Abstract
Background
Bone marrow stromal cells (BMSCs) have shown potential to treat chronic pain, although much still needs to be learned about their efficacy and mechanisms of action under different pain conditions. Here, we provide further convergent evidence on the effects of BMSCs in rodent pain models.
Results
In an orofacial pain model involving injury of a tendon of the masseter muscle, BMSCs attenuated behavioral pain conditions assessed by von Frey filaments and a conditioned place avoidance test in female Sprague-Dawley rats. The antihyperalgesia of BMSCs in females lasted for <8 weeks, which is shorter than that seen in males. To relate preclinical findings to human clinical conditions, we used human BMSCs. Human BMSCs (1.5 M cells, i.v.) attenuated mechanical and thermal hyperalgesia induced by spinal nerve ligation and suppressed spinal nerve ligation-induced aversive behavior, and the effect persisted through the 8-week observation period. In a trigeminal slice preparation, BMSC-treated and nerve-injured C57B/L mice showed reduced amplitude and frequency of spontaneous excitatory postsynaptic currents, as well as excitatory synaptic currents evoked by electrical stimulation of the trigeminal nerve root, suggesting inhibition of trigeminal neuronal hyperexcitability and primary afferent input by BMSCs. Finally, we observed that GluN2A (N-methyl-D-aspartate receptor subunit 2A) tyrosine phosphorylation and protein kinase Cgamma (PKCγ) immunoreactivity in rostral ventromedial medulla was suppressed at 8 weeks after BMSC in tendon-injured rats.
Conclusions
Collectively, the present work adds convergent evidence supporting the use of BMSCs in pain control. As PKCγ activity related to N-methyl-D-aspartate receptor activation is critical in opioid tolerance, these results help to understand the mechanisms of BMSC-produced long-term antihyperalgesia, which requires opioid receptors in rostral ventromedial medulla and apparently lacks the development of tolerance.
Keywords: Orofacial pain, tendon ligation, spinal nerve injury, chronic constriction injury, trigeminal neuron, mesenchymal stromal cells
Introduction
Studies have shown that bone marrow stromal cells (BMSCs), a major type of multipotent mesenchymal stromal (or stem) cells, appear to have potential to treat chronic pain conditions.1,2 In a randomized, blinded, placebo-controlled veterinary clinical trial, autologous adipose-derived mesenchymal stem cells significantly improved pain scores in dogs with chronic osteoarthritis of the hip.3 BMSCs have been shown to improve tendon injuries in racehorses.4 Recent studies indicate that ex vivo expanded mesenchymal stem cells have pain-relieving properties in preclinical pain models and do not show adverse side effects.5–13
In a myogenic orofacial pain rat model involving the ligation injury of the tendon of the masseter muscle,14 we have shown that intravenous infusion of BMSCs derived from the rat produced long-term attenuation of mechanical pain hypersensitivity in male rats.9 In the present study, we aimed to provide further convergent evidence to support the BMSC-produced antihyperalgesia. We have extended the observation to female rats and spinal nerve injury models and used additional measures of nociception including nociceptive thermal responses, conditioned place avoidance (CPA), and trigeminal neuronal synaptic activity. Further, we observed that the long-term antihyperalgesic effect of BMSCs was associated with suppression of N-methyl-D-aspartate (NMDA) receptor 2A subunit (GluN 2A) phosphorylation in the pain modulatory circuitry.
Methods
Animals and pain models
Sprague-Dawley rats (200–250 g) and C57BL/6 mice (≈20 g) were purchased (Harlan, Indianapolis, IN). Animals involved in the present study were housed on the ninth floor of the University of Maryland School of Dentistry. The facility is an approved, registered research site (USDA #MD-R-118) and accredited by Association for Assessment and Accreditation of Laboratory Animal Care. Animals were kept under controlled environment conditions (≈22℃), relative humidity 40–60%, 12 h/12 h light–dark cycles, and food and water ad libitum. The animals were allowed to acclimate post arrival for 3–5 days before experiments. They were housed in cages (2–3 rats and 4–5 mice per cage) with floors covered by soft bedding materials to minimize potential painful contact with a hard surface. The animals’ conditions were monitored continuously throughout the course of studies, which include body weight, grooming, locomotion, ambulant activity, and condition of the wound. The behavioral studies involve stimulation that produces only momentary additional pain or discomfort and the rats can escape from the stimuli at any time.
The following three rodent pain models were used: (1) Tendon ligation (TL) was achieved via an intraoral approach as described elsewhere.14 Briefly, on the left intraoral site, a 5-mm long incision was made posterior–anteriorly lateral to the gingivobuccal margin in the buccal mucosa, beginning immediately next to the first molar. The tendon of the anterior superficial part of the rat masseter muscle was gently freed and tied with two chromic gut (4.0) ligatures, 2-mm apart. (2) L5 spinal nerve ligation (SNL) was performed per Kim and Chung.15 Briefly, the left paraspinal muscles were separated with a hemostat from the spinous process at the L4–S2 levels. The L5 spinal nerve was isolated and tightly ligated with one silicon-treated silk (9.0) suture. (3) Chronic constriction injury of the infraorbital nerve (CCI-ION) was performed in mice according to Wei et al.16 A 5–7 mm long incision was made along the left gingivobuccal margin in the buccal mucosa. The ION is freed from surrounding connective tissues. At 3–4 mm from the nerve where its branches emerge from the infraorbital fissure, the ION was loosely tied with two chromic gut (4.0) ligatures, 2-mm apart. All surgical procedures were performed under pentobarbital sodium (20–50 mg/kg i.p.) anesthesia. Animals were randomly assigned to experimental groups. The number of animals for different experimental conditions was determined by our previous studies and a power analysis.
The vaginal smears of the female rats were taken by a physiological saline lavage with an eyedropper. The solution was examined under a microscope for the cell content of the vaginal canal. The stage of the estrous cycle was determined by relative frequency of leukocytes, cornified, and nucleated epithelial cells.17
All experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (National Institute of Health Publications No. 80–23) and approved by the University of Maryland, Baltimore, Institutional Animal Care and Use Committee. The report was prepared following the ARRIVE guidelines.
BMSC procedures
Primary cultures of BMSCs were obtained from donor rats as described.9,18 The rats were sacrificed with CO2, both ends of the tibiae, femurs, and humerus were cut off by scissors. A syringe fitted with an 18-gauge needle was inserted into the shaft of the bone and bone marrow was flushed out with culture medium (alpha-modified Eagle medium, Gibco, Carlsbad, CA, USA; 10% fetal bovine serum, Hyclone, Logan, UT, USA). The bone marrow was then mechanically dissociated and the suspension passed through a 100-m cell strainer to remove debris. The cells were incubated at 37℃ in 5% CO2 in tissue-culture flasks (100 × 200 mm) (Sarstedt, Nümbrecht, Germany), and non-adherent cells were removed by replacing the medium. At day 7, when the cultures reached 80% confluence, the cells were washed with PBS and harvested. The cell numbers were calculated by the Hemacytometer. For intravenous administration, 1.5 × 106 cells (1.5 M) in 0.2 ml PBS were slowly injected into one tail vein of the anesthetized rat over a 2-minute period using a 22-gauge needle. The property of expanded cells was assessed by flow cytometry with conventional markers.9 Flow cytometry analyses were performed at the University of Maryland Greenbaum Cancer Center Shared Flow Cytometry Facility.
Cryopreserved human primary BMSCs (hBMSCs) were purchased (KT-002, RoosterBio, Inc., Frederick, MD, USA). hBMSCs vial from liquid nitrogen was immediately thawed in 37℃ water bath. Cells were removed from water bath once only a small bit of ice was remaining. Cells were aseptically transferred into 50-ml centrifuge tube, and 4 ml of culture media were slowly added. Cells were centrifuged 200 × g for 10 min and the supernatant was carefully removed without disturbing the pellet. The cells were then resuspended in 45 ml of culture media, mixed well, and seeded into three T75 vessels. After reaching 90% confluence, the cells were passed into six 10-cm culture plates. The cells were collected after reaching more than 90% confluence and 1.5 M cells infused to the animals.
Behavioral testing
All behavioral tests were conducted under blind conditions. Mechanical sensitivity of the orofacial region was assessed as described.14,19 A series of calibrated von Frey filaments were applied to the skin above the injured tendon or the corresponding contralateral side. An active withdrawal of the head from the probing filament was defined as a response. Each von Frey filament was applied five times at intervals of 5–10 seconds. The response frequencies [(number of responses/number of stimuli) × 100%] to a range of von Frey filament forces were determined, and a stimulus-response frequency (S-R) curve was plotted. After a non-linear regression analysis, an EF50 value, defined as the effective von Frey filament force (g) that produces a 50% response frequency, was derived from the S-R curve (Prism, GraphPad).20 A leftward shift of the S-R curve, resulting in a reduction of EF50, occurred after TL or CCI-ION. This shift of the curve suggests the development of mechanical hypersensitivity or presence of mechanical hyperalgesia and allodynia since there was an increase in response to suprathreshold stimuli and a decreased response threshold for nocifensive behavior.
For the hind paw nocifensive behavioral test, an electronic von Frey unit (EVF3, Bioseb) was used to assess mechanical sensitivity according to Ren.19 The measurement range of the unit is 0 to 500 g with 0.1 g resolution. The probe was applied against the lateral edge of the hind paw with gradually increasing force. The force (g) that induced paw withdrawal was digitized by the unit and used as the threshold for mechanical nociception. Hind paw thermal hyperalgesia was assessed by measuring the latency of paw withdrawal in response to a radiant heat source.21 The paw withdrawal latency (PWL) was determined by an electronic clock circuit. A 20-s cutoff was used to prevent tissue damage.
For measurement of CPA behavior,22 animals were placed within a 40.5 × 30.5 × 16 cm Plexiglas chamber positioned on top of a mesh screen. One half of the chamber was painted white (light area) and the other half was painted black (dark area). During behavioral testing, animals were allowed unrestricted movement throughout the test chamber for the duration of a 30-min test period. Testing began immediately with suprathreshold mechanical stimulation (758 mN von Frey monofilament) applied to the facial skin at 15-s intervals. The mechanical stimulus was applied to the skin above the injured tendon when the animal was within the preferred dark area and the facial skin on the non-injured side when the animal was within the non-preferred light area of the test chamber. Pre-injury naïve animals are mechanically stimulated in an identical manner as the injured groups. Based on the location of the animal at each 15-s interval, the mean percentage of time spent in each side of the chamber was calculated for the entire test period.
Whole-cell patch-clamp recording
The male mouse brain stem was dissected and horizontally sliced at a thickness of 0.4 mm. Slices were kept in a 50-ml glass beaker containing carbogenated protective artificial cerebrospinal fluid at 33℃. After at least a 1-hour recovery, slices were transferred into a recording chamber and continuously perfused (10–15 ml/min) with oxygenated Krebs’ solution (composition in mM: NaCl 126, NaHCO3 26, glucose 10, KCl 2.5, CaCl2 2, MgCl2 1.2, and NaH2PO4 1.25) at room temperature. We recorded from lamina II neurons of the subnucleus caudalis of the spinal trigeminal nucleus (Vc). Spontaneous excitatory postsynaptic currents (sEPSCs) were recorded continuously at a holding potential of −70 mV in the absence of tetrodotoxin. The recording electrode resistance was 7–10 MΩ. To block inhibitory transmission, 1 mM strychnine sulfate and 10 mM bicuculline methiodide were included in the superfusate in all experiments. Whole-cell voltage-clamp recordings were performed with series resistance compensation using an EPC 10 amplifier.
Western blot
Rats were anesthetized with isoflurane (3%) and quickly decapitated. The brainstem tissue block that included the rostral ventromedial medulla (RVM) was harvested by taking punches with a 15-gauge needle. The tissues were homogenized in solubilization buffer (50 mM Tris.HCl, pH 8.0; 150 mM NaCl, 1 mM EDTA, 1% NP40, 0.5% deoxycholic acid, 0.1% SDS, 1 mM Na3VO4, 1 U/ml aprotinin, 20 µg/ml leupeptin, 20 µg/ml pepstatin A). The homogenate was centrifuged at 20,200 × g for 10 min at 4℃. The supernatant was removed. The protein concentration was determined using a detergent-compatible protein assay with a bovine serum albumin standard. For detecting the immunoreactivity with near-infrared fluorescence using the Odyssey Infrared Imaging System (Odyssey®CLx, LI-COR, Lincoln, NE), 50 µg protein samples were denatured by boiling for 5 min and loaded onto 4%–20% Bis-Tris gels (Invitrogene). After electrophoresis, proteins were transferred to nitrocellulose membranes. The membranes were blocked for 1 h with Odyssey Blocking Buffer and then incubated with primary antibodies: anti-phosphotyr 1246-GluN2A antibody (1:1000, #4206, Cell Signaling, Danvers, MA) and anti-PKCγ antibody (1:1000, ab4145, abcam, Cambridge, MA), diluted in Odyssey Blocking Buffer at 4℃ overnight, followed by washing with PBS containing 0.1% Tween 20 (PBST) three times. The membranes were then incubated for 1 h with IRDye800CW-conjugated goat anti-rabbit IgG and IRDye680-conjugated goat anti-mouse IgG secondary antibodies (LI-COR) diluted in Odyssey Blocking Buffer. The blots were further washed three times with PBST and rinsed with PBS. Proteins were visualized by scanning the membrane with 700 - and 800-nm channels. The loading and blotting of the amount of protein was verified by reprobing the membrane with anti-β-actin and with Coomassie blue staining.
Immunohistochemistry
Rats were deeply anesthetized with pentobarbital sodium (100 mg/kg, i.p.) and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer at pH 7.4. The brain stem was removed, post-fixed, and transferred to 25% sucrose (w/v) for cryoprotection. Transverse sections (20 -µm) were cut with a cryostat. The free-floating sections were incubated with relevant antibodies with 1% normal goat sera and 0.3% Triton x-100 overnight at 4℃. After washes in PBS, the sections were incubated with relevant IgGs conjugated to Cy3 or Cy2 (1:500; Jackson ImmunoResearch, West Grove, PA) for 4 h at room temperature or overnight at 4℃. Double immunofluorescent staining was performed for PKCγ (abcam) with NeuN (Chemicon) or GluN2A (Santa Cruz). Following washes, the stained sections were mounted on gelatin-coated slides and coverslipped with Vectashield (Vector Laboratories). Slides were examined with a Nikon fluorescence microscope and images were captured with a CCD Spot camera. Control sections were processed with the same method except that the primary antisera are omitted.
Data analysis
Data are presented as mean (95% confidence interval) for von Frey filament force data (EF50s) and mean ± SEM for other data. Statistical comparisons were made by the use of analysis of variance (ANOVA). For unbalanced sample size, multiple regressions were performed and Type III sum-of-squares was used to calculate p values (GraphPad Prism). The Tukey test was performed for post-hoc comparisons. For animals that were subject to repeated testing, ANOVA with repeated measures was used. p < 0.05 was considered significant for all cases.
Results
BMSC attenuated hyperalgesia in females with tendon injury
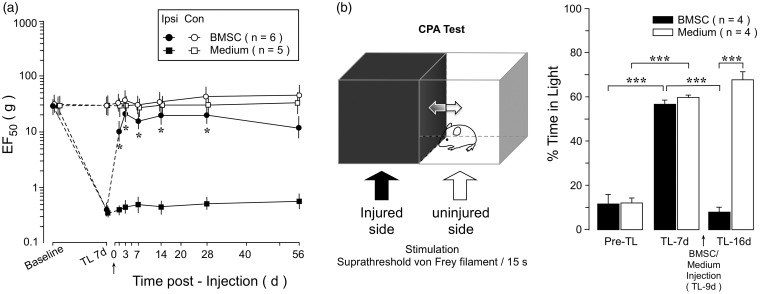
Women exhibit a significantly higher prevalence of temporomandibular pain than men.23 However, it is unclear whether BMSCs produce long-lasting attenuation of orofacial pain in females as that in males.9 Thus, we examined the effect of rat BMSCs in female animals in the TL model.14 The TL rats groomed normally and displayed normal locomotor activity. They maintained their weight and explored their environment and interacted with cagemates. BMSCs did not produce adverse effects on the animals. As shown in Figure 1(a), EF50s of von Frey filaments, a measure of mechanical sensitivity, were significantly reduced at 7 days post-TL on the ipsilateral injured side (p < 0.001) compared to the baseline, indicating the development of mechanical hyperalgesia and allodynia after TL. Following infusion of BMSCs (1.5 M cells, n = 6), the ipsilateral EF50s were significantly elevated (Figure 1(a)). The increased EF50s lasted for at least 28 days post-BMSC. At 56 days post-BMSC, EF50 showed more variability and the increase did not reach statistical significance. Infusion of culture medium (n = 5) did not have an effect and BMSCs did not affect EF50 on the contralateral non-injured side (Figure 1(a)).
Figure 1.
Effect of BMSCs on hyperalgesia in female rats. (a) At 7 days after TL, BMSCs (1.5 M) were infused through a tail vein (arrow). Compared to culture medium-injected rats, EF50s on the injured ipsilateral (Ipsi) side were increased in BMSC-treated rats, indicating attenuation of hyperalgesia. BMSCs did not affect EF50 on the non-injured contralateral (Con) side. Error bars are 95% confidence intervals of EF50. *, p < 0.05, vs. medium. (b) TL-induced aversive behavior and the effects of BMSCs in the conditioned place avoidance (CPA) test (left). Compared to Pre-TL, the percentage of time rats spent within the light area was increased after TL (7 days). Infusion of BMSCs resulted in a reduction of TL-induced aversive behavior, indicated by a decrease in time spent in the light chamber (TL-16 days). Error bars are SEM (***, p < 0.001). ANOVA with post-hoc comparisons.
Pain involves both sensory discriminative and emotional/aversive components. We also assessed aversive behavior in a separate set of female rats during persistent orofacial pain with the CPA behavior test.22 During the test, rats were placed within a chamber equally divided into a light and a dark area. Rats normally prefer to stay in the dark area although they are allowed to move between the chambers. In TL-treated rats, the injured side was stimulated with a suprathreshold von Frey filament while the rat stayed in the dark area (Figure 1(b)). When the rat moved to the light side, the corresponding non-injured side was stimulated. Thus, staying in the preferred dark side was associated with a painful stimulus and the rat was forced to make a decision to remain there or avoid the painful stimulus. TL induced an increase in time spent in the light area. For example, in the BMSC group, rats only spent 11.5 ± 4.3% of time in the light chamber before injury, but stayed there for 56.7 ± 1.8% of time at 7 days after TL (Figure 1(b)). At 9 days post-TL, BMSCs (1.5 M) were infused i.v. and the CPA tested again at 7 days after BMSCs (TL-16 days). BMSC-treated rats spent significantly less time (7.7 ± 2.4%) in the light area of the chamber, compared to medium-treated rats (67.7 ± 3.6%), suggesting suppression of aversive behavior (Figure 1(b)).
The effect of human BMSCs on neuropathic pain
We next extended our observations to a spinal model of neuropathic pain with human BMSCs (hBMSCs) treatment. L5 SNL injury was produced in male rats, and hBMSCs (1.5 M cells) were infused at 7 days after nerve injury when persistent hyperalgesia had developed. We tested the effect of hBMSCs in rats after spinal nerve injury on mechanical and thermal nociception. As shown in Figure 2(a), hBMSC (1.5 M cells) significantly increased mechanical nociceptive threshold of the rat hind paw measured with an electronic von Frey unit, compared to that before infusion of BMSCs (SNL 7 days). The thermal pain hypersensitivity was also significantly attenuated as shown by a significant increase in PWL to a noxious thermal stimulus (Figure 2(b)). These effects persisted over the 8-weeks testing period, similar to that in the orofacial pain models with rat BMSCs.9 Rat BMSCs also produced antihyperalgesia in the SNL rats (data not shown). Both hBMSCs and rat BMSCs also reversed aversive behavior in rats with SNL, as indicated by a significant reduction of time spent in the light chamber after injury in the CPA test (Figure 2(c)).
Figure 2.
Effects of human BMSCs (hBMSC) on neuropathic pain. (a) L5 spinal nerve injury (SNL) was produced in male rats. Mechanical sensitivity was assessed with an electronic von Frey unit. Primary hBMSCs were infused i.v. at 0.2 ml with 1.5 M cells at 7 days after SNL. Compared to medium control, the mechanical nocifensive response threshold (Ipsi) of BMSC-treated rats was increased, indicating attenuation of hyperalgesia. (b) Thermal nocifensive responses, expressed by paw withdrawal latency (PWL) to a noxious thermal stimulus, of SNL rats were inhibited in hBMSC-treated rats, indicating attenuation of thermal hyperalgesia. ***, p < 0.001, versus Medium, ANOVA with post-hoc comparisons. (c) The effects of hBMSCs and rat BMSCs (rBMSCs) on SNL-induced aversive behavior. Compared to naïve rats, the percentage of time spent within the light area was increased after SNL in medium-treated rats. Infusion of BMSCs resulted in a reduction of SNL-induced aversive behaviour, indicated by a decrease in time spent in the light chamber. ***, p < 0.001, versus Medium. ###, p < 0.001, versus naïve. Error bars are SEM.
The effect of BMSCs on trigeminal neuronal activity
In addition to multiple behavioral endpoints, we examined the effects of rat BMSCs on trigeminal neuronal activity. We chose to use an ex vivo trigeminal slice adult mouse preparation recently adapted in our lab24,25 since the trigeminal nerve root can be included for stimulation. A similar preparation of the subnucleus caudalis of the spinal trigeminal nucleus (Vc) with trigeminal nerve root attached is unavailable from the adult rat. For this mouse preparation, the CCI-ION was produced.16 We have previously shown that BMSCs attenuated pain hypersensitivity in the CCI-ION model in rats.9 A total of 12 male mice were used in neuronal recordings (Naïve: 3, Medium: 3, BMSC: 6). One to two slices were taken from one animal and one neuron was recorded from one slice.
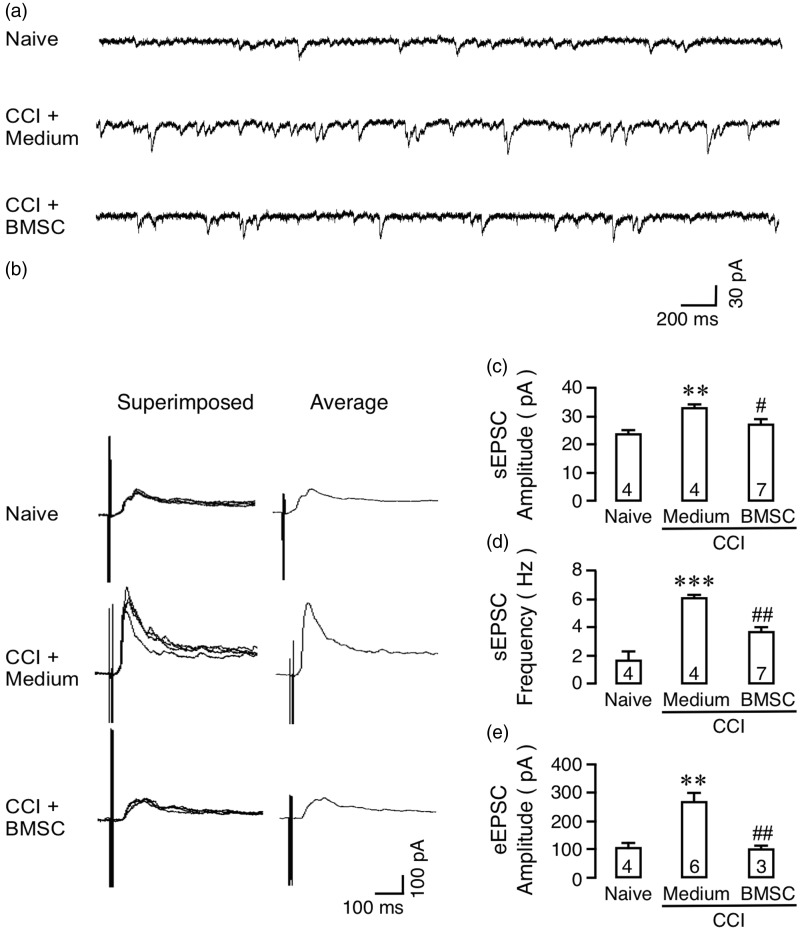
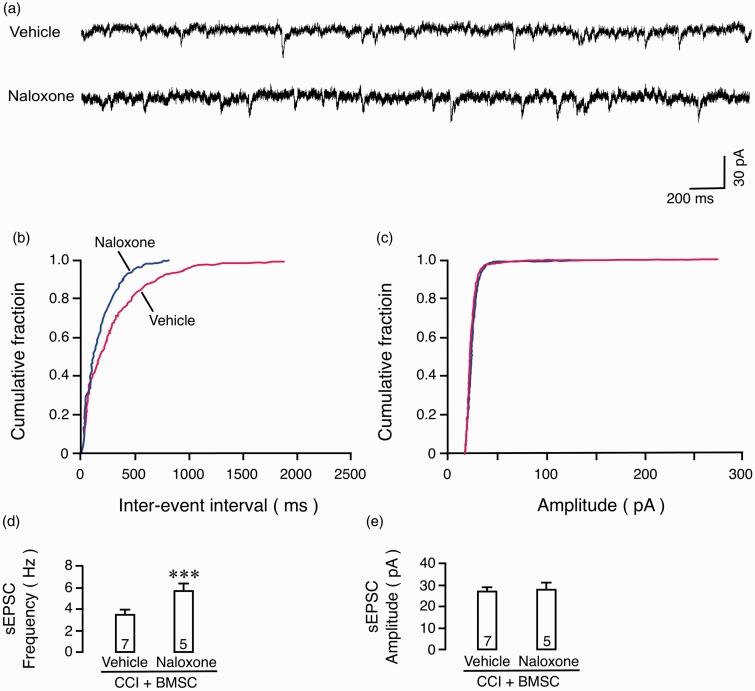
At 14 days after CCI-ION, male mice received BMSCs (1.5 M cells) or medium control infusion from a tail vein. At 1–6 days after BMSCs, we recorded from lamina II neurons of the Vc (medullary dorsal horn). The effects of BMSCs were consistent during the first week after BMSC administration, and cells were combined in each group for analysis. Compared to that from culture medium-treated mice, the Vc neurons in BMSC-treated animals showed significantly reduced amplitude and frequency of sEPSCs (Figure 3(a), (c), and (d)). In slices from BMSC-treated mice, naloxone, an opioid receptor antagonist (1 μM), rekindled the frequency of sEPSCs compared to vehicle treated slices (Figure 4(a), (b), and (d)) without an effect on the amplitude of sEPSCs (Figure 4(a), (c), and (e)), suggesting a presynaptic action of BMSC-induced endogenous opioids.9,26 The effect of naloxone was consistent with behavioral observation and suggests the involvement of endogenous opioids in BMSC’s antihyperalgesia.
Figure 3.
Effects of BMSCs on synaptic activity of trigeminal neurons. Whole cell patch clamp recordings were performed at 1 to 6 days after infusion of rat BMSCs from horizontal slices of the Vc with attached trigeminal nerve root prepared from adult mouse (5–8 weeks). (a) Examples of spontaneous excitatory postsynaptic current (sEPSC). (b) NMDA receptor-mediated synaptic current, or eEPSC, was obtained by electrical stimulation of the nerve root at suprathreshold intensity (0.2 mA, 0.1 ms pulse) after depolarizing the cell to +40 mV. The evoked current was induced in the presence of CNQX (10 M), strychnine (1 M), and bicuculline (10 M), but was abolished when AP-5 (0.02–1 mM) or tetrodotoxin (1 m) was added to the bath. The eEPSC was increased in CCI mice receiving medium but significantly reduced in BMSC-treated animals. Mg2+ was absent in the external solution. (c to e) Summary of the effect of BMSCs on sEPSC amplitude (C), frequency (D), and eEPSC (E). Holding potential = −70 mV. Number in the bars indicates the number of neurons in each group. **, p < 0.01, ***, p < 0.001, versus naïve; #, p < 0.05, ##, p < 0.01, versus CCI. ANOVA with post-hoc comparisons.
Figure 4.
Effect of naloxone on sEPSCs. (a) Examples of sEPSC recordings. (b, c) Cumulative fraction curves of sEPSC inter-event intervals (b) and amplitude (c). Note a left-ward shift of the inter-event interval curve in naloxone-treated mice, suggesting a reduction in the event interval or an increase in sEPSC frequency. (d, e) Summary of the effect of naloxone on sEPSC frequency (d) and amplitude (e) in CCI mice treated with BMSCs. Number in the bars indicates the number of neurons in each group. ***, p < 0.001, versus vehicle, Student t-test.
Electrical stimulation of the trigeminal nerve root at suprathreshold intensity (0.2 mA, 0.1 ms pulse) after depolarizing the cell to +40 mV evoked NMDA receptor-mediated synaptic currents or eEPSCs in lamina II neurons of Vc (Figure 3(b)). The evoked current was induced in the presence of CNQX (10 M), an AMPA/kainate receptor antagonist, strychnine (1 M), a glycine receptor antagonist, and bicuculline (10 M), a GABAA receptor antagonist, but was abolished when AP-5 (0.02–1 mM), an NMDA receptor antagonist, or tetrodotoxin (1 m), a sodium channel blocker, was added to the bath. Compared to naïve mice, the NMDA receptor-mediated eEPSCs were increased in mice (14 days after CCI-ION) receiving the medium treatment, but returned to the naïve level in BMSC-treated animals (Figure 3(b), 3(e)). These results suggest inhibition of trigeminal neuronal hyperexcitability and primary nociceptive afferent input at the Vc level after BMSC treatment.
The effect of BMSCs on NMDA receptor phosphorylation and protein kinase Cgamma (PKCγ)
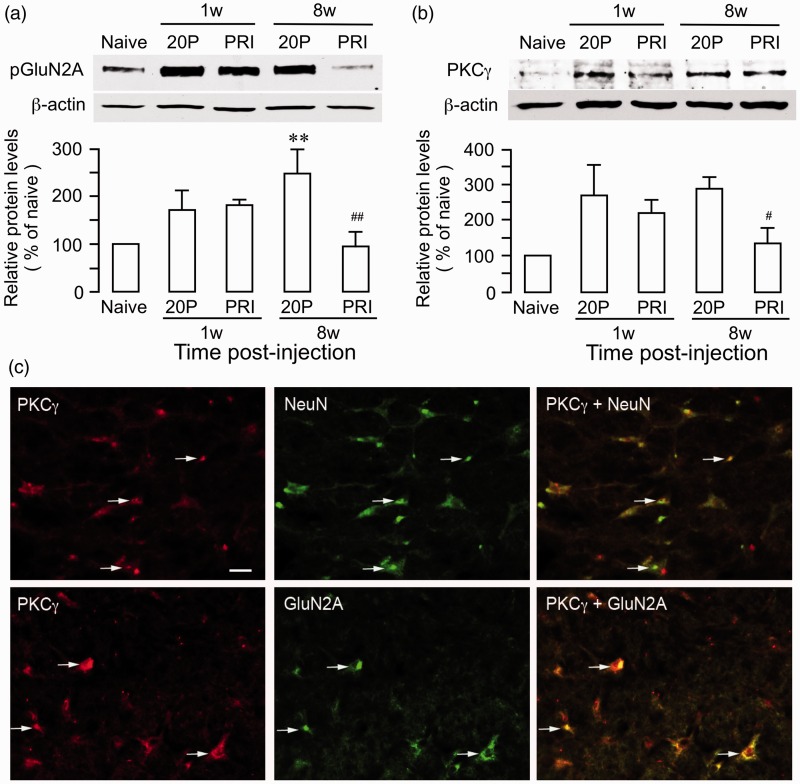
Our previous studies indicate that endogenous opioid receptors in the RVM, an important site of the pain modulatory circuitry, contribute to BMSC-produced long-term antihyperalgesia.9 While tolerance to endogenous opioids may develop after repeated opioid receptor activation,27 surprisingly, analgesia tolerance did not develop after infusion of BMSCs.9 Since the NMDA receptor is involved in the development of opiate tolerance,28,29 we examined the effect of BMSCs on NMDA receptor activity in the RVM. TL was produced in male rats and BMSCs (1.5 M) was infused i.v. at 7 days post TL. RVM tissues were collected at 1 week and 8 weeks after BMSC infusion. We used 20-passage BMSCs as a control since they did not produce antihyperalgesia.9
We have shown that the GluN2A subunit of the NMDA receptor in the RVM is important for descending pain facilitation.30 Using an antibody against phosphotyrosine 1246 of the GluN2A, compared to 20-passage BMSCs (248 ± 51% of naive), we observed that GluN2A tyrosine phosphorylation (pGluN2A) in the RVM tissue was suppressed at 8 weeks (96 ± 29% of naive) after primary rat BMSC treatment in TL rats (Figure 5(a)). There is evidence that PKCγ activity related to NMDA receptor activation plays critical role in opioid tolerance.31,32 Consistently, PKCγ expression in the RVM was also decreased at 8 weeks (133 ± 46% of naive) after primary BMSC treatment compared to 20-passage BMSC-treated rats (289 ± 52% of naive) (Figure 5(b)). Double immunofluorescent staining showed that PKCγ-like immunoreactivity colocalized with NeuN, a neuronal marker, and GluN2A (Figure 5(c)). These results suggest that BMSC-produced long-term antihyperalgesia involve an effect on NMDA receptors and signaling pathways associated with opioid tolerance.
Figure 5.
Effect of BMSC on NMDA receptor phosphorylation and PKCγ in RVM. (a) Total proteins from RVM tissues were isolated and GluN2A (NR2A) subunit tyrosine phosphorylation was determined with anti-phosphotyrosine 1246 GluN2A (pGluN2A). At 8 weeks after primary (PRI) BMSC treatment, pGluN2A was increased in 20 P-BMSC samples (**, p < 0.01, vs. navie, n = 3). The BMSC treatment led to a return of the P-GluN2A to the naïve level (##, p < 0.01, vs. 20 P/8 weeks). There was a trend for increase in pGluN2A at 1 week after BMSC treatment, which was likely an effect of injury. (b) The levels of PKCγ were reduced in PRI BMSC-treated rats at the 8-week time point (#, p < 0.05, vs. 20 P/8 weeks). β-actin was a loading control. N = 3/group, ANOVA with post-hoc comparisons. (c) Localization of PKCγ- and GluN2A-like immunoreactivity in RVM neurons. Brainstem tissue sections were processed for PKCγ and GluN2A double immunofluorescence staining. PKCγ immunoreactivity (red, Cy3) is colocalized with NeuN (green, Cy2). Overlap of images of the left and middle columns reveals double fluorescence (right, yellow/orange). Arrows indicate examples of double-labeled neurons. Scale = 0.25 µm.
Discussion
Proper treatment of chronic pain conditions continues to be an unmet challenge. Recent studies have shown potential of mesenchymal stromal cells for the management of chronic pain, although much still needs to be learned about their efficacy and mechanisms of action in the CNS under different pain conditions. The present work provides further convergent evidence that favor the use of mesenchymal stromal cells for pain relief. We show that both rat and human BMSCs, a major type of mesenchymal stromal cells, produced long-lasting pain attenuation and inhibited trigeminal neuronal hyperexcitability in rodent models of persistent pain and that BMSCs suppressed NMDA receptor phosphorylation and PKCγ levels in the RVM pain modulatory circuitry.
We recently developed a myogenic orofacial pain model involving a tendon of the masseter muscle.14 The model is particularly useful in studying the chronicity of pain associated with temporomandibular joint disorders. In the present study, we used this model in female rats to evaluate the effect of BMSCs in order to compare it to the effects in male rats conducted in a previous study.9 The results showed reversal of sensory and emotional pain hypersensitivity in these rats, consistent with previous report.9 The antihyperalgesia of BMSCs is unlikely related to an effect on motor behavior as shown in previous reports.8,9,12 These results further demonstrate that a single infusion of BMSCs produced long-lasting antihyperalgesia. In the same TL model, analgesia produced by a single dose of morphine and duloxetine, two commonly used analgesics, waned in less than two hours.14 The long-lasting pain relieving effect of BMSCs have also been shown in other studies12,33 and the underlying cellular mechanisms require further investigation.
We noticed that the increase in EF50s in female rats only reached statistical significance at the first four weeks after the BMSC treatment. Thus, the pain-attenuating effect of BMSCs in the female rats appeared to last shorter (<8 weeks) compared to that in male rats (up to five months).9 Considering the involvement of RVM mu-opioid receptors in BMSC-produced antihyperalgesia,9 this result is unexpected as female rats have been shown to be more sensitive to the mu-opioid agonist injected into the periaqueductal gray.34 Although we monitored the estrous status of the rats, we did not separate female rats based on the estrous status since there were no significant cycle-related differences in baseline responses as we showed previously.35 In fact, “there is no good meta-evidence that the cyclicity of female rodents is a strong systematic modulator of pain sensitivity”.36 Thus, the female gonadal hormonal status is unlikely a factor that interrupts BMSC-produced effect. Considering different involvement of immune cells in pain in males and females,37 it is worth pursuing that whether there is sex difference in BMSC-produced immune regulation related to their pain relieving effect.
We show that post-injury treatment with hBMSCs produced long-lasting antihyperalgesia in rats with spinal nerve injury similar to that of trigeminal tissue or nerve injury.9 Both thermal and mechanical hypersensitivity as well as aversive behavior were attenuated after systemic infusion of hBMSCs. The use of human cells is important for relating preclinical findings to clinical conditions. Only a few preclinical studies have reported pain-attenuating effect of hBMSCs7,8 or human adipose-derived mesenchymal stem cells [10; also see 1 for a review). The antihyperalgesia of hBMSCs seen in the present study seems more prominent compared to other reports,8,10 although a direct comparison is not justified due to different species (rat vs. mouse), nerve injury models (SNL vs. spared nerve injury and CCI), and the use of primary versus passage cells. In contrast to hBMSCs and adipose-derived cells, human umbilical cord blood-derived mesenchymal stem cells did not produce a significant effect on pain hypersensitivity after spinal cord injury in mice,38 suggesting phenotypic differences between different mesenchymal stem cell types. The available evidence favors the use of primary hBMSCs or adipose-derived mesenchymal stem cells in pain relief.
Utilizing an ex vivo Vc slice preparation from adult mice, we show that BMSCs inhibited hyperexcitability of trigeminal medullary dorsal horn neurons after nerve injury, which has not been observed previously. In slices from BMSC-treated mice, nerve injury-induced increases in frequency and amplitude of sEPSCs were significantly reduced, suggesting reduced presynaptic glutamate release and postsynaptic hyperexcitaility.39 Further, NMDA receptor-mediated eEPSC was enhanced in CCI-ION mice but this increase was abolished after treatment with BMSCs, supporting that BMSCs produce a decrease of primary afferent nociceptive input. The inhibition of trigeminal neuronal activity is consistent with the pain-relieving effect of BMSCs. Chen et al.12 show that BMSC-produced antihyperalgesia involves transforming growth factor beta1 (TGFβ1) and direct application of TGFβ inhibited sEPSC of spinal neurons. Our results also suggest that BMSC may engage endogenous opioid receptors to control nociceptive transmission in the medullary dorsal horn. Similar to an effect on behavioral hyperalgesia,9 naloxone rekindled sEPSC frequency in BMSC-treated mice. This electrophysiological evidence not only complements behavioral measure of nociception but also demonstrates that BMSC exert a selective effect on sensory transmission pathways.
We finally examined the effect of BMSCs on NMDA receptor activity in RVM. We show that BMSCs suppressed GluN2A phosphorylation in RVM at a later (8 weeks) but not early (1 week) time point of treatment. Thus, the later, but not early effect of BMSCs involves an action on NMDA receptors. The NMDA receptor is a major mediator of central sensitization and hyperalgesia, while it also is involved in the development of opiate tolerance.28,29 Chronic morphine treatment upregulates NMDA receptor subunit expression.40 Since RVM opioid receptors are required for BMSC-produced antihyperalgesia and the analgesic tolerance does not develop as suggested by the long-term effect of BMSCs,9 the late suppression of NMDA receptor activity would be consistent with inhibition of the development of opioid tolerance. Interestingly, mesenchymal stem cells inhibit NMDA receptor expression and protect against glutamate excitotoxicity in culture.41 Related to this, amniotic epithelial stem cells attenuated GluN1 (NR1) subunit phosphorylation and alleviated mechanical allodynia after spinal cord injury in rats.38 PKCγ activity related to NMDA receptor activation is essential for the development of opioid tolerance.31,32 We show that PKCγ expression in RVM was also decreased at 8w after BMSC treatment. These results suggest that signaling pathways related to the development of opioid tolerance, particularly at the late phase of BMSC-produced antihyperalgesia, are interrupted in BMSC-treated animals. These results suggest a promising analgesic profile of BMSC: to inhibit NMDA receptor activation and promote opioid analgesia while suppressing the development of tolerance.
The present work adds convergent evidence to the growing preclinical literature that support the use of BMSCs in pain control. It is interesting that BMSCs produced similar antihyperalgesic effect under diverse conditions that potentially involve multiple mechanisms. Future studies will identify cellular mechanisms that are crucial in BMSCs’ pain relieving effect. We show that BMSCs have antihyperalgesic efficacy in a number of animal persistent pain models by multiple endpoints. Clinical studies have also been promising. In a proof of concept clinical study, adipose mesenchymal stromal cells reduced pain in females with trigeminal pain.42 A single local administration of autologous bone marrow concentrate cells, mainly mesenchymal stromal cells, significantly reduced lumbar discogenic pain up to 12 months.33 Since BMSCs are easy to isolate and expand ex vivo and relatively safe in clinical use,43 their therapeutic use may provide a novel direction in the management of chronic pain conditions.
Acknowledgments
We thank Dr. George T-J Huang for his advise on the early stages of this project.
Authors' contributions
WG contributed to the conception and design, collection, and assembly of behavioral and Western blot data, data analysis and interpretation, and manuscript writing; Y-XC contributed to the design experiments, collection, and assembly of patch clamp data; SI contributed to the conception and design, BMSC cultures, data analysis and interpretation, and manuscript writing; J-LY contributed to the collection and assembly of behavioral data; SZ contributed to the collection and assembly of behavioral data; ZM contributed to the collection and assembly of behavioral data; FW contributed to the conception and design, data analysis and interpretation, and manuscript writing; RD contributed to the conception and design, data analysis and interpretation, and manuscript writing; KR contributed to the conception and design, assembly of data, data analysis and interpretation, and manuscript writing. All authors gave final approval of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Maryland Stem Cell Foundation grant 2014-MSCRFI-0584 (KR); National Institutes of Health grants: DE025137 (KR), NS019296 (FW), DE021804 (RD).
References
- 1.Franchi S, Castelli M, Amodeo G, et al. Adult stem cell as new advanced therapy for experimental neuropathic pain treatment. Biomed Res Int 2014; 2014: 470983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo W, Imai S, Dubner R, et al. Multipotent stromal cells for arthritic joint pain therapy and beyond. Pain Manag 2014; 4: 153–162. [DOI] [PubMed] [Google Scholar]
- 3.Black LL, Gaynor J, Gahring D, et al. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther 2007; 8: 272–284. [PubMed] [Google Scholar]
- 4.Godwin EE, Young NJ, Dudhia J, et al. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J 2012; 44: 25–32. [DOI] [PubMed] [Google Scholar]
- 5.Musolino PL, Coronel MF, Hökfelt T, et al. Bone marrow stromal cells induce changes in pain behavior after sciatic nerve constriction. Neurosci Lett 2007; 418: 97–101. [DOI] [PubMed] [Google Scholar]
- 6.Abrams MB, Dominguez C, Pernold K, et al. Multipotent mesenchymal stromal cells attenuate chronic inflammation and injury-induced sensitivity to mechanical stimuli in experimental spinal cord injury. Restor Neurol Neurosci 2009; 27: 307–321. [DOI] [PubMed] [Google Scholar]
- 7.Siniscalco D, Giordano C, Galderisi U, et al. Intra-brain microinjection of human mesenchymal stem cells decreases allodynia in neuropathic mice. Cell Mol Life Sci 2010; 67: 655–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siniscalco D, Giordano C, Galderisi U, et al. Long-lasting effects of human mesenchymal stem cell systemic administration on pain-like behaviors, cellular, and biomolecular modifications in neuropathic mice. Front Integr Neurosci 2011; 5: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo W, Wang H, Zou S, et al. Bone marrow stromal cells produce long-term pain relief in rat models of persistent pain. Stem Cells 2011; 29: 1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacerdote P, Niada S, Franchi S, et al. Systemic administration of human adipose-derived stem cells reverts nociceptive hypersensitivity in an experimental model of neuropathy. Stem Cells Dev 2013; 22: 1252–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Buul GM, Siebelt M, Leijs MJ, et al. Mesenchymal stem cells reduce pain but not degenerative changes in a mono-iodoacetate rat model of osteoarthritis. J Orthop Res 2014; 32: 1167–1174. [DOI] [PubMed] [Google Scholar]
- 12.Chen G, Park CK, Xie RG, et al. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-b secretion. J Clin Invest 2015; 125: 3226–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe S, Uchida K, Nakajima H, et al. Early transplantation of mesenchymal stem cells after spinal cord injury relieves pain hypersensitivity through suppression of pain-related signaling cascades and reduced inflammatory cell recruitment. Stem Cells 2015; 33: 1902–1914. [DOI] [PubMed] [Google Scholar]
- 14.Guo W, Wang H, Zou S, et al. Long lasting pain hypersensitivity following ligation of the tendon of the masseter muscle in rats: A model of myogenic orofacial pain. Mol Pain 2010; 6: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992; 50: 355–363. [DOI] [PubMed] [Google Scholar]
- 16.Wei F, Guo W, Zou S, et al. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci 2008; 28: 10482–10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asdell SA. Patterns of mammalian reproduction, Ithaca, NY: Cornell University Press, 1964. [Google Scholar]
- 18.Shen LH, Li Y, Chen J, et al. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience 2006; 137: 393–399. [DOI] [PubMed] [Google Scholar]
- 19.Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav 1999; 67: 711–716. [DOI] [PubMed] [Google Scholar]
- 20.Guo W, Wei F, Zou S-P, et al. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J Neurosci 2004; 24: 9161–9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 22.LaBuda CJ, Fuchs PN. A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol 2000; 163: 490–494. [DOI] [PubMed] [Google Scholar]
- 23.Isong U, Gansky SA, Plesh O. Temporomandibular joint and muscle disorder-type pain in U.S. adults: the National Health Interview Survey. J Orofac Pain 2008; 22: 317–322. [PMC free article] [PubMed] [Google Scholar]
- 24.Choi IS, Cho JH, Jang IS. A1 receptors inhibit glutamate release in rat medullary dorsal horn neurons. Neuroreport 2011; 22: 711–715. [DOI] [PubMed] [Google Scholar]
- 25.Kim YS, Chu Y, Han L, et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 2014; 81: 873–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell SL, Mathew SS, Hablitz JJ. Pre- and postsynaptic effects of kainate on layer II/III pyramidal cells in rat neocortex. Neuropharmacology 2007; 53: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandran P, Sluka KA. Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration. Pain 2003; 102: 195–201. [DOI] [PubMed] [Google Scholar]
- 28.Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science 1991; 251: 85–87. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Muñoz M, Sánchez-Blázquez P, Vicente-Sánchez A, et al. The mu-opioid receptor and the NMDA receptor associate in PAG neurons: implications in pain control. Neuropsychopharmacology 2012; 37: 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo W, Robbins MT, Wei F, et al. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci 2006; 26: 126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim G, Wang S, Zeng Q, et al. Expression of spinal NMDA receptor and PKCgamma after chronic morphine is regulated by spinal glucocorticoid receptor. J Neurosci 2005; 25: 11145–11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang YN, Tsai RY, Lin SL, et al. Amitriptyline attenuates astrocyte activation and morphine tolerance in rats: role of the PSD-95/NR1/nNOS/PKCγ signaling pathway. Behav Brain Res 2012; 229: 401–411. [DOI] [PubMed] [Google Scholar]
- 33.Pettine KA, Murphy MB, Suzuki RK, et al. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells 2015; 33: 146–156. [DOI] [PubMed] [Google Scholar]
- 34.Tershner SA, Mitchell JM, Fields HL. Brainstem pain modulating circuitry is sexually dimorphic with respect to mu and kappa opioid receptor function. Pain 2000; 85: 153–159. [DOI] [PubMed] [Google Scholar]
- 35.Ren K, Wei F, Murphy A, et al. Progesterone attenuates persistent inflammatory hyperalgesia in female rats: involvement of spinal NMDA receptor mechanisms. Brain Res 2000; 865: 272–277. [DOI] [PubMed] [Google Scholar]
- 36.Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain 2005; 117: 1–5. [DOI] [PubMed] [Google Scholar]
- 37.Sorge RE, Mapplebeck JC, Rosen S, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015; 18: 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roh DH, Seo MS, Choi HS, et al. Transplantation of human umbilical cord blood or amniotic epithelial stem cells alleviates mechanical allodynia after spinal cord injury in rats. Cell Transplant 2013; 22: 1577–1590. [DOI] [PubMed] [Google Scholar]
- 39.Baba H, Doubell TP, Woolf CJ. Peripheral inflammation facilitates Abeta fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord. J Neurosci 1999; 19: 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Ma W, Chabot JG, et al. Calcitonin gene-related peptide as a regulator of neuronal CaMKII-CREB, microglial p38-NFκB and astroglial ERK-Stat1/3 cascades mediating the development of tolerance to morphine-induced analgesia. Pain 2010; 151: 194–205. [DOI] [PubMed] [Google Scholar]
- 41.Voulgari-Kokota A, Fairless R, Karamita M, et al. Mesenchymal stem cells protect CNS neurons against glutamate excitotoxicity by inhibiting glutamate receptor expression and function. Exp Neurol 2012; 236: 161–170. [DOI] [PubMed] [Google Scholar]
- 42.Vickers ER, Karsten E, Flood J, et al. A preliminary report on stem cell therapy for neuropathic pain in humans. J Pain Res 2014; 7: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med 2015; 3: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]