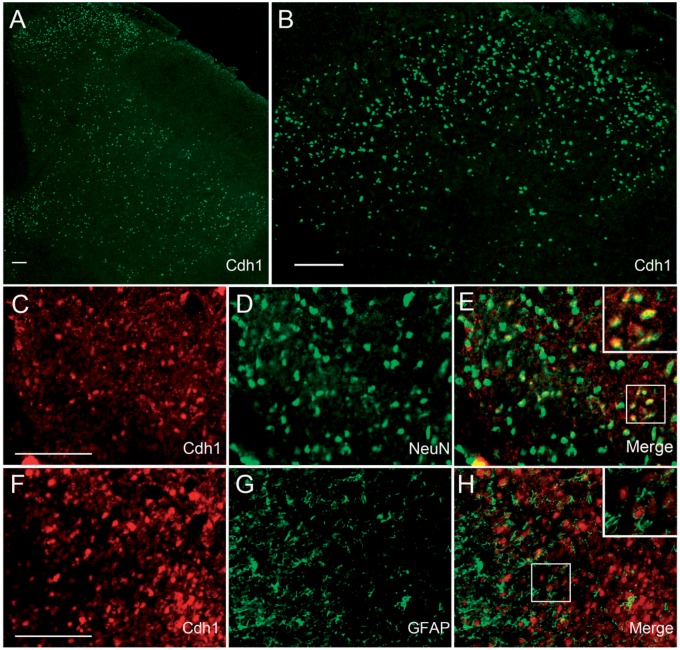
To examine the distribution of Cdh1 in the spinal cord, immunofluorescent staining was performed. We found that Cdh1 immunoreactivity was distributed in the spinal dorsal horn in adult rats, with higher density in the superficial layer (Figure 1(a) and (b)). Double-labelling of Cdh1 with neuronal marker NeuN or astrocytic marker GFAP suggested that Cdh1 was expressed in dorsal horn neurons (Figure 1(c)–(e)) but not astrocyte (Figure 1(f)–(h)) in normal rats.
Figure 1.
Expression of Cdh1 in the spinal cords of normal rats. (a) Immunostaining showed a higher density of Cdh1 immunoreactivity (green) in the superficial layer of the dorsal horn. (b) Higher magnification image of (a). (c–h) Double-staining for Cdh1 (red) and the cell-specific markers: neuron-specific nuclear protein (NeuN; c–e, green; neurons) and glial fibrillary acidic protein (GFAP; f–h, green; astrocytes). Note that Cdh1 was colocalised with NeuN (e) but not with GFAP (h) in the superficial laminae of the dorsal horn. Scale bars: A, B, C and F: 200 µm.
Downregulation of Cdh1 expression in the ipsilateral spinal cord after SNI
As expected, behavioural mechanical allodynia developed after SNI in rats. Compared with sham-operated rats, SNI-treated rats exhibited lower MWTs in the ipsilateral hind paw at 3 days, 7 days and 14 days after SNI (Figure 2(a); n = 10, P < 0.001), similar with the previous report in mice.29 No significant changes of the MWTs were observed in sham-operated rats (Figure 2(a); n = 10, P > 0.05).
The expressions of APC2 and Cdh1 were examined by Western blotting and immunofluorescent staining on 14 days post nerve injury, the time of maximal expression of mechanical allodynia for SNI-treated rats.29 APC2 is one of the core subunits of APC/C and forms a catalytic E3 ubiquitin ligase core with the APC11 subunit.35 Our result showed that there were no significant changes in APC2 expression in the ipsilateral or contralateral spinal cord in the SNI group as compared with that in the sham-operated group (Figure 2(b); n = 3, P > 0.05). The expression levels of total Cdh1 in the ipsilateral spinal cord in SNI animals were significantly lower than those in sham-operated animals (Figure 2(c); n = 3, P < 0.05), but were comparable in the contralateral spinal cord between the two groups (Figure 2(c); n = 3, P > 0.05). No cross-reaction was observed in Western blotting experiments between anti-Cdh1 antibodies and CDC20 protein.36 Similarly, a weaker Cdh1 immunoreactivity was observed in the ipsilateral superficial dorsal horn at 14 days after SNI (Figure 2(d); n = 3). These results suggest that Cdh1 protein level is downregulated at 14 days after nerve injury, although there is no change in APC2 expression in the ipsilateral spinal dorsal horn.
Attenuation of mechanical allodynia after intrathecal treatment with lentivirus encoding Cdh1 in SNI-treated rats
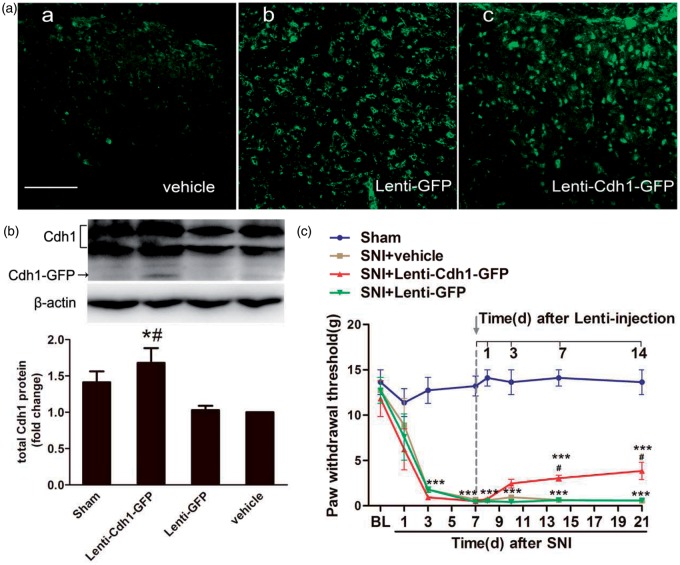
We next sought to assess whether upregulation of spinal Cdh1 expression could alleviate the established pain behaviours induced by SNI. We performed intrathecal injection of recombinant lentivirus encoding Cdh1 (Lenti-Cdh1-GFP), which has been shown to effectively increase the total expression of Cdh1 in vitro.32 Firstly, after Lenti-Cdh1-GFP was intrathecally injected, we confirmed its efficiency by examining GFP expression and quantitatively assessing Cdh1 expression through Western blotting in the spinal cord tissue. At three days after intrathecal injection of Lenti-Cdh1-GFP and Lenti-GFP, a wide GFP expression was observed in many cells of spinal cord (Figure 3(a)), indicating a successful transfection of the recombinant lentivirus in spinal cord. Then, we detected the fusion protein of Cdh1–GFP in spinal cord of rats at three days after intrathecal injection of Lenti-Cdh1-GFP. In addition, intrathecal injection of Lenti-Cdh1-GFP induced a remarkable upregulation of total Cdh1 protein in spinal cord as compared to that either in the vehicle group or Lenti-GFP group (Figure 3(b); n = 3, P < 0.05).
Figure 3.
Effects of intrathecal administration of recombinant lentivirus encoding Cdh1 (Lenti-Cdh1-GFP) on mechanical allodynia in the ipsilateral hind paws of SNI-treated rats. (a) GFP immunostaining was obviously observed in the superficial laminae of the dorsal horn at three days after intrathecal injection of Lenti-Cdh1-GFP (4.0 × 108 TU/mL; 10 µL) (c) and control lentivirus (Lenti-GFP; 4.0 × 108 TU/mL; 10 µL) (b), but not vehicle (0.9% saline;10 µL) (a). (b) Representative bands (top) for the expression of Cdh1 in the spinal cord of rats at three days after intrathecal injection of Lenti-Cdh1-GFP, Lenti-GFP or vehicle. Quantitative data (bottom) for Western blotting bands (n = 3 in each group) are shown. *P < 0.05 versus Lenti-GFP; #P < 0.05 versus vehicle; one-way ANOVA followed by Fisher’s LSD post hoc test. Note that the fusion protein of Cdh1–GFP was detected in the spinal cord tissues at three days after Lenti-Cdh1-GFP infection. (c) MWTs of ipsilateral hind paws to von Frey filament probing were measured before and at 1 day, 3 days, 7 days and 14 days after intrathecal treatment with Lenti-Cdh1-GFP, Lenti-GFP or vehicle. Intrathecal administration of recombinant lentivirus was performed immediately after behavioural determinations on day 7. Data were expressed in mean ± SEM (n = 6 in each group). ***P < 0.001 versus sham; #P < 0.05 versus SNI + vehicle; two-way ANOVA followed by Fisher’s LSD post hoc test. BL: baseline. Scale bars: 100 µm.
Furthermore, intrathecal treatment with Lenti-Cdh1-GFP significantly attenuated SNI-induced mechanical hypersensitivity, starting on 7 days and persisting to 14 days after treatment, as compared with vehicle treatment (Figure 3(c); n = 6, P < 0.05). Intrathecal treatment with Lenti-GFP did not cause significant changes in the MWTs of SNI rats as compared with vehicle treatment (Figure 3(c); n = 6, P > 0.05). These data demonstrated that rescued expression of Cdh1 in spinal cord by intrathecal treatment with lentivirus encoding Cdh1 significantly attenuated mechanical allodynia induced by peripheral nerve injury in rats.
SNI-induced increase of the surface expression of the GluR1 in the spinal cord and its relationship with Cdh1
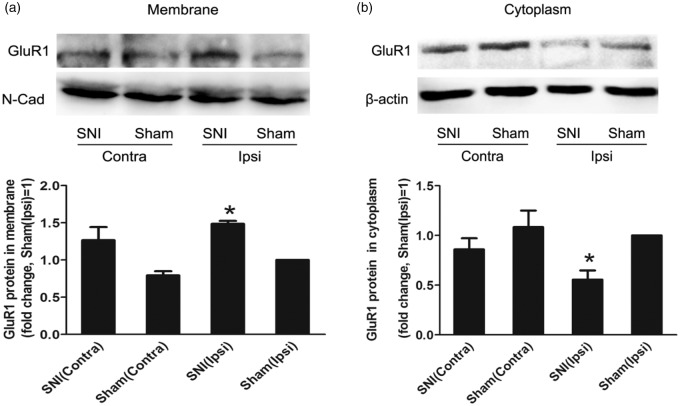
Then, a series of experiments were performed to identify whether GluR1 was a potential target of Cdh1 in the spinal cord during the maintenance of neuropathic pain. First, we found that SNI induced significant changes in both the surface expression and cytosolic levels of GluR1 protein in the spinal cord tissue. At 14 days after SNI, the surface expression of GluR1 in the ipsilateral spinal cord was significantly upregulated as compared with that in sham-operated animals (Figure 4(a); n = 3, P < 0.05). In contrast, the cytosolic expression of GluR1 was downregulated after SNI (Figure 4(b); n = 3, P < 0.05). In these SNI-treated animals, GluR1 levels on the membrane and in the cytosol were comparable between the ipsilateral and contralateral fractions (Figure 4(a) and (b); n = 3, P > 0.05). These data suggest that the surface delivery of GluR1 was increased in the spinal cord neurons after nerve injury.
Figure 4.
Changes in the surface delivery of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptor subunit GluR1 in the spinal cord after nerve injury. (a) Representative Western blotting bands (top) and quantitative data (bottom) for analysis (n = 3 in each group) show the protein expression levels of GluR1 in the membrane fraction of the bilateral spinal cord at 14 days after SNI. N-Cadherin (N-Cad) was used as the internal references for the plasma membrane fraction. (b) Representative Western blotting bands (top) and quantitative data (bottom) for analysis (n = 3 in each group) show the protein expression levels of GluR1 in the cytosolic fraction of different groups. β-actin was used as the internal references for the cytosolic fraction.*P < 0.05 versus sham (Ipsi); one-way ANOVA followed by Fisher’s LSD post hoc test. Contra: contralateral; Ipsi: ipsilateral.
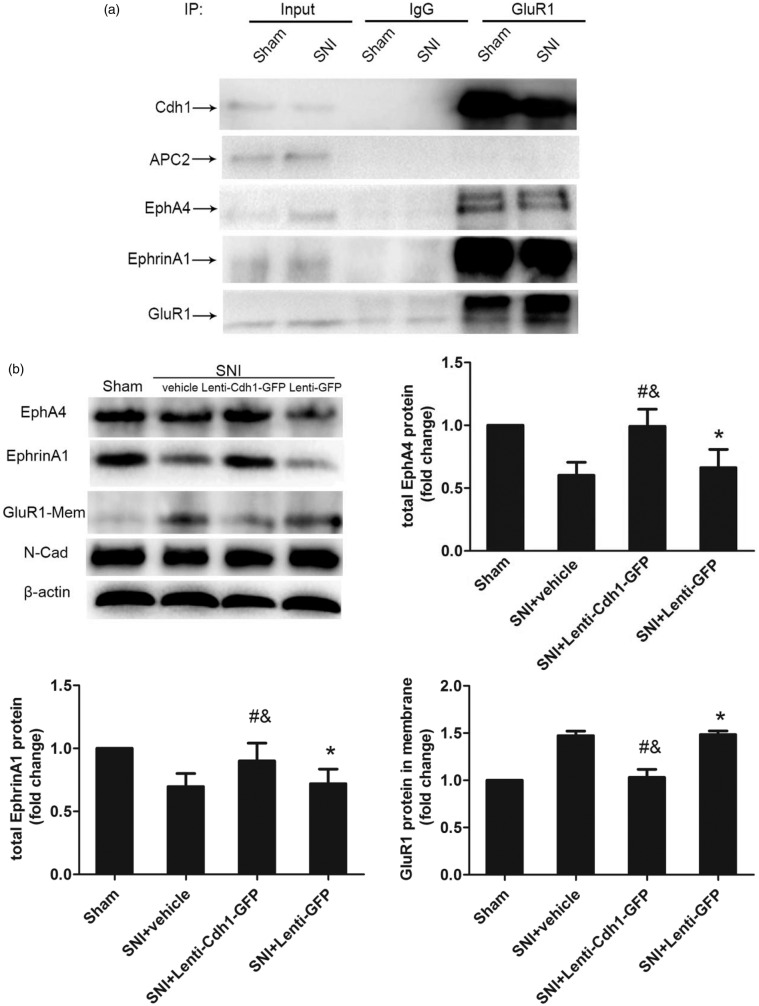
Next, Cdh1, EphA4 and EphrinA1 were co-immunoprecipitated by anti-GluR1 antibody in the ipsilateral spinal cord at 14 days after SNI or in that from sham-operated animals (Figure 5(a)), indicating that a complex of EphA4, EphrinA1, Cdh1 and GluR1 exists in spinal cord and is also linked to SNI-induced neuropathic pain.
Figure 5.
Effects of intrathecal administration of Lenti-Cdh1-GFP on the expression of surface GluR1 subunit, erythropoietin-producing human hepatocellular receptor A4 (EphA4) and EphrinA1 in the ipsilateral dorsal horn of SNI rats. (a) Interactions between GluR1, Cdh1, EphA4, EphrinA1 and APC2 in the ipsilateral spinal cord were determined by co-immunoprecipitation analysis. (b) Western blotting analysis of changes in GluR1, EphA4 and EphrinA1 in the ipsilateral spinal cord at 14 days after intrathecal injection of recombinant lentivirus or vehicle in SNI model rats (n = 3 for GluR1 in each group; n = 4 for EphA4 and EphrinA1 in each group). *P < 0.05 versus sham; #P < 0.05 versus SNI + vehicle; &P < 0.05 versus SNI + Lenti-GFP; one-way ANOVA followed by Fisher’s LSD post hoc test. IP: immunoprecipitation; GluR1-Mem: GluR1 in the plasma membrane fraction.
To further identify the potential role of Cdh1 in the regulation of GluR1 expression during the maintenance of neuropathic pain, we tested whether Cdh1 overexpression in the spinal cord affected the enhanced GluR1 expression in the plasma membrane fraction after SNI. Immunoblot analysis showed that at 14 days after intrathecal administration, Lenti-Cdh1-GFP, but not Lenti-GFP, prevented the enhanced surface expression of GluR1 (Figure 5(b); n = 3, P < 0.05) and the decrease in the EphA4-EphrinA1 signalling complex (Figure 5(b); n = 3, P < 0.05) induced by SNI in the ipsilateral spinal cord. Taken together, these results suggest that Cdh1 regulates the surface expression of GluR1 in spinal cord neurons during the maintenance of neuropathic pain by interacting with the EphA4-EphrinA1 signalling complex, and that GluR1 may contribute to spinal Cdh1-mediated mechanisms underlying the maintenance of neuropathic pain.
Discussion
In this study, we found that Cdh1 was strongly expressed in neurons of the superficial dorsal horn. The expression of Cdh1 was downregulated in the ipsilateral dorsal horn during the maintenance of neuropathic pain after SNI. Through intrathecal injection of lentivirus encoding Cdh1, we observed that the rescued Cdh1 expression in the spinal cord attenuated mechanical allodynia induced by SNI. These data suggest that SNI-induced downregulation of Cdh1 may be involved in spinal mechanisms of behavioural hypersensitivity. Furthermore, our co-immunoprecipitation data showed interaction of Cdh1 with GluR1 in the spinal dorsal horn. For SNI-treated rats, overexpression of spinal Cdh1 increased the protein levels of GluR1-related EphA4 and EphrinA1 and prevented the enhanced expression of membrane GluR1. These results suggest that the downregulation of Cdh1 in the spinal cord may result in increased surface expression of GluR1 by reducing the activity of EphA4-EphrinA1 signalling cascades, which is involved in mechanisms underlying the maintenance of neuropathic pain.
Cdh1 is a WD40-domain co-activator protein of APC/C. For fully exerting its functions, APC/C needs to associate with its co-activators that determine substrate specificity of the APC/C and regulate the activity of APC/C.37 Emerging evidence has demonstrated that APC/C-Cdh1 is involved in the regulation of cell cycle progression,11 cell differentiation38 and a variety of neurobiological processes35 through ubiquitination and degradation of specific substrates. However, the functions of APC/C-Cdh1 in neurological disorders are not completely understood. Three studies have described changes in the activities and protein levels of Cdh1 in cerebral ischemia32,33 and spinal cord injury.27 The somatosensory nociceptive system is composed of primary nociceptive neurons, intricate networks at the level of the spinal dorsal horn and brainstem and a large array of brain regions, which together generate the multidimensional experience of pain.23 Our previous study found that rescued expression of Cdh1 in the anterior cingulate cortex (ACC) alleviated SNI-induced mechanical allodynia,34 suggesting the involvement of Cdh1 protein in the ACC in the development of neuropathic pain. However, the potential functions of spinal Cdh1 on the pain processing remain unclear in that study. The present study further demonstrated the roles of spinal Cdh1 in neuropathic pain and extended the knowledge of APC/C-Cdh1 in neurological disorders.
Previous studies have demonstrated that the AMPA receptor GluR1 subunit is an important downstream substrate of APC/C-Cdh1 in neurons of invertebrates and mammals during the processing of neurodevelopment.20,21,39 Furthermore, trafficking of the AMPA receptor GluR1 subunit in the dorsal horn contributes to the induction and development of nociceptive sensory signals.40,41 In this study, we found that a complex of GluR1, Cdh1, EphA4 and EphrinA1 existed in the spinal cords of adult rat at 14 days after nerve injury. The present study also showed that Cdh1 overexpression in the spinal cord by lentiviral delivery significantly prevented the enhanced surface expression of GluR1 associated with the increased levels of EphA4 and its ligand EphrinA1 induced by neuropathic pain. These findings are consistent with a previous report in which the expression of Cdh1 is shown to be required for EphA4-dependent downregulation of GluR1 in homeostatic plasticity.21 Thus, these data indicate that Cdh1 expression in the spinal cord of rats also regulate the trafficking and function of GluR1 during the maintenance of neuropathic pain. In addition to regulating the degradation of GluR1 in neuronal patterning and connectivity, the APC/C-Cdh1 targets a large array of substrates for ubiquitin-mediated degradation in the central nervous system. For instance, Liprinα operates downstream of APC/C-Cdh1 in the regulation of synapses.39 SnoN and Id2 are also identified to be two ubiquitin substrates of neuronal APC/C-Cdh1 in the regulation of axon growth.35 Even though the changes of other relevant targets of Cdh1 in spinal cord were not evaluated after neuropathic pain, the results from the present study suggested that the trafficking of GluR1-containing AMPA receptors contributes to Cdh1-related neuropathic pain modulation in the spinal cord, which may be not the unique but acceptable explanation for Cdh1 function in the development of neuropathic pain.
Our previous study found that rescued expression of Cdh1 in the ACC alleviated SNI-induced mechanical allodynia,34 and the analgesic effect was thought to be more notable compared with that in this study. A recent study identified a descending projection trail from the pyramidal cells in the deep layers of the ACC to the superficial layer of spinal dorsal horn.42 Descending projection system provides possible pathway for ACC to modulate spinal sensory transmission. These data prompt us to hypothesise that APC/C-Cdh1 signalling in spinal cord may be regulated by descending modulation system during the development of neuropathic pain. Additional mechanistic investigation of the effects of APC/C-Cdh1 signalling on neural circuit is required.
APC/C is composed of three subcomplexes: the scaffolding subcomplex, the tetratricopeptide repeat arm and the catalytic subcomplex.43 APC2, a cullin family-related protein, is one of the components of the catalytic subcomplex.35 Although APC2 is important for the activity of APC/C, the catalytic subcomplex does not have substrate specificity.44 Moreover, two previous studies suggest that Cdh1 may possess additional functions beyond its APC/C-dependent roles.45,46 Taken together, these results could explain why there were no significant changes in the expression of APC2 in the spinal cords of rats at 14 days after nerve injury. Additionally, in contrast to a previous study in which APC2 was shown to be associated with GluR1 in the rat brain in vivo,21 our co-immunoprecipitation data showed that GluR1 did not interact with APC2 but did associate with Cdh1, EphA4 and EphrinA1 in the spinal cords of adult rats. However, in the previous study, co-immunoprecipitation assays were performed in the brain to target APC2, not in the spinal cord to target GluR1, as did in our study.
Conclusions
Our data demonstrated that overexpression of spinal Cdh1 by intrathecal administration of lentivirus encoding Cdh1 significantly attenuated SNI-induced mechanical allodynia, accompanied by a decrease in the surface expression of AMPA GluR1 subunit in the spinal cord. The present study provides insights into the role of APC/C-Cdh1 in the dorsal horn neurons of the spinal cord in molecular mechanisms underlying the maintenance of neuropathic pain.
Author contributions
CZ and WLY contributed to the study conception and design. RH, WT and DJL performed the experiments and contributed to data analysis and interpretation. RH and WLY wrote the manuscript. LL, YZ, CHZ and LW provided critical revisions of the article. RH and WLY were responsible for final article revision. All authors read and discussed the results, commented on the manuscript and approved the final version to be submitted.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81000476, 81100819 and 81171158).
References
- 1.Jensen TS, Baron R, Haanpaa M, et al. A new definition of neuropathic pain. Pain 2011; 152: 2204–2205. [DOI] [PubMed] [Google Scholar]
- 2.Hegde AN. The ubiquitin-proteasome pathway and synaptic plasticity. Learn Mem 2010; 17: 314–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem 2001; 70: 503–533. [DOI] [PubMed] [Google Scholar]
- 6.Ossipov MH, Bazov I, Gardell LR, et al. Control of chronic pain by the ubiquitin proteasome system in the spinal cord. J Neurosci 2007; 27: 8226–8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed AS, Ahmed M, Li J, et al. Proteasome inhibitor MG132 modulates inflammatory pain by central mechanisms in adjuvant arthritis. Int J Rheum Dis. Epub ahead of print 5 April 2014. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed AS, Li J, Ahmed M, et al. Attenuation of pain and inflammation in adjuvant-induced arthritis by the proteasome inhibitor MG132. Arthritis Rheum 2010; 62: 2160–2169. [DOI] [PubMed] [Google Scholar]
- 5.Moss A, Blackburn-Munro G, Garry EM, et al. A role of the ubiquitin-proteasome system in neuropathic pain. J Neurosci 2002; 22: 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierre S, Maeurer C, Coste O, et al. Toponomics analysis of functional interactions of the ubiquitin ligase PAM (Protein Associated with Myc) during spinal nociceptive processing. Mol Cell Proteomics 2008; 7: 2475–2485. [DOI] [PubMed] [Google Scholar]
- 7.Cachemaille M, Laedermann CJ, Pertin M, et al. Neuronal expression of the ubiquitin ligase Nedd4-2 in rat dorsal root ganglia: modulation in the spared nerve injury model of neuropathic pain. Neuroscience 2012; 227: 370–380. [DOI] [PubMed] [Google Scholar]
- 8.Laedermann CJ, Cachemaille M, Kirschmann G, et al. Dysregulation of voltage-gated sodium channels by ubiquitin ligase NEDD4-2 in neuropathic pain. J Clin Invest 2013; 123: 3002–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol 2006; 7: 644–656. [DOI] [PubMed] [Google Scholar]
- 11.Visintin R, Prinz S, Amon A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science 1997; 278: 460–463. [DOI] [PubMed] [Google Scholar]
- 12.Gieffers C, Peters BH, Kramer ER, et al. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc Natl Acad Sci USA 1999; 96: 11317–11322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konishi Y, Stegmuller J, Matsuda T, et al. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science 2004; 303: 1026–1030. [DOI] [PubMed] [Google Scholar]
- 15.Lasorella A, Stegmuller J, Guardavaccaro D, et al. Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature 2006; 442: 471–474. [DOI] [PubMed] [Google Scholar]
- 13.Kannan M, Lee SJ, Schwedhelm-Domeyer N, et al. The E3 ligase Cdh1-anaphase promoting complex operates upstream of the E3 ligase Smurf1 in the control of axon growth. Development 2012; 139: 3600–3612. [DOI] [PubMed] [Google Scholar]
- 16.Almeida A, Bolanos JP, Moreno S. Cdh1/Hct1-APC is essential for the survival of postmitotic neurons. J Neurosci 2005; 25: 8115–8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harmey D, Smith A, Simanski S, et al. The anaphase promoting complex induces substrate degradation during neuronal differentiation. J Biol Chem 2009; 284: 4317–4323. [DOI] [PubMed] [Google Scholar]
- 18.Yao W, Qian W, Zhu C, et al. Cdh1-APC is involved in the differentiation of neural stem cells into neurons. Neuroreport 2010; 21: 39–44. [DOI] [PubMed] [Google Scholar]
- 20.Juo P, Kaplan JM. The anaphase-promoting complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Curr Biol 2004; 14: 2057–2062. [DOI] [PubMed] [Google Scholar]
- 19.Fu AK, Hung KW, Fu WY, et al. APC(Cdh1) mediates EphA4-dependent downregulation of AMPA receptors in homeostatic plasticity. Nat Neurosci 2011; 14: 181–189. [DOI] [PubMed] [Google Scholar]
- 21.Pick JE, Malumbres M, Klann E. The E3 ligase APC/C-Cdh1 is required for associative fear memory and long-term potentiation in the amygdala of adult mice. Learn Mem 2013; 20: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuner R. Central mechanisms of pathological pain. Nat Med 2010; 16: 1258–1266. [DOI] [PubMed] [Google Scholar]
- 23.Luo C, Kuner T, Kuner R. Synaptic plasticity in pathological pain. Trends Neurosci 2014; 37: 343–355. [DOI] [PubMed] [Google Scholar]
- 47.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science 2000; 288: 1769–1772. [DOI] [PubMed] [Google Scholar]
- 24.Xing GG, Liu FY, Qu XX, et al. Long-term synaptic plasticity in the spinal dorsal horn and its modulation by electroacupuncture in rats with neuropathic pain. Exp Neurol 2007; 208: 323–332. [DOI] [PubMed] [Google Scholar]
- 25.Qi YH, Yao WL, Zhang CH, et al. Effect of lentivirus-mediated RNA interference of APC-Cdh1 expression on spinal cord injury in rats. Genet Mol Res 2014; 13: 1366–1372. [DOI] [PubMed] [Google Scholar]
- 44.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 26.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 2000; 87: 149–158. [DOI] [PubMed] [Google Scholar]
- 45.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 46.Malkmus SA, Yaksh TL. Intrathecal catheterization and drug delivery in the rat. Methods Mol Med 2004; 99: 109–121. [DOI] [PubMed] [Google Scholar]
- 28.Qiu J, Zhang C, Lv Y, et al. Cdh1 inhibits reactive astrocyte proliferation after oxygen-glucose deprivation and reperfusion. Neurochem Int 2013; 63: 87–92. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Yao W, Qiu J, et al. The involvement of down-regulation of Cdh1-APC in hippocampal neuronal apoptosis after global cerebral ischemia in rat. Neurosci Lett 2011; 505: 71–75. [DOI] [PubMed] [Google Scholar]
- 42.Tan W, Yao WL, Hu R, et al. Alleviating neuropathic pain mechanical allodynia by increasing Cdh1 in the anterior cingulate cortex. Mol Pain 2015; 11: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puram SV, Bonni A. Novel functions for the anaphase-promoting complex in neurobiology. Semin Cell Dev Biol 2011; 22: 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kramer ER, Gieffers C, Hölzl G, et al. Activation of the human anaphase-promoting complex by proteins of the CDC20/Fizzy family. Curr Biol 1998; 8: 1207–1210. [DOI] [PubMed] [Google Scholar]
- 30.Almeida A. Regulation of APC/C-Cdh1 and its function in neuronal survival. Mol Neurobiol 2012; 46: 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delgado-Esteban M, Garcia-Higuera I, Maestre C, et al. APC/C-Cdh1 coordinates neurogenesis and cortical size during development. Nat Commun 2013; 4: 2879. [DOI] [PubMed] [Google Scholar]
- 35.van Roessel P, Elliott DA, Robinson IM, et al. Independent regulation of synaptic size and activity by the anaphase-promoting complex. Cell 2004; 119: 707–718. [DOI] [PubMed] [Google Scholar]
- 36.Tao YX. Dorsal horn alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking in inflammatory pain. Anesthesiology 2010; 112: 1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Wu J, Wu Z, et al. Regulation of AMPA receptors in spinal nociception. Mol Pain 2010; 6: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen T, Koga K, Descalzi G, et al. Postsynaptic potentiation of corticospinal projecting neurons in the anterior cingulate cortex after nerve injury. Mol Pain 2014; 10: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schreiber A, Stengel F, Zhang Z, et al. Structural basis for the subunit assembly of the anaphase-promoting complex. Nature 2011; 470: 227–232. [DOI] [PubMed] [Google Scholar]
- 39.Tang Z, Li B, Bharadwaj R, et al. APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Mol Biol Cell 2001; 12: 3839–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao D, Inuzuka H, Korenjak M, et al. Cdh1 regulates cell cycle through modulating the claspin/Chk1 and the Rb/E2F1 pathways. Mol Biol Cell 2009; 20: 3305–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan L, Zou W, Gao D, et al. Cdh1 regulates osteoblast function through an APC/C-independent modulation of Smurf1. Mol Cell 2011; 44: 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]