Abstract
PURPOSE
We aimed to present our biopsy method and retrospectively evaluate the results, upgrade rate, and follow-up findings of stereotactic vacuum-assisted breast biopsy (VABB) procedures performed in our clinic.
METHODS
Two hundred thirty-four patients with mammographically detected nonpalpable breast lesions underwent VABB using a 9 gauge biopsy probe and prone biopsy table. A total of 195 patients (median age 53 years, range 32–80 years) with 198 microcalcification-only lesions with a follow-up of at least one year were included in the study. The location of the lesion relative to the needle was determined from the postfire images, and unlike the conventional technique, tissue retrieval was predominantly performed from that location, followed by a complete 360° rotation, if needed.
RESULTS
The median core number was 8.5. Biopsy results revealed 135 benign, 24 atypical, and 39 malignant lesions. The total upgrade rate at surgery was 7.7% (6.1% for ductal carcinomas in situ and 10.5% for atypical lesions). Patients with benign lesions were followed up for a median period of 27.5 months, with no interval change. At the follow-up, scar formation was seen in 23 patients (17%); three of the scars were remarkable for resembling a malignancy.
CONCLUSION
Our biposy method is fast and practical, and it is easily tolerated by patients without compromising accuracy. Patients with a diagnosis of atypia still need to undergo a diagnostic surgical procedure and those with a malignancy need to undergo curative surgery, even if the lesion is totally excised at biopsy. VABB may leave a scar in the breast tissue, which may resemble a malignancy, albeit rarely.
Currently, the gold standard for breast biopsies is open excision of the suspected lesion. However, an excisional biopsy inevitably leaves a scar behind. The cost and morbidity associated with this procedure have prompted many physicians to evaluate less invasive and alternative procedures (1–3).
Clustered suspicious microcalcifications can be a very early sign of malignancy, particularly typical for ductal carcinoma in situ (DCIS). Microcalcifications are usually detected on screening mammographies, and most of them cannot be identified on ultrasonography (US). Lesions detected only on mammography require stereotactic guidance for biopsy, and vacuum-assisted breast biopsy (VABB) is currently the biopsy method of choice for stereotactic biopsies. VABB is considered to be a safe procedure and is comparable to surgical biopsy for the characterization of microcalcifications (4, 5). Studies have shown that it is a very accurate biopsy method and is characterized by high-quality specimens, high calcification retrieval, and low rates of false-negative results (6–10). In case of microcalcifications, complete percutaneous excision is frequently possible, which leads to a decrease in sampling error as well as a decrease in upgrade rate, imaging-pathology discordance, and re-biopsy rate. The likelihood of subsequent growth on follow-up is also diminished. VABB is not a therapeutic procedure for malignant lesions; however, using VABB for diagnosis rather than surgical biopsy decreases the number of operations required (11–13).
Vacuum biopsy can be used for the histopathologic diagnosis of all nonpalpable breast lesions; however, it is mostly reserved for mammographically detected lesions, mainly microcalcifications. In this paper, we aim to present our biopsy method and retrospectively evaluate the results, upgrade rate, and follow-up findings of stereotactic-guided VABB procedures performed over a six-year period in our clinic.
Methods
Between January 2008 and January 2014, 238 patients were referred to our clinic for VABB. Biopsy could not be performed in four patients because the breast was not thick enough in two patients and the location of the microcalcifications was too deep in one and too superficial in the other. Microcalcifications were moderately suspicious in two of them, and they underwent surgical biopsy. A six-month follow-up was recommended for the other two patients because the microcalcifications resembled fat necrosis. Biopsy was successfully performed in 234 patients. Thirty-one patients were excluded because they were followed up for less than a year. Another eight patients were excluded because biopsy was performed for mass/architectural distortion lesions and not microcalcifications.
The remaining 195 patients (median age 53 years, range 32–80 years) with 198 microcalcification lesions, who underwent surgery or had at least one year of follow-up, were included. Biopsy was performed for 198 lesions, all of which were mammographically detected and could not be identified on US. Informed consent forms regarding the procedure of biopsy and academic use of data were signed by all patients. The ethics committee did not ask for any further approval as this is a retrospective analysis of the results.
Before biopsy, lesions were classified according to the new edition of mammographic BI-RADS classification published in 2013 (14). Forty lesions comprising amorphous calcifications (20.2%, considered mildly suspicious in the former edition) and 133 lesions comprising coarse heterogeneous calcifications (67.18%, considered moderately suspicious in the former edition) were classified as BI-RADS category 4b (87.38% in total). Twenty-five lesions comprising fine pleomorphic, fine linear, or fine linear branching calcifications were classified as BI-RADS category 4c/5 (12.63%, highly suspicious of a malignancy). The number of BI-RADS 4c/5 lesions was relatively low because surgeons tended to prefer wire-guided surgical excision with onsite pathologic evaluation for the diagnosis of very suspicious lesions.
Fisher’s exact test was used for statistical evaluation. A P value of <0.05 was considered to be significant.
Biopsy
Stereotactic vacuum-assisted biopsy was performed using a prone biopsy table (Multicare Platinum; Hologic) and 9 gauge (G) biopsy device (Suros ATEC; Hologic). All VABB procedures were performed under local anesthesia.
Craniocaudal and mediolateral mammograms were taken before targeting the lesion. Following appropriate prone positioning, the scout view and stereotactic paired images were used for accurate needle placement using the x-y-z coordinates determined by the machine. If the calculations showed that the procedure was possible, a local anesthetic (2% prilocaine hydrochloride with no adrenaline) was applied and the needle was inserted in the breast. Further pre- and postfire stereo images were obtained. According to the needle–lesion relation in these postfire images, the position of the lesion relative to the needle was determined, and unlike the conventional technique, tissue retrieval was predominantly performed from that location, followed by a complete 360° rotation if needed. This way, the lesion was better targeted and less biopsy specimens were required (Figs. 1, 2). Specimen graphy was acquired in all lesions. At least two cores with at least five calcium specks were regarded sufficient (15). Our aim was total excision for lesions smaller than 1 cm. The biopsy cavity was irrigated with saline after the procedure, and a radiopaque marker (Suros Surgical Systems) was placed if the lesion seen at mammography was totally or near totally removed or if a large area was sampled and if documenting the precise site of biopsy was desired. Post-clip mammograms were then taken to ensure accurate clip deployment.
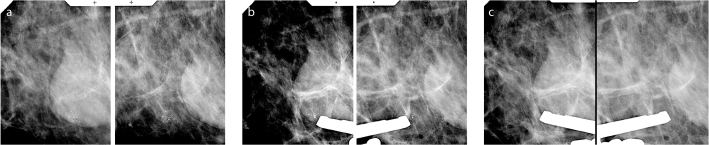
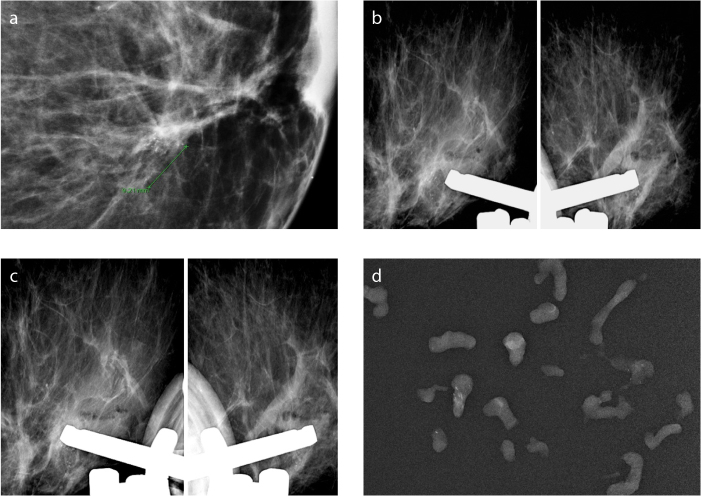
Figure 1.
a–c. Stereo (a) and prefire (b) images of a 5 mm cluster of coarse heterogeneous microcalcifications. Microcalcifications were located at the 12 o’clock position on postfire images (c). Tissue retrieval was started from this location, and consecutive samples were taken from there, followed by a round tour from other locations.
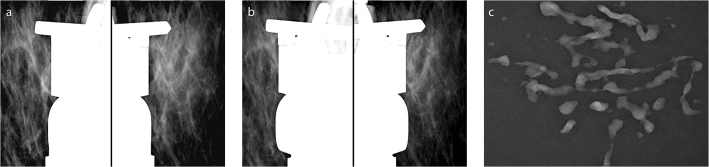
Figure 2.
a–c. Microcalcifications in a linear distribution in a 55-year-old patient. Pathology revealed low-grade DCIS. Pre- (a) and postfire (b) images and specimen graphy (c) obtained during stereotactic 9G directional vacuum-assisted biopsy. According to the postfire images, tissue retrieval was predominantly performed from the 4 o’clock position, more specimens were obtained from 4–6 o’clock positions, and then, the 360° tour was completed.
The median duration of the procedure was 27.5 min (range, 20–40 min). It became shorter as our experience increased, and our modified biopsy method allowed us to finish the procedure in a short period of time. The actual specimen retrieval time was around or under half a minute. The procedure was very well tolerated by almost all patients. Later, ice compression was locally applied, and patients were advised to apply intermittent ice compression during the rest of the day. Specimens that contained calcifications were determined from the specimen graphy and those with and without calcifications were sent in two separate labeled formalin containers for pathologic examination.
Management of patients after biopsy
Surgical excision was recommended to all patients with a histologic diagnosis of infiltrating or in situ carcinoma and lesions with atypia. Excision was recommended for all cases of discordance between histologic and mammographic results (such as BI-RADS 4c lesions with a benign diagnosis) after a discussion at the multidisciplinary team meeting. Mammographic follow-up was recommended for the remaining patients with concordant benign biopsy results. The median follow-up time in patients with benign lesions was 27 months (range, 13–84 months). All patients had at least one year of follow-up.
Results
Pathology results of biopsy specimens revealed 135 benign lesions (68.2%), 39 malignant lesions (19.7%), and 24 atypical lesions (12.1%). Follow-up data was available for all benign lesions. Biopsy results are summarized in Table 1.
Table 1.
Histologic diagnoses on vacuum-assisted breast biopsy
| Histologic diagnoses on VABB | n |
|---|---|
| Benign lesions (n=135) | |
| Fibrosis-adenosis | 44 |
| Fibrocyctic changes | 32 |
| Columnar cell changes | 23 |
| Fibroadenoma and fibroadenomotoid lesions | 11 |
| Sclerosing adenosis | 13 |
| Dystrophic calcifications and benign breast tissue | 11 |
| Lactational changes | 1 |
| Malignant lesions (n=39) | |
| DCIS (one with lobular cancerization and one with papilloma) | 33 |
| IDC | 6 |
| Atypical lesions (n=24) | |
| ADH | 16 |
| Atypical columnar changes and hyperplasia | 3 |
| Pure flat epithelial atypia | 3 |
| LCIS | 1 |
| Pure ALH | 1 |
VABB, vacuum-assisted breast biopsy; DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; ADH, atypical ductal hyperplasia; LCIS, lobular carcinoma in situ; ALH, atypical lobular hyperplasia.
In 177 lesions (89.4%), sampling was performed once, in 20 lesions (10.1%) twice, and in one lesion (0.5%) three times. Microcalcifications were confirmed with specimen graphies of 195 lesions (98.5%), while they were pathologically confirmed in all biopsy specimens. Therefore, vacuum-assisted biopsy was considered to be successful in all lesions. The microcalcifications were completely excised in 87 of 198 lesions (43.94%). In 92 lesions (46.5%), a radiopaque marker was placed. The relation between lesion characteristics and total removal at biopsy is given in detail in Table 2. The number of lesions in each subgroup was low, and for statistical analysis, the lesions were grouped into two according to the number of calcifications (<15 and >15) and lesion size (≤1cm and >1cm) (Table 3). Fisher’s exact test analysis revealed that lesions with <15 microcalcifications and those that were <1 cm were more likely to be totally excised by VABB and that the difference was highly significant (P < 0.001). There were 112 lesions that were ≤1 cm, and 69 of these (61.6%) were totally excised at biopsy. On the other hand, there were 108 lesions with <15 calcifications, 66 of which (61.1%) were totally excised.
Table 2.
Relationship between characteristics of microcalcifications and total removal at biopsy
| Lesion characteristics | Total removal n=87 (44) |
Partial removal n=111 (56) |
|---|---|---|
| Size of the lesion | ||
| ≤10 mm | 69 (34.8) | 43 (21.7) |
| 11–25 mm | 18 (9.1) | 33 (16.7) |
| >25 mm | 35 (17.7) | |
| Number of calcium specks | ||
| <5 | 18 (9.1) | 4 (2) |
| 5–15 | 48 (24.2) | 38 (19.2) |
| 15–30 | 20 (10.1) | 27 (13.6) |
| >30 | 1 (0.5) | 42 (21.2) |
Data are presented as n (%).
Table 3.
Relationship between characteristics of microcalcifications and total removal at biopsy
| Lesion characteristics | Total removal 87 (44) |
Partial removal 111 (56) |
P |
|---|---|---|---|
| Size of the lesion | |||
| ≤10 mm | 69 | 43 | <0.001 |
| >10 mm | 18 | 68 | |
| Number of calcium specks | P | ||
| <15 | 66 | 42 | <0.001 |
| >15 | 21 | 69 | |
Data are presented as n (%) or n.
The median core number that we collected was 8.5 (range, 5–16). The specimens that contained and did not contain calcium were separately assessed on histopathologic evaluation. In 184 of 198 lesions (92.93%), the histopathologic findings were similar. In 14 lesions (7.1%), the correct diagnosis was achieved only on specimens with microcalcifications, while the histologic evaluation of specimens without calcifications revealed benign breast tissue. Ten of 14 lesions (71.4%) were malignant or atypical and four (28.57%) were benign.
There were no major complications during procedures in any patients. Mild pain and bruising were experienced by approximately one-third of the patients. Only one patient experienced severe pain, and the procedure had to be terminated after taking five specimens. Sufficient number of microcalcifications to make a diagnosis was retrieved even in this patient. One patient experienced moderate pain at the biopsy site after the procedure without any hematoma formation. It continued for a few weeks and spontaneously resolved. Hematoma occurred in five patients (2.52%). The hematomas in four of the patients measured between 1 and 2 cm and were conservatively treated; surgical intervention was not required. In one patient, there was bleeding from the biopsy site a few hours after the procedure. She had a 3 cm hematoma that regressed by compression.
Follow-up data was available for all 135 benign lesions. One of these lesions was operated on because it was categorized as BI-RADS 4c and the biopsy result was fibrocystic changes. The final pathology was similar. There was no discordance among other lesions, and none of the patients underwent surgery. No suspicious lesions were detected in any patient during the follow-up period (false negative rate, 0%).
On the follow-up mammograms or sonograms, there was scar formation at the biopsy site in 23 patients (17%). Most of these were minimal distortions or linear scars that did not cause any diagnostic problems. No further evaluation was required. Three patients had marked scars. Of the marked scars, one was only seen on US. It was a distortion requiring tissue diagnosis to rule out a malignancy, and the pathologic examination reported benign findings. Two lesions were seen on both follow-up mammograms and sonograms: one was an irregular mass (Fig. 3) and the other was an indistinct hypoechoic nodule with asymmetrical density. These patients underwent breast magnetic resonance imaging (MRI). No contrast enhancement was detected at MRI, and the patients did not accept further interventional procedure. No interval change was detected in the follow-up physical exam, US, or mammography.
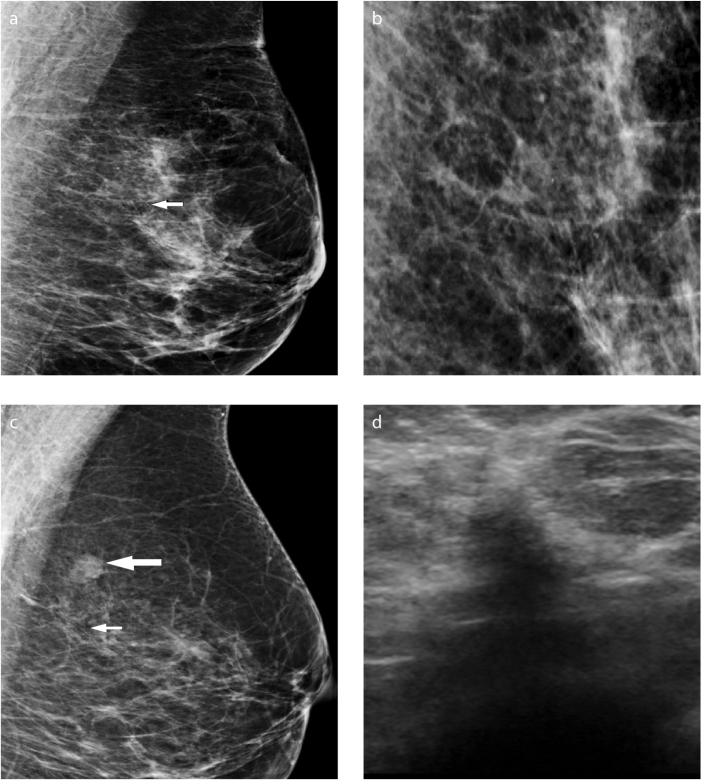
Figure 3.
a–d. Marked scar after VABB. Mediolateral oblique (MLO) (a) and MLO magnification (b) images of amorphous microcalcifications a, (arrow) in the upper outer quadrant in the left breast in a 52-year-old patient with a strong family history. Histopathology revealed adenosis and fibrosis. Follow-up mammogram (c) shows an irregular nodule at the biopsy site (thick arrow). There were a few residual calcifications; therefore, a marker was not required (thin arrow). Irregular hypoechoic scar is seen on sonogram (d). MRI was normal; the lesion did not change in the following years.
All patients with malignant biopsy results underwent surgery. Six of 39 malignant lesions were invasive cancers. Only one of these was >1 cm. All calcifications were excised in two patients with invasive cancer; and at surgery there was no residual tumor in either of them. Four patients had residual microcalcifications and residual tumor (Fig. 4). Thirty-three lesions were identified as DCIS at biopsy. Calcifications were totally excised in 18 lesions, but 12 patients had residual tumor at surgery. Among all patients with malignant lesions, calcifications were totally excised at biopsy in 20 lesions and partially excised in 19. At surgery, 17 of 19 partially excised (89.5%) and 12 of 20 totally excised (60%) lesions had residual tumor. The rate of malignant lesions totally excised at biopsy was 25.6% (10/39).
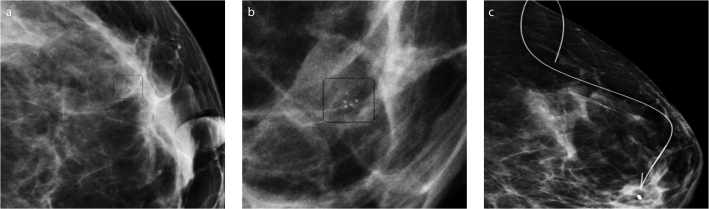
Figure 4.
a–c. Craniocaudal mammogram (a) and magnified MLO image (b) show a 5 mm cluster of coarse heterogeneous microcalcifications. The lesion was partially excised and histopathology revealed IDC. The patient underwent surgery after wire localization of the marker left at the biopsy site (c). There was residual disease at the final pathologic examination.
Among 33 DCIS lesions, 24 were high grade and nine were low or moderate grade. Patients with two high-grade DCIS lesions were upgraded to invasive ductal carcinoma (IDC) at surgery (Fig. 5). The upgrade ratio for DCIS was 6.1% (2/33). The final pathology report of two low-grade DCIS lesions was moderate-grade DCIS and one was high-grade DCIS; however, these were not considered as an upgrade.
Figure 5.
a–d. Craniocaudal magnification image (a) of a 9 mm cluster of pleomorphic microcalcifications in a 62-year-old patient. High-grade DCIS was determined at biopsy. The lesion was upgraded to IDC at the final pathologic examination. Pre- (b) and postfire (c) images and specimen graphy (d) are presented.
Only 19 of 24 lesions with atypia were treated by surgery. Five patients with atypical ductal hyperplasia (ADH) whose calcifications were completely excised were not operated per their surgeon’s decision. Lesions were <1 cm in these patients. No suspicious findings were detected in the 2–3 year follow-up mammograms. They are still under regular follow-up. Among the operated patients, one with flat epithelial atypia (FEA) and one with ADH were upgraded to high-grade DCIS. In both, the calcifications were partially excised. The upgrade ratio for atypia was 10.5% (2/19). The calcifications were completely excised in 10 of 24 lesions with atypia. Upgrade was not determined in any of them. Patients with 15 of 19 lesions that were operated had residual atypia at surgery.
Because the number of patients with upgrade at surgery was very low, we could not detect any significant association between lesion characteristics or the total removal rate at biopsy and upgrade detected at surgery. The relationship between lesion characteristics and removal type versus upgrade among the 52 patients who underwent surgery after the diagnosis of atypia or DCIS is summarized in Table 4.
Table 4.
Relationship between lesion characteristics and type of removal versus upgrade at surgery after a diagnosis of atypia or DCIS
| Lesion characteristics | Upgrade (+) 4 (7.8) |
Upgrade (−) 48 (92.3) |
|---|---|---|
| Size of the lesion (n=52) | ||
| ≤10 mm | 1 (1.9) | 27 (51.9) |
| 11–25 mm | - | 12 (23.1) |
| >25 mm | 3 (5.8) | 9 (17.3) |
| Number of calcium specks (n=52) | ||
| <5 | - | 1 (1.9) |
| 5–15 | 1 (1.9) | 23 (44.2) |
| 15–30 | - | 14 (26.9) |
| >30 | 3 (5.8) | 10(19.2) |
| Type of removal (n=52) | Upgrade (+) 4 (9.6) |
Upgrade (−) 48 (90.4) |
| Complete | 1 (1.9) | 20 (38.5) |
| Partial | 3 (5.8) | 28 (51.9) |
Data are presented as n (%).
DCIS, ductal carcinoma in situ.
According to the final pathology results, malignancy rates for BI-RADS categories were 9.8% for BI-RADS 4b (5% for amorphous microcalcifications and 11.3% for coarse heterogeneous microcalcifications), 96% for BI-RADS 4c/5, and 20.7% overall (41/198).
Discussion
Stereotactic VABB is a highly accurate technique for sampling nonpalpable breast lesions. It is particularly advantageous in the assessment of calcified lesions (7–10, 16–18). This technique uses only one puncture, with the probe of the device remaining within the breast, at the site of interest, throughout the sampling. Meyer et al. (8) showed 90.8% of microcalcifications in samples obtained using standard automated needle devices compared with 95%–100% of microcalcifications in samples extracted by the vacuum-assisted device (19, 20). Accordingly, in our series, microcalcifications were identified in specimen radiographs of 98.5% of lesions. They were histopathologically identified in all lesions.
VABB frequently allows the complete removal of a mammographic lesion. In the literature, the percentage of lesions completely removed by VABB is variable. Liberman et al. (21) showed total removal of 13% of microcalcifications among 108 procedures. More recent studies showed higher (48%–53%) rates of total removal (6, 12). This variability is mainly due to the different patient selection criteria. Some studies have included only microcalcifications, while others have included masses and distortions. In our study, VABB showed complete removal of the suspicious calcifications in 43.9% of the lesions. The complete removal of microcalcifications leads to a decrease in sampling error, upgrade rate, imaging-pathology discordance, and re-biopsy rate. The likelihood of subsequent growth on follow-up is also diminished. However, VABB cannot be considered a therapeutic procedure, even in case of complete removal of microcalcifications (22). Liberman et al. (23) reported that in patients with complete removal of microcalcifications by VABB, there was still residual cancer in almost 80% of patients at surgery. In our study, among the 20 cancer patients whose calcifications were completely removed, there was residual disease in 12 of them (60%).
The demonstration of calcification retrieval on specimen radiography is important for a successful biopsy. However, sometimes it is not possible, even with multiple samplings. Even if we see no calcifications on specimen graphy, they can be detected on histopathologic evaluation, which is sufficient for making a diagnosis. When calcifications are detected on specimen graphy, a separate evaluation of calcium-containing cores may assist the pathologist, who can evaluate these cores with additional sections (24). In our study, we wanted to evaluate if there were any differences regarding histopathologic information between specimens with and without calcifications. We found that the histopathologic diagnosis was made solely on the pots with microcalcifications in only 14 lesions (7.1%). In the remaining 184 lesions (92.9%), diagnosis was similar for both pots. Gümüş et al. (25) showed that in 87% of patients, an accurate diagnosis can still be made even if the targeted microcalcifications are missed. However, in our study, 10 out of these 14 lesions were either malignant or atypical, which means that in 15.6% of patients (10/63) with significant lesions, diagnosis was possible only in those specimens with calcifications. We think that separating specimens with calcifications is still advantageous to pathologists.
Obtaining large numbers of specimens may not prove to be useful for an accurate diagnosis, but an adequate number of correctly targeted specimens is essential (26, 27). In the study by Lomoschitz et al. (28), at 11G vacuum-assisted biopsy, the highest diagnostic yield was achieved with 12 specimens per lesion, independent of the mammographic appearance of the lesion. They showed that even with the standardized retrieval of 20 specimens per lesion, underestimation of the disease still occurs. The mean specimen number of 13 studies that they reviewed was 12.6. In the literature, it is mostly advised to take out 12–14 specimens per lesion. In our study, the median number of cores was 8.5, and this is lower than that in literature. We routinely assessed the position of microcalcifications relative to the needle in the postfire images and started taking samples from that location and obtained most of the tissue from the related position. Starting the biopsy from that location enables the most suspicious tissue to be directly drawn toward the needle, and this way we can finish the procedure faster, taking out a fewer number of specimens. It is also very helpful when the position of the microcalcifications change due to the injection of local anesthetic material. We think that this practical approach increases patient tolerance, decreases complication rates, and provides more accurate sampling. Although the average core number was low in our study, the false negative rate was 0% and total excision rate was almost 44%. The procedure was very well tolerated by almost all patients, and our complication rate was very low. Even in the patient who had severe pain and from whom only five specimens were removed, an adequate number of calcifications were retrieved due to this approach. In our study, one sampling was enough in 89.4% of patients. Those who needed 2–3 samplings were mostly those from the first year when we started performing vacuum-assisted biopsy, a period when we had less experience.
Stereotactic VABB seems to be almost as accurate as open surgical biopsy, but with lower complication rates (29). Mammographic changes after surgical biopsy, such as architectural distortion, parenchymal scar, calcifications, fat necrosis, and asymmetric glandular tissue defects, have been well described and may mimic the mammographic signs of a malignancy. However, radiologic findings after vacuum-assisted biopsy are not well known. Generally, it is believed that needle biopsies leave no scars on the breast. Lamm et al. (30) showed that the biopsy needle tract was evident in only 2% of the lesions for which 11G directional vacuum-assisted biopsy was used and none of the lesions for which 14G directional vacuum-assisted biopsy was used. On the other hand, Yazıcı et al. (31) reported that scar formation was detected in 4.3% of the lesions for which 11G directional vacuum-assisted biopsy was used (six minimal, two moderate, and one marked scar in 210 lesions). Our total scar rate (17%) is higher than that in the literature, probably because we used 9G needles and also because we performed routine US examination in the annual follow-ups. The scars were more apparent on US examinations. In our study, we detected three marked scars that were interpreted to be suspicious for a malignancy. Sampling was done twice in two of these three lesions, and there may be a relationship between sampling number and marked scars. Radiologists interpreting follow-up mammograms and/or sonograms of these patients should be aware of this possibility.
A histological upgrade has important consequences for patient management. When sampling microcalcifications, DCIS is the most common diagnosed malignancy. A core biopsy diagnosis of DCIS is upgraded to invasive disease at surgery in 15%–36% of patients, whereas this is reported in only 10% of patients undergoing VABB (10). Our DCIS underestimation rate (2/33, 6.1%) is lower than that in the literature. The number of specks of microcalcification, size of the cluster, and grade of DCIS are factors associated with an increased likelihood of upgrade. Clusters that contain more than 40 specks of calcification have a 48% chance of invasion at final histology compared with the 15% chance for clusters with <40 specks of calcification. Clusters that are <11 mm in diameter have an 18% chance of invasion at final histology compared with the 35% chance for clusters ≥60 mm in diameter (32). Thirteen percent of patients initially diagnosed with low-grade DCIS will be upgraded to invasive disease compared with 36% of those with high-grade DCIS (33). Upgrade to invasive cancer is important because all patients with invasive disease will require sentinel lymph node biopsy, while only 31% of those with a final diagnosis of DCIS have their nodal status ascertained at the time of breast surgery (34). In our study, only two DCIS lesions were upgraded to IDC. Interestingly, one of them was only 9 mm in diameter and included 8–10 microcalcifications, which were completely removed; however, it was a high-grade DCIS. The final pathology report of one low-grade DCIS came out as a high-grade DCIS. This may be important because some surgeons prefer to perform sentinel node biopsy in high-grade DCIS lesions.
Surgical excision was recommended for all patients with the diagnosis of atypia after stereotactic VABB. Underestimation rates are lower for VABB than for core needle biopsy (11%–35% vs. 44%–56%, respectively) (11). Although upgrade to malignancy is lower compared to core biopsy, it is still possible even if all mammographic calcifications have been removed. In the literature, upgrade rate ranges from 0% to 17% (35). Kohr et al. (36) found that there were no significant differences in upgrade rates based on whether the determinant mammographic calcifications were completely removed at stereotactic VABB. However, they found that upgrade to DCIS or invasive carcinoma was significantly less likely when ADH involved <3 foci than when it involved ≥3 foci. Villa et al. (35) reviewed the data from nine different studies and concluded that in experienced centers, patients with no residual calcifications can be conservatively managed with six-month mammographic follow-up and then with an annual mammographic follow-up, with an overall underestimation rate under 2%. In our study, of 16 patients with ADH, one was upgraded to DCIS. Although we recommended excision for all of them, five patients with ADH, whose calcifications were totally excised, did not undergo surgical excision because their surgeons did not want them to. During the 2–3 years of follow-up, no interval changes were detected in these patients. FEA is recognized as a precursor of breast cancer, and its management (surgical excision or intensive follow-up) remains unclear after diagnosis on core needle biopsy. In some series, the upgrade rate of FEA is as high as 10% (37). Lavoué et al. (38) indicate that the presence of FEA on core needle biopsy, even in isolation, warrants follow-up excision. However, some recent studies report that women with FEA without residual microcalcifications after VABB can be conservatively managed. Villa et al. (39) note that 9G VABB is associated with a lower percentage of residual microcalcifications than a 11G device, but it is safe to follow patients with FEA if all calcifications are removed. In our study, one of three patients with pure flat atypia was upgraded to high-grade DCIS, and the upgrade ratio among all atypical lesions was 10.5%, which was higher than the upgrade ratio of DCIS. Fifteen of 19 patients who underwent surgery (78.9%) had findings of atypia in the final pathology report. We believe that surgical excision should be performed in all patients with atypia at biopsy.
There were some limitations in our study. One of them was the low number of patients. Although there are numerous publications about VABB with a large series of patients, our biopsy method is slightly different from the conventional technique. The relatively low number of patients limited the statistical powering of our study. Another limitation is the lack of surgery in some atypical lesions. Although we recommended excision for all atypical lesions, some referring surgeons chose follow-up for lesions that were completely excised, probably in line with the patient’s wishes. However, conservative management by follow-up is not standard for atypical lesions, and we can still encounter malignancy in these patients in the following years. Another limitation was the learning curve. We started performing vacuum-assisted biopsy with the conventional method, and after a short period of time, we realized that starting with and taking more samples from the position where the microcalcifications were actually located allowed us to take out more representative samples in a shorter period of time, with almost no discomfort for the patient. However, we did not compare the actual number of specimens or the procedure time between the two methods.
In conclusion, VABB is a very accurate diagnostic technique with no major complications. In experienced hands it can be performed rapidly and efficiently; it is also very well tolerated by patients. Determining the position of the lesion in relation to the needle on postfire images and taking more samples from that position enable a faster procedure and increase patient tolerance without compromising the accuracy. Patients with a benign diagnosis can be safely followed up without any surgical interventions, while those with a malignant diagnosis benefit from a less invasive diagnostic technique that can expedite definitive treatment. Although VABB is more accurate than core needle biopsy and the likelihood of upgrade at surgery is low, patients with a diagnosis of atypia still need to undergo a diagnostic surgical procedure, even if the lesion is totally excised at biopsy. Radiologists should be aware that VABB may leave a scar in the breast tissue, which may rarely resemble a malignancy.
Main points.
We introduce a different biopsy technique, where the location of the lesion relative to the needle is determined from the postfire images and tissue retrieval is performed predominantly from that location, followed by a complete 360° rotation if needed.
Scar formation can be seen after VABB, especially on follow-up US examinations, and marked scars that resemble malignancy may be encountered, albeit rarely.
Lesions with less than 15 microcalcifications and those that are smaller than 1 cm were significantly more likely to be excised totally by VABB.
Although VABB frequently allows complete removal of the mammographic lesions, 60% of patients with complete lesion removal still had residual disease at surgery in our study.
In our study, total upgrade rate at surgery (7.69%) was lower than the rate reported in the literature, probably due to our method of biopsy. Nevertheless, diagnosis of atypia at VABB warrants excisional biopsy.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Parker SH, Jobe WE, Dennis MA, et al. US-guided automated large-core breast biopsy. Radiology. 1993;187:507–511. doi: 10.1148/radiology.187.2.8475299. http://dx.doi.org/10.1148/radiology.187.2.8475299. [DOI] [PubMed] [Google Scholar]
- 2.Liberman L, Feng TL, Dershaw DD, et al. US-guided core breast biopsy: use and cost-effectiveness. Radiology. 1998;208:717–723. doi: 10.1148/radiology.208.3.9722851. http://dx.doi.org/10.1148/radiology.208.3.9722851. [DOI] [PubMed] [Google Scholar]
- 3.Smith DN, Rosenfield Darling ML, Meyer JE, et al. The utility of ultrasonographically guided large-core needle biopsy: results from 500 consecutive breast biopsies. J Ultrasound Med. 2001;20:43–49. doi: 10.7863/jum.2001.20.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Pfarl G, Helbich TH, Riedl CC, et al. Stereotactic 11-gauge vacuum-assisted breast biopsy: a validation study. AJR Am J Roentgenol. 2002;179:1503–1507. doi: 10.2214/ajr.179.6.1791503. http://dx.doi.org/10.2214/ajr.179.6.1791503. [DOI] [PubMed] [Google Scholar]
- 5.Sigal-Zafrani B, Muller K, El Khoury C, et al. Vacuum-assisted large-core needle biopsy (VLNB) improves the management of patients with breast microcalcifications: analysis of 1009 cases. Eur J Surg Oncol. 2008;34:377–381. doi: 10.1016/j.ejso.2007.05.006. http://dx.doi.org/10.1016/j.ejso.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Burbank F, Parker SH, Fogarty TJ. Stereotactic breast biopsy: improved tissue harvesting with the Mammotome. Am Surg. 1996;62:738–744. [PubMed] [Google Scholar]
- 7.Liberman L, Smolkin JH, Dershaw DD, et al. Calcification retrieval at stereotactic, 11-gauge, directional vacuum-assisted breast biopsy. Radiology. 1998;208:251–260. doi: 10.1148/radiology.208.1.9646821. http://dx.doi.org/10.1148/radiology.208.1.9646821. [DOI] [PubMed] [Google Scholar]
- 8.Meyer JE, Smith DN, Di Piro PJ, et al. Stereotactic breast biopsy of clustered microcalcifications with a directional vacuum-assisted device. Radiology. 1997;204:575–576. doi: 10.1148/radiology.204.2.9240556. http://dx.doi.org/10.1148/radiology.204.2.9240556. [DOI] [PubMed] [Google Scholar]
- 9.Jackman RJ, Burbank F, Parker SH, et al. Atypical ductal hyperplasia diagnosed at stereotactic breast biopsy: improved reliability with a 14-gauge directional vacuum biopsy. Radiology. 1997;204:485–488. doi: 10.1148/radiology.204.2.9240540. http://dx.doi.org/10.1148/radiology.204.2.9240540. [DOI] [PubMed] [Google Scholar]
- 10.Jackman RJ, Burbank F, Parker SH, et al. Stereotactic breast biopsy of nonpalpable lesions: determinants of ductal carcinoma in situ underestimation rates. Radiology. 2001;218:497–502. doi: 10.1148/radiology.218.2.r01fe35497. http://dx.doi.org/10.1148/radiology.218.2.r01fe35497. [DOI] [PubMed] [Google Scholar]
- 11.Kettritz U, Rotter K, Schreer I, et al. Stereotactic vacuum assisted breast biopsy in 2874 patients: a multicenter study. Cancer. 2004;100:245–251. doi: 10.1002/cncr.11887. http://dx.doi.org/10.1002/cncr.11887. [DOI] [PubMed] [Google Scholar]
- 12.Liberman L, Kaplan JB, Morris EA, et al. To excise or to sample the mammographic target: what is the goal of stereotactic 11-gauge-vacuum-assisted breast biopsy? AJR Am J Roentgenol. 2002;179:679–683. doi: 10.2214/ajr.179.3.1790679. http://dx.doi.org/10.2214/ajr.179.3.1790679. [DOI] [PubMed] [Google Scholar]
- 13.Liberman L, Gougoutas CA, Zakowski MF, et al. Calcifications highly suggestive of malignancy comparison of breast biopsy methods. AJR Am J Roentgenol. 2001;177:165–172. doi: 10.2214/ajr.177.1.1770165. http://dx.doi.org/10.2214/ajr.177.1.1770165. [DOI] [PubMed] [Google Scholar]
- 14.ACR BI-RADS Atlas. 5th ed. [Accessed February 4, 2014]. available at: http://www.acr.org/Quality-Safety/Resources/
- 15.Gill HK, Ioffe OB, Berg WA. When is a diagnosis of sclerosing adenosis acceptable at core biopsy? Radiology. 2003;228:50–57. doi: 10.1148/radiol.2281020447. http://dx.doi.org/10.1148/radiol.2281020447. [DOI] [PubMed] [Google Scholar]
- 16.Cangiarella J, Waisman J, Symmans WF, et al. Mammotome core biopsy for mammary microcalcifications: analysis of 160 biopsies from 142 women with surgical and radiologic follow-up. Cancer. 2001;91:173–177. doi: 10.1002/1097-0142(20010101)91:1<173::aid-cncr22>3.0.co;2-9. http://dx.doi.org/10.1002/1097-0142(20010101)91:1<173::AID-CNCR22>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 17.This reference appears to belong to an old abstract. Please replace it with a current abstract (no older than 2 years) or an original article.
- 18.Reynolds HE, Poon CM, Goulet RJ, et al. Biopsy of breast microcalcifications using an 11-gauge directional vacuum-assisted device. AJR Am J Roentgenol. 1998;171:611–613. doi: 10.2214/ajr.171.3.9725283. http://dx.doi.org/10.2214/ajr.171.3.9725283. [DOI] [PubMed] [Google Scholar]
- 19.Meyer JE, Smith DN, Lester SC, et al. Large-core needle biopsy of nonpalpable breast lesions. JAMA. 1999;281:1638–1641. doi: 10.1001/jama.281.17.1638. http://dx.doi.org/10.1001/jama.281.17.1638. [DOI] [PubMed] [Google Scholar]
- 20.Jackman RJ, Rodriguez-Soto J. Breast microcalcifications: retrieval failure at prone stereotactic core and vacuum breast biopsy—frequency, causes, and outcome. Radiology. 2006;239:61–70. doi: 10.1148/radiol.2383041953. http://dx.doi.org/10.1148/radiol.2383041953. [DOI] [PubMed] [Google Scholar]
- 21.Liberman L, Hann LE, Dershaw DD, et al. Mammographic findings after stereotactic 14-gauge vacuum-assisted biopsy. Radiology. 1997;203:343–347. doi: 10.1148/radiology.203.2.9114086. http://dx.doi.org/10.1148/radiology.203.2.9114086. [DOI] [PubMed] [Google Scholar]
- 22.Penco S, Rizzo S, Bozzini AC, et al. Stereotactic vacuum-assisted breast biopsy is not a therapeutic procedure even when all mammographically found calcifications are removed: analysis of 4,086 procedures. AJR Am J Roentgenol. 2010;195:1255–1260. doi: 10.2214/AJR.10.4208. http://dx.doi.org/10.2214/AJR.10.4208. [DOI] [PubMed] [Google Scholar]
- 23.Liberman L, Kaplan JB, Morris EA, et al. To excise or to sample the mammographic target: what is the goal of stereotactic 11-gauge vacuum-assisted breast biopsy? AJR Am J Roentgenol. 2002;179:679–683. doi: 10.2214/ajr.179.3.1790679. http://dx.doi.org/10.2214/ajr.179.3.1790679. [DOI] [PubMed] [Google Scholar]
- 24.Margolin FR, Kaufman L, Jacobs RP, et al. Stereotactic core breast biopsy of malignant calcifications: diagnostic yield of cores with and cores without calcifications on specimen radiographs. Radiology. 2004;233:251–254. doi: 10.1148/radiol.2331031680. http://dx.doi.org/10.1148/radiol.2331031680. [DOI] [PubMed] [Google Scholar]
- 25.Gümüş H, Mills P, Fish D, Gümüş M, et al. Breast microcalcification: diagnostic value of calcified and non-calcified cores on specimen radiographs. Breast J. 2013;19:156–161. doi: 10.1111/tbj.12069. http://dx.doi.org/10.1111/tbj.12069. [DOI] [PubMed] [Google Scholar]
- 26.Zagouri F, Sergentanis TN, Nonni A, et al. Vacuum-assisted breast biopsy: the value and limitations of cores with microcalcifications. Pathol Res Pract. 2007;203:563–566. doi: 10.1016/j.prp.2007.05.001. http://dx.doi.org/10.1016/j.prp.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Gümüş H, Gümüş M, Devalia H, et al. Causes of failure in removing calcium in microcalcification-only lesions using 11-gauge stereotactic vacuum-assisted breast biopsy. Diagn Interv Radiol. 2012;18:354–359. doi: 10.4261/1305-3825.DIR.5024-11.1. [DOI] [PubMed] [Google Scholar]
- 28.Lomoschitz FM, Helbich TH, Rudas M, et al. Stereotactic 11-gauge vacuum-assisted breast biopsy: influence of number of specimens on diagnostic accuracy. Radiology. 2004;232:897–903. doi: 10.1148/radiol.2323031224. http://dx.doi.org/10.1148/radiol.2323031224. [DOI] [PubMed] [Google Scholar]
- 29.Bruening W, Fontanarosa J, Tipton K, et al. Systematic review: comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions. Ann Intern Med. 2010;152:238–246. doi: 10.7326/0003-4819-152-1-201001050-00190. http://dx.doi.org/10.7326/0003-4819-152-1-201001050-00190. [DOI] [PubMed] [Google Scholar]
- 30.Lamm RL, Jackman RJ. Mammographic abnormalities caused by percutaneous stereotactic biopsy of histologically benign lesions evident on follow-up mammograms. AJR Am J Roentgenol. 2000;174:753–756. doi: 10.2214/ajr.174.3.1740753. http://dx.doi.org/10.2214/ajr.174.3.1740753. [DOI] [PubMed] [Google Scholar]
- 31.Yazici B, Sever AR, Mills P, et al. Scar formation after stereotactic vacuum-assisted core biopsy of benign breast lesions. Clin Radiol. 2006;61:619–624. doi: 10.1016/j.crad.2006.03.008. http://dx.doi.org/10.1016/j.crad.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Bagnall MJ. Predicting invasion in mammographically detected microcalcification. Clin Radiol. 2001;56:828–832. doi: 10.1053/crad.2001.0779. http://dx.doi.org/10.1053/crad.2001.0779. [DOI] [PubMed] [Google Scholar]
- 33.O’Flynn EA, Morel JC, Gonzalez J, et al. Prediction of the presence of invasive disease from the measurement of extent of malignant microcalcification on mammography and ductal carcinoma in situ grade at core biopsy. Clin Radiol. 2009;64:178–183. doi: 10.1016/j.crad.2008.08.007. http://dx.doi.org/10.1016/j.crad.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 34.NHS Breast Screening Programme & Association of Breast Surgery at BASO. An audit of screen-detected breast cancers for the year of screening April 2008 to March 2009. 2010. [Accessed January 20, 2010]. Available at: http://www.cancerscreening.nhs.uk/index.html.
- 35.Villa A, Tagliafico A, Chiesa F, et al. Atypical ductal hyperplasia diagnosed at 11-gauge vacuum-assisted breast biopsy performed on suspicious clustered microcalcifications: could patients without residual microcalcifications be managed conservatively? AJR Am J Roentgenol. 2011;197:1012–1018. doi: 10.2214/AJR.11.6588. http://dx.doi.org/10.2214/AJR.11.6588. [DOI] [PubMed] [Google Scholar]
- 36.Kohr JR, Eby PR, Allison KH, et al. Risk of upgrade of atypical ductal hyperplasia after stereotactic breast biopsy: effects of number of foci and complete removal of calcifications. Radiology. 2010;255:723–730. doi: 10.1148/radiol.09091406. http://dx.doi.org/10.1148/radiol.09091406. [DOI] [PubMed] [Google Scholar]
- 37.Khoumais NA, Scaranelo AM, Moshonov H, et al. Incidence of breast cancer in patients with pure flat epithelial atypia diagnosed at core-needle biopsy of the breast. Ann Surg Oncol. 2013;20:133–138. doi: 10.1245/s10434-012-2591-0. http://dx.doi.org/10.1245/s10434-012-2591-0. [DOI] [PubMed] [Google Scholar]
- 38.Lavoué V, Roger CM, Poilblanc M, et al. Pure flat epithelial atypia (DIN 1a) on core needle biopsy: study of 60 biopsies with follow-up surgical excision. Breast Cancer Res Treat. 2011;125:121–126. doi: 10.1007/s10549-010-1208-1. http://dx.doi.org/10.1007/s10549-010-1208-1. [DOI] [PubMed] [Google Scholar]
- 39.Villa A, Chiesa F, Massa T, et al. Flat epithelial atypia: comparison between 9-gauge and 11-gauge devices. Clin Breast Cancer. 2013;13:450–454. doi: 10.1016/j.clbc.2013.08.008. http://dx.doi.org/10.1016/j.clbc.2013.08.008. [DOI] [PubMed] [Google Scholar]