Abstract
We performed this study to longitudinally compare rates of stunting, wasting and underweight among low birthweight (LBW), non-LBW, and/or small-for-gestational age (SGA) and non-SGA infants in Leyte, The Philippines and factors that predicted catch up. Birthweights of 357 infants born in Leyte, The Philippines were obtained within 48 hours of delivery and infants were evaluated at one, six and 12 months. Newborns were classified as LBW, SGA, or both. We derived length-for-age, weight-for-length and weight-for-age Z-scores using WHOAnthro. Generalized estimating equations models were used to compare the differences in prevalence and mean Z-scores for these growth and nutritional outcomes, with separate models made with LBW and SGA as distinct primary predictors. We compared the longitudinal risk of stunting, wasting and underweight during infancy among LBW versus non-LBW and SGA versus non-SGA infants, while also evaluating key potential confounding, explanatory and modifying covariates. Overall, 9.0% of infants were born prematurely, 14.0% of infants were LBW and 22.9% were SGA. LBW infants had significantly increased odds of stunting, wasting and underweight persisting to 12 months of age, and SGA infants had significantly increased odds of stunting and underweight. LBW and SGA infants had higher rates of weight-for-length gain in the first month of life. Maternal educational attainment and exclusive breastfeeding decreased the risk of stunting and undernutrition. In this setting, LBW and SGA infants have higher rates of growth stunting and undernutrition during the first year of life and do not exhibit catch-up growth by 12 months of age.
Clinical Trial Registration
Introduction
In low resource settings, undernutrition during infancy is thought to greatly increase the risk of infant and early childhood mortality [1]. Poor growth during infancy has been shown to result in increased risk of short stature among adults, which is associated with decreased work productivity and higher rates of adverse birth outcomes for women of reproductive age [2].
Studies have demonstrated that low birthweight (LBW) and small-for-gestational age (SGA) are risk factors for both linear growth stunting and undernutrition among young children [3]. Specifically, prospective studies conducted in Cebu, The Philippines demonstrated that LBW infants are at higher risk of stunting for the first two years of life than normal birthweight infants, with the greatest effect during the first year of life [3]. LBW has additionally been associated with other adverse health outcomes during infancy and adulthood in low, middle and high-income nations [4–8].
The prevalence of LBW deliveries ranges from 12–25% in low-income nations compared to 7% in higher-income nations. In affluent populations, most LBW infants are born premature, while in low-income countries the majority are full-term infants who have experienced growth restriction in utero, often culminating in an SGA newborn [6, 9, 10]. Thus, understanding the post-natal growth consequences of SGA and factors modifying this relationship is of great importance in the low and middle income country (LMIC) context.
A significant challenge to our understanding of the influence of SGA on post-natal morbidity and growth trajectories is that these newborns represent a heterogeneous group. Specifically, most studies define SGA as a birthweight that is less than the 10th percentile of a healthy reference curve for the newborn’s sex and gestational age (GA). SGA may occur, however, due to the pathologic process of intrauterine growth restriction (IUGR), whereby a fetus does not reach its in utero growth potential, or as a result of normal variability whereby a fetus achieves its in utero growth potential, which is constitutionally small. The availability of the International Fetal and Newborn Growth Consortium for the 21st Century standard (INTERGROWTH-21th) to determine SGA, rather than reliance on United States derived reference curves, has been demonstrated to decrease the percentage of newborns categorized as SGA [11, 12]. This approach may better capture SGA that is due to a pathologic process in the LMIC setting.
The primary objectives of this study were to compare rates of stunting, wasting and underweight at one, six and 12-months-old among LBW, non-LBW, SGA and non-SGA infants born in Leyte, The Philippines. Additional objectives included i) assessment of differences in growth velocity across birthweight status groups during specific age “windows,” ii) determining the timing of growth and nutritional catch-up where this occurred, and iii) elucidating other risk factors (mode of feeding, maternal nutritional status and educational attainment) for growth faltering and undernutrition during infancy. Quantification of these relationships may further emphasize the need for prenatal interventions to reduce the risk of LBW and SGA births, as well as post-natal interventions to optimize catch-up growth and nutrition in resource poor settings.
Methods
Study Population
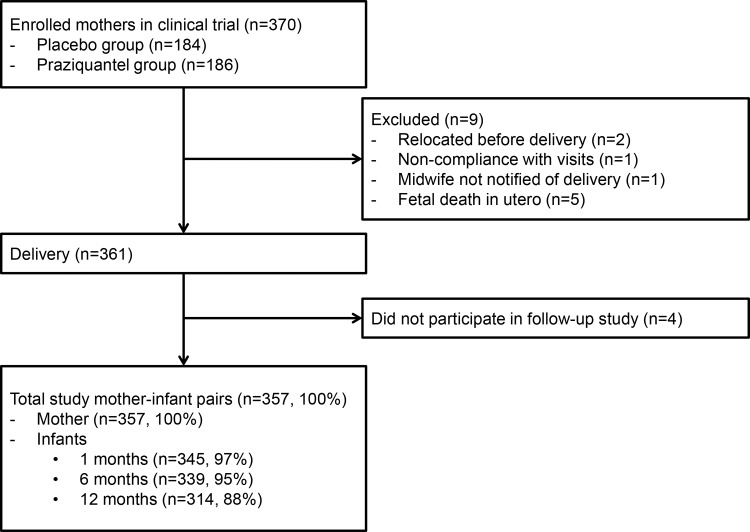
This study utilizes data collected as part of an NIH-funded double blind randomized controlled trial (RCT) of Praziquantel given at 12–16 weeks gestation (ClinicalTrials.gov, NCT00486863) and a Thrasher Fund supported study of the infants born to these women. The RCT enrolled 370 otherwise healthy pregnant women from rice farming villages in northeastern Leyte, The Philippines with singleton pregnancies who were infected with Schistosomiasis japonicum as described [13]. As part of screening procedures for enrollment, women underwent a transabdominal ultrasound to determine GA, viability of fetus and singleton pregnancy. Maternal anthropometric measures were also made in the first-trimester and used for statistical analyses. Women were deemed healthy and eligible to participate based on history, physical examination, and laboratory studies. Women were randomized 1:1 to placebo or Praziquantel. All women were provided with prenatal vitamins with iron at enrollment and reported compliance was 99.7%. Malaria is not endemic and the prevalence of HIV is <0.1% [14]. Of note, Praziquantel did not significantly impact birthweight or risk of LBW or SGA (manuscript in review), such that this was not included in these post-natal analyses. All newborn live births (n = 357) were eligible to participate (Fig 1). At the time of close out from the NIH trial, when the newborn was 28 days of age, mothers were asked to enroll in this separate follow-up study of their infants.
Fig 1. Flow of participants.
Newborn Measures
As part of the study protocol, all mothers gave birth at a municipal health center or were referred to Remedios Trinidad Romualdez Hospital if indicated. Newborns were weighed within 48 hours of delivery on a Tanita model BD-585 portable scale (Arlington Heights, MD) accurate to 10g, and all birthweights with the exception of one were obtained within 24 hours. LBW was defined as weight <2.5 kg and prematurity as birth <37 weeks gestation. SGA was defined as birthweight <10th percentile for GA using the INTERGROWTH-21th [11]. Ultrasound derived GA was used to determine prematurity and SGA status.
Infant Follow-Up
Infants were assessed at RTR hospital at one, six and 12-months-old. They were seen by the study pediatrician who conducted a history, physical examination and assessed length, weight and head circumference. Recumbent length was measured using a pediatric stadiometer (Ellards Instrumentation LTD, Monroe, WA) as per Gibson [15]. WHOAnthro was used to derive length-for-age (LAZ), weight-for-length (WLZ) and weight-for-age (WAZ) Z-scores at one, six and 12-months-old [15, 16]. Stunting, wasting and underweight were defined as LAZ, WLZ or WAZ <-2.0, respectively.
Assessment of Potential Confounders and Modifying Covariates
Maternal educational status was defined as having attained a high school degree or greater level of education versus less than high school completion. At the one, six and 12-month follow-up visits, mothers were asked about infant feeding practices, which were categorized as exclusive breastfeeding, bottle feeding or mixed.
Statistical Analyses
Group differences (LBW vs. non-LBW; SGA vs. non-SGA) were determined by using a Student’s t test or Wilcoxon rank-sum test for continuous variables and Fisher’s exact or Chi-square test for categorical variables. To assess the effects of LBW and SGA on LAZ, WLZ, and WAZ and length, weight-for-length, and weight gains, as well as the probability of stunting, wasting and underweight, we implemented generalized estimating equations (GEE) models with an exchangeable correlation structure and robust standard error estimation. This approach was used to compare differences in growth and nutritional parameters longitudinally during infancy, comparing infants born in distinct birthweight categories while adjusting for within-subject correlation. These models capture distinct outcomes (eg, stunting) at three timepoints for each infants and relate each of these outcomes to time varying predictors at the corresponding timepoint, as well as non-time varying covariates such as sex. Finally, we employed GEE models to assess the effects of LBW and SGA on absolute gains in length and weight as well as weight for weight-for-length in the first year of life.
GEE models were also used to identify other risk factors for adverse growth and nutritional outcomes as well as effect modifiers of the relationship between birthweight category and risk of stunting, wasting and underweight throughout infancy. Distinct models with LBW or SGA as the primary predictor were evaluated for each of the nutritional outcome measures, as we could not include the non-independent variables of SGA/LBW in the same models. LBW and SGA status (in distinct models) and potential confounders with P values <0.2 in the univariable models were considered for inclusion in multivariable models. Multivariable models for stunting, wasting and underweight were built by performing a backward elimination process until only significant variables remained.
All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC). P values <0.05 were considered to be significant, except in univariable analyses.
Ethical Considerations
Infants with an acute or chronic medical condition or malnutrition diagnosed during the newborn period or during the infant follow-up study were referred for care. The pregnancy trial and the infant follow-up studies were separately approved by both the Rhode Island Hospital Institutional Review Board in Providence, RI and the Ethics Review Board of the Research Institute of Tropical Medicine in Manila, The Philippines. All maternal participants of the study provided written informed consent approved by both review boards.
Results
Of 370 pregnant women enrolled, there were five fetal deaths in utero. Birthweight was ascertained for 361 of 365 live births and 357 infants enrolled in the infant follow up study. Of these, 14.0% were LBW and 22.9% were SGA (Table 1). Approximately 80.0% of LBW infants were SGA and 48.8% of SGA infants were LBW. There were 32 infants (9.0%) who were premature. Importantly, among the LBW deliveries, only 12 (24%) were premature, such that most LBW was due to IUGR, rather than prematurity. Given the relatively small number of newborns who were premature-SGA (n = 7) or premature-LBW (n = 12), we did not analyze these sub-groups separately. Between one and six-months-old, there were significantly more non-LBW infants (93.2%) who were exclusively breastfed than LBW infants (84.0%).
Table 1. Basic descriptive data by birthweight and size for gestational agea.
| Covariate | Birthweight | Size for gestational age | ||||
|---|---|---|---|---|---|---|
| Low birthweightb (n = 50) | Non-low birthweight (n = 307) | P valuec | Small-for-gestational aged (n = 82) | Non-small-for-gestational age (n = 275) | P value | |
| Birth data | ||||||
| Female, % | 44.0 (30.2–57.8) | 47.2 (41.6–52.8) | 0.67 | 40.2 (29.6–50.8) | 48.7 (42.8–54.6) | 0.17 |
| Length, cm | 44.9 (44.0–45.8) | 47.2 (46.8–47.5) | <0.001 | 46.0 (45.4–46.7) | 47.1 (46.7–47.5) | <0.001 |
| Weight-for-length | 0.049 (0.047–0.051) | 0.063 (0.062–0.064) | <0.001 | 0.053 (0.052–0.055) | 0.063 (0.062–0.064) | <0.001 |
| Weight, kg | 2.19 (2.09–2.28) | 2.96 (2.93–3.00) | <0.001 | 2.45 (2.38–2.51) | 2.98 (2.93–3.02) | <0.001 |
| Gestational age, wk | 37.5 (36.8–38.2) | 38.7 (38.6–38.8) | <0.001 | 39.0 (38.7–39.3) | 38.4 (38.2–38.6) | <0.001 |
| Small-for-gestational age, % | 80.0 (68.9–91.1) | 20.0 (15.5–24.5) | <0.001 | |||
| Low birthweight, % | 48.8 (38.0–59.6) | 3.6 (1.4–5.8) | <0.001 | |||
| Feeding | ||||||
| Exclusively breastfeeding, % | ||||||
| Birth to 1month | 90.0 (81.7–98.3) | 94.5 (91.9–97.1) | 0.21 | 90.2 (83.8–96.6) | 94.9 (92.3–97.5) | 0.12 |
| 1 to 6 months | 84.0 (73.8–94.2) | 93.2 (90.4–96.0) | 0.045 | 86.6 (79.2–94.0) | 93.5 (90.6–96.4) | 0.046 |
| 6 to 12 months | 82.0 (71.4–92.6) | 90.6 (87.3–93.9) | 0.07 | 85.4 (77.8–93.0) | 90.6 (87.2–94.0) | 0.18 |
| First-trimester maternal data | ||||||
| Age, y | 25.3 (23.2–27.4) | 26.2 (25.5–26.9) | 0.19 | 26.0 (24.5–27.6) | 26.1 (25.3–26.8) | 0.76 |
| Parity, no | 3.1 (2.5–3.8) | 3.7 (3.4–3.9) | 0.027 | 3.4 (3.0–3.9) | 3.7 (3.4–3.9) | 0.31 |
| Height, cm | 146.6 (145.2–148.0) | 147.6 (147.0–148.2) | 0.26 | 146.1 (144.9–147.3) | 147.9 (147.2–148.5) | 0.011 |
| Body mass index, kg/m2 | 21.7 (20.8–22.5) | 21.9 (21.6–22.3) | 0.56 | 21.9 (21.3–22.5) | 21.9 (21.5–22.2) | 0.77 |
| Weight, kg | 46.6 (44.7–48.5) | 47.7 (46.9–48.5) | 0.28 | 46.7 (45.3–48.0) | 47.8 (46.9–48.7) | 0.34 |
| Education (≥ high school) | 66.0 (52.9, 79.1) | 56.4 (50.9, 62.0) | 0.20 | 57.3 (46.6–68.0) | 57.8 (52.0–63.6) | 0.94 |
aValues are means or proportions (95% confidence intervals), n = 357.
bDefined as birthweight <2.5kg.
cTested by Student’s t test or Wilcoxon’s rank-sum test for continuous variables and Fisher’s exact or Chi-square test for categorical variables.
dDefined as birthweight <10th percentile for gestational age.
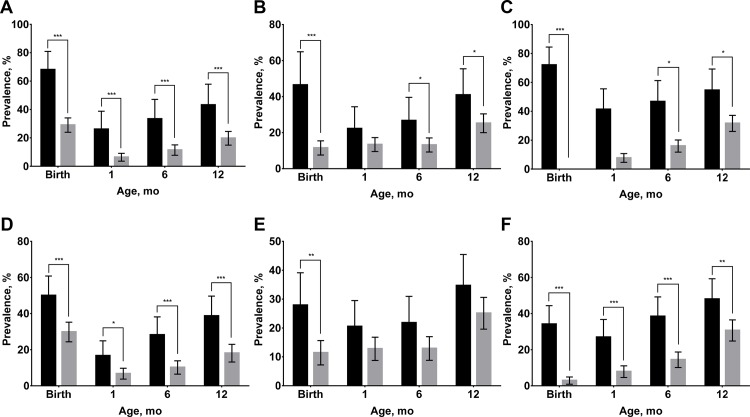
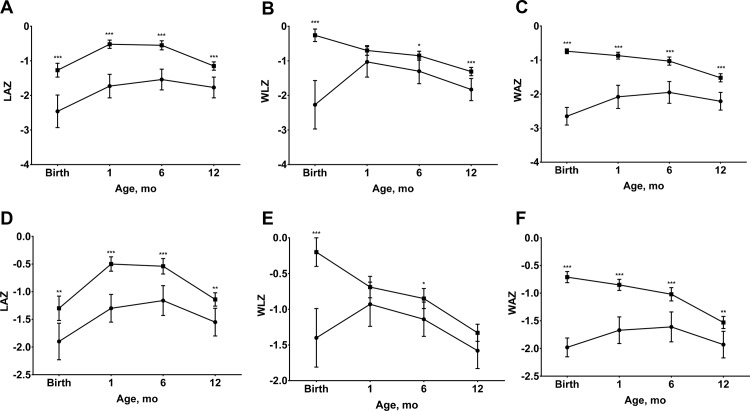
At each age, significantly more LBW infants remained stunted and underweight compared to non-LBW infants and the same patterns were observed among SGA and non-SGA infants (Fig 2). Significantly more LBW infants were wasted at birth, six and 12-months-old compared to non-LBW infants, however, the only significant difference in the prevalence of wasting between SGA and non-SGA infants was observed at birth. Similarly, significant differences were found at most timepoints for mean LAZ and WAZ between LBW and non-LBW infants and between SGA and non-SGA infants (Fig 3). The only exception was the convergence of mean LAZ for SGA and non-SGA infants by 12-months-old (Fig 3D). Non-LBW infants had significantly higher WLZ than LBW infants at birth, six and 12-months-old, while differences in WLZ between SGA and non-SGA infants were found at birth and six-months-old.
Fig 2.
Stunting, wasting, and underweight from birth to 12 months of age by birthweight (A, stunting; B, wasting; C, underweight) and size for gestational age (D, stunting; E, wasting; F, underweight). Values are prevalence and 95% confidence intervals, n = 357. In A, B, and C, black bars indicate low birthweight; gray bars indicate non-low birthweight. In D, E, and F, black bars indicate small-for-gestational age; gray bars indicate non-small-for-gestational age. Stunting, wasting, and underweight were defined as height-for-age Z score <-2.0, weight-for-height Z score <-2.0 and weight-for-age Z score <-2.0, respectively. Low birthweight was defined as birthweight <2.5kg. Small-for-gestational age was defined as birthweight <10th percentile for gestational age. *P <0.05, ** P <0.01, *** P <0.001 different from low birthweight or small-for-gestational age group.
Fig 3.
Length-for-age (LAZ), weight-for-length (WLZ) and weight-for-age (WAZ) z scores from birth to 12 months of age by birthweight (A, LAZ; B, WLZ; C, WAZ) and size for gestational age (D, LAZ; E, WLZ; F, WAZ). Values are means and 95% confidence intervals, n = 357. In A, B, and C, square symbols indicate low birthweight; circle symbols indicate non-low birthweight. In D, E, and F, square symbols indicate small-for-gestational age; circle symbols indicate non-small-for-gestational age. Low birthweight was defined as birthweight <2.5kg. Small-for-gestational age was defined as birthweight <10th percentile for gestational age. *P <0.05, ** P <0.01, *** P <0.001 different from low birthweight or small-for-gestational age group.
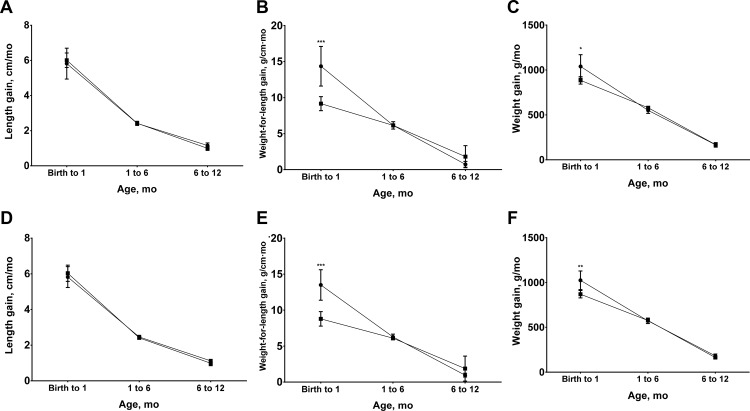
The significantly higher weight-for-length gains of LBW and SGA infants between birth and one month of life (Fig 4) diminished differences in the prevalence of wasting (Fig 2B and 2E) and reduced differences in WLZ after one-month-old (Fig 3B and 3E). Significant, but small differences in weight gain in the neonatal period, however, did not fully mitigate the significant differences in the prevalence of underweight infants (Fig 2C and 2F) among LBW and SGA newborns and differences in WAZ (Fig 3C and 3F). This is likely due to the fact that WAZ captures both linear growth faltering and wasting. Finally, neither LBW nor SGA infants experienced higher linear growth velocities (Fig 4A and 4D), such that infants in these disadvantaged birth categories had persistently higher prevalence of stunting and lower mean HLZ throughout infancy (Figs 2 and 3A and 3B).
Fig 4.
Length, weight-for-length and weight gains from birth to 12 months of age by birthweight (A, length gain; B, weight-for-length gain; C, weight gain) and size for gestational age (D, length gain; E, weight-for-length gain; F, weight gain). Values are means and 95% confidence intervals, n = 357. In A, B, and C, square symbols indicate low birthweight; circle symbols indicate non-low birthweight. In D, E, and F, square symbols indicate small-for-gestational age; circle symbols indicate non-small-for-gestational age. Low birthweight was defined as birthweight <2.5kg. Small-for-gestational age was defined as birthweight <10th percentile for gestational age. *P <0.05, ** P <0.01, *** P <0.001 different from non-LBW or non-SGA group.
In the univariable GEE models, the odds of stunting throughout infancy were significantly increased when infants were male, LBW, SGA and bottle or mixed fed versus exclusively breastfed (Table 2). The odds of stunting were decreased when infants had a higher GA at birth and when mothers were taller. The probability of wasting was increased for infants who were LBW, SGA or had mothers who were older or had lower educational attainment. Underweight was significantly associated with the infant’s sex, GA and LBW and SGA status, as well as with maternal age, height and weight.
Table 2. Univariable generalized estimating equations models predicting stunting, wasting and underweight at three time points during infancya.
| Covariate | Reference | Stuntingb | Wastingc | Underweightd | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| Sex (male) | Female | 1.78 (1.12, 2.85) | 0.015 | 1.27 (0.88, 1.83) | 0.20 | 2.07 (1.37, 3.11) | <0.001 |
| Gestational age, wk | 0.69 (0.61, 0.79) | <0.001 | 0.98 (0.85, 1.14) | 0.81 | 0.70 (0.62, 0.80) | <0.001 | |
| Low birthweight (LBW)e | Non-LBW | 3.82 (2.29, 6.37) | <0.001 | 2.12 (1.35, 3.33) | 0.001 | 4.30 (2.58, 7.16) | <0.001 |
| Small for gestational age (SGA)f | Non-SGA | 2.98 (1.88, 4.72) | <0.001 | 1.73 (1.16, 2.58) | 0.008 | 2.90 (1.88, 4.47) | <0.001 |
| Feeding (breastfeeding or bottle formula) | Exclusively breastfeeding | 2.30 (1.38, 3.81) | 0.001 | 0.97 (0.53, 1.76) | 0.92 | 1.46 (0.86, 2.46) | 0.16 |
| Maternal age (≥30 y old) | <30 years old | 1.05 (0.65, 1.71) | 0.83 | 1.71 (1.18, 2.49) | 0.005 | 1.63 (1.09, 2.44) | 0.018 |
| Maternal height, cm | 0.95 (0.92, 0.99) | 0.007 | 0.99 (0.96, 1.03) | 0.59 | 0.95 (0.91, 0.99) | 0.008 | |
| Maternal body mass index, kg/m2 | 0.98 (0.91, 1.05) | 0.56 | 0.97 (0.91, 1.04) | 0.44 | 0.97 (0.91, 1.03) | 0.34 | |
| Maternal weight, kg | 0.97 (0.94, 1.00) | 0.035 | 0.98 (0.96, 1.01) | 0.22 | 0.96 (0.94, 0.99) | 0.008 | |
| Maternal education (<high school) | ≥high school | 1.16 (0.74, 1.80) | 0.52 | 1.50 (1.04, 2.16) | 0.031 | 1.43 (0.96, 2.12) | 0.08 |
aValues are odds ratios (ORs) [95% confidence intervals (CIs)], n = 357.
bDefined as length-for-age Z score <-2.0.
cDefined as weight-for-length Z score <-2.0.
dDefined as weight-for-age Z score <-2.0.
eDefined as birthweight <2.5kg.
fDefined as birthweight <10th percentile for gestational age.
In the multivariable GEE analyses (Table 3), the final model with LBW as the primary predictor for risk of stunting during infancy retained the following covariates; sex, GA, LBW, feeding type, and maternal height. The stunting model with SGA included sex, SGA, feeding type, and maternal height. The two distinct final models with LBW or SGA for the outcome of wasting included the same confounders, maternal age and maternal educational attainment. The model for underweight with LBW included sex, GA, LBW, and maternal age, while the SGA model included sex, and maternal age. Also of note, parity and maternal age were highly correlated such that we could not retain both in the final models. Parity, however, was significantly related to both wasting and underweight when maternal age was removed from the model (data not shown). There were no significant interactions between either birthweight category and other predictors.
Table 3. Multivariable generalized estimating equations models predicting stunting, wasting and underweight during infancya.
| Covariate | Reference | Stuntingb | Wastingc | Underweightd | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| Low birthweight (LBW) Model | |||||||
| Sex (male) | Female | 2.06 (1.24, 3.42) | 0.005 | 2.21 (1.41, 3.45) | <0.001 | ||
| Gestational age, wke | 0.72 (0.63, 0.83) | <0.001 | 0.74 (0.65, 0.85) | <0.001 | |||
| LBWf | Non-LBW | 2.64 (1.53, 4.53) | <0.001 | 2.33 (1.47, 3.70) | <0.001 | 3.76 (2.21, 6.40) | <0.001 |
| Feeding (breastfeeding or bottle formula) | Exclusively breastfeeding | 2.46 (1.44, 4.20) | 0.001 | ||||
| Maternal age (≥30 y old) | <30 years old | 1.73 (1.18, 2.52) | 0.005 | 1.88 (1.23, 2.86) | 0.003 | ||
| Maternal height, cm | 0.95 (0.91, 0.99) | 0.007 | |||||
| Maternal education (<high school) | ≥high school | 1.56 (1.08, 2.27) | 0.019 | 1.52 (1.01, 2.31) | 0.047 | ||
| Small for gestational age (SGA) Model | |||||||
| Sex (male) | Female | 1.81 (1.13, 2.89) | 0.014 | 2.01 (1.33, 3.04) | 0.001 | ||
| SGAg | Non-SGA | 2.60 (1.62, 4.19) | <0.001 | 1.75 (1.16, 2.64) | 0.007 | 2.82 (1.81, 4.37) | <0.001 |
| Feeding (breastfeeding or bottle formula) | Exclusively breastfeeding | 2.27 (1.36, 3.79) | 0.002 | ||||
| Maternal age (≥30 y old) | <30 years old | 1.70 (1.17, 2.48) | 0.006 | 1.73 (1.15, 2.61) | 0.009 | ||
| Maternal height, cm | 0.95 (0.92, 0.99) | 0.017 | |||||
| Maternal education (<high school) | 1.47 (1.02, 2.13) | 0.040 | |||||
aLBW and SGA models were built with low birthweight and small-for-gestational age as primary predictor respectively. Values are odds ratios (ORs) [95% confidence intervals (CIs)], n = 357. Maternal height was considered in the multivariable model for stunting, and maternal body mass index for wasting and underweight.
bDefined as length-for-age Z score <-2.0.
cDefined as weight-for-length Z score <-2.0.
dDefined as weight-for-age Z score <-2.0.
eEvaluated only for LBW model.
fBirthweight < 2.5 kg.
gDefined as birthweight <10th percentile for gestational age.
Discussion
Our results suggest that LBW and SGA infants in this setting do not catch up to non-LBW and non-SGA infants, even by age 12 months, with respect to most measures of linear growth and nutritional status. A slightly greater weight gain among both LBW and SGA infants was limited to the first month of life, explaining some convergence in WAZ and WLZ by one–month-old, but with little catch up thereafter. The lack of any significant differences in linear growth velocity during any window led to significant difference in risk of stunting and LAZ throughout infancy. This differential growth pattern is likely due to rapid soft tissue gain in the first few months of life, that did not, however, allow catch up growth [17]. These findings contrast those conducted in industrialized nations, where a large proportion of infants born SGA achieve weight and length catch up growth during infancy [18–20]. This is likely due to the availability of human milk fortifiers, the availability of formulas with higher kilocalories per ounce, and maternal nutritional status, with better nourished mothers providing greater volume of breast milk with higher fat content [21, 22]. In addition, early weaning to complementary foods with low protein and fat content, such as rice, may also hinder catch up growth.
The persistently increased odds of growth stunting and undernutrition in LBW and SGA infants that we observed are consistent with studies conducted in other resource-constrained settings in Africa and Asia [3, 23–26]. In a cohort study in Cebu, The Philippines, LBW was a predictor of stunting until at least two years of age [3]. In a separate study in Metro Cebu, LBW status increased the odds of stunting at six and 12-months-old [25]. A Tanzanian cohort found that newborns with birthweight <10th percentile had over twice the risk of stunting and 1.45 times the risk of wasting compared to the other newborns throughout the first 18 months of life [24].
Importantly, studies in the United States have shown that IUGR and SGA infants have lower nutritional Z-scores during infancy and early childhood than infants with the same birthweight who were born prematurely [9, 27–30]. This suggests that prematurity results in a less permanent growth impairment than IUGR, with the latter process beginning in utero [27]. These findings are especially relevant to infants in LMIC settings, where a greater proportion of LBW deliveries are consequent to IUGR than prematurity, though this is difficult to determine with certainty due to limitations of GA determination in this setting [9, 31]. Given this, and the fact that 80% of the LBW newborns in this cohort were SGA, in the LMIC setting LBW status likely captures a significant proportion of SGA newborns who are more easily identified and remain at risk.
In addition to LBW and SGA, our study identified other risk factors for stunting, wasting and underweight. Breastfeeding was shown to be a protective factor for decreasing odds of stunting among all infants regardless of birthweight status. This is consistent with the well-described benefits of exclusive breastfeeding in Filipino infants and other infants in LMIC settings [8, 25, 32–35]. This is likely due to poor nutritional value of supplementary foods in these regions and lack of clean water sources for formula, which increases the risk of diarrheal illness and other infections [36]. Importantly, already at risk LBW infants were somewhat less likely to be exclusively breastfed, which supports the findings of previous studies in Cebu [37].
Male sex was also a significant risk factor for stunting in LBW infants and for underweight in LBW and SGA infants. In previous studies in Filipino infants, males were more likely to become stunted in the first year of life, and females in the second year of life [3]. Males may be more susceptible to impairments in growth in early life due to their more rapid growth trajectory than girls over this period, which is likely the result of a sex-specific epigenetic process that begins in utero [18, 38, 39]. As expected, maternal height was a risk factor for stunting in both LBW and SGA infants. Similarly, previous studies in LMIC settings describe maternal short stature as a predictor for both LBW and stunting during infancy [40–43]. Maternal age (≥30 years) was a risk factor for wasting and underweight in both groups, yet not for stunting. By contrast, a recent study showed that extremes of low (<30 years) and high (>45 years) maternal age increases the risk of stunting during infancy [44]. Importantly, age may also be capturing the effects of parity, as we found that parity was significantly related to risk of wasting and underweight when maternal age, with which it was highly correlated, was removed from models. This is likely due to greater food insecurity for mothers and the infant participants in homes with more children,
Importantly, other studies of LBW infants in the Philippines have reported that postnatal growth patterns are significantly related to social economic status [25]. Our results show that lower maternal educational attainment was a risk factor for undernutrition during infancy, particularly wasting. This emphasizes the key role of maternal education in addition to the key public health messaging needed to encourage exclusive breast feeding, as suggested by other studies [8, 17].
Though previous studies have examined growth and nutritional outcomes in the LMIC setting, this study adds a determination of SGA that is more likely to be accurate based on the use of 12–16 week ultrasound for GA determination. The use of the INTERGROWTH-21th rather than Western-based reference curve for SGA also makes it more likely that these SGA infants experienced IUGR. In addition, the longitudinal design allowed for better assessment of causality in determining risk factors for undernutrition and growth faltering during infancy.
Limitations of this study include the fact that mothers and infants were from a rural region of The Philippines, which may limit generalizability. In addition, though we considered SGA births as newborns who likely experienced IUGR during pregnancy, it is possible that some of these newborns, in fact, reached their in utero growth potential and did not experience IUGR. As above, use of a healthy reference curve comprised of newborns from multiple different nations, including LMICs, mitigates this concern somewhat. With respect to etiology of SGA, limitations with respect to our ability to diagnose infections, particularly viral infections that might impact growth in utero, preclude identifying these common etiologies. In addition, the definition of pre-eclampsia employed at the time of study inception required two elevated blood pressures separated by four hours and most refused to wait. Though many women were diagnosed at delivery with pre-eclampsia based on elevated blood pressure, lack of application of standard definition did not allow us to determine the percent of newborns who were SGA due to this very common etiology. Finally, these infants were only followed until 12-months-old, however, additional studies of these children at five years of age will further elucidate long-term growth and nutritional catch up.
Though LBW and SGA infants in this setting exhibit increased weight and weight-for-length velocity in the first month of life, they remain at significantly higher risk of undernutrition and do not catch up to non-LBW and non-SGA infants by 12-months-old. Importantly, infants who were LBW were actually less likely to be exclusively breastfed at six-months-old. Lower maternal educational attainment continues to influence the risk of undernutrition in this setting among all infants, emphasizing the need for education regarding exclusive breastfeeding and health literacy in order to the decrease the risk of stunting and undernutrition in these high risk groups.
Acknowledgments
We thank our study staff and participants from the Philippines.
Data Availability
Data are from the efficacy and safety study of praziquantel (http://dx.doi.org/10.1016/ S1473-3099(15)00345-X). Both legal and ethical restrictions prohibit the authors from making the minimal data set publicly available. Given that the data used for this paper involve human subjects, data use will be considered for qualified researchers upon request and upon institutional review board approval from the research sites. Requests should be submitted to Jennifer F Friedman (jennifer_friedman@brown.edu).
Funding Statement
All phases of this study were supported by NIH/NIAID S. japonicum and pregnancy outcomes: An RCT (Grant #U01AI066050: https://www.niaid.nih.gov/researchfunding/grant/Pages/default.aspx), Thrasher Research Fund Infant hemoglobin, nutritional status and neuro-cognitive development following maternal treatment for S. japonicum during pregnancy (Grant #02826-5: https://www.thrasherresearch.org/default.aspx), Alpert Medical School Summer Assistantship, American Society of Tropical Medicine & Hygiene’s Benjamin Kean Travel Fellowship, Asian Pacific American Medical Student Association’s Global Health Fellowship and Rhode Island Medical Women’s Association.
References
- 1.Pelletier DL, Frongillo EA Jr, Schroeder DG, Habicht JP. The effects of malnutrition on child mortality in developing countries. Bull World Health Organ. 1995;73(4):443–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Norgan NG. Long-term physiological and economic consequences of growth retardation in children and adolescents. Proc Nutr Soc. 2000;59(2):245–56. . [DOI] [PubMed] [Google Scholar]
- 3.Adair LS, Guilkey DK. Age-specific determinants of stunting in Filipino children. J Nutr. 1997;127(2):314–20. . [DOI] [PubMed] [Google Scholar]
- 4.Christian P, Murray-Kolb LE, Tielsch JM, Katz J, LeClerq SC, Khatry SK. Associations between preterm birth, small-for-gestational age, and neonatal morbidity and cognitive function among school-age children in Nepal. BMC Pediatr. 2014;14(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mwaniki MK, Atieno M, Lawn JE, Newton CRJC. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: A systematic review. Lancet. 2012;379(9814):445–52. 10.1016/S0140-6736(11)61577-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizzo G, Arduini D. Intrauterine growth restriction: Diagnosis and management. A review. Minerva Ginecol. 2009;61(5):411–20. [PubMed] [Google Scholar]
- 7.Guellec I, Lapillonne A, Renolleau S, Charlaluk ML, Roze JC, Marret S, et al. Neurologic outcomes at school age in very preterm infants born with severe or mild growth restriction. Pediatrics. 2011;127(4):e883–e91. 10.1542/peds.2010-2442 [DOI] [PubMed] [Google Scholar]
- 8.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–51. 10.1016/S0140-6736(13)60937-X . [DOI] [PubMed] [Google Scholar]
- 9.Villar J, Belizan JM. The relative contribution of prematurity and fetal growth retardation to low birth weight in developing and developed societies. Am J Obstet Gynecol. 1982;143(7):793–8. [DOI] [PubMed] [Google Scholar]
- 10.Manning F. Intrauterine growth retardation. Fetal medicine Principles and practice. 1995:317. [Google Scholar]
- 11.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–68. 10.1016/S0140-6736(14)60932-6 . [DOI] [PubMed] [Google Scholar]
- 12.Kozuki N, Katz J, Christian P, Lee AC, Liu L, Silveira MF, et al. Comparison of US Birth Weight References and the International Fetal and Newborn Growth Consortium for the 21st Century Standard. JAMA Pediatr. 2015;169(7):e151438 10.1001/jamapediatrics.2015.1438 . [DOI] [PubMed] [Google Scholar]
- 13.Olveda RM, Acosta LP, Tallo V, Baltazar PI, Lesiguez JL, Estanislao GG, et al. Efficacy and safety of praziquantel for the treatment of human schistosomiasis during pregnancy: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2016;16(2):199–208. 10.1016/S1473-3099(15)00345-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United States Agency for International Development. Health Profile: Philippines. HIV/AIDS. USAID ED USAID from the American People. 2005.
- 15.Gibson R. Principles of Nutritional Assessment. New York: Oxford University Press; 1990. [Google Scholar]
- 16.WHO Anthro 2005. Beta version Feb 17th, 2006: Software for assessing growth and development of the world's children Geneva: WHO, 2006. Available: http://www.who.int/childgrowth/software/en/. [Google Scholar]
- 17.Ashworth A, Morris SS, Lira PIC. Postnatal growth patterns of full-term low birth weight infants in northeast Brazil are related to socioeconomic status. J Nutr. 1997;127(10):1950–6. [DOI] [PubMed] [Google Scholar]
- 18.Harding JE, McCowan LME. Perinatal predictors of growth patterns to 18 months in children born small for gestational age. Early Hum Dev. 2003;74(1):13–26. [DOI] [PubMed] [Google Scholar]
- 19.Karlberg JPE, Albertsson-Wikland K, Kwan EYW, Lam BCC, Low LCK. The timing of early postnatal catch-up growth in normal, full-term infants born short for gestational age. Horm Res. 1997;48(SUPPL. 1):17–24. [DOI] [PubMed] [Google Scholar]
- 20.Hokken-Koelega ACS, De Ridder MAJ, Lemmen RJ, Den Hartog H, Keizer-Schrama SMPFDM, Drop SLS. Children born small for gestational age: Do they catch up? Pediatr Res. 1995;38(2):267–71. [DOI] [PubMed] [Google Scholar]
- 21.Emmett PM, Rogers IS. Properties of human milk and their relationship with maternal nutrition. Early Hum Dev. 1997;49 Suppl:S7–28. . [DOI] [PubMed] [Google Scholar]
- 22.Brown KH, Robertson AD, Akhtar NA. Lactational capacity of marginally nourished mothers: infants' milk nutrient consumption and patterns of growth. Pediatrics. 1986;78(5):920–7. . [PubMed] [Google Scholar]
- 23.Kebede A, Larson C. The health consequences of intrauterine growth retardation in southwestern Ethiopia. Trop Doct. 1994;24(2):64–9. . [DOI] [PubMed] [Google Scholar]
- 24.Sania A, Spiegelman D, Rich-Edwards J, Hertzmark E, Mwiru RS, Kisenge R, et al. The contribution of preterm birth and intrauterine growth restriction to childhood undernutrition in tanzania. Matern Child Nutr. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricci JA, Becker S. Risk factors for wasting and stunting among children in Metro Cebu, Philippines. Am J Clin Nutr. 1996;63(6):966–75. . [DOI] [PubMed] [Google Scholar]
- 26.Christian P, Lee SE, Donahue Angel M, Adair LS, Arifeen SE, Ashorn P, et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int J Epidemiol. 2013;42(5):1340–55. 10.1093/ije/dyt109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binkin NJ, Yip R, Fleshood L, Trowbridge FL. Birth weight and childhood growth. Pediatrics. 1988;82(6):828–34. [PubMed] [Google Scholar]
- 28.Low JA, Galbraith RS, Muir D, Killen H, Pater B, Karchmar J. Intrauterine growth retardation: A study of long-term morbidity. Am J Obstet Gynecol. 1982;142(6 Pt 1):670–7. [DOI] [PubMed] [Google Scholar]
- 29.Ounsted M, Moar V, Scott A. Growth in the first four years: II. Diversity within groups of small-for-dates and large-for-dates babies. Early Hum Dev. 1982;7(1):29–39. [DOI] [PubMed] [Google Scholar]
- 30.Cruise MO. A longitudinal study of the growth of low birth weight infants. I. Velocity and distance growth, birth to 3 years. Pediatrics. 1973;51(4):620–8. [PubMed] [Google Scholar]
- 31.Bollen KA, Noble MD, Adair LS. Are gestational age, birth weight, and birth length indicators of favorable fetal growth conditions? A structural equation analysis of Filipino infants. Stat Med. 2013;32(17):2950–61. 10.1002/sim.5771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hengstermann S, Mantaring JBV, Sobel HL, Borja VE, Basilio J, Iellamo AD, et al. Formula feeding is associated with increased hospital admissions due to infections among infants younger than 6 months in Manila, Philippines. J Hum Lact. 2010;26(1):19–25. 10.1177/0890334409344078 [DOI] [PubMed] [Google Scholar]
- 33.Onsa ZO, Khair Ahmed NM. Impact of exclusive breast feeding on the growth of sudanese children (0–24 Months). Pak J Nutr. 2014;13(2):99–106. [Google Scholar]
- 34.Daniels MC, Adair LS. Breast-feeding influences cognitive development in Filipino children. J Nutr. 2005;135(11):2589–95. [DOI] [PubMed] [Google Scholar]
- 35.Yoon PW, Black RE, Moulton LH, Becker S. Effect of not breastfeeding on the risk of diarrheal and respiratory mortality in children under 2 years of age in Metro Cebu, the Philippines. Am J Epidemiol. 1996;143(11):1142–8. [DOI] [PubMed] [Google Scholar]
- 36.Onayade AA, Abiona TC, Abayomi IO, Makanjuola ROA. The first six month growth and illness of exclusively and non-exclusively breast-fed infants in Nigeria. East Afr Med J. 2004;81(3):146–53. [DOI] [PubMed] [Google Scholar]
- 37.Adair LS, Popkin BM. Low birth weight reduces the likelihood of breast-feeding among Filipino infants. J Nutr. 1996;126(1):103–12. [DOI] [PubMed] [Google Scholar]
- 38.Hack M, Klein NK, Taylor HG. Long-term developmental outcomes of low birth weight infants. Future Child. 1995;5(1):176–96. [PubMed] [Google Scholar]
- 39.Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJP. Boys live dangerously in the womb. Am J Hum Biol. 2010;22(3):330–5. 10.1002/ajhb.20995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varela-Silva MI, Azcorra H, Dickinson F, Bogin B, Frisancho AR. Influence of maternal stature, pregnancy age, and infant birth weight on growth during childhood in Yucatan, Mexico: A test of the intergenerational effects hypothesis. Am J Hum Biol. 2009;21(5):657–63. 10.1002/ajhb.20883 [DOI] [PubMed] [Google Scholar]
- 41.Zottarelli LK, Sunil TS, Rajaram S. Influence of parental and socioeconomic factors in stunting in children under 5 years in Egypt. East Mediterr Health J. 2007;13(6):1330–42. [DOI] [PubMed] [Google Scholar]
- 42.Martorell R, Zongrone A. Intergenerational influences on child growth and undernutrition. Paediatr Perinat Epidemiol. 2012;26(SUPPL. 1):302–14. 10.1111/j.1365-3016.2012.01298.x [DOI] [PubMed] [Google Scholar]
- 43.Britto RPDA, Floren̂ TMT, Benedito Silva AA, Sesso R, Cavalcante JC, Sawaya AL. Influence of maternal height and weight on low birth weight: A cross-sectional study in poor communities of northeastern Brazil. PLoS ONE. 2013;8(11):e80159 10.1371/journal.pone.0080159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fenske N, Burns J, Hothorn T, Rehfuess EA. Understanding child stunting in India: A comprehensive analysis of socio-economic, nutritional and environmental determinants using additive quantile regression. PLoS ONE. 2013;8(11):e78692 10.1371/journal.pone.0078692 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are from the efficacy and safety study of praziquantel (http://dx.doi.org/10.1016/ S1473-3099(15)00345-X). Both legal and ethical restrictions prohibit the authors from making the minimal data set publicly available. Given that the data used for this paper involve human subjects, data use will be considered for qualified researchers upon request and upon institutional review board approval from the research sites. Requests should be submitted to Jennifer F Friedman (jennifer_friedman@brown.edu).