Abstract
Gestational weight gain is known to influence fetal growth. However, it is unclear whether the associations between gestational weight gain and fetal growth vary by trimester. In a diverse cohort of 8,977 women who delivered a singleton between 2011 and 2013, we evaluated the associations between trimester-specific gestational weight gain and infant size for gestational age. Gestational weight gain was categorized per the 2009 Institute of Medicine (IOM) recommendations; meeting the recommendations was the referent. Large for gestational age and small for gestational age were defined as birthweight > 90th percentile or <10th percentile, respectively, based on a national reference standard birthweight distribution. Logistic regression models estimated the odds of having a large or small for gestational age versus an appropriate for gestational age infant. Only gestational weight gain exceeding the IOM recommendations in the 2nd and 3rd trimesters independently increased the odds of delivering a large for gestational age infant (Odds Ratio (95% Confidence Interval): 1st: 1.17 [0.94, 1.44], 2nd: 1.47 [1.13, 1.92], 3rd: 1.70 [1.30, 2.22]). Gestational weight gain below the IOM recommendations increased the likelihood of having a small for gestational age infant in the 2nd trimester only (1.76 [1.23, 2.52]). There was effect modification, and gestational weight gain below the IOM recommendations increased the likelihood of having a small for gestational age infant in the 2nd trimester and only among women with a pre-pregnancy body mass index from 18.5–24.9 kg/m2 (2.06 [1.35, 3.15]). These findings indicate that gestational weight gain during the 2nd and 3rd trimesters is more strongly associated with infant growth. Interventions to achieve appropriate gestational weight gain may optimize infant size at birth.
Introduction
Gestational weight gain (GWG) is a known driver of fetal growth. In 2009, the Institute of Medicine (IOM) released updated guidelines on GWG in part to account for the increasing prevalence of obesity among women of reproductive age. The IOM report highlighted the need for further research on how patterns of GWG throughout the course of pregnancy impact neonatal outcomes. Infants born either LGA or SGA may be more likely to accumulate excess fat in early childhood [1] and are more likely to become obese later in life [2–4]. However, it is currently unclear whether there are critical windows in pregnancy when GWG most strongly influences fetal growth.
Only two prior studies looked at GWG in all three trimesters and birthweight in the context of the current obesity epidemic, and none specifically examined size for gestational age, which is an important predictor of later life obesity [5,6]; therefore, we need to clarify the associations of patterns of GWG throughout the entire course of pregnancy on infant size at birth. Additionally, most prior studies lacked information on measured pre-pregnancy weight and were unable to determine 1st trimester GWG. A better understanding of the timing of when GWG influences growth will help inform future lifestyle interventions designed to optimize fetal growth. The aim of this cohort study was to determine whether trimester-specific gestational weight gain impacts infant size for gestational age and to explore whether maternal pre-pregnancy body mass index (BMI) modifies this relationship.
Materials and Methods
The study setting was Kaiser Permanente Northern California, a large group practice prepaid health plan which provides comprehensive medical services to members residing in a 14-county region of Northern California (approximately 30% of the surrounding population). The demographic, racial/ethnic, and socioeconomic makeup of the Kaiser Permanente Northern California membership is well representative of the population residing in the same geographic area, except that the very poor and the very wealthy are under-represented [7,8].
Cohort identification
We identified 12,662 female Kaiser Permanente Northern California members who completed a detailed health survey as part of the Kaiser Permanente Research Program on Genes, Environment, and Health (RPGEH) between 2007 and 2012 and delivered a singleton livebirth between 2011 and 2013. Pregnancies were identified through the electronic medical record (EMR). We then excluded 163 women with preexisting diabetes (either type 1 or type 2) and limited the cohort to full-term births (gestational age ≥ 37 weeks), resulting in 11,643 women. We restricted the cohort to women who had a pre-pregnancy weight as well as a final pregnancy weight within four weeks of delivery available (n = 10,285). We then limited the cohort to women whose deliveries occurred after the RPGEH health survey was completed and for whom infant birthweight information was available (n = 9,153). Due to the small number of underweight women (BMI < 18.5 kg/m2) (n = 176), they were excluded from the cohort. The final analytic cohort consisted of 8,977 women.
This study was approved by the Kaiser Permanente Northern California Institutional Review Board, who waived the requirement for obtaining written informed consent from study participants. Patient records were anonymized and de-identified prior to analysis.
Maternal characteristics
Self-reported maternal race/ethnicity [Non-Hispanic white, hereafter referred to as white, 2) African American, 3) Asian/Pacific Islander, 4) Hispanic, and 5) Other/Unknown] and educational attainment were obtained from the RPGEH survey.
Pre-pregnancy dietary pattern
The RPGEH survey included 20 food categories, including sugar-sweetened beverages. To identify major dietary patterns, principal components analysis was used on the 20 foods to identify factors that accounted for much of the variance. The food groups (factors) were rotated using an orthogonal transformation, resulting in uncorrelated, independent factors. The factor score for each factor (pattern) was calculated by summing intakes of food groups weighted by factor loading, and each individual was assigned a score for each identified pattern. Individuals with a high score for a pattern compared with individuals with lower scores have a stronger tendency to follow that pattern. We identified two distinct dietary patterns: Prudent and Western. The dietary pattern scores were then categorized by tertiles.
Pre-pregnancy physical activity
Volume of total METs was calculated as minutes per week based on four questions. The first three questions assessed the following forms of physical activity during the previous 7 days: walking, moderate recreational activity and vigorous recreational activity. A fourth question assessed sedentary behaviors. Women reported the average minutes per week she spent doing each activity. These questions were adopted from validated scales.
Gestational diabetes
Gestational diabetes status was assessed through the Kaiser Permanente Northern California Pregnancy Glucose Tolerance Registry [9] and defined as having at least two plasma glucose values on the 100-g, 3-hour oral glucose tolerance test meeting or exceeding the Carpenter-Coustan thresholds [10].
Exposure ascertainment
We searched the EMR data for a pre-pregnancy weight measured within 12 months of conception. For those missing a measured pre-pregnancy weight (19.0%), a self-reported pre-pregnancy weight was used for 15.0%, and an early pregnancy measured weight was used for 4.0%. To validate this method of estimating pre-pregnancy weight, we compared the self-reported pre-pregnancy weight to a weight measured within 12 months of the last menstrual period among the 4,723 women for whom both measurements were available. The intra-class correlation coefficient between the two weights was 0.975.
Pre-pregnancy BMI was calculated as pre-pregnancy weight (kilograms) divided by height (meters) squared. BMI categories were created in accordance with the 2009 Institute of Medicine (IOM) GWG recommendations as follows: normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30.0 kg/m2). Total gestational weight gain was calculated as the difference between the last measured pregnancy weight and pre-pregnancy weight, in kilograms; the last weight measured during pregnancy was obtained from the EMR and measured within four weeks of delivery.
We calculated 1st trimester GWG to be the difference between the last available weight measurement during the 1st trimester (gestational weeks, mean (SD): 13.6 (1.3)) and pre-pregnancy weight. Among the 8,977 women in the cohort, 78.5% women had a 1st trimester weight measurement. We calculated 2nd trimester GWG rate as the difference between the early 3rd trimester weight (gestational weeks, mean (SD): 29.9 (1.3)) and the last 1st trimester weight measurement, divided by the gestational weeks between the two measurements. Finally, we calculated 3rd trimester GWG rate to be the difference between the last pregnancy weight (gestational weeks, mean (SD): 38.7 (1.3)) and the early 3rd trimester weight measurement, divided by the gestational weeks between the two measurements.
The 2009 IOM recommendations suggest a range of absolute weight gain in the first trimester and a range of pregravid BMI-specific rates of weight gain per week for the 2nd and 3rd trimesters [11]. We determined whether each woman met, exceeded or was below the IOM recommendations for trimester-specific weight gain, by calculating whether she met recommended absolute amount of weight gain in the first trimester (1.1–4.4 pounds) for all women. For the 2nd and 3rd trimester, we determined whether the rate of GWG in each respective trimester met the IOM range of recommended weight gain for her pre-pregnancy BMI.
For total GWG weight gain, we determined whether each woman met, exceeded or was below the IOM recommendations based on the last measured weight in pregnancy minus her pregnancy weight. We subtracted 13 weeks from the gestational age at the delivery and multiplied this value by the BMI-specific recommended rate of weight gain for the 2nd and 3rd trimesters. Weight gained after the first trimester was then added to the BMI-specific absolute weight gain recommended by the IOM for the first trimester.
Offspring characteristics
We used sex- and gestational age-specific references for size for gestational age from Oken et al. [12], a United States national reference standard. Size for gestational age was categorized as follows: large for gestational age if birthweight greater than the 90th percentile, small for gestational age if birthweight less than the 10th percentile, and appropriate for gestational age if birthweight between the 10th and 90th percentiles, inclusive. Sex and birth weight data for the children were obtained from the EMR.
Statistical analysis
Unconditional logistic regression analysis was used to obtain odds ratios (ORs) and confidence intervals (CIs) estimating the odds of a small or large for gestational age infant (both versus appropriate for gestational age) associated with maternal GWG for both total GWG and trimester-specific GWG, as well as a single model including trimester-specific GWG in all three trimesters simultaneously. We estimated the odds of LGA or SGA associated with exceeding and gaining below the recommendations, as compared to meeting the recommendations.
Covariates in the fully adjusted model included those of a priori interest based on existing literature (maternal age at delivery, race/ethnicity, gestational age at delivery, parity (number of previous livebirths), pre-pregnancy BMI, gestational diabetes status, infant sex (with male as the referent), pre-pregnancy physical activity (in tertiles of total MET-minutes/week, with the lowest tertile as the referent), pre-pregnancy Western dietary pattern score (in tertiles, with the lowest tertile as the referent), and pre-pregnancy Prudent dietary pattern score (in tertiles, with the lowest tertile as the referent).
To assess the potential modifying effect of maternal pre-pregnancy BMI in each trimester, we included separate cross product (interaction) terms in each regression model, using trimester-specific gestational weight gain (Exceeded vs Met/Below) as the exposure. Finally, we conducted the following sensitivity analyses: 1) excluding women with self-reported weights, and 2) excluding women with conditions that may affect gestational weight gain. SAS version 9.3 (SAS Institute Inc., Cary, NC) was used for all analyses.
Results
Table 1 summarizes the demographic characteristics of the 8,977 women in the cohort. Women were, on average, 33 years old at delivery, and the majority (65.6%) had the equivalent of a college degree (Table 1). The cohort was diverse (43.0% were racial/ethnic minorities), and 63.9% were multiparous, while 46.6% were overweight or obese. The proportions of SGA, AGA and LGA infants were 8.4%, 81.6%, and 10.0%, respectively. Overall, 11.1% fell below, 22.8% met and 66.1% exceeded the IOM recommendations for total GWG; however, the percent exceeding the IOM recommendations for rate of GWG in each trimester were quite a bit higher (1st: 46.9%, 2nd: 72.3%, 3rd: 63.6%) (Table 2).
Table 1. Characteristics of the 8,977 Pregnant Women at Kaiser Permanente Northern California Who Delivered Between 2011 and 2013.
| Characteristic | No. | % |
|---|---|---|
| Maternal | ||
| Education (years) | ||
| High School Graduate or Less | 1,079 | 12.0 |
| Some College | 1,570 | 17.5 |
| College Graduate or Higher | 5,885 | 65.6 |
| Other/Unknown | 443 | 4.9 |
| Race/Ethnicity | ||
| Non-Hispanic White | 4,626 | 51.5 |
| African American | 340 | 3.8 |
| Asian/Pacific Islander | 1,927 | 21.5 |
| Hispanic | 1,585 | 17.7 |
| Other/Unknown | 499 | 5.6 |
| Parity | ||
| 0 | 3,204 | 35.7 |
| 1 | 3,712 | 41.4 |
| ≥ 2 | 2,015 | 22.5 |
| Unknown | 46 | 0.5 |
| Pre-pregnancy body mass index (kg/m2) | ||
| 18.5–24.9 (Normal Weight) | 4,798 | 53.5 |
| 25.0–29.9 (Overweight) | 2,435 | 27.1 |
| ≥ 30.0 (Obese) | 1,744 | 19.4 |
| Had gestational diabetes | 624 | 7.0 |
| Child | ||
| Male infant sex | 4, 566 | 50.9 |
| Size for gestational age | ||
| Small for gestational age | 756 | 8.4 |
| Appropriate for gestational age | 7,322 | 81.6 |
| Large for gestational age | 899 | 10.0 |
| Mean (SD) | ||
| Maternal age at delivery (years) | 33.0 (4.8) | |
| Infant birth weight (grams) | 3,495.6 (460.3) | |
| Infant’s gestational age at delivery (weeks) | 39.2 (1.1) | |
| Pre-pregnancy volume of physical activity, MET-mins/week | 799.2 (912.0) | |
Table 2. Adjusteda Odds Ratios (ORs) and 95% Confidence Intervals (CIs) for Size for Gestational Age Associated With Total and Trimester-Specific Gestational Weight Gain (GWG).
| Pregnancy Risk Factor | N (%) | Small for gestational age (SGA) | Large for gestational age (LGA) | ||
|---|---|---|---|---|---|
| Total | OR | 95% CI | OR | 95% CI | |
| IOM GWG Recommendations | |||||
| Below | 995 (11.08) | 1.55 | [1.17, 2.07] | 0.58 | [0.41, 0.81] |
| Met | 2,049 (22.82) | Reference | Reference | ||
| Exceeded | 5, 933 (66.09) | 0.64 | [0.51, 0.80] | 2.12 | [1.75, 2.58] |
| 1st Trimester | |||||
| IOM GWG Recommendations | |||||
| Below | 2,311 (32.79) | 1.02 | [0.76, 1.36] | 0.80 | [0.63, 1.01] |
| Met | 1,432 (20.32) | Reference | Reference | ||
| Exceeded | 3,305 (46.89) | 0.82 | [0.62, 1.08] | 1.17 | [0.94, 1.44] |
| 2nd Trimester | |||||
| IOM GWG Recommendations | |||||
| Below | 781 (12.92) | 1.76 | [1.23, 2.52] | 0.79 | [0.54, 1.15] |
| Met | 893 (14.78) | Reference | Reference | ||
| Exceeded | 4,369 (72.30) | 0.70 | [0.51, 0.95] | 1.47 | [1.13, 1.92] |
| 3rd Trimester | |||||
| IOM GWG Recommendations | |||||
| Below | 1,719 (23.90) | 0.98 | [0.71, 1.36] | 0.93 | [0.68, 1.27] |
| Met | 900 (12.52) | Reference | Reference | ||
| Exceeded | 4,572 (63.58) | 0.81 | [0.60, 1.09] | 1.70 | [1.30, 2.22] |
aAdjusted for race/ethnicity, maternal age at delivery, gestational age at delivery, pre-pregnancy BMI (kg/m2), gestational diabetes status, infant sex, parity, pre-pregnancy physical activity (in tertiles of MET-mins/week), pre-pregnancy Prudent dietary pattern score (in tertiles), and pre-pregnancy Western dietary pattern score (in tertiles).
Total GWG
Table 2 displays the odds ratios (ORs) and 95% confidence intervals (CIs) associated with SGA and LGA infants, by trimester-specific rate of GWG and total rate of GWG.
Overall, women who gained below the IOM recommendations for total GWG were more likely to deliver an SGA infant (OR (95% CI): 1.55 [1.17, 2.07]) and less likely to deliver an LGA infant (0.58 [0.41, 0.81]), compared to women with met the IOM recommendations. In contrast, women who exceeded the IOM recommendations were less likely to deliver SGA infants and more likely to deliver LGA infants (SGA: 0.64 [0.51, 0.80]; LGA: 2.12 [1.75, 2.58]).
Trimester-specific GWG
In models assessing each trimester separately (Table 2), excess GWG increased the likelihood of an LGA infant in the 2nd and 3rd trimesters only (1st: 1.17 [0.94, 1.44], 2nd: 1.47 [1.13, 1.92], 3rd: 1.70 [1.30, 2.22]). In the second trimester only, excess GWG was associated with decreased risk of having an SGA infant, and gaining below the IOM recommendations was associated with increased risk of an SGA infant.
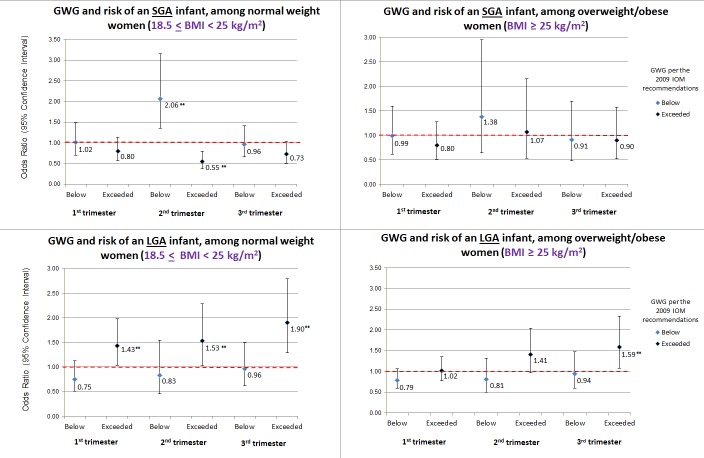
There was significant effect modification by maternal pre-pregnancy BMI for 2nd trimester GWG and SGA only (P-value interaction term = 0.0016); there was no significant effect modification by BMI for SGA in the 1st or 3rd trimester, as well as during any trimester for GWG and LGA. Normal weight women whose 2nd trimester GWG was below the IOM recommendations had a twofold increased likelihood of having an SGA infant (2.06 [1.35, 3.15], while those with excess 2nd trimester GWG were less likely to have an SGA infant (0.55 [0.37, 0.80]). In contrast, for overweight and obese women, GWG outside of the IOM recommendations was not associated with SGA (Fig 1). Excess GWG was associated with LGA in all three trimesters for normal weight women, and in the 3rd trimester only for overweight and obese women.
Fig 1. Odds ratios and 95% confidence intervals from multivariable* models of trimester-specific GWG and risk of SGA or LGA infants, by maternal pre-pregnancy BMI.
*Adjusted for race/ethnicity, maternal age at delivery, gestational age at delivery, parity, pre-pregnancy BMI, gestational diabetes status, infant sex, pre-pregnancy physical activity (in tertiles of MET-minutes/week), pre-pregnancy Western dietary pattern score (in tertiles), and pre-pregnancy Prudent dietary pattern score (in tertiles); ** = indicates statistical significance (p-value <0.05).
In a model adjusting for all three trimesters simultaneously, excess GWG in the 2nd and 3rd trimesters was associated with having an LGA infant (1st: 1.09 (0.87–1.37, 2nd: 1.33 (1.01–1.74), 3rd: 1.63 (1.21–2.20)). There was no significant association during any trimester between GWG below the IOM recommendations and having an LGA infant (1st: 0.78 (0.61–1.00), 2nd: 0.81 (0.55–1.17), 3rd: 1.04 (0.74–1.46)). In the 2nd trimester only, GWG below the IOM recommendations was associated with increased odds of an SGA infant (1st: 1.07 (0.78–1.46), 2nd: 1.74 (1.22–2.49), 3rd: 1.00 (0.70–1.44)), while excess 2nd GWG was associated with decreased odds of an SGA infant (1st: 0.88 (0.66–1.18), 2nd: 0.71 (0.52–0.99), 3rd: 0.91 (0.65–1.27)).
Results were also similar for the sensitivity analysis that was restricted to women with a measured weight (n = 7,285) as well as for a separate sensitivity analysis that excluded 1,781 women with certain conditions that may impact gestational weight gain (gestational diabetes, bariatric surgery, any thyroid disorder, and preeclampsia or gestational hypertension) (data not shown).
Discussion
Overall, gaining below the current IOM GWG recommendations increased the risk of SGA and decreased the risk of LGA. In contrast, excess GWG increased the risk of LGA and decreased the risk of SGA. These associations varied by trimester. Only excess rate of GWG in the 2nd and 3rd trimesters significantly increased risk of LGA, and GWG below the IOM recommendation significantly increased risk of SGA in the 2nd trimester only. We found significant effect modification by pre-pregnancy BMI; the association between 2nd trimester GWG below the IOM recommendations and SGA was only significant among normal weight women. Excess GWG was associated with LGA in all three trimesters among normal weight women, but only in the 3rd trimester among overweight/obese women.
There is some evidence from previous studies that in comparison to the 1st trimester, the 2nd and/or 3rd trimesters may form the period when GWG most strongly influences fetal growth and birth weight [13–18]. Gaillard et al found a positive association between standard deviation of change in GWG per week and size for gestational age in all three trimesters, but the strongest associations were in the 2nd and 3rd trimesters [19]. In a prospective cohort study, Margerison-Zilco et al found that that both total GWG and GWG in all three trimesters were positively associated with birthweight, with the impact stronger, though not significantly, in the 2nd trimester [14]. Abrams et al found that maternal GWG was positively associated with birthweight in all three trimesters, with the strongest association occurring in the 2nd trimester [13].
We found that in the 2nd trimester only, gaining below the IOM recommendations was associated with having an SGA infant among normal weight women (pre-pregnancy BMI: 18.5–24.9 kg/m2), but not among overweight and obese women. Consistent with our findings, Drehmer et al found an overall association between GWG below the IOM recommendations and SGA in the 2nd, but not the 3rd trimester, but they did not look at the association stratified by pre-pregnancy BMI [20]. Additionally, while excess GWG was associated with LGA in all three trimesters for normal weight women, only excess 3rd trimester GWG was associated with LGA for overweight and obese women. A prior study found that GWG in the 2nd and 3rd trimesters of pregnancy, but not first trimester, was associated with risk of large for gestational age infants; however, they did not examine the associations by pre-pregnancy BMI [21]. Previous research [22] has found that the association between maternal GWG and birthweight is attenuated in obese women compared with normal weight women, suggesting that GWG is not as important of a contributor to fetal growth in obese versus non-obese women.
One possible consequence of excess GWG is the transfer of excess nutrients across the placenta; the influx of excess nutrients, particularly maternal glucose and lipids/fatty acids, can accelerate fetal growth and fat accretion and lead to an LGA infant [23,24]. On the other hand, calorie restriction and maternal undernutrition are thought to impede fetal growth; thus, it is biologically plausible for excess GWG to reduce the risk of having an SGA infant, and for low GWG to increase the risk of SGA. We found that the impact of GWG on infant size for gestational age varied by trimester, which may be due in part to the fact that both the composition of GWG and the biological development of the fetus differ by trimester [25,26]. GWG during the first trimester is disproportionately maternal fat and may influence maternal glucose metabolism later in pregnancy [25,27]. The fetal-placental glucose utilization rates peak in the 2nd trimester [28], and excess GWG may further accelerate glucose utilization and thereby increase fetal growth. The 3rd trimester is when the placenta most actively shunts nutrients to the fetus, and the majority of fetal growth [29] and fat accretion [30] occurs; therefore, it is unsurprising that 3rd trimester GWG was associated with fetal growth and infant size at birth.
The strengths of the present study include the large, diverse cohort, the historical prospective cohort study design, and the availability of measured pre-pregnancy weight in the majority of participants (81%), as well as our ability to assess GWG in all three trimesters. We were also able to adjust for pre-pregnancy dietary pattern and physical activity; although both measures were assessed before pregnancy, they are likely a good surrogate for women’s lifestyle during pregnancy. However, one limitation is that the diet and physical activity components of the RPGEH survey are not validated measures. We also lacked information on the composition of maternal weight gain (e.g., fat, placental weight, fluid, etc.), as well as information on infant body composition at birth, which may be important given that neonatal adiposity may be a stronger predictor of subsequent obesity factors than size for gestational age alone [31,32].
In summary, GWG according to the 2009 IOM recommendations is associated with size for gestational age, and our data suggest that the timing of the GWG may be important, as associations between excess GWG and LGA appear to be stronger in the 2nd and 3rd trimesters. We also found that there was no significant association in any trimester between GWG below the current recommendations and SGA among women who are overweight or obese before pregnancy. This suggests that perhaps less weight gain may be safe for overweight and obese women, but this needs to be confirmed in future studies that include other outcomes as well. Clarifying how and when GWG is driving fetal growth will help to inform future prevention strategies.
Acknowledgments
These data were previously presented as an abstract at the annual meeting of The Obesity Society in Los Angeles, CA, November 2–7, 2015. The authors report no conflict of interest. This research was supported by a research grant to MMH from the Health Resources and Services Administration (http://www.hrsa.gov/index.html)(R40MC21515).
Data Availability
The data used for this study contain protected health information (PHI) and are therefore available upon request. Kaiser Permanente IRB policies prohibit releasing PHI. Data are available from the Kaiser Permanente Division of Research for researchers who meet the criteria for access to confidential data. Interested researchers should contact: Monique Hedderson, Kaiser Permanente Division of Research, monique.m.hedderson@kp.org.
Funding Statement
This research was supported by a research grant to MMH from the Health Resources and Services Administration (http://www.hrsa.gov/index.html)(R40MC21515). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hediger ML, Overpeck MD, McGlynn A, Kuczmarski RJ, Maurer KR, Davis WW (1999) Growth and fatness at three to six years of age of children born small- or large-for-gestational age. Pediatrics 104: e33 [DOI] [PubMed] [Google Scholar]
- 2.Dickinson S, Colagiuri S, Faramus E, Petocz P, Brand-Miller JC (2002) Postprandial hyperglycemia and insulin sensitivity differ among lean young adults of different ethnicities. J Nutr 132: 2574–2579. [DOI] [PubMed] [Google Scholar]
- 3.Starling AP, Brinton JT, Glueck DH, Shapiro AL, Harrod CS, Lynch AM, et al. (2015) Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr 101: 302–309. 10.3945/ajcn.114.094946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ornoy A (2011) Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol 32: 205–212. S0890-6238(11)00151-1 [pii]; 10.1016/j.reprotox.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 5.Sridhar SB, Darbinian J, Ehrlich SF, Markman MA, Gunderson EP, Ferrara A, et al. (2014) Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am J Obstet Gynecol 211: 259–8. S0002-9378(14)00172-0 [pii]; 10.1016/j.ajog.2014.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stettler N (2007) Nature and strength of epidemiological evidence for origins of childhood and adulthood obesity in the first year of life. Int J Obes (Lond) 31: 1035–1043. [DOI] [PubMed] [Google Scholar]
- 7.Krieger N (1992) Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health 82: 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. (2001) Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285: 2370–2375. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara A, Kahn HS, Quesenberry C, Riley C, Hedderson MM (2004) An increase in the incidence of gestational diabetes mellitus: Northern California, 1991–2000. Obstet Gynecol 103: 526–533. [DOI] [PubMed] [Google Scholar]
- 10.2011) Committee opinion no. 504: screening and diagnosis of gestational diabetes mellitus. Obstet Gynecol 118: 751–753. 10.1097/AOG.0b013e3182310cc3 [DOI] [PubMed] [Google Scholar]
- 11.IOM (Institutes of Medicine) (2009) Weight Gain During Pregnancy: Reexamining the Guidelines. [PubMed]
- 12.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW (2003) A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 3: 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abrams B, Selvin S (1995) Maternal weight gain pattern and birth weight. Obstet Gynecol 86: 163–169. [DOI] [PubMed] [Google Scholar]
- 14.Margerison-Zilko CE, Shrimali BP, Eskenazi B, Lahiff M, Lindquist AR, Abrams BF (2012) Trimester of maternal gestational weight gain and offspring body weight at birth and age five. Matern Child Health J 16: 1215–1223. 10.1007/s10995-011-0846-1 [DOI] [PubMed] [Google Scholar]
- 15.Sekiya N, Anai T, Matsubara M, Miyazaki F (2007) Maternal weight gain rate in the second trimester are associated with birth weight and length of gestation. Gynecol Obstet Invest 63: 45–48. [pii]; 10.1159/000095286 [DOI] [PubMed] [Google Scholar]
- 16.Strauss RS, Dietz WH (1999) Low maternal weight gain in the second or third trimester increases the risk for intrauterine growth retardation. J Nutr 129: 988–993. [DOI] [PubMed] [Google Scholar]
- 17.Hickey CA, Cliver SP, McNeal SF, Hoffman HJ, Goldenberg RL (1996) Prenatal weight gain patterns and birth weight among nonobese black and white women. Obstet Gynecol 88: 490–496. [DOI] [PubMed] [Google Scholar]
- 18.Karachaliou M, Georgiou V, Roumeliotaki T, Chalkiadaki G, Daraki V, Koinaki S, et al. (2015) Association of trimester-specific gestational weight gain with fetal growth, offspring obesity, and cardiometabolic traits in early childhood. Am J Obstet Gynecol 212: 502–514. S0002-9378(14)02497-1 [pii]; 10.1016/j.ajog.2014.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaillard R, Durmus B, Hofman A, Mackenbach JP, Steegers EA, Jaddoe VW (2013) Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring) 21: 1046–1055. [DOI] [PubMed] [Google Scholar]
- 20.Drehmer M, Duncan BB, Kac G, Schmidt MI (2013) Association of second and third trimester weight gain in pregnancy with maternal and fetal outcomes. PLoS One 8: e54704 10.1371/journal.pone.0054704;PONE-D-12-25751 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karachaliou M, Georgiou V, Roumeliotaki T, Chalkiadaki G, Daraki V, Koinaki S, et al. (2015) Association of trimester-specific gestational weight gain with fetal growth, offspring obesity, and cardiometabolic traits in early childhood. Am J Obstet Gynecol 212: 502–514. S0002-9378(14)02497-1 [pii]; 10.1016/j.ajog.2014.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viswanathan M, Siega-Riz AM, Moos A, Deierlein S, Mumford J, Knaack PTLJLK (2008) Outcomes of Maternal Weight Gain, Evidence Report/Techonology Assessment No. 168. AHRQ Publication No. 08-E-09. [PMC free article] [PubMed]
- 23.Desoye G, Gauster M, Wadsack C (2011) Placental transport in pregnancy pathologies. Am J Clin Nutr 94: 1896S–1902S. [pii]; 10.3945/ajcn.110.000851 [DOI] [PubMed] [Google Scholar]
- 24.Scholl TO, Sowers M, Chen X, Lenders C (2001) Maternal glucose concentration influences fetal growth, gestation, and pregnancy complications. Am J Epidemiol 154: 514–520. [DOI] [PubMed] [Google Scholar]
- 25.van Raaij JM, Peek ME, Vermaat-Miedema SH, Schonk CM, Hautvast JG (1988) New equations for estimating body fat mass in pregnancy from body density or total body water. Am J Clin Nutr 48: 24–29. [DOI] [PubMed] [Google Scholar]
- 26.Hytten, Frank E., Chamberlain, Geoffrey, and Hytten, Frank E. Physiology of human pregnancy (1980) Clinical physiology in obstetrics. Oxford; Boston St. Louis: Blackwell Scientific Publications; Distributors, U.S.A., Blackwell Mosby.
- 27.Hedderson MM, Gunderson EP, Ferrara A (2010) Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol 115: 597–604. 10.1097/AOG.0b013e3181cfce4f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hay WW (2006) Early postnatal nutritional requirements of the very preterm infant based on a presentation at the NICHD-AAP workshop on research in neonatology. J Perinatol 26 Suppl 2: S13–S18. [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine (1990) Nutrition during pregnancy. Part I Weight gain.
- 30.Brook CG (1972) Evidence for a sensitive period in adipose-cell replication in man. Lancet 2: 624–627. [DOI] [PubMed] [Google Scholar]
- 31.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, et al. (2009) Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr 90: 1303–1313. 10.3945/ajcn.2008.27416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crozier SR, Inskip HM, Godfrey KM, Cooper C, Harvey NC, Cole ZA, et al. (2010) Weight gain in pregnancy and childhood body composition: findings from the Southampton Women's Survey. Am J Clin Nutr 91: 1745–1751. 10.3945/ajcn.2009.29128 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for this study contain protected health information (PHI) and are therefore available upon request. Kaiser Permanente IRB policies prohibit releasing PHI. Data are available from the Kaiser Permanente Division of Research for researchers who meet the criteria for access to confidential data. Interested researchers should contact: Monique Hedderson, Kaiser Permanente Division of Research, monique.m.hedderson@kp.org.