Abstract
Background
Neuropathic pain in small-fiber neuropathy results from injury to and sensitization of nociceptors. Functional prostatic acid phosphatase (PAP) acts as an analgesic effector. However, the mechanism responsible for the modulation of PAP neuropathology, which leads to loss of the analgesic effect after small-fiber neuropathy, remains unclear.
Results
We used a resiniferatoxin (RTX)-induced small-fiber neuropathy model to examine whether functional PAP(+) neurons are essential to maintain the analgesic effect. PAP(+) neurons were categorized into small to medium neurons (25th–75th percentile: 17.1–23.7 µm); these neurons were slightly reduced by RTX (p = 0.0003). By contrast, RTX-induced activating transcription factor 3 (ATF3), an injury marker, in PAP(+) neurons (29.0% ± 5.6% vs. 0.2% ± 0.2%, p = 0.0043), indicating PAP neuropathology. Moreover, the high-affinity nerve growth factor (NGF) receptor (trkA) colocalized with PAP and showed similar profiles after RTX-induced neuropathy, and the PAP/trkA ratios correlated with the degree of mechanical allodynia (r = 0.62, p = 0.0062). The NGF inducer 4-methylcatechol (4MC) normalized the analgesic effects of PAP; specifically, it reversed the PAP and trkA profiles and relieved mechanical allodynia. Administering 2.5S NGF showed similar results to those of administering 4MC. This finding suggests that the analgesic effect of functional PAP is mediated by NGF-trkA signaling, which was confirmed by NGF neutralization.
Conclusions
This study revealed that functional PAP(+) neurons are essential for the analgesic effect, which is mediated by NGF-trkA signaling.
Keywords: Resiniferatoxin, prostatic acid phosphatase, nerve growth factor, 4-methylcatechol, trkA receptor, small-fiber neuropathy
Background
Prostatic acid phosphatase (PAP), a glycoprotein synthesized by the prostate gland, is traditionally used to diagnose prostate cancer. PAP has two isoforms, secreted and transmembrane isoforms,1,2 and, more than in the prostate gland, is also extensively expressed in nonprostate tissue2,3 such as nervous tissues.1,4,5. Recent studies have identified PAP is molecular identity to thiamine monophosphatase (TMPase)1 and, historically, TMPase, with the acid phosphatase characteristics, has been widely used for labeling nociceptive dorsal root ganglia (DRG) neurons. PAP hydrolyzes adenine monophosphate (AMP), particularly in acidic environments,3,6 to adenosine that acts as an analgesic effector.1,7 Moreover, small nociceptive DRG neurons, including peptidergic and nonpeptidergic neurons, coexpress PAP,4 suggesting that PAP profiles correlate with pain modulation. For example, a growing body of evidence has shown that intrathecal injection of recombinant PAP counteracts inflammatory pain6 and enhances neuropathic pain in PAP-knockout mice.1,7,8 These findings confirm that PAP acts as an analgesic modulator. PAP expression is higher in small nonpeptidergic DRG than in peptidergic DRG neurons.4 Previously, we have established a mouse model of small-fiber neuropathy,9 in which small sensory nerves were specifically depleted by resiniferatoxin (RTX), a capsaicin analog acting on the transient receptor potential vanilloid receptor 1 (TRPV1).10–12 This RTX-induced neuropathy model mimics human small-fiber neuropathy; that is, the model exhibits the degeneration of cutaneous sensory nerves and neuropathic pain. Furthermore, RTX-induced neuropathy with skin denervation markedly increases the expression of activating transcription factor 3 (ATF3), a marker of neuronal injury, in nonpeptidergic P2X3 neurons, which is correlated with neuropathic pain.10 These observations suggest that PAP exerts analgesic effects for small-fiber neuropathy. Whether the expression profiles of this subpopulation of PAP neurons correlate with injury-induced neuropathic pain should be determined, and the mechanism responsible for modulating the analgesic potential in injury-induced neuropathic pain should be investigated.
The compound 4-methylcatechol (4MC) enhances the synthesis of neurotrophins such as nerve growth factor (NGF) and promotes skin reinnervation.13 Cutaneous sensory nerves function as the peripheral receptors of small DRG neurons; thus, inducing ATF3 expression in these neurons caused skin denervation and neuropathic pain in our RTX-induced neuropathy model.10 NGF is trophic to small DRG neurons and modulates neuropathic pain through its high-affinity receptor, trkA; a reduction in NGF and trkA expression is accompanied by various peripheral neuropathies.14,15 Whether 4MC modulates PAP expression profiles through NGF-trkA signaling is unclear.
RTX-induced neuropathy is an excellent model for determining how the loss of the analgesic effect of PAP leads to injury-induced neuropathic pain. In the present study, we investigated the intervention of PAP profiles to neuropathic pain and the effect of 4MC on reversing neuropathic pain through NGF-trkA signaling.
Materials and methods
Experimental design and groups
This study investigated PAP-trkA signaling-mediated pain behavior in RTX-induced neuropathy by investigating whether PAP neuropathology is correlated with pain development and whether 4MC reverses pain and PAP profiles in RTX-induced neuropathy in an NGF-trkA signaling-dependent manner. Neuropathy was induced by the intraperitoneal (i.p.) administration of a single dose of RTX (50 µg/kg, Sigma, St. Louis, MO) (the RTX group).9,10,16 Mice received either daily i.p. injections of 4MC (10 µg/kg, Wako, Osaka, Japan) immediately after RTX treatment for seven consecutive days (the 4MC group),9,13 or an equal volume of vehicle as a control (the vehicle group). To confirm the effect of 4MC, 2.5S NGF and anti-NGF antisera were i.p. administered. Briefly, 2.5S NGF (Alomone Labs, Jerusalem, Israel) was dissolved in Dulbecco’s Modified Eagle Medium, and mice received NGF (1 µg/10 g) immediately after RTX treatment (the NGF group).17 Some mice in the 4MC group received additional anti-NGF antisera (30 µg/kg, Sigma)18 to neutralize NGF induced by 4MC (the abNGF group). To compare each experimental approach, 2.5S NGF and anti-NGF antisera were administered consecutively for seven days.
After treatment, the mice were housed in plastic cages in a 12-h light/dark cycle and were given access to water and food ad libitum. All procedures were conducted in accordance with ethical guidelines for laboratory animals,19 and the protocol was approved by the Institutional Animal Care and Use Committee of Kaohsiung Medical University. All experimental procedures were performed carefully, and all efforts were made to minimize animal suffering.
Evaluation of mechanical allodynia
The changes in the mechanical threshold of each group were assessed using the up-and-down method with different calibers of von Frey monofilaments (Somedic Sales AB, Hörby, Sweden) in accordance with our established protocol.10,16 Briefly, a series of monofilaments was applied to the plantar region of the hindpaw. If paw withdrawal occurred, a monofilament of a smaller caliber was applied. In the absence of paw withdrawal, a monofilament of a larger caliber was applied. Four additional stimuli with monofilaments of various calibers were applied on the basis of the preceding responses, and the mechanical thresholds were calculated using a formula published in a previous study.20
Double immunofluorescence staining of DRG neurons
Animals were sacrificed by intracardiac perfusion with 0.1 M phosphate buffer (PB) followed by 4% paraformaldehyde (4 P) in 0.1 M PB. After perfusion, the 4th and 5th (L4 and L5, respectively) lumbar DRGs were removed carefully and postfixed in 4P for another 6 h. The DRGs were cryoprotected with 30% sucrose in 0.1 M PB overnight, and 8 -µm-thick cryosections were obtained using a cryostat (CM1850, Leica, Wetzlar, Germany). For adequate sampling, two ganglia (L4/L5) per mouse and 5 to 8 sections per DRG tissue (at 80-µm intervals) were immunostained. The following primary antisera were used in this study: anti-PAP (chicken, 1:600, Aves Labs, Tigard, OR), anti-ATF3 (rabbit, 1:100, Santa Cruz Biotechnology, Santa Cruz, CA), anti-peripherin (rabbit, 1:800, Chemicon, Temecula, CA), anti-neurofilament SMI32 (mouse, 1:600, Covance, Emeryville, CA), anti-P2X3 (rabbit, 1:400, Neuromics, Edina, MN), and anti-trkA (rabbit, 1:200, Santa Cruz). Briefly, sections were incubated overnight at 4℃ with one of the primary antiserum combinations: P2X3/PAP, PAP/peripherin, PAP/SMI32, ATF3/PAP, and PAP/trkA. Thereafter, the sections were incubated with Texas red or fluorescein isothiocyanate-conjugated secondary antisera (1:100, Jackson ImmunoResearch, West Grove, PA) corresponding to the appropriate primary antisera for 1 h. Sections were mounted with Vectashield (Vector, Burlingame, CA) for quantification.
Quantification of different phenotypic DRG neurons
To quantify the various phenotypic DRG neurons, each DRG section was systematically photographed at 200 × under a fluorescence microscope (Axiophot microscope, Zeiss, Oberkochen, Germany) to produce a montage of the entire DRG section according to an established procedure.9,10,16 To avoid density bias, only neurons with a clear nuclear profile were counted, and only sections containing neuronal ganglia were measured using ImageJ version 1.44d (National Institutes of Health [NIH], Bethesda, MD). For morphometric analysis of PAP neuropathology, the diameters of PAP(+) neurons and ATF3(+)/PAP(+) neurons were measured using Image Pro-Plus (Media Cybernetics, Bethesda, MD) and plotted as a histogram.
Statistical analysis
To minimize individual bias, five to seven mice were included in each group, and the coding information was masked during the behavioral tests and quantification procedures. All data are expressed as mean ± standard derivation (SD) of the mean. A t test was performed to analyze the data following a Gaussian distribution. For data which did not follow a Gaussian distribution, a nonparametric Mann–Whitney test was applied. Results with p < 0.05 were considered statistically significant.
Results
Phenotypic characteristics of PAP(+) neurons in RTX-induced neuropathy
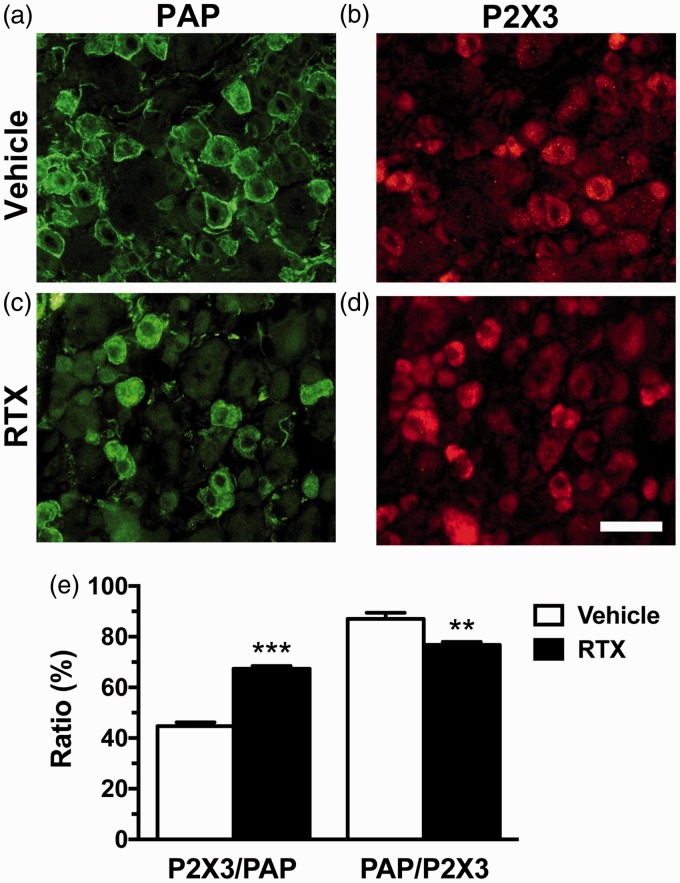
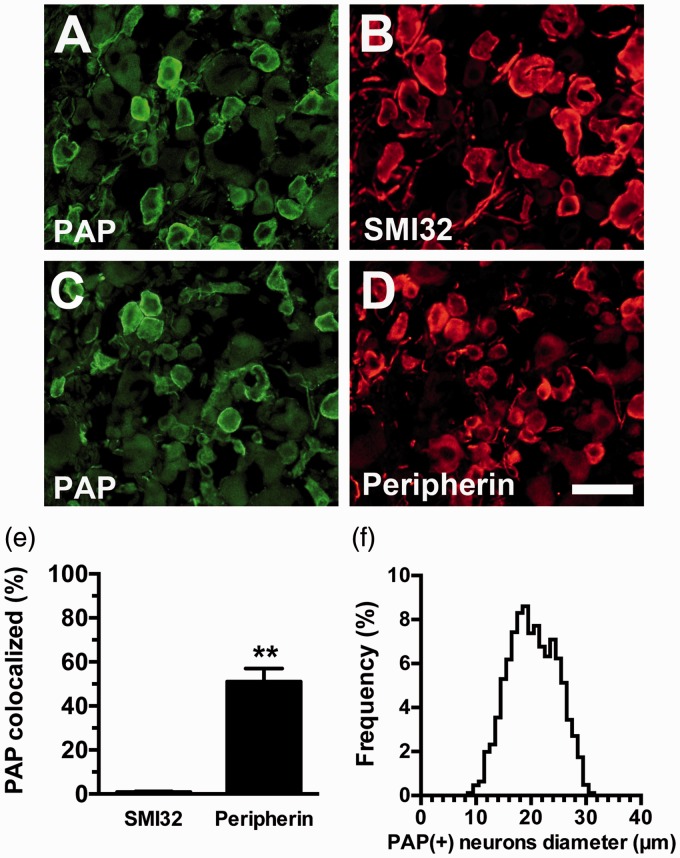
In a previous study, we developed an RTX-induced small-fiber neuropathy mouse model, in which sensitized P2X3 purinociceptors-mediated mechanical allodynia.10 In the present study, RTX affected PAP expression, as demonstrated by the coexpression of P2X3 and PAP (Figure 1). Notably, PAP and P2X3 showed inverse patterns after neuropathy; specifically, there was an increase in P2X3(+) and decrease in PAP(+) neurons, resulting in an increased P2X3/PAP ratio (67.4% ± 2.1% vs. 44.7% ± 3.4%, p < 0.0001) and decreased PAP/P2X3 ratio (87.1% ± 5.4% vs. 76.8% ± 2.3%, p = 0.001) (Figure 1(e)). Furthermore, PAP expression was higher in peripherin(+) neurons than in neurofilament (SMI32)(+) neurons (Figure 2). The diameter histograms confirmed that the PAP(+) neurons were small to medium DRG neurons (25th–75th percentile: 17.1–23.7 µm) (Figure 2(f)). These results indicate that RTX potentially changes the expression profiles of PAP.
Figure 1.
Colocalization of prostatic acid phosphatase (PAP)(+) and P2X3(+) dorsal root ganglion neurons in resiniferatoxin (RTX)-induced neuropathy. (a–d) Double immunofluorescence staining was performed with the following primary antiserum combinations: anti-PAP (a, c) and anti-P2X3 (b, d) antisera in the vehicle (a, b) and RTX (c, d) groups. (e) The graph indicates the changes of colocalized ratios of P2X3(+)/PAP(+) and PAP(+)/P2X3(+) neurons in the vehicle (opened bar) and RTX (filled bar) group according to Panels (a) to (d).
Bar, 50 µm. **p < 0.01, ***p < 0.001.
Figure 2.
Expression of prostatic acid phosphatase (PAP)(+) by the peripherin(+) and neurofilament (SIM32)(+) dorsal root ganglion (DRG) neurons. Double immunofluorescence staining of the DRG sections was performed with the following two primary antisera combinations: (a, b) PAP/SMI32 and PAP/peripherin (c, d). (a, b) The micrographs show extremely low colocalization of PAP (a) and SMI32 (b). (c, d) The micrographs show high colocalization of PAP (c) and peripherin (d). (e) The graph quantifies the colocalized ratios of PAP(+)/SMI32(+) (open bar) and PAP(+)/peripherin(+) neurons (filled bar) according to Panels (a) to (d). (f) The graph shows the diameter histogram of PAP(+) neurons, indicating that PAP(+) neurons are small to medium neurons.
Bar, 50 µm. **p < 0.01
PAP expression profiles were correlated with mechanical allodynia
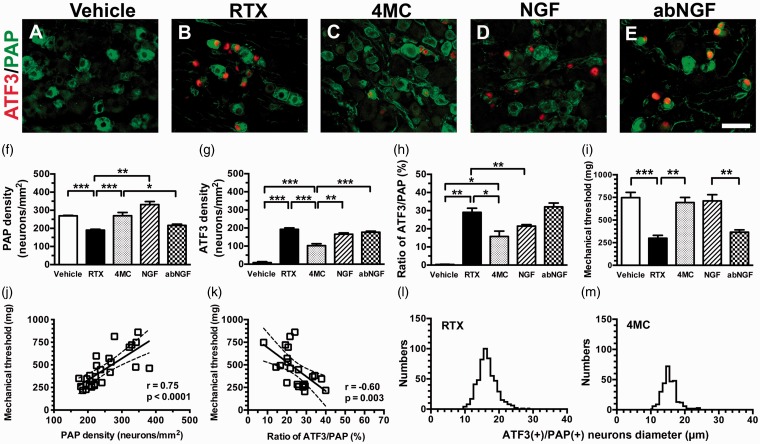
To understand the expression of PAP and its relationship with ATF3, an injury marker, we performed double immunofluorescence staining of DRG neuronal sections in RTX-induced neuropathy (Figure 3(a) to (e)). PAP(+) neurons were mildly lower in the RTX group than in the vehicle group (190.6 ± 12.2 vs. 269.5 ± 6.9 neurons/mm2, p = 0.0003) (Figure 3(f)). By contrast, ATF3(+) neurons were markedly higher in the RTX group than in the vehicle group (192.3 ± 18.0 vs. 9.0 ± 13.1 neurons/mm2, p < 0.0001) (Figure 3(g)). Moreover, ATF3 was induced preferentially in PAP(+) neurons (Figure 3(a) to (e)), resulting in an increased ratio of ATF3(+)/PAP(+) neurons in the RTX group (29.0% ± 5.6% vs. 0.2% ± 0.2%, p = 0.0043) (Figure 3(h)).
Figure 3.
Phenotypic changes in prostatic acid phosphatase (PAP)(+) dorsal root ganglion neurons in resiniferatoxin (RTX)-induced neuropathy. (a–e) Double immunofluorescence staining was performed with anti-activating transcription factor 3 (ATF3; a–e in red) and anti-PAP (a–e in green) antisera in the vehicle (a), RTX (b), 4-methylcatechol (4MC) (c), NGF (RTX + 2.5S NGF; d), and (e) abNGF (4MC + anti-NGF antisera) groups. (f–h) The graphs quantify the density changes in (f) PAP(+) and (g) ATF3(+) neurons and (h) the ratio changes in ATF3(+)/PAP(+) neurons according to Panels (a) to (e). (i) The mechanical thresholds were assessed using the up-and-down method with von Frey monofilaments, as described in the Materials and Methods. The graph indicates the changes in the mechanical thresholds in the vehicle (open bar), RTX (filled bar), 4MC (grey bar), NGF (slashed bar), and abNGF (dotted bar) groups. (j, k) The graphs indicate that the mechanical thresholds correlated with PAP expression, that is, a linear correlation with (j) PAP densities and (k) an inverse correlation with the ratio of ATF3(+)/PAP(+) neurons. (l, m) The graphs show the diameter histogram of ATF3(+)/PAP(+) neurons in the RTX (l) and 4MC (M) groups. The diameter histogram shows no difference between the RTX and 4MC groups, but higher numbers of ATF3(+)/PAP(+) neurons in the RTX group.
Bar, 50 µm. *p < 0.05, **p < 0.01, ***p < 0.001.
Mice in the RTX group showed mechanical allodynia compared with the vehicle group (747.1 ± 156.0 vs. 299.6 ± 70.6 mg, p = 0.0025) (Figure 3(i)). This behavioral change correlated with the PAP profiles; that is, the densities of PAP(+) neurons (r = 0.75, p < 0.0001; Figure 3(j)) and the ratios of ATF3(+)/PAP(+) neurons (r = −0.60, p = 0.003; Figure 3(k)) were inversely linear to the mechanical thresholds. Collectively, these findings indicate that PAP expression had a strong influence in mediating pain development.
4MC and NGF relieved mechanical allodynia correlated with the patterns of trkA expression in RTX-induced neuropathy
After neuropathy was induced using RTX, 4MC normalized PAP expression and relieved mechanical allodynia; specifically, the PAP(+) neuronal density (269.7 ± 48.8 neurons/mm2, p = 0.61) (Figure 3(c) and (f)) and mechanical allodynia (693.8 ± 146.0 mg, p = 0.54) (Figure 3(i)) in the 4MC group were comparable with those in the vehicle group. Furthermore, although 4MC reduced the ATF3(+) neuronal density (102.4 ± 27.8 neurons/mm2, p < 0.0001) and ratio of ATF3(+)/PAP(+) neurons (15.8% ± 5.9%, p = 0.0095), the ratios of ATF3(+)/PAP(+) neurons were still higher in the 4MC group than in the vehicle group (p = 0.016; Figure 3(c), (g), (h)). Morphometric analysis demonstrated that 4MC reduced the absolute number of ATF3(+)/PAP(+) neurons but did not have a significant effect on their mean diameter (15.5 ± 2.2 vs. 16.5 ± 2.8 µm, p > 0.05) (Figure 3(l) vs. (m)).
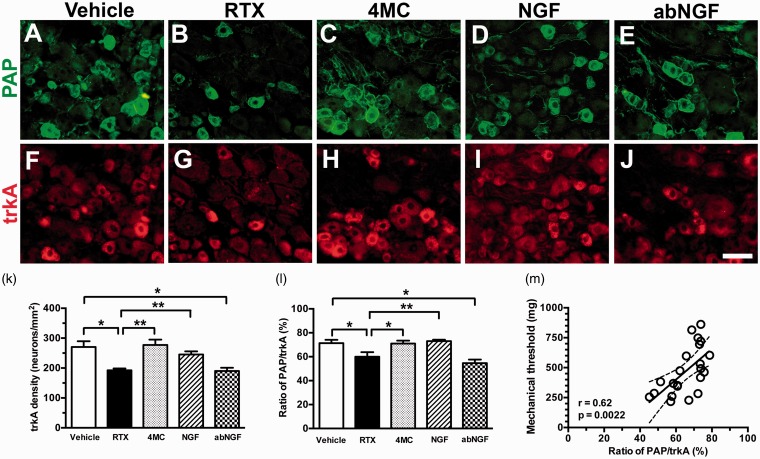
To understand the putative mechanisms of pain relief by 4MC, we examined the coexpression of PAP(+)/trkA(+) neurons (Figure 4). For each group, PAP(+) neuronal density was similar to trkA(+) neuronal density (Figure 3(f) vs. 4(k)). The PAP/trkA ratios correlated linearly with the mechanical threshold (r = 0.62, p = 0.0062; Figure 4(m)). To confirm the effect of 4MC, the mice in the NGF group were systemically (i.p.) administered 2.5S NGF. The NGF group exhibited the same effect as the 4MC group; that is, PAP and trkA expression profiles were reversed, ATF3 induction was normalized, and mechanical allodynia relieved. Those findings, however, were not observed in the abNGF group (administered anti-NGF antisera to neutralize NGF) (Figures 3 and 4). These findings suggest that 4MC regulates PAP profiles through NGF-trkA signaling.
Figure 4.
Colocalization of prostatic acid phosphatase (PAP) and high-affinity nerve growth factor (trkA) receptor after resiniferatoxin (RTX)-induced neuropathy. (a–j) Double immunofluorescence staining of dorsal root ganglia sections was performed with anti-PAP (a–e) and trkA (f-j) in the vehicle (a, f), RTX (b, g), 4-methylcatechol (4MC; c, h), (d, i) NGF (RTX + 2.5S NGF), and (e, j) abNGF (4MC + anti-NGF antisera) groups. (k, l) The graphs show the changes in (k) trkA density and (l) the colocalized ratios of PAP(+)/trkA(+) neurons in the vehicle (open bar), RTX (filled bar), 4MC (grey bar), NGF (slashed bar), and abNGF groups (dotted bar) according to Panels (a) to (j). (m) The graph shows that the mechanical thresholds correlate linearly with the ratios of PAP(+)/trkA(+) neurons.
Bar, 50 µm. *p < 0.05, **p < 0.01.
Discussion
This is the first report documenting the intervention of PAP and NGF-trkA signaling, which mediates neuropathic pain. We speculate one possible mechanism: neuropathic pain in RTX-induced small-fiber neuropathy develops because of PAP neuropathology and the decrease in NGF-trkA signaling.
PAP downregulation was correlated with pain perception and molecular significance of PAP neuropathology
RTX is specifically neurotoxic to TRPV1(+) neurons; it also induces small-fiber neuropathy, such as skin denervation, which results in thermal hypoalgesia,9 small-diameter neuron injury,10 and peripheral sensitization of P2X3 purinoceptors, leading to mechanical allodynia.16 ATF3 is both an injury marker and a marker of pain; for example, the ratios of P2X3(+)/ATF3(+) neurons correlate linearly with the degree of neuropathic pain.10 PAP is susceptible to RTX because of the high ratios of PAP(+)/P2X3(+) and PAP(+)/peripherin(+) neurons. Thus, injured PAP neurons are critical for modulating neuropathic pain; that is, the ratios of ATF3(+)/PAP(+) neurons are inversely linear to the mechanical threshold.
The balance of extracellular purine nucleotides such as adenine nucleotides is maintained by functional membrane-bound ectonucleotidase activity, which responded for the cellular pathophysiological homeostasis.21–24 The potential role of PAP downregulation in nociceptive transmission is unclear. The ectonucleotidase activity of PAP may facilitate the hydrolysis of AMP to adenosine, which acts as an analgesic ligand.6,7 PAP downregulation leads to the imbalance of AMP/adenosine ratios, inhibiting the analgesic effect of adenosine. Taken together, these findings suggest the neuronal soma underlies the irritation and/or injury coincident with the metabolic imbalance of extracellular nucleotides, resulting in the loss of the analgesic effect of PAP. Additional studies should investigate the effects of altered purine metabolism to clarify the cellular responses to neuronal soma irritation. A previous study demonstrated that PAP-mediated neuropathic pain was TRPV1-dependent.8 In our RTX-induced neuropathy model, TRPV1 was completely depleted,9,10 and the present study provides evidence of an alternative nociceptive pathway to the TRPV1-dependent nociceptive pathway; the loss of the analgesic effect of PAP is mediated by neuronal soma injury and/or irritation. Furthermore, pain development correlated with trkA profiles; for example, the ratios of PAP(+)/trkA(+) neurons were linearly correlated with the degree of mechanical allodynia.
Amelioration of neuropathic pain by 4MC through NGF-trkA signaling
This report demonstrates the amelioration of neuropathic pain by 4MC through the intervention of PAP and NGF-trkA signaling. In addition to previous studies demonstrating that 4MC promotes skin reinnervation,9,13 the present study demonstrated that 4MC could facilitate the recovery irritated and/or injured small-diameter neuronal soma, as indicated by the reduced ratios of ATF3(+)/PAP(+) neurons. Conversely, NGF-sensitized sensory nerves resulted in pain perception25 and targeting of NGF-trkA signaling26 is a new direction for pain therapy.27,28 Coincidentally, several studies have suggested that the interaction of NGF and TRPV1 is critical for pain transmission.17,29–32 The present study shows contrary outcomes; for example, 4MC-promoted NGF played the role of a modulator in the recovery of PAP and PAP(+)/trkA(+) neuronal profiles coinciding with the normalization of mechanical allodynia. 4MC appears to have a pleiotropic effect in modulating neuronal activity; for example, it also increases the synthesis of the brain-derived neurotrophic factor (BDNF)33,34 and enhances the phosphorylation of the trk family and activation of the MAPK/ERK cascade.35 Regarding the targeted signaling pathway of 4MC in RTX-induced neuropathy, BDNF modulates the function of the motor36,37 and myelinated sensory neurons,37 but no effect was observed in this current neuropathic model because (1) RTX specifically affects small-diameter sensory neurons with no pathologic alternations in the motor and myelinated sensory neurons,9 and (2) 4MC has no effect on trkB and/or trkC signaling because there are no changes in BDNF and neurotrophic 3 (NT3) expression in the peripheral tissues, as determined in our previous study.13 Regarding the possible mechanism of the effect of 4MC on pain-relief in RTX-induced neuropathy, this report provides immunohistochemical evidence of the potential effect of 4MC, as demonstrated by the PAP/trkA ratios of approximately 70%. Collectively, these data reinforce our speculations: 4MC regulates PAP pathology through NGF-trkA signaling, which was confirmed through alternative NGF administration and NGF neutralization experiments. Combining with the 4MC reduced the ATF3(+)/PAP(+) neurons; this study suggests that 4MC has a pleiotropic effect on both the recovery of irritated and/or injured neuronal soma and restores NGF-trkA signaling. In summary, ATF3 might be an upstream molecule initiating the loss of the analgesic effect of PAP, and PAP is a downstream molecule of NGF-trkA signaling, restoring the analgesic effect of PAP in a trkA-dependent manner (Figure 5).
Figure 5.
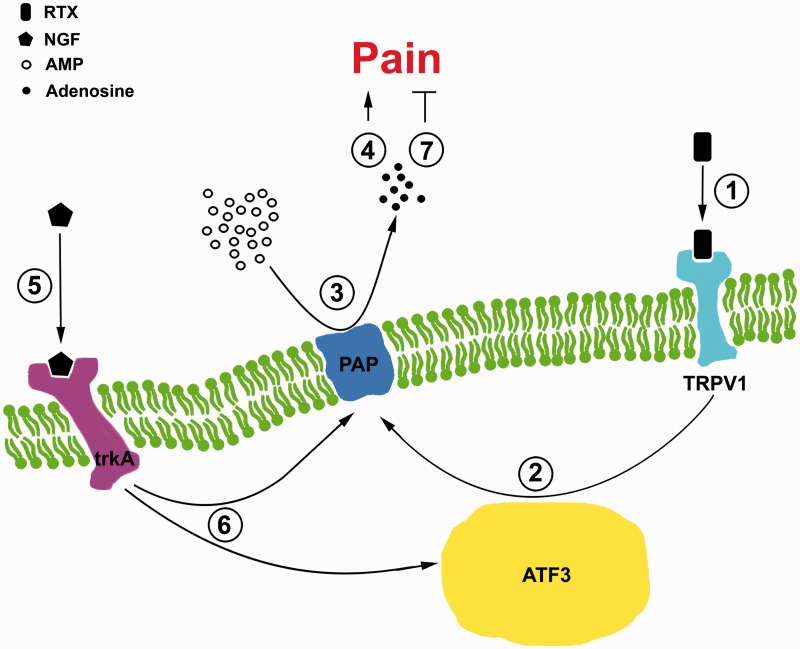
Diagram of prostatic acid phosphatase (PAP) pathology modulates the pain perception in resiniferatoxin (RTX) neuropathy. This diagram illustrates the PAP pathology mediating pain development follows a NGF-trkA-dependent manner, as described in the following steps: (1) RTX sensitizes the transient receptor potential vanilloid subtype 1 (TRPV1). (2) RTX then induces activating transcription factor 3 (ATF3) upregulation on PAP(+) neurons, indicating the PAP pathology. (3) The PAP pathology reduces the AMP hydrolysis. (4) This reduction evokes pain perception because of the decrease in the adenosine analgesic effect. (5) Nerve growth factor (NGF) binds to high-affinity NGF receptor (trkA) and (6) reverses the induction of the ATF3 and PAP pathology. (7) This recovers the PAP-mediated analgesic effect by returning the ability of AMP hydrolysis.
Conclusion
For pain development, this study provides evidence of an alternative pathway to conventional small nociceptor sensitization-induced neuropathic pain; the loss of analgesic effect of PAP is mediated by neuronal soma irritation and/or injury. Alternatively, restoring the analgesic ligand may be a new direction for targeting painful neuropathy, that is, maintenance of steady-static adenosine content and functional PAP ectonucleotidase activity at the neuronal soma level.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Science Council (NSC100-2320-B-037-018 and NSC102-2320-B-037-009); Ministry of Science and Technology (MOST103-2320-B-037-015-MY3), Taiwan; and Kaohsiung Medical University Hospital (KMUH104-4M19).
References
- 1.Zylka MJ, Sowa NA, Taylor-Blake B, et al. Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron 2008; 60: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quintero IB, Araujo CL, Pulkka AE, et al. Prostatic acid phosphatase is not a prostate specific target. Cancer Res 2007; 67: 6549–6554. [DOI] [PubMed] [Google Scholar]
- 3.Araujo CL, Quintero IB, Kipar A, et al. Prostatic acid phosphatase is the main acid phosphatase with 5'-ectonucleotidase activity in the male mouse saliva and regulates salivation. Am J Physiol Cell Physiol 2014; 306: C1017–C1027. [DOI] [PubMed] [Google Scholar]
- 4.Taylor-Blake B, Zylka MJ. Prostatic acid phosphatase is expressed in peptidergic and nonpeptidergic nociceptive neurons of mice and rats. PLoS One 2010; 5: e8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Street SE, Kramer NJ, Walsh PL, et al. Tissue-nonspecific alkaline phosphatase acts redundantly with PAP and NT5E to generate adenosine in the dorsal spinal cord. J Neurosci 2013; 33: 11314–11322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sowa NA, Vadakkan KI, Zylka MJ. Recombinant mouse PAP has pH-dependent ectonucleotidase activity and acts through A(1)-adenosine receptors to mediate antinociception. PLoS One 2009; 4: e4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Street SE, Walsh PL, Sowa NA, et al. PAP and NT5E inhibit nociceptive neurotransmission by rapidly hydrolyzing nucleotides to adenosine. Mol Pain 2011; 7: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sowa NA, Street SE, Vihko P, et al. Prostatic acid phosphatase reduces thermal sensitivity and chronic pain sensitization by depleting phosphatidylinositol 4,5-bisphosphate. J Neurosci 2010; 30: 10282–10293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh YL, Chiang H, Tseng TJ, et al. Enhancement of cutaneous nerve regeneration by 4-methylcatechol in resiniferatoxin-induced neuropathy. J Neuropathol Exp Neurol 2008; 67: 93–104. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh YL, Chiang H, Lue JH, et al. P2X3-mediated peripheral sensitization of neuropathic pain in resiniferatoxin-induced neuropathy. Exp Neurol 2012; 235: 316–325. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh YL, Lin CL, Chiang H, et al. Role of peptidergic nerve terminals in the skin: reversal of thermal sensation by calcitonin gene-related peptide in TRPV1-depleted neuropathy. PLoS One 2012; 7: e50805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000; 288: 306–313. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh YL, Lin WM, Lue JH, et al. Effects of 4-methylcatechol on skin reinnervation: promotion of cutaneous nerve regeneration after crush injury. J Neuropathol Exp Neurol 2009; 68: 1269–1281. [DOI] [PubMed] [Google Scholar]
- 14.Anand P. Neurotrophic factors and their receptors in human sensory neuropathies. Prog Brain Res 2004; 146: 477–492. [DOI] [PubMed] [Google Scholar]
- 15.Indo Y. Neurobiology of pain, interoception and emotional response: lessons from nerve growth factor-dependent neurons. Eur J Neurosci 2014; 39: 375–391. [DOI] [PubMed] [Google Scholar]
- 16.Lin CL, Fu YS, Hsiao TH, et al. Enhancement of purinergic signalling by excessive endogenous ATP in resiniferatoxin (RTX) neuropathy. Purinergic Signal 2013; 9: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frias B, Charrua A, Avelino A, et al. Transient receptor potential vanilloid 1 mediates nerve growth factor-induced bladder hyperactivity and noxious input. BJU Int 2012; 110: E422–E428. [DOI] [PubMed] [Google Scholar]
- 18.Zhang QL, Qiao LY. Regulation of IGF-1 but not TGF-beta1 by NGF in the smooth muscle of the inflamed urinary bladder. Regul Pept 2012; 177: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 20.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 21.Tsuda M, Tozaki-Saitoh H, Inoue K. Pain and purinergic signaling. Brain Res Rev 2010; 63: 222–232. [DOI] [PubMed] [Google Scholar]
- 22.Burnstock G, Knight GE, Greig AV. Purinergic signaling in healthy and diseased skin. J Invest Dermatol 2012; 132: 526–546. [DOI] [PubMed] [Google Scholar]
- 23.Burnstock G, Novak I. Purinergic signalling and diabetes. Purinergic Signal 2013; 9: 307–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnstock G. Purinergic mechanisms and pain – an update. Eur J Pharmacol 2013; 716: 24–40. [DOI] [PubMed] [Google Scholar]
- 25.Mizumura K, Murase S. Role of nerve growth factor in pain. Handb Exp Pharmacol 2015; 227: 57–77. [DOI] [PubMed] [Google Scholar]
- 26.Mantyh PW, Koltzenburg M, Mendell LM, et al. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 2011; 115: 189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bannwarth B, Kostine M. Targeting nerve growth factor (NGF) for pain management: what does the future hold for NGF antagonists? Drugs 2014; 74: 619–626. [DOI] [PubMed] [Google Scholar]
- 28.Leite VF, Buehler AM, El Abd O, et al. Anti-nerve growth factor in the treatment of low back pain and radiculopathy: a systematic review and a meta-analysis. Pain Physician 2014; 17: E45–E60. [PubMed] [Google Scholar]
- 29.De Angelis F, Marinelli S, Fioretti B, et al. M2 receptors exert analgesic action on DRG sensory neurons by negatively modulating VR1 activity. J Cell Physiol 2014; 229: 783–790. [DOI] [PubMed] [Google Scholar]
- 30.Shinoda M, Asano M, Omagari D, et al. Nerve growth factor contribution via transient receptor potential vanilloid 1 to ectopic orofacial pain. J Neurosci 2011; 31: 7145–7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eskander MA, Ruparel S, Green DP, et al. Persistent nociception triggered by nerve growth factor (NGF) is mediated by TRPV1 and oxidative mechanisms. J Neurosci 2015; 35: 8593–8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malek N, Pajak A, Kolosowska N, et al. The importance of TRPV1-sensitisation factors for the development of neuropathic pain. Mol Cell Neurosci 2015; 65: 1–10. [DOI] [PubMed] [Google Scholar]
- 33.Ishikawa K, Yasuda S, Fukuhara K, et al. 4-Methylcatechol prevents derangements of brain-derived neurotrophic factor and TrkB-related signaling in anterior cingulate cortex in chronic pain with depression-like behavior. Neuroreport 2014; 25: 226–232. [DOI] [PubMed] [Google Scholar]
- 34.Fukuhara K, Ishikawa K, Yasuda S, et al. Intracerebroventricular 4-methylcatechol (4-MC) ameliorates chronic pain associated with depression-like behavior via induction of brain-derived neurotrophic factor (BDNF). Cell Mol Neurobiol 2012; 32: 971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sometani A, Nomoto H, Nitta A, et al. 4-Methylcatechol stimulates phosphorylation of Trk family neurotrophin receptors and MAP kinases in cultured rat cortical neurons. J Neurosci Res 2002; 70: 335–339. [DOI] [PubMed] [Google Scholar]
- 36.Baydyuk M, Xu B. BDNF signaling and survival of striatal neurons. Front Cell Neurosci 2014; 8: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richner M, Ulrichsen M, Elmegaard SL, et al. Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system. Mol Neurobiol 2014; 50: 945–970. [DOI] [PubMed] [Google Scholar]