Introduction
AF is a common arrhythmia associated with large burden of morbidity and mortality.[1] In areas with a high prevalence of rheumatic heart disease, valve disease is the most common substrate for the occurrence of AF and this problem assumes greater importance because the resulting escalation in morbidity and mortality involves relatively younger population. As is true of the general population, the prevalence of AF in patients with rheumatic mitral valve disease (RMVD) increases with advancing age. When compared to patients with mitral valve disease without AF, those with AF are at a higher NYHA class, have more severe left ventricular dysfunction and show greater left atrial enlargement. Mitral valve is the most commonly involved valve among patients with AF with valvular heart disease. Mitral stenosis, Mitral regurgitation and Tricuspid regurgitation comprise 70% of valvular heart disease related to AF. Diker et al in an Echo Doppler study had found AF in 29% of patients with isolated mitral stenosis, in 16% with isolated mitral regurgitation, in 52% in combined mitral stenosis and regurgitation but in only 1% of patients with aortic valvular disease.[2]
Pathophysiology And Electrophysiology
While the mechanisms of non-valvular AF have been extensively studied, the literature is sparse concerning pathophysiological mechanisms leading to AF in patients with underlying valvular diseases. There are apparent differences in the pathological findings in these two subsets of patients. Occurrence of AF is known to correlate with LA size ; the incidence of AF rises from 3% when the left atrial diameter is < 40mm to 54% if the left atrial diameter is > 40 mm.[3] Mitral valve disease is associated with large left atria, and the elevated left atrial pressure causes myocardial stretch, which in turn results in slow conduction velocities, increased dispersion of refractoriness and increased automaticity, all of which create the milieu for initiating and perpetuating sustained AF.
A large postmortem study on patients with AF and associated organic heart disease showed a spectrum of histologic abnormalities that diffusely involved both the right and left atria. It was postulated that fibrosis and degeneration of the atrial myocardium in valvular heart disease, especially those of rheumatic etiology, disturb impulse propagation in the atria and lead to AF.[4] Atrial fibrosis probably contributes to persistent AF after balloon valvuloplasty or surgical valve replacement and repair. AF also occurs more frequently when mitral valve is calcified or is prolapsing.[5]
An insight into the role of substrate in perpetuation of AF in patients with mitral stenosis was provided in an elegant study by Fan et al.[6] The regional ERPs in the atria increased after mitral valvuloplasty in patients with sinus rhythm and in AF; but in those with AF the increase was heterogenous, while in those with sinus rhythm it was homogenous. A study of a small group of patients with rheumatic AF, who had undergone balloon mitral valvuloplasty, had revealed that there was an organized atrial activity most often at the Os of the Coronary Venous Sinus preceding initiation of AF, with no evidence of focal firing from the pulmonary veins.[7]
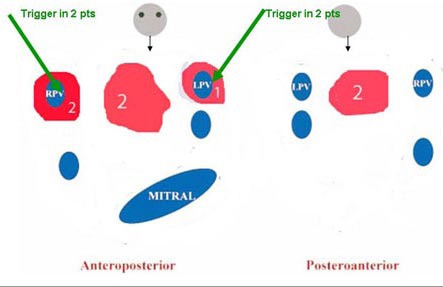
Intuitively, left atrial mapping in these patients should throw light on the substrate perpetuating the fibrillation. However, literature is sparse in this regard. In our small series, the electroanatomic maps showed extensive left atrial scarring of diverse patterns [[Fig 1]]. The significance of this finding remains speculative at this point and merits further investigation.
Figure 1. Schematic diagram showing the varied scar pattern in the 5 rheumatic AF patients who underwent ablation. The overlap in numbers is due to patients having scar in more than one area. scars are coloured in red.
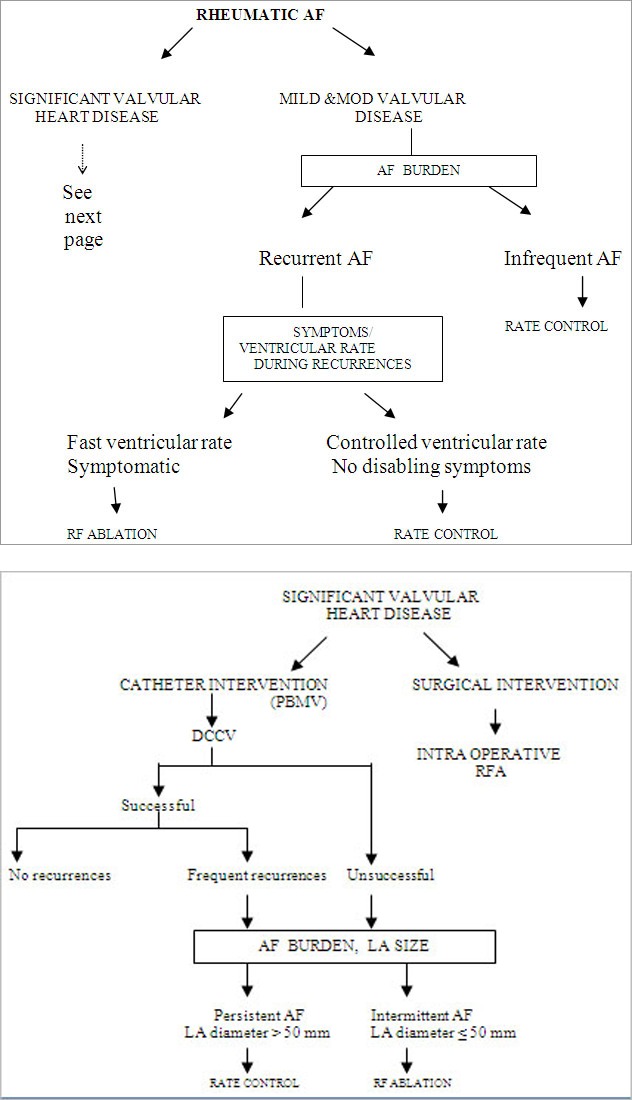
Suggested Management Algorithm.
While the exact mechanism of development AF is yet to be fully elucidated the impact of it on the patient, especially patients with mitral stenosis has been studied. The impact almost entirely depends on the ventricular rate. As the ventricular rate increases the diastole decreases, therefore mitral flow increases. In MS increased mitral flow causes increased left atrial and pulmonary venous pressure. The loss of atrial contraction per se has minimal impact on the patient with significant MS. Unlike the situation in normal patients, atrial contraction does not cause an increase in flow across an obstructed mitral valve. This reflected as a loss of the A wave in the M –mode in echocardiogram of MS patients who are in sinus rhythm.[8]
Thrombo- Embolism and Anticoagulation
AF is a major cause of systemic thrombo-embolism and in patients over the age of 65 years, it is responsible for more than one-third of all strokes.[9] Advancing age, history of previous thromboembolic event, presence of mitral valve disease, congestive heart failure, enlarged left atrium, previous MI, hypertension and left atrial thrombus on transesophageal echocardiography predict occurrence of embolic strokes in patients with AF.[10] The presence of AF multiplies the risk of stroke 5 times in a patient with structurally normal heart, and increases by a factor of 17 in those with mitral valvular disease. The lifetime recurrence rates for strokes in these patients may be as high as 30%–75%.[11] The risk of recurrent strokes appears to be similar with chronic and paroxysmal AF. Transesophageal echocardiographic studies have shown that the presence of significant mitral regurgitation is associated with a lower incidence of spontaneous echo contrast in the left atrium and thus with a lower risk of thrombi and embolization as compared to Rheumatic mitral stenosis.[12] A particular study has demonstrated that 20% of patients with mitral stenosis and none with mitral regurgitation show left atrial thrombi.[13] More importantly, 28 of the 30 patients (93%) with atrial thrombi showed AF, demonstrating the role of rhythm disturbance in the generation of left atrial thrombus. In patients with mitral valve disease, thrombi are found not only in the left atrial appendage but also in the body of the left atrium. This is in contrast to nonvalvular AF in which thrombi form pre dominantly (90%) in the left atrial appendage.[14]
In a surgical clinicopathologic study in patients with AF, the prevalence of left atrial clot with predominant mitral regurgitation was 8.3% in comparison with 54% in patients with predominant mitral stenosis (p < 0.0001) 12 In sinus rhythm, the prevalence of left atrial clot was 0% in predominant mitral regurgitation and 14.3% in patients with mitral stenosis (p < 0.001). None of the patients with AF and severe mitral regurgitation had left atrial clot.[12,15]
All patients with Rheumatic AF need to be anticoagulated in the absence of contraindications. The use and timing of anticoagulation for patients in sinus rhythm with mitral stenosis is still a moot point as the risk of thromboembolism is unrelated to the severity of the disease. However, successful balloon valvuloplasty results in resolution of echo contrast and decrease in thromboembolic risk.[16] Correction of the valvular lesion thus should be undertaken whenever feasible. There are no doseranging trials to guide anticoagulant therapy in patients with AF in valvular heart disease. Based on single-center studies in patients with valvular disease and on the results of large multi-center studies involving patients with AF of non-valvular etiology, however, similar recommendations can be made.
Management
The natural history of non-valvular AF is extremely variable. A good number of patients with nonvalvular AF have paroxysmal episodes for long periods that become chronic or persistent in only a few. On the other hand, the initial attacks of AF in valvular heart disease are paroxysmal, but almost invariably progress to chronic AF. The symptoms are related to the irregular and rapid ventricular rate, development of heart failure and thromboembolic complications. These complications are related to the duration of AF and occur more often in AF associated with valvular heart disease. While the treatment of the underlying valvular disease is of primary importance, the management of the arrhythmia is aimed at either control of ventricular rate without attempting to restore sinus rhythm, or to restoration of sinus rhythm with followup aggressive therapy to maintain it. As embolic complications are the major cause of morbidity, chronic anticoagulant therapy is important in all patients with AF and valvular heart disease.
Correction of Underlying Disorder
Treatment of the underlying valvular abnormality should be considered, e.g. surgical repair or replacement of mitral or tricuspid valve in severe regurgitant lesions, or valvuloplasty in mitral stenosis. However, in patients with enlarged and dysfunctional atria, despite correction of the underlying valvular lesion, AF often persists. As a general rule in all these patients correction of reversible factors like thyrotoxicosis and alcohol intake must be addressed.
Delayed correction of underlying disorder would bring down the chance of maintaining these patients in sinus rhythm. This is partly reflected in the American Heart Association practice guidelines. It recommends mitral valve surgery in asymptomatic patients with severe MS as a Class II b indication, if there is new onset AF. In patients with severe MR it is a Class II a indication. However, this is based on consensus opinion and not on substantial data.[17]
Control of Ventricular Rate
The control of ventricular rate is one of the main goals of the treatment of patients with all forms of AF when sinus rhythm cannot be restored immediately. This strategy remains the mainstay in patients with valvular heart disease as most of them have chronic AF not readily amenable to rhythm conversion. Of the several drugs used Digitalis, which was most often used earlier, is very often ineffective during exercise because its electrophysiologic action is mediated largely through augmentation of vagal tone on the AV node. Beta-blockers such as propranolol, metoprolol and atenolol, as well as negative chronotropic calcium-channel blockers such as verapamil and diltiazem, are effective agents for control of ventricular rate with a low incidence of adverse effects.[18] Studies have shown that combining digoxin and a beta-blocker that has intrinsic sympathomimetic activity keeps ventricular rates at peak exercise low while minimizing the effects of these drugs when heart rates are slowest, as is usually seen during the night.[18,19] Catheter ablation of the AV junction and implantation of a rate-responsive ventricular permanent pacemaker should be considered in drugrefractory patients or patients who cannot take beta-blockers and calcium-channel blockers.[20]
Rhythm Control
The clinical advantage of maintaining patients in sinus rhythm following corrective procedures for mitral valvular disease has been demonstrated in a few studies. Vaturi et al showed worse functional class and increased transmitral gradients in patients with atrial fibrillation compared to those in sinus rhythm following Mitral Replacement Surgery.[21]
In a study by Leon et al, patients with AF, BMV resulted in inferior immediate and long-term outcomes, as reflected in a smaller post-BMV mitral valve area (1.7 6 0.7 vs. 2 6 0.7 cm2; p, 0.0001) and a lower event free survival (freedom of death, redo-PMV and mitral valve surgery) at a mean follow-up time of 60 months (32% vs. 61%; p, 0.0001). AF by itself does not unfavorably influence the outcome, but is a marker for clinical and morphologic features associated with inferior results after PMV.[22]
Maatouk et al compared outcomes at ten years in a fairly large group of patients with and without AF who underwent balloon mitral commissurotomy. They reported a lower ten year survival and a lower ten year event free survival in the AF group. The AF group also had higher rate of restenosis. However the cause of death were not reported and the events described were reinterventions and mitral valve replacements.[23]
Rheumatic AF has also been shown to increase the incidence of prosthetic valve thrombosis in a study that was primarily looking at the results of thrombolytic therapy in patients with prosthetic valve thrombosis.[24]
In patients with valvular AF, conversion and maintenance of sinus rhythm is difficult due to valvular abnormalities, large left atria and the presence of unhealthy substrate. Cardioversion to sinus rhythm may be achieved by chemical means or by electrical cardioversion. Chemical agents are less effective as in most cases the AF is of long duration. Antiarrhythmic agents of Vaughan Williams classes IA, IC or III are effective. Success rates in the range of 60% have been reported with flecainide, propafenone and amiodarone[25,26] Newer class III agents such as intravenous ibutilide and intravenous or oral dofetilide are most effective in atrial flutter and fibrillation of recent onset. Short-term amiodarone with or without electrical cardioversion has been shown to be effective in the restoration of sinus rhythm in chronic AF after mitral valve surgery.[25] Prophylactic use of oral amiodarone and sotalol has been shown to prevent AF immediately following cardiac surgery.[27]
The debate on preference of rate over rhythm control that was addressed by the AFFIRM,[28] RACE,[29] STAF[30] trials predominantly involved non-valvular AF patients. These trials failed to demonstrate superiority of rhythm control strategy. However further analysis of the AFFIRM data showed that that the presence of AF was associated with a 47% increased mortality compared with sinus rhythm and the use of an antiarrhythmic medication was associated with a 49% increased mortality , suggesting that any mortality benefit from the maintenance of sinus rhythm was offset by increased mortality from currently available antiarrhythmics.[31] The more recently published randomized trial by Roy et al showed no benefit of rhythm control over rate control in patients with LV dysfunction.[32] Non pharmacological methods, that have evolved from the surgical to radiofrequency catheter based pulmonary vein isolation with and without linear lesions have shown reasonable success in maintenance of sinus rhythm[33] Trials comparing these modalities against rate control need to be conducted for determining the guidelines for the best modality of management.
Both pharmacological and non pharmacological methods of conversion and maintenance of sinus rhythm which have been studied in non- valvular AF have also been studied in valvular/rheumatic AF albeit in smaller and less well conducted studies. Similar to non-valvular AF there is no conclusive data to determine the best modality of management in rheumatic AF.
CRAAFT[34] trial was a prospective study of 144 rheumatic valvular patients comparing rate control (using Diltiazem) and Rhythm control (Amiodarone versus placebo). Besides demonstrating a mortality benefit with rhythm control, the study showed a improvement in NYHA class, quality of life and exercise capacity on achievement of sinus rhythm. There was no difference in rates of hospitalization or thromboembolism or bleeds between the two groups. In contrast to the trials involving non- valvular AF, this study had individuals of young age (mean age 39 yrs), and only those who sustained sinus rhythm at one year (69%) were compared with the rate control group. The mortality observed in the rate control arm was due to prosthetic valve thrombosis. The other major limitation of the study was its small sample size, a dropout of 13% and a relatively short follow up. Another study that compared both modalities in patients undergoing balloon mitral valvuloplasty showed that the six minute walk test improved significantly in patients in whom sinus rhythm was maintained.[35]
Maze surgery and its modifications have been successfully attempted by many investigators to restore sinus rhythm in RVHD and atrial fibrillation patients.[36]
Patients undergoing mechanical valve replacement and concomitant AF surgery, the incidence of stroke 5 years after surgery is lower than in those who undergo mitral valve replacement alone.[37,38] Although initial studies had shown insufficient rates of sinus rhythm restoration (59%) for the Maze procedure in AF associated with rheumatic valve disease,[39] subsequent studies by other investigators have shown comparable conversion rates with acceptable operative risk to that of nonvalvular AF.[36] Patwardhan et al[40] pioneered the technique of radiofrequency bipolar maze for atrial fibrillation during valve surgery. There was 80% freedom from atrial fibrillation at five months along with restoration of atrial transport function. Guang et al[41] have also had similar experience with radiofrequency maze during mitral valve surgery, with a longer follow up of 3 years wherein 77% of patients remained in sinus rhythm. The outcome of surgical maze for atrial fibrillation is similar in rheumatic and non-rheumatic atrial fibrillation in terms of sinus rhythm achievement and restoration of left-atrial function. Lee et al[42] showed that the maze procedure is equally effective in AF of rheumatic and non rheumatic etiology in terms of sinus conversion rate. Patwardhan’s group[43] recently evaluated the efficacy of three different methods of ablative procedures - biatrial lesions, left atrial lesions and pulmonary vein isolation - and found them all comparable in a group of rheumatic patients.
Patients undergoing mechanical valve replacement and concomitant AF surgery, the incidence of stroke 5 years after surgery is lower than in those who undergo mitral valve replacement alone.37, 38 Although initial studies had shown insufficient rates of sinus rhythm restoration (59%) for the Maze procedure in AF associated with rheumatic valve disease,39 subsequent studies by other investigators have shown comparable conversion rates with acceptable operative risk to that of nonvalvular AF.36 Patwardhan et al[40] pioneered the technique of radiofrequency bipolar maze for atrial fibrillation during valve surgery. There was 80% freedom from atrial fibrillation at five months along with restoration of atrial transport function. Guang et al[41] have also had similar experience with radiofrequency maze during mitral valve surgery, with a longer follow up of 3 years wherein 77% of patients remained in sinus rhythm. The outcome of surgical maze for atrial fibrillation is similar in rheumatic and non-rheumatic atrial fibrillation in terms of sinus rhythm achievement and restoration of left-atrial function. Lee et al[42] showed that the maze procedure is equally effective in AF of rheumatic and non rheumatic etiology in terms of sinus conversion rate. Patwardhan’s group[43] recently evaluated the efficacy of three different methods of ablative procedures - biatrial lesions, left atrial lesions and pulmonary vein isolation - and found them all comparable in a group of rheumatic patients.
Multiple approaches for catheter ablation of AF are under clinical investigation, and although preliminary results are encouraging, indications, safety and long-term success are still not well defined; it is particularly less well studied in rheumatic AF.
A small study among patients with AF and rheumatic heart disease has shown that in a good number the arrhythmia is a relatively organized rhythm with earliest atrial activity near the os of the coronary sinus.7 Catheter ablation in this area was successful in restoring sinus rhythm in most of these patients. All these patients were on amiodarone but details of long term follow up of these patients are not available.
Furthermore a recent study showed efficacy of Hybrid Therapy of Radiofrequency Catheter Ablation and BMV in Patients with Atrial Fibrillation and Mitral Stenosis. Twenty consecutive patients with drug-resistant AF and rheumatic MS underwent RFA combined with a BMV or transthoracic direct cardioversion (DC) following a BMV. During a mean follow-up period of 4.0 +/- 2.7 years, 8 patients (80%) in the RFA group were maintained in SR, as compared to 1 (10%) in the DC group. However if this efficacy translated into better clinical outcomes is not known.[44]
Conclusions
In geographical regions where rheumatic heart disease is prevalent AF is an important health care issue affecting younger population. It significantly contributes to mortality and morbidity and constitutes a burden on healthcare resources of the society.
The benefit of long term anticoagulation is well established. Whether the rate control or rhythm control constitutes a better strategy is not clearly determined in non-valvular AF. Compared to patients with non-valvular AF maintenance of sinus rhythm in rheumatic AF patients appears to be more beneficial, particularly among those undergoing mitral valve surgery.
However, the benefit of restoring sinus rhythm are not clear in rheumatic heart disease patients who are haemodynamically stable and do not require valvular surgery. Although small studies have shown benefit in terms of functional class it remains to be seen if it will significantly alter important clinical endpoints.
Pharmacological methods of rhythm control have drawbacks and it appears prudent to compare nonpharmacological methods of rhythm control against rate control, considering the advancements of these modalities and their success rates in maintenance of sinus rhythm.
Despite lack of large supportive evidence it seems reasonable to attempt conversion to sinus rhythm in rheumatic heart disease in patients undergoing corrective valve surgery. However the best strategy of achieving it is not well established.
Disclosures
None.
References
- 1.Kannel W B, Abbott R D, Savage D D, McNamara P M. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N. Engl. J. Med. 1982 Apr 29;306 (17):1018–22. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 2.Diker E, Aydogdu S, Ozdemir M, Kural T, Polat K, Cehreli S, Erdogan A, Göksel S. Prevalence and predictors of atrial fibrillation in rheumatic valvular heart disease. Am. J. Cardiol. 1996 Jan 01;77 (1):96–8. doi: 10.1016/s0002-9149(97)89145-x. [DOI] [PubMed] [Google Scholar]
- 3.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of non rheumatic atrial fibrillation [Abstr]. J Am Coll Cardiol. 1993;0:0–0. [Google Scholar]
- 4.Bailey G W, Braniff B A, Hancock E W, Cohn K E. Relation of left atrial pathology to atrial fibrillation in mitral valvular disease. Ann. Intern. Med. 1968 Jul;69 (1):13–20. doi: 10.7326/0003-4819-69-1-13. [DOI] [PubMed] [Google Scholar]
- 5.Selzer A, Katayama F. Mitral regurgitation: clinical patterns, pathophysiology and natural history. Medicine (Baltimore) 1972 Sep;51 (5):337–66. [PubMed] [Google Scholar]
- 6.Fan Katherine, Lee Kathy L, Chow Wing-Hing, Chau Elaine, Lau Chu-Pak. Internal cardioversion of chronic atrial fibrillation during percutaneous mitral commissurotomy: insight into reversal of chronic stretch-induced atrial remodeling. Circulation. 2002 Jun 11;105 (23):2746–52. doi: 10.1161/01.cir.0000018441.64861.de. [DOI] [PubMed] [Google Scholar]
- 7.Nair M, Shah P, Batra R, Kumar M, Mohan J, Kaul U, Arora R. Chronic atrial fibrillation in patients with rheumatic heart disease: mapping and radiofrequency ablation of flutter circuits seen at initiation after cardioversion. Circulation. 2001 Aug 14;104 (7):802–9. doi: 10.1161/hc3201.094228. [DOI] [PubMed] [Google Scholar]
- 8.Arani D T, Carleton R A. The deleterious role of tachycardia in mitral stenosis. Circulation. 1967 Oct;36 (4):511–6. doi: 10.1161/01.cir.36.4.511. [DOI] [PubMed] [Google Scholar]
- 9.Wipf J E, Lipsky B A. Atrial fibrillation. Thromboembolic risk and indications for anticoagulation. Arch. Intern. Med. 1990 Aug;150 (8):1598–603. doi: 10.1001/archinte.150.8.1598. [DOI] [PubMed] [Google Scholar]
- 10.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch. Intern. Med. 1994 Jul 11;154 (13):1449–57. [PubMed] [Google Scholar]
- 11.Morris D C, Hurst J W. Atrial fibrillation. Curr Probl Cardiol. 1980 Apr;5 (1):1–51. doi: 10.1016/0146-2806(80)90020-1. [DOI] [PubMed] [Google Scholar]
- 12.Karatasakis G T, Gotsis A C, Cokkinos D V. Influence of mitral regurgitation on left atrial thrombus and spontaneous echocardiographic contrast in patients with rheumatic mitral valve disease. Am. J. Cardiol. 1995 Aug 01;76 (4):279–81. doi: 10.1016/s0002-9149(99)80081-2. [DOI] [PubMed] [Google Scholar]
- 13.Hwang J J, Chen J J, Lin S C, Tseng Y Z, Kuan P, Lien W P, Lin F Y, Chu S H, Hung C R, How S W. Diagnostic accuracy of transesophageal echocardiography for detecting left atrial thrombi in patients with rheumatic heart disease having undergone mitral valve operations. Am. J. Cardiol. 1993 Sep 15;72 (9):677–81. doi: 10.1016/0002-9149(93)90884-f. [DOI] [PubMed] [Google Scholar]
- 14.JORDAN R A, SCHEIFLEY C H, EDWARDS J E. Mural thrombosis and arterial embolism in mitral stenosis; a clinico-pathologic study of fifty-one cases. Circulation. 1951 Mar;3 (3):363–7. doi: 10.1161/01.cir.3.3.363. [DOI] [PubMed] [Google Scholar]
- 15.Wanishsawad C, Weathers L B, Puavilai W. Mitral regurgitation and left atrial thrombus in rheumatic mitral valve disease. A clinicopathologic study. Chest. 1995 Sep;108 (3):677–81. doi: 10.1378/chest.108.3.677. [DOI] [PubMed] [Google Scholar]
- 16.Leung D Y, Black I W, Cranney G B, McCredie R M, Hopkins A P, Walsh W F. Resolution of left atrial spontaneous echocardiographic contrast after percutaneous mitral valvuloplasty: implications for thromboembolic risk. Am. Heart J. 1995 Jan;129 (1):65–70. doi: 10.1016/0002-8703(95)90044-6. [DOI] [PubMed] [Google Scholar]
- 17.Bonow Robert O, Carabello Blase A, Kanu Chatterjee, de Leon Antonio C, Faxon David P, Freed Michael D, Gaasch William H, Lytle Bruce Whitney, Nishimura Rick A, O'Gara Patrick T, O'Rourke Robert A, Otto Catherine M, Shah Pravin M, Shanewise Jack S, Smith Sidney C, Jacobs Alice K, Adams Cynthia D, Anderson Jeffrey L, Antman Elliott M, Faxon David P, Fuster Valentin, Halperin Jonathan L, Hiratzka Loren F, Hunt Sharon A, Lytle Bruce W, Nishimura Rick, Page Richard L, Riegel Barbara. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006 Aug 01;114 (5):e84–231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 18.Roth A, Harrison E, Mitani G, Cohen J, Rahimtoola S H, Elkayam U. Efficacy and safety of medium- and high-dose diltiazem alone and in combination with digoxin for control of heart rate at rest and during exercise in patients with chronic atrial fibrillation. Circulation. 1986 Feb;73 (2):316–24. doi: 10.1161/01.cir.73.2.316. [DOI] [PubMed] [Google Scholar]
- 19.James M A, Channer K S, Papouchado M, Rees J R. Improved control of atrial fibrillation with combined pindolol and digoxin therapy. Eur. Heart J. 1989 Jan;10 (1):83–90. doi: 10.1093/oxfordjournals.eurheartj.a059386. [DOI] [PubMed] [Google Scholar]
- 20.Scheinman M M, Morady F, Hess D S, Gonzalez R. Catheter-induced ablation of the atrioventricular junction to control refractory supraventricular arrhythmias. JAMA. 1982 Aug 20;248 (7):851–5. [PubMed] [Google Scholar]
- 21.Vaturi M, Sagie A, Shapira Y, Feldman A, Fink N, Strasberg B, Adler Y. Impact of atrial fibrillation on clinical status, atrial size and hemodynamics in patients after mitral valve replacement. J. Heart Valve Dis. 2001 Nov;10 (6):763–6. [PubMed] [Google Scholar]
- 22.Leon M N, Harrell L C, Simosa H F, Mahdi N A, Pathan A, Lopez-Cuellar J, Inglessis I, Moreno P R, Palacios I F. Mitral balloon valvotomy for patients with mitral stenosis in atrial fibrillation: immediate and long-term results. J. Am. Coll. Cardiol. 1999 Oct;34 (4):1145–52. doi: 10.1016/s0735-1097(99)00310-1. [DOI] [PubMed] [Google Scholar]
- 23.Maatouk Faouzi, Betbout Fethi, Ben-Farhat Mohamed, Addad Faouzi, Gamra Habib, Ben-Hamda Khaldoun, Dridi Zohra, Merchaoui Najet, Hammami Sonia, Maaoui Sabri, Hendiri Taoufik, Boughanmi Hatem. Balloon mitral commissurotomy for patients with mitral stenosis in atrial fibrillation: ten-year clinical and echocardiographic actuarial results. J. Heart Valve Dis. 2005 Nov;14 (6):727–34. [PubMed] [Google Scholar]
- 24.Gupta D, Kothari S S, Bahl V K, Goswami K C, Talwar K K, Manchanda S C, Venugopal P. Thrombolytic therapy for prosthetic valve thrombosis: short- and long-term results. Am. Heart J. 2000 Dec;140 (6):906–16. doi: 10.1067/mhj.2000.111109. [DOI] [PubMed] [Google Scholar]
- 25.Skoularigis J, Röthlisberger C, Skudicky D, Essop M R, Wisenbaugh T, Sareli P. Effectiveness of amiodarone and electrical cardioversion for chronic rheumatic atrial fibrillation after mitral valve surgery. Am. J. Cardiol. 1993 Aug 15;72 (5):423–7. doi: 10.1016/0002-9149(93)91134-4. [DOI] [PubMed] [Google Scholar]
- 26.Daoud E G, Strickberger S A, Man K C, Goyal R, Deeb G M, Bolling S F, Pagani F D, Bitar C, Meissner M D, Morady F. Preoperative amiodarone as prophylaxis against atrial fibrillation after heart surgery. N. Engl. J. Med. 1997 Dec 18;337 (25):1785–91. doi: 10.1056/NEJM199712183372501. [DOI] [PubMed] [Google Scholar]
- 27.Gomes J A, Ip J, Santoni-Rugiu F, Mehta D, Ergin A, Lansman S, Pe E, Newhouse T T, Chao S. Oral d,l sotalol reduces the incidence of postoperative atrial fibrillation in coronary artery bypass surgery patients: a randomized, double-blind, placebo-controlled study. J. Am. Coll. Cardiol. 1999 Aug;34 (2):334–9. doi: 10.1016/s0735-1097(99)00213-2. [DOI] [PubMed] [Google Scholar]
- 28.Wyse D G, Waldo A L, DiMarco J P, Domanski M J, Rosenberg Y, Schron E B, Kellen J C, Greene H L, Mickel M C, Dalquist J E, Corley S D. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002 Dec 05;347 (23):1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 29.Hagens Vincent E, Van Gelder Isabelle C, Crijns Harry J G M. The RACE study in perspective of randomized studies on management of persistent atrial fibrillation. Card Electrophysiol Rev. 2003 Jun;7 (2):118–21. doi: 10.1023/a:1027439430017. [DOI] [PubMed] [Google Scholar]
- 30.Wyse D George. Rhythm management in atrial fibrillation: less is more. J. Am. Coll. Cardiol. 2003 May 21;41 (10):1703–6. doi: 10.1016/s0735-1097(03)00331-0. [DOI] [PubMed] [Google Scholar]
- 31.Corley Scott D, Epstein Andrew E, DiMarco John P, Domanski Michael J, Geller Nancy, Greene H Leon, Josephson Richard A, Kellen Joyce C, Klein Richard C, Krahn Andrew D, Mickel Mary, Mitchell L Brent, Nelson Joy Dalquist, Rosenberg Yves, Schron Eleanor, Shemanski Lynn, Waldo Albert L, Wyse D George. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004 Mar 30;109 (12):1509–13. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 32.Roy Denis, Talajic Mario, Nattel Stanley, Wyse D George, Dorian Paul, Lee Kerry L, Bourassa Martial G, Arnold J Malcolm O, Buxton Alfred E, Camm A John, Connolly Stuart J, Dubuc Marc, Ducharme Anique, Guerra Peter G, Hohnloser Stefan H, Lambert Jean, Le Heuzey Jean-Yves, O'Hara Gilles, Pedersen Ole Dyg, Rouleau Jean-Lucien, Singh Bramah N, Stevenson Lynne Warner, Stevenson William G, Thibault Bernard, Waldo Albert L. Rhythm control versus rate control for atrial fibrillation and heart failure. N. Engl. J. Med. 2008 Jun 19;358 (25):2667–77. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 33.O'Neill Mark D, Jaïs Pierre, Hocini Mélèze, Sacher Frédéric, Klein George J, Clémenty Jacques, Haïssaguerre Michel. Catheter ablation for atrial fibrillation. Circulation. 2007 Sep 25;116 (13):1515–23. doi: 10.1161/CIRCULATIONAHA.106.655738. [DOI] [PubMed] [Google Scholar]
- 34.Vora Amit, Karnad Dilip, Goyal Venkat, Naik Ajay, Gupta Anup, Lokhandwala Yash, Kulkarni Hema, Singh Bramah. Control of rate versus rhythm in rheumatic atrial fibrillation: a randomized study. Indian Heart J. 2004 Sep 21;56 (2):110–6. [PubMed] [Google Scholar]
- 35.Hu C L, Jiang H, Tang Q Z, Zhang Q H, Chen J B, Huang C X, Li G S. Comparison of rate control and rhythm control in patients with atrial fibrillation after percutaneous mitral balloon valvotomy: a randomised controlled study. Heart. 2006 Aug;92 (8):1096–101. doi: 10.1136/hrt.2005.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim K B, Cho K R, Sohn D W, Ahn H, Rho J R. The Cox-Maze III procedure for atrial fibrillation associated with rheumatic mitral valve disease. Ann. Thorac. Surg. 1999 Sep;68 (3):799–803. doi: 10.1016/s0003-4975(99)00777-8. [DOI] [PubMed] [Google Scholar]
- 37.Bando Ko, Kobayashi Junjiro, Kosakai Yoshio, Hirata Mitsuhiro, Sasako Yoshikado, Nakatani Satoshi, Yagihara Toshikatsu, Kitamura Soichiro. Impact of Cox maze procedure on outcome in patients with atrial fibrillation and mitral valve disease. J. Thorac. Cardiovasc. Surg. 2002 Sep;124 (3):575–83. doi: 10.1067/mtc.2002.124392. [DOI] [PubMed] [Google Scholar]
- 38.Jatene M B, Marcial M B, Tarasoutchi F, Cardoso R A, Pomerantzeff P, Jatene A D. Influence of the maze procedure on the treatment of rheumatic atrial fibrillation - evaluation of rhythm control and clinical outcome in a comparative study. Eur J Cardiothorac Surg. 2000 Feb;17 (2):117–24. doi: 10.1016/s1010-7940(00)00326-2. [DOI] [PubMed] [Google Scholar]
- 39.Fukada J, Morishita K, Komatsu K, Sato H, Shiiku C, Muraki S, Tsukamoto M, Abe T. Is atrial fibrillation resulting from rheumatic mitral valve disease a proper indication for the maze procedure? Ann. Thorac. Surg. 1998 Jun;65 (6):1566–9. doi: 10.1016/s0003-4975(98)00135-0. [DOI] [PubMed] [Google Scholar]
- 40.Patwardhan A M, Dave H H, Tamhane A A, Pandit S P, Dalvi B V, Golam K, Kaul A, Chaukar A P. Intraoperative radiofrequency microbipolar coagulation to replace incisions of maze III procedure for correcting atrial fibrillation in patients with rheumatic valvular disease. Eur J Cardiothorac Surg. 1997 Oct;12 (4):627–33. doi: 10.1016/s1010-7940(97)00222-4. [DOI] [PubMed] [Google Scholar]
- 41.Guang Yang, Zhen-jie Cai, Yong Liu Wei, Tong Li, Ying Li. Evaluation of clinical treatment of atrial fibrillation associated with rheumatic mitral valve disease by radiofrequency ablation. Eur J Cardiothorac Surg. 2002 Feb;21 (2):249–54. doi: 10.1016/s1010-7940(01)01118-6. [DOI] [PubMed] [Google Scholar]
- 42.Lee Jae Won, Park Nam Hee, Choo Suk Jung, Jo Min Seop, Song Hyun, Song Meong Gun. Surgical outcome of the maze procedure for atrial fibrillation in mitral valve disease: rheumatic versus degenerative. Ann. Thorac. Surg. 2003 Jan;75 (1):57–61. doi: 10.1016/s0003-4975(02)04319-9. [DOI] [PubMed] [Google Scholar]
- 43.Jekic Mihaela, Foster Eric L, Ballinger Michelle R, Raman Subha V, Simonetti Orlando P. Cardiac function and myocardial perfusion immediately following maximal treadmill exercise inside the MRI room. J Cardiovasc Magn Reson. 2008 Jan 15;10 () doi: 10.1186/1532-429X-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xing Mingzhao. [Comments on the Ito et al. article (Endocr J. 2008 Oct 8. Epub ahead of print)] The lack of clinicopathological correlation of BRAF mutation in papillary thyroid cancer needs to be interpreted with caution. Endocr. J. 2009;56 (2):305–6. doi: 10.1507/endocrj.k08e-335. [DOI] [PubMed] [Google Scholar]


