Abstract
This paper highlights an updated anatomy of parametrial extension with emphasis on magnetic resonance imaging (MRI) assessment of disease spread in the parametrium in patients with locally advanced cervical cancer. Pelvic landmarks were identified to assess the anterior and posterior extensions of the parametria, besides the lateral extension, as defined in a previous anatomical study. A series of schematic drawings and MRI images are shown to document the anatomical delineation of disease on MRI, which is crucial not only for correct image-based three-dimensional radiotherapy but also for the surgical oncologist, since neoadjuvant chemoradiotherapy followed by radical surgery is emerging in Europe as a valid alternative to standard chemoradiation.
There are two main treatment options in patients with cervical cancer: radical surgery, including trachelectomy or radical hysterectomy, which is usually performed in early stage disease as suggested by the International Federation of Gynecology and Obstetrics (FIGO stages IA, IB1, and IIA), or primary radiotherapy with concurrent administration of platinum-based chemotherapy (CRT) for patients with bulky FIGO stage IB2/IIA2 tumors (> 4 cm) or locally advanced disease (FIGO stage IIB or greater). Some authors suggested the use of CRT followed by surgery for bulky tumors or locally advanced disease (1). Others proposed resection of the Müllerian compartment (fallopian tubes, uterus, proximal and middle vagina, enveloped by peritoneal and subperitoneal mesotissue known as mesometrium) and pelvic lymph node dissection by total mesometrial resection, without adjuvant radiation in FIGO stages IB, IIA, and selected IIB (2), following their ontogenetic theory of locoregional cancer spread (3–6). In all cases, pretreatment assessment of tumor extension and presence of parametrial invasion are of paramount importance to help define an appropriate management strategy. Staging of cervical cancer is still based on FIGO criteria, which are based on clinical findings. Its accuracy is limited in the advanced stages (7). Magnetic resonance imaging (MRI) has been shown to be the most reliable imaging technique in local staging, treatment planning, and follow-up of cervical cancer (8, 9), with staging accuracy ranging from 75% to 96% (10). In 2010, National Comprehensive Cancer Network (NCCN-2010) included MRI in the basic work-up of patients suffering from cervical cancer for stages greater than IB1.
The aim of this paper is to show MRI anatomy of the parametrium, paying special attention to the pelvic landmarks, using a series of T2-weighted and diffusion-weighted imaging (DWI) findings that are useful to identify its complete extension (i.e., anterior and posterior extensions, in addition to the lateral extension).
Technical suggestions
The MRI protocol for cervical cancer usually includes anatomical and morphologic sequences of the pelvis, such as T1-weighted imaging in the axial plane and T2-weighted imaging in the axial and sagittal planes, and high spatial resolution axial oblique (short axis of cervix) and coronal oblique (long axis of cervix) T2-weighted imaging with small field-of-view, which improve identification of parametrial invasion (12). Large field-of-view axial T1- and/or T2-weighted imaging of the abdomen is applied to identify enlarged lymph nodes and hydronephrosis. Dynamic multiphase contrast-enhanced three-dimensional T1-weighted imaging sequence is not routinely used for staging cervical carcinoma, unless the tumor is small and the patient is considered for fertility-sparing surgery or to distinguish between cervical or endometrial origin in cases of biopsy-proven adenocarcinoma. DWI sequences are also usually performed, at different b values (0 and 600 s/mm2; 0 and 800 s/mm2; 0 and 1000 s/mm2) (13, 14). In our department two b values are usually used (0 and 800 s/mm2). DWI is acquired in a straight axial plane (the same plane of the axial T2-weighted imaging) using a single shot echo-planar imaging. Apparent diffusion coefficient (ADC) maps are calculated on a pixel-by-pixel basis using a software, drawing a region of interest (ROI) within the solid tumor components. ADC value is measured in mm2/s and it is calculated by the slope of the line of the natural algorithm of signal intensity versus b values. In high b value DWI, areas of restricted diffusion appear bright. ADC maps are usually displayed as gray-scale images. Areas of restricted diffusion have lower ADC values and appear as a darker shade of gray on the ADC maps compared with areas of freely moving water. Technical details of our MRI examination in cervical cancer staging are presented in the Table.
Table.
MRI protocol for evaluation of patients with cervical carcinoma
| Parameter | Axial T1-w | Sagittal T2-w | Axial T2-w | Axial DWI | Axial oblique T2-w | Axial oblique DWI | Coronal oblique T2-w | Axial upper abdomen T2-w |
|---|---|---|---|---|---|---|---|---|
| Sequences | FSE- XL | FRFSE- XL | FRFSE- XL | EPI | FRFSE | EPI | FRFSE-XL | FRFSE- XL |
| Echo time (ms) | Min full | 85 | 85 | Minimum | 85 | Minimum | 85 | 84 |
| No. of signals acquired (NEX) | 2 | 2 | 2 | 6 | 4 | 6 | 4 | 1 |
| Repetition time (ms) | 470 | 4500 | 4500 | 5425 | 4500 | 5000 | 4500 | 1850 |
| No. of sections | 30 | 26 | 30 | 30 | 16 | 16 | 16 | 48 |
| Receiver bandwidth (kHz) | 31.25 | 41.67 | 31.25 | 41.67 | 41.67 | 41.67 | ||
| Echo train length | 3–5 | 16–24 | 16–24 | 16–24 | 16–24 | 17 | ||
| Field of view (mm) | 240 | 240 | 240 | 280 | 220 | 220 | 24 | 46 |
| Section thickness (mm) | 4 | 4 | 4 | 4 | 3 | 3 | 4 | 5 |
| Section spacing (mm) | 0.5 | 0.4 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 1 |
| Matrix size | 448×288 | 384×256 | 384×256 | 128×128 | 384×256 | 128×128 | 384×256 | 256×256 |
| b value (s/mm2) | --- | --- | --- | 800 | --- | 800 | --- | --- |
| Phase direction | A/P | S/I | A/P | R/L | Unswap | R/L | Unswap | R/L |
| Imaging options | NPW | NPW | NPW | … | … | … | NPW | FC |
| Acquisition time (min:s) | 03:21 | 02:47 | 01:53 | 04:20 | 03:05 | 02:43 | 03:05 | 02:13 |
T1-w, T1-weighted; T2-w, T2-weighted; DWI, diffusion-weighted imaging; FSE, fast spin-echo; XL, extra large (increased gradients); FRFSE, fast recovery fast spin-echo; EPI, echo planar imaging; NEX, number of excitations; A/P, anterior/posterior; S/I, superior/inferior; R/L, right/left; NPW, no phase wrap; FC, flow compensation.
MRI anatomy of parametrium
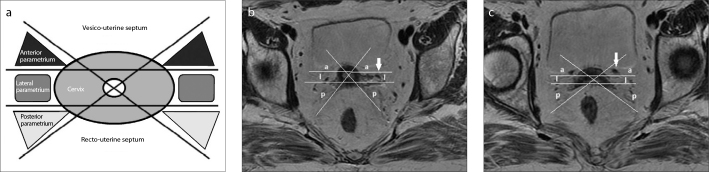
Parametrium and paracervix consist of connective areolar tissue enveloping the visceral branches of hypogastric vessels during their course toward the uterus and vagina. In the international anatomical nomenclature (Fig. 1), parametrium refers to the tissue located cranially to the ureter, between the uterine corpus and the pelvic sidewall, surrounding the uterine artery. The parametrium includes the superficial uterine pedicle (uterine artery and superficial uterine vein) and related connective tissue and lymphatic channels. The connective tissue crossing below the ureter is considered paracervix, which can be identified with the cardinal ligament. Structure named by surgeons as paracolpum is usually included in the term paracervix (11). On MRI, as well as in the FIGO classification, the parametrium is usually related to the fatty tissue surrounding both the uterine corpus and the cervix, bilaterally. In this paper the term parametrium will be used for indicating both the parametrium and the paracervix. To correctly assess the parametrial extension on MRI, some fixed pelvic structures need to be known. The main pelvic structure is the cardinal ligament, which is not directly visualized on MRI but it can be inferred from the location of the uterine artery and the uterine vein. Then, on T2-weighted imaging (Fig. 2) the cranial-caudal extension of parametrium can be recognized in the anatomical plane between uterine vessels joining on the uterus at the isthmus level superiorly, and the ureter opening in bladder inferiorly. Furthermore, the ureter is an anatomical landmark for the lateral parametrium. In the international anatomical nomenclature the lateral parametrium is assessed in a proximal part consisting of connective tissue and a lateral part that reaches the pelvic wall, and is made of fatty tissue and lymph nodes surrounding the vessels and nerves (15). On MRI, the proximal lateral parametrium is identified medially to the ureter, while the distal lateral parametrium is identified laterally to the ureter (Fig. 3). In their study, Ercoli et al. (11) also described the anterior and posterior parametria, which were proven to extend ahead and behind the uterine cervix (Fig. 4). The authors defined the “anterior parametrium” as the constant bilateral anterior expansion of parametrium around the cervico-vescical branches of the uterine artery, or the superficial portion of the vesi-co-uterine ligament, which is composed of vascular and fatty tissue connecting the bladder to the cervix and contributing to the roof of the ureteral tunnel near the bladder (16). Since the vesico-uterine ligament is not always easily recognized on MRI, the site of the roof of the ureteral tunnel near the bladder can be considered as the structure identifying the anterior extension of the parametrium. The authors defined the “posterior parametrium” as the posterior expansion of the parametrium at the uterosacral and rectovaginal ligament level; the posterior parametrium is composed of dense connective fibers originating from the posterior-lateral pelvic sidewalls and converging towards the upper dorsolateral portions of the uterine cervix (i.e., uterine torus) and the dorsolateral portions of the superior and middle vagina, respectively. Therefore, the upper part of the posterior parametrium corresponds to the uterosacral ligament joining the uterine torus, while its lower part extends until the superior vaginal walls (16). On MRI, the anterior and posterior extensions of the parametrium can be identified by drawing two oblique lines almost perpendicular to each other, centered on the cervical canal and tangent to the uterosacral ligaments. In this way, the “anterior parametrium” can be identified as a triangular space laterally to the vesico-uterine cleavage plane, including the iuxta-vesical tract of the ureters, while the “posterior parametrium” is identified as a triangular space at the uterosacral ligament level (Fig. 5).
Figure 1.
Classical anatomical scheme of parametrium location and extension. The frontal section through the uterus and parametrium shows tissue surrounding the uterine corpus and cervix, extending to the pelvic sidewall, and uterine vessels within the parametrium penetrating the uterus at the isthmus level. U, ureter; V, uterine vessels; black star, parametrium; void star, paracervix.
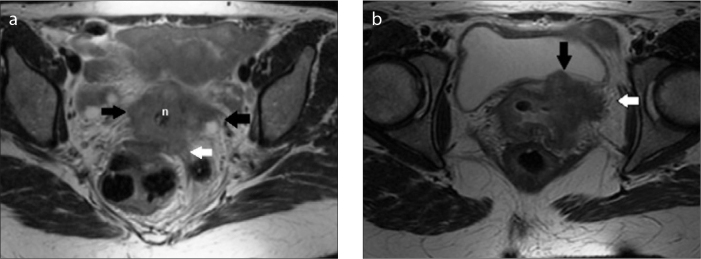
Figure 2.
a–f. Cranial-caudal extension of the parametrium on coronal and corresponding axial T2-weighted images. On coronal T2-weighted image (a), uterine vessels (arrows) identify the cranial limit of the parametrium. The horizontal lines drawn on coronal T2-weighted image (b) indicate parametrial cranio-caudal extension corresponding to axial T2-weighted images at different levels (c–f). The course of the ureter (c–f, white arrows) is crucial within the parametrium. n, small cervical cancer, confined within the stroma.
Figure 3.

Axial T2-weighted image showing the lateral extension of the parametrium.
Ureters (arrows) are the anatomical landmark for the lateral parametrium. The proximal lateral parametrium is identified medially to the ureter (white stars), while the distal lateral parametrium is identified laterally to the ureter (black stars).
Figure 4.
Schematic drawing of complete parametrial extension (lateral, anterior, and posterior extensions) in the sagittal view. USL, utero-sacral ligament; VUL, vesico-uterine ligament; U, ureter; UV, uterine vessels.
Figure 5.
a–c. Identification of the anterior and posterior parametria extension. The schematic (a) and axial T2-weighted images (b, c) show the parametrial extension in the axial plane. Two oblique lines are drawn almost perpendicular to each other, centered on the cervical lumen and tangent to the utero-sacral ligaments cranially (b) and to the site of the vesico-uterine ligament, which can be located at the level of juxta-vesical tract of the ureter distally (c); two parallel lines are also drawn ahead and behind the cross of oblique lines at the cervical lumen level. In this way, the upper (b) and lower parts (c) of anterior, lateral, and posterior parametrium can be identified. Cranially, the ureter is the anatomical landmark for the lateral parametrium (b, arrow), while distally it represents the anatomical landmark for the anterior parametrium (c, arrow). a, anterior parametrium; l, lateral parametrium; p, posterior parametrium.
T2-weighted MRI in the diagnosis of parametrial invasion
On T2-weighted imaging, cervical cancer appears to have intermediate signal intensity in contrast to the low signal intensity of the cervical stromal ring, while signal intensity of normal parametrium is the same as the pelvic fat tissue. On T2-weighted imaging, disruption of low signal intensity of the cervical stromal ring is considered a sign of stage IIB disease, since it indicates full-thickness stromal invasion suggesting microscopic parametrial invasion. Additional MRI key features, such as a spiculated tumor-parametrium interface, soft-tissue extension into the parametria, or encasement of the periuterine vessels, permit to make a confident diagnosis of macroscopic parametrial invasion (10) (Fig. 6).
Figure 6.
a, b. Parametrial spread in locally advanced cervical cancer. Axial T2-weighted images (a, b) show uterine cervix neoplasm invading bilateral proximal lateral parametria (a, black arrows) and the left posterior parametrium (a, white arrow); distally, invasion of the ureteral tunnel (b, black arrow) as well as invasion of the left anterior parametrium (b, white arrow) are also shown. n, cervical cancer.
DWI and ADC map to avoid pitfalls
There are some important pitfalls in delineating tumor margins and evaluating parametrial invasion on T2-weighted imaging (10). At baseline MRI evaluation, inflammation surrounding the tumor as well as the granulation tissue (post-biopsy changes) can lead to an overestimation of cervical invasion on T2-weighted imaging, because they are characterized by high signal intensity, similar to cancer. Overestimation of parametrial invasion on T2-weighted imaging occurs more frequently in large tumors (70% accuracy) compared with the smaller ones (96% accuracy). This may be related to the stromal and parametrial edema caused by tumor compression or inflammation (10), which can lead to an overestimation of the tumor size, as well. The reported T2-weighted imaging sensitivity and specificity in the evaluation of parametrial invasion are 69% and 93%, respectively, and the overall accuracy in staging cervical cancer is approximately 90%, with very high negative predictive value, but considerably lower positive predictive value (17). To overcome pitfalls on T2-weighted imaging, DWI should be routinely used, since T2-weighted imaging findings could be confirmed but also significantly rejected (Fig. 7). The advantage of DWI is to reflect the increasing tumor cellularity without being influenced by edematous changes and increasing vascularity. Increasing cellularity and architectural distortion in tumor tissue lead to reduction in extracellular space. These changes are identified by an area of restricted diffusion, which is hyperintense on DWI and is seen “at a glance.” However, much attention should be paid during DWI evaluation. First of all, “T2 shine-through effect” has to be excluded. “T2 shine-through effect” is seen in lesions with high fluid content showing a very long T2 relaxation time and demonstrating high signal intensity even at high b values. This pitfall may be avoided by referring to the ADC map, which is characterized by reduced ADC value in hypercellular lesions. Moreover, special attention must be paid in drawing the ROI within the lesion on the ADC map. Areas of necrosis should be carefully avoided since tumor necrosis, which is an indicator of poor differentiation, results in facilitated diffusion. From the above, it is intuitive that isolated viewing of DWI without correlation to ADC maps is not recommended. In addition, DWI and ADC maps, having poor anatomic detail and low spatial resolution, should always be analyzed in conjunction with higher-resolution anatomic MRI images. Some limitations in the use of DWI must be noted. Huge post-biopsy laceration of the cervix, resulting in air-trapping phenomena and intra-corporeal materials (i.e., hip prosthesis), both producing susceptibility artifacts and image distortion, can interfere with high-quality DWI. In addition, cytotoxic edema after CRT shows hyperintensity on T2-weighted imaging and decreased ADC values on DWI, which can be confused with residual cancer after therapy (18).
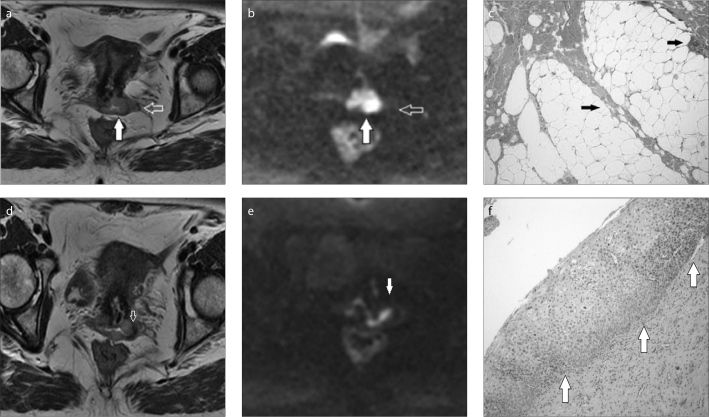
Figure 7.
a–f. Cervical cancer invading the vaginal fornix without parametrial spread. The axial T2-weighted image (a) shows cervical cancer (a, white arrow) with a suspicious left proximal lateral parametrial spread (a, void arrow). On corresponding high b value axial diffusion-weighted image (b), the cervical cancer appears as a bright area of restricted diffusion (b, white arrow), and seems confined within the cervix. No area of restricted diffusion is seen in the parametrium (b, void arrow). Histopathology confirmed the absence of parametrial involvement, showing fibrous and adipose tissue within the left parametrium (c, arrows). Distally, axial T2-weighted (d, arrow) and corresponding diffusion-weighted (e, arrow) images are suggestive for neoplastic invasion of the left fornix. Histopathology documents cervical intraepithelial neoplasia II, moderate dysplasia with abnormal changes in the basal layers of the squamous epithelial tissues in the posterior wall of the vagina (f, arrows).
Implications of MRI on surgical treatment
In Europe, particularly in Italy and France, neoadjuvant CRT followed by radical surgery is emerging as a valid alternative to standard CRT in locally advanced cervical cancer, improving the overall survival rates (75.5% vs. 62%–70%) (1). Tailoring of radical surgery might play an important role in diminishing the overall rate of complications and improving the quality of life of these patients. In their classification of radical hysterectomy, Querleu and Morrow (15) included four types of resections, depending on the extension of the disease (15). Different surgical approaches may be suggested and, in specific settings, nerve preservation surgery may be considered. Recently, different theories are emerging in cervical cancer treatment. Following their ontogenetic theory of cancer spread, Höckel et al. (2) proposed exclusive surgery, without adjuvant radiation, in FIGO stages IB, IIA, and selected cases of IIB. Resection of the entire Müllerian compartment (fallopian tubes, uterus, proximal and middle vagina enveloped by peritoneal and subperitoneal mesotissue known as mesometrium) together with pelvic lymph node dissection by total mesometrial resection, resulted in high locoregional tumor control with low morbidity in their series (2). In their compartmental theory, the authors stated that tissue at risk of locoregional tumor spread is the mature tissue derived from the corresponding morphogenetic field in the embryo. According to this theory, early stage cervical carcinoma is confined to its developmental compartment and the entire compartment of the same embryologic origin should be removed. Tumor-free resection margins are not sufficient. Tumor permeation within ontogenetic compartments explains the emergence of local tumor relapses despite successful surgical treatment and the lack of predictive robustness of surgical margin width (5). The surgical resection of the entire compartment seems to be essential for pelvic tumor control. The identification of pelvic viscero-parietal compartment serves as a template lymph-node basin to be cleared, while extracompartmental nonlymphatic tissues (i.e., pelvic autonomic nerved and bladder vessels) can remain in situ. Non-Müllerian paracervical and paravaginal tissues can be left in situ despite close proximity to the tumor, if the smooth compartment borders can be exposed (2). Phenotype changes, which are generally associated with advanced malignant progression, can make transgression into adjacent compartments from different embryonic precursor tissues. In more advanced disease, Höckel et al. (4) proposed a laterally extended endopelvic resection, which achieved locoregional tumor control both with central disease and with tumor fixed to the pelvic side wall, in their series. The authors based this therapeutic option on their ontogenetic theory of cancer field suggesting that during malignant progression, neoplasm permeates the adult tissue domains in retrograde sequence with respect to development. The neoplasm originating in the subcompartment (cervical epithelia and stroma) first infiltrates the mature Müllerian compartment, which is established by weeks 8–9 (i.e., cervical stroma and any of the following tissues: paracervix, proximal vagina, paracolpos, corpus, paracorpus, fallopian tubes except fimbria and mesosalpinx); then the late mature genital metacompartment, which is established by weeks 5–6, is infiltrated (i.e., Müllerian compartment and any of following several tissues: mesometrium, mesobladder, uterovaginal suspensory, dorsal lamina muscolaris of the bladder, distal vagina, dorsal uretra, rectovaginal septum, fimbria, ovary, and mesovar, uterine peritoneum); thereafter the early mature urogenital metacompartments, which are established after week 4, are involved (i.e., genital metacompartment and several tissues: bladder mucosa, ureter and mesoureter, dorsolateral urogenital mesentery, anterior rectal wall and mesorectum, pelvic peritoneum) (6). In their series (4), tumor always infiltrated the Müllerian compartment in patients with advanced disease. The authors stated that the probability of tumor infiltrating an ontogenetically different compartment by local permeation depends on the degree of developmental kinship between the two compartments, which can be estimated from the ontogenetic pathway. Among non-Müllerian compartments, the bladder was another favorite site of tumor infiltration because Müllerian compartment and the bladder trigone are derived from the same metacompartment, while rectal infiltration was less frequent since the mesorectum is derived from a different one. The authors explained fixation of the tumor to the pelvic wall by inflammatory reaction accompanying the tumor front and producing fibrotic adherences of the tumor to adjacent compartments that are not infiltrated yet.
In this scenario of different therapeutic approaches to cervical cancer, MRI can be usefully employed in the preliminary assessment of disease spread. In particular, the knowledge of MRI anatomy of parametrial extension can help to correctly assess the local involvement of disease by imaging. The lateral, anterior, and posterior parametrial extensions are easily identified on T2-weighted imaging, while DWI can help the diagnosis of parametrial neoplastic involvement. Both radiotherapists and surgeons could draw benefit from the preliminary evaluation of cervical cancer by MRI. However, a limitation of MRI technique should be reported. Some studies suggested the potential role of adjuvant hysterectomy in reducing recurrence rate after both suboptimal and complete response to neoadjuvant CRT. When this therapeutic approach is chosen, it would be helpful to correctly assess whether residual parametrial involvement after neoadjuvant CRT is present by MRI. This would be useful as a “road map” to guide surgical treatment in order to achieve a less aggressive approach, while identification of parametrial residual involvement could represent a fundamental element to plan the extent of radical adjuvant surgery (19–22). However, cytotoxic edema and fibrosis after CRT, which are characterized by decreasing ADC values at increasing radiation doses (18), can mimic residual cancer.
Conclusion
A comprehensive anatomical MRI knowledge is mandatory to correctly identify parametrial extension, which is useful for accurate evaluation of the extent of disease in cervical cancer.
Main points.
Different treatment options have been described in patients with cervical cancer, depending on the local extension of the disease. The pretreatment assessment of tumor extension and, particularly, the presence of parametrial invasion are of paramount importance to help define the appropriate management strategy.
The anatomical delineation of disease is essential for choosing the right therapeutic approach. MRI has been shown to be the most reliable imaging technique in local staging, treatment planning, and follow-up of cervical cancer so that in 2010, National Comprehensive Cancer Network (NCCN-2010) included MRI in the basic work-up of patients suffering from cervical cancer for stages greater than IB1.
The anatomical extension of parametria is precisely assessed by morphologic T2-weighted imaging. DWI and ADC map are also useful to better assess parametrial spread of the disease and avoid pitfalls.
Footnotes
This paper was presented at RSNA 2012 as an Educational Exhibit (space LL-OBE 2247).
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Ferrandina G, Margariti PA, Smaniotto D, et al. Long-term analysis of clinical outcome and complications in locally advanced cervical cancer patients administered concomitant chemoradiation followed by radical surgery. Gynecol Oncol. 2010;119:404–410. doi: 10.1016/j.ygyno.2010.08.004. http://dx.doi.org/10.1016/j.ygyno.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Höckel M, Horn LC, Manthey N, et al. Resection of the embryologically defined uterovaginal (Müllerian) compartment and pelvic control in patients with cervical cancer: a prospective analysis. Lancet Oncol. 2009;10:683–692. doi: 10.1016/S1470-2045(09)70100-7. http://dx.doi.org/10.1016/S1470-2045(09)70100-7. [DOI] [PubMed] [Google Scholar]
- 3.Höckel M, Horn LC, Fritsch H. Association between the mesenchymal compartment of uterovaginal organogenesis and local tumour spread in stage IB-IIB cervical carcinoma: a prospective study. Lancet Oncol. 2005;6:751–756. doi: 10.1016/S1470-2045(05)70324-7. http://dx.doi.org/10.1016/S1470-2045(05)70324-7. [DOI] [PubMed] [Google Scholar]
- 4.Höckel M, Horn LC, Einenkel J. (Laterally) extended endopelvic resection: surgical treatment of locally advanced and recurrent cancer of the uterine cervix and vagina based on ontogenetic anatomy. Gynecol Oncol. 2012;127:297–302. doi: 10.1016/j.ygyno.2012.07.120. http://dx.doi.org/10.1016/j.ygyno.2012.07.120. [DOI] [PubMed] [Google Scholar]
- 5.Höckel M, Hentschel B, Horn LC. Association between developmental steps in the organogenesis of the uterine cervix and locoregional progression of cervical cancer: a prospective clinicopathological analysis. Lancet Oncol. 2014;15:445–456. doi: 10.1016/S1470-2045(14)70060-9. http://dx.doi.org/10.1016/S1470-2045(14)70060-9. [DOI] [PubMed] [Google Scholar]
- 6.Höckel M. Morphogenetic fields of embryonic development in locoregional cancer spread. Lancet Oncol. 2015;16:148–151. doi: 10.1016/S1470-2045(14)71028-9. http://dx.doi.org/10.1016/S1470-2045(14)71028-9. [DOI] [PubMed] [Google Scholar]
- 7.Thomeer MG, Gerestein C, Spronk S, van Doorn HC, van der Ham E, Hunink MG. Clinical examination versus magnetic resonance imaging in the pretreatment staging of cervical carcinoma: systematic review and meta-analysis. Eur Radiol. 2013;23:2005–2018. doi: 10.1007/s00330-013-2783-4. http://dx.doi.org/10.1007/s00330-013-2783-4. [DOI] [PubMed] [Google Scholar]
- 8.Manfredi R, Maresca G, Smaniotto D, et al. Cervical cancer response to neo-adjuvant therapy: MR imaging assessment. Radiology. 1993;209:819–824. doi: 10.1148/radiology.209.3.9844681. http://dx.doi.org/10.1148/radiology.209.3.9844681. [DOI] [PubMed] [Google Scholar]
- 9.Cellini N, Smaniotto D, Scambia G, et al. Chemoradiation with concomitant boost followed by radical surgery in locally advanced cervical cancer: a dose-escalation study. Am J Clin Oncol. 2008;31:280–284. doi: 10.1097/COC.0b013e31815aff03. http://dx.doi.org/10.1097/COC.0b013e31815aff03. [DOI] [PubMed] [Google Scholar]
- 10.Sala E, Wakely S, Senior E, Lomas D. MRI of malignant neoplasms of the uterine corpus and cervix. AJR Am J Roentgenol. 2007;188:1577–1587. doi: 10.2214/AJR.06.1196. http://dx.doi.org/10.2214/AJR.06.1196. [DOI] [PubMed] [Google Scholar]
- 11.Ercoli A, Delmas V, Fanfani F, et al. Terminologia anatomica versus unofficial descriptions and nomenclature of the fasciae and ligaments of the female pelvis: a dissection-based comparative study. Am J Obstet Gynecol. 2005;193:1565–1573. doi: 10.1016/j.ajog.2005.05.007. http://dx.doi.org/10.1016/j.ajog.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Sala E, Rockall AG, Freeman SJ, Mitchell DG, Reinhold C. The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: what the radiologist needs to know. Radiology. 2013;266:717–740. doi: 10.1148/radiol.12120315. http://dx.doi.org/10.1148/radiol.12120315. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Zhang Y, Liang B, Yang Z. The utility of diffusion-weighted MR imaging in cervical cancer. Eur J Radiol. 2010:101–106. doi: 10.1016/j.ejrad.2009.04.025. http://dx.doi.org/10.1016/j.ejrad.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Kuang F, Ren J, Zhong Q, Liyuan F, Huan Y, Chen Z. Eur Radiol. 2013:1050–1058. doi: 10.1007/s00330-012-2681-1. http://dx.doi.org/10.1007/s00330-012-2681-1. [DOI] [PubMed] [Google Scholar]
- 15.Querleu D, Morrow CP. Classification of radical hysterectomy. Lancet Oncol. 2008;9:297–303. doi: 10.1016/S1470-2045(08)70074-3. http://dx.doi.org/10.1016/S1470-2045(08)70074-3. [DOI] [PubMed] [Google Scholar]
- 16.Touboul C, Fauconnier A, Zareski E, Bouhanna P, Daraï E. The lateral infraureteral parametrium: myth or reality? Am J Obstet Gynecol. 2008;199:242.e1–6. doi: 10.1016/j.ajog.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Kaur H, Silverman PM, Iyer RB, Verschraegen CF, Eifel PJ, Charnsangavej C. Diagnosis, staging, and surveillance of cervical carcinoma. AJR Am J Roentgenol. 2003;180:1621–1631. doi: 10.2214/ajr.180.6.1801621. http://dx.doi.org/10.2214/ajr.180.6.1801621. [DOI] [PubMed] [Google Scholar]
- 18.Hein PA, Kremser C, Judmaier W, et al. Diffusion-weighted magnetic resonance imaging for monitoring diffusion changes in rectal carcinoma during combined, preoperative chemoradiation: preliminary results of a prospective study. Eur J Radiol. 2003;45:214–222. doi: 10.1016/s0720-048x(02)00231-0. http://dx.doi.org/10.1016/S0720-048X(02)00231-0. [DOI] [PubMed] [Google Scholar]
- 19.Walji N, Chue AL, Yap C, et al. Is there a role for adjuvant hysterectomy after suboptimal concurrent chemoradiation in cervical carcinoma? Clin Oncol (R Coll Radiol) 2010;22:140–146. doi: 10.1016/j.clon.2009.11.006. http://dx.doi.org/10.1016/j.clon.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Morice P, Rouanet P, Rey A, et al. Results of the GYNECO 02 study, an FNCLCC phase III trial comparing hysterectomy with no hysterectomy in patients with a (clinical and radiological) complete response after chemoradiation therapy for stage IB2 or II cervical cancer. Oncologist. 2012;17:64–71. doi: 10.1634/theoncologist.2011-0276. http://dx.doi.org/10.1634/theoncologist.2011-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yabuki Y, Asamoto A, Hoshiba T, Nishimoto H, Kitamura S. Dissection of the cardinal ligament in radical hysterectomy for cervical cancer with emphasis on the lateral ligament. Am J Obstet Gynecol. 1991;164:7–14. doi: 10.1016/0002-9378(91)90614-w. http://dx.doi.org/10.1016/0002-9378(91)90614-W. [DOI] [PubMed] [Google Scholar]
- 22.Colombo PE, Bertrand MM, Gutowski M, et al. Total laparoscopic radical hysterectomy for locally advanced cervical carcinoma (stages IIB, IIA and bulky stages IB) after concurrent chemoradiation therapy: surgical morbidity and oncological results. Gynecol Oncol. 2009;114:404–409. doi: 10.1016/j.ygyno.2009.05.043. http://dx.doi.org/10.1016/j.ygyno.2009.05.043. [DOI] [PubMed] [Google Scholar]