Introduction
High dose chemotherapy with autologous hematopoietic cell transplantation (AutoHCT) has demonstrated both improvement in event free survival and overall survival in patients with multiple myeloma (MM)(1). Despite improved outcomes with novel therapies and AutoHCT, neither strategy provides prolonged disease control in MM. Only allogeneic hematopoietic cell transplantation (AlloHCT), in part due to a graft versus myeloma effect, can provide a potentially curative option (2). However, the use of AlloHCT has been limited by its toxicity and treatment related mortality. Historically, AlloHCT was used in patients with aggressive myeloablative conditioning regimens with treatment related mortality between 15-40 %(3). In more recent years, improvements in supportive care and new conditioning regimens have improved these numbers (4). Rapid immune reconstitution as evidenced by lymphocyte and monocyte recovery has been associated with improved survival in patients after AlloHCT in hematological malignancies such as acute leukemia (5). In this study, we sought to find out if lymphocyte and monocyte recovery are similarly associated with overall survival in patients undergoing AlloHCT in MM.
We performed an IRB-approved retrospective analysis of consecutive patients who underwent AlloHCT for MM between 2002 and 2013 at a tertiary medical center. Patients received either a myeloablative or non-myeloablative conditioning regimen. The non-myeloablative regimen consisted of low dose total body irradiation (TBI) either alone or with other drugs, fludarabine (Flu) with cyclophosphamide (CyFlu) or TBI with fludarabine (FluTBI) or combination of melphalan with fludarabine (FluMel). Cyclophosphamide with TBI (CyTBI) was the myeloablative conditioning regimen used. All patients underwent prior AutoHCT except for one who had an upfront AlloHCT. Graft versus host disease (GVHD) prophylaxis consisted of methotrexate and tacrolimus. Patient received antimicrobial prophylaxis with ciprofloxacin, acyclovir, fluconazole/voriconzaole and trimethoprim-sulfamethaxozole/dapsone.
The primary endpoint of the study was overall survival and we assessed the effect of peripheral blood lymphocyte and monocyte count recovery at day +100 on overall survival (OS). Two patients died before day 100 and were excluded from the analysis, i.e. this was a day 100 landmark analysis. The absolute lymphocyte count (ALC) and absolute monocyte count (AMC) were obtained from a complete blood count done at +15, +30, +60 and +100 days after transplant. For survival analyses, a 100 day ALC cut-off of 500 × 106 cells/L and AMC cut-off of 300 × 106 cells/L was used. Overall survival was assessed from the time of transplantation to last follow-up or death due to any cause. Overall survival estimates were obtained using the Kaplan- Meier estimator. Development of acute and chronic GVHD and relapse were treated as time dependent co-variates. Cox proportional hazards model was used for univariate and multivariable analysis. Multivariable analysis was done with forward stepwise model selection approach to identify significant risk factors. Each step of model building contained the main effect: day 100 ALC and AMC. All analysis were performed in SAS 9.3 (SAS Institute, Cary, NC).
Seventy-eight patients underwent AlloHCT for MM at our institution between 2002 and 2013. Patient, disease and transplant characteristics are shown in table 1. The median age at transplant was 53 years (range 23-69) with 64% male predominance. At the time of AlloHCT, 32% of patients had achieved response very good partial response or better and the majority had at least a partial response. The related and unrelated donors were 70(90%) and 8(10%), respectively. The majority received non-myeloabalative conditioning regimens. The median follow up was 49.4 months (2.3-129.3).
Table 1.
Cohort description
| Characteristic | Total, N=78 (%) |
|---|---|
| Median age at transplant, years (range) | 53 (23-69) |
| Median time from diagnosis to transplant, months (range) | 12 (7-81) |
| Gender -Male -Female |
50 (64) 28 (36) |
| International Staging System -I -II -III -Missing |
27 (35) 21 (27) 28 (36) 2 (2) |
| IMWG Risk Status -Standard risk -Intermediate Risk -High Risk -Missing |
22 (28) 29 (37) 13 (17) 14 (18) |
| Disease Status at Transplant -sCR, CR or VGPR -PR -Stable or Progressive disease |
25 (32) 48 (62) 5 (6) |
| Donor type -Related* -Unrelated |
70 (90) 8 (10) |
| Type of transplant -Myeloablative -Non-myeloablative |
11 (14) 67 (86) |
| Conditioning regimen -Low dose TBI +/− others -Cyclophosphamide/Fludarabine -Cyclophosphamide/TBI -Fludarabine/Melphalan -Fludarabine/TBI -Others# |
46 (59) 7 (9) 4 (5) 11 (14) 6 (8) 4 (5) |
| Median CD34 cells infused, × 106 cells/kg (range) | 7.3 (1.1-32.5) |
| Acute GVHD -None -Grade 1 -Grade 2 -Grade 3 -Grade 4 -Missing |
41 (53) 14 (18) 14 (18) 5 (6) 3 (4) 1 (1) |
| Chronic GVHD -Present -Absent |
29 (37) 49 (63) |
| CMV reactivation | 14 (18) |
| Received DLI infusion | 9 (12) |
| Relapse/progression of myeloma | 27 (35) |
| Cause of death (N=34) -Disease -Transplant related mortality |
14 (41) 20 (59) |
| Baseline ALC ≥500 × 106 cells/L | 62 (80) |
| Baseline AMC ≥300 × 106 cells/L | 54 (69) |
| Day 100 ALC ≥500 × 106 cells/L | 48 (62) |
| Day 100 AMC ≥300 × 106 cells/L | 45 (58) |
| Median follow up of surviving patients, months (range) | 49.4 (2.3 – 129.3) |
Related- 1-syngeneic, 1-haploidentical
Others- Mel- 1, FUMEP- 2, Flu-Bu- 1
IMWG- International Myeloma Working Group, sCR- stringent complete response, CR- somplete response, VGPR- very good partial response, PR- partial response, CMV- cytomegalovirus, DLI- donor lymphocyte infusion
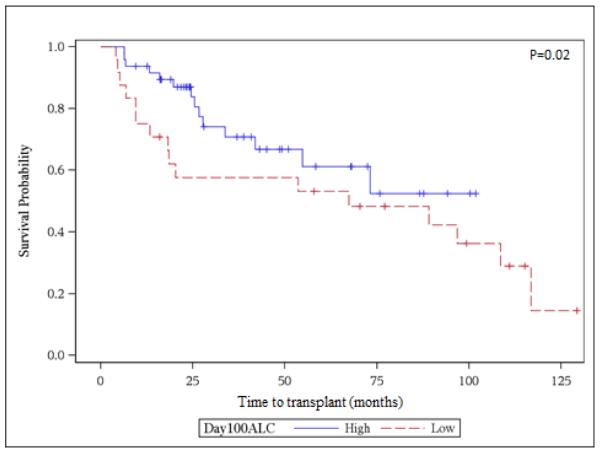
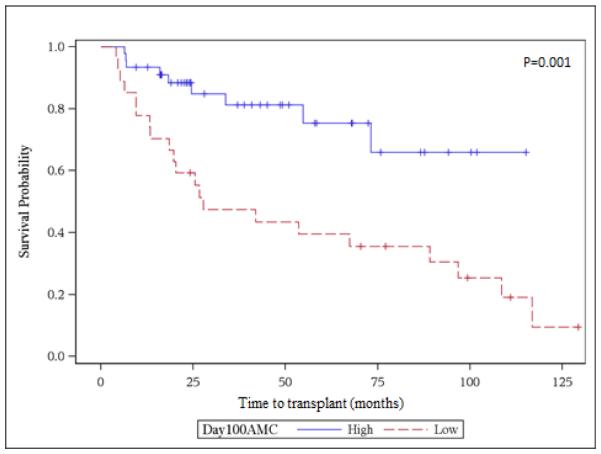
Very early ALC and AMC recovery at days +15 and +30 were not associated with improved OS on unadjusted analysis. However, by day +60, AMC > 300 × 106 was strongly associated with decreased risk of dying (HR 0.47, 95% CI 0.28-0.8, p=0.0002). Day +60 recovery of ALC >500 x106 was not associated with better survival. On univariate analysis, achievement of ALC >500 × 106 and AMC >300 × 106 at day +100 were associated with improved survival (HR 0.47, 95% CI 0.28 – 0.8, p= 0.02) and (HR 0.42, 95% CI 0.24-0.74, p=0.001), respectively. Figures 1A and 1B show the Kaplan Meier curves for overall survival (landmark analysis) of the groups with >=500 vs. <500 ALC and >=300 vs. <300 AMC day 100 count recovery. The 5-year survival was 75 (95% CI 54.4-87.7) % and 27.3 (95% CI 11.3-46.1) % for the high day 100 AMC and low day 100 AMC groups, respectively. Other variables associated with overall survival in unadjusted analysis included age at transplant, disease status at transplant, type of conditioning regimen, number of CD34+ cells infused and relapse or progression of MM after transplant. These factors were then tested in the multivariable models in addition to having AMC and ALC recovery as the main effects. Only two factors were associated with survival on multivariate analysis. Patients with a day 100 AMC count of < 300 had increased mortality with an adjusted hazard ratio of 4.5 (95% confidence interval 1.7-12.0, p-value 0.003). Additionally, increasing age at transplant was also associated with higher mortality (for 1 year increase in age, HR 1.05, 95% confidence interval 1.01-1.10, p-value 0.01). Chronic GVHD did not have an effect on survival in both the univariate and multivariable analysis in our study. One year post-transplant mortality was 18%. The cumulative incidence of cGVHD was 28.8 (95% CI 20.2-41)% and 33.4 (95% CI 24.2-46.1)% at 1 and 3 years, respectively.
Figure 1A.
Univariate survival analysis for day100 ALC recovery. High ALC ≥500 × 106 cells/L, Low ALC <500 × 106 cells/L
Figure 1B.
Univariate survival analysis for day100 AMC recovery. High AMC ≥300 × 106 cells/L, Low ALC <300 × 106 cells/L
Rapid post-alloHCT immune reconstitution is shown to be essential for long term survival in acute leukemia (5). Both lymphocyte and monocyte recovery have been shown to have prognostic significance in acute leukemia (6). Monocytopenia has previously been associated with development of invasive fungal infections between 40 and 100 days post-alloHCT, although it did not achieve significance on multivariable testing (7). Consequently, severe monocytopenia < 100 ×106/dl is associated with reduced survival after diagnosis of invasive fungal infections post allogeneic stem cell transplant(8). In a cohort of patients mainly with acute leukemia and MDS, monocyte recovery of >300×106 was associated with improved survival after allogeneic stem cell transplant in both myeloablative and reduced intensity conditioning (5,6). Similar to these studies, the advantage of our findings is that this information can be easily derived from complete blood count used in routine clinical practice; however, our analysis is limited by the inability to determine the phenotypic and functional changes within the lymphocyte and monocyte populations that may be responsible for this effect seen on survival. Previous studies have shown the deleterious effect of monocyte recovery in patients after autoHCT in lymphoma (9). This could be due to the fact that immunologic tolerance is not required for autologous graft versus disease effect (5). Though the exact mechanism remains unclear, monocytes are known to promote tumorigenesis and angiogenesis and suppress the host immune response to cancer (10). This explains the negative prognostic effect of elevated monocyte counts on solid tumors like gastric cancer, head and neck cancer and hepatocellular carcinoma (11). Monocytes in the circulation are an important source of soluble mediators, which may help support the evolution of malignant cells (12). However, in another study, recovery of NK cells and certain subsets of monocyte cells (CD14+ and not CD16+) were shown to have longer progression free survival in patients with multiple myeloma after autoHCT (13). Animal models have shown that transplanted bone marrow derived cells particularly F4/80+Gr1+(inflammatory monocytes) and F480+Gr1-(resident monocytes) contribute to mucosal regeneration after bone transplantation resulting in the favorable outcomes (14). Recent studies have demonstrated that monocytes may contribute to wound healing from vascular injury, spinal cord injury and myocardial infarction (15). Monocytes are responsible to lead inflammatory cascade and secrete inductive cytokines responsible for revascularization and tissues regeneration at the injury sites. Whether such mechanism is responsible for favorable outcomes after AlloHCT in humans is purely speculative and warrants further investigation (14). Monocytopenia could be due to various reasons one of which is poor graft function. Chimerism studies and subset analysis of white cell recovery at day 100 may have helped in further delineating graft function further in our study.
In conclusion, we show that monocyte re-constitution is associated with superior survival post AlloHCT in MM and could be used as a prognostic marker for outcomes. Lymphocyte recovery was not found to be similarly prognostic on multivariate analysis. Whether strategies to help improve AMC counts in patients with poor AMC recovery by day 60-100 will result in improved outcomes is unclear, but would be worth further study in a larger clinical context.
Acknowledgments
This project was supported by the National Center for Research Resources, The National Center for Advancing Translational Sciences, and the Office of the Director, National Institutes of Health through 8UL1TR000055. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Dr. D’Souza is supported by Institutional Research Grant # 86-004-26 from the American Cancer Society.
Footnotes
The authors disclose no conflicts of interest.
References
- 1.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vekemans MC, Michaux L, Van Den Neste E, Ferrant A. Long-term survival after allogeneic stem cell transplantation for advanced stage multiple myeloma. Br J Haematol. 2014;166:616–618. doi: 10.1111/bjh.12881. [DOI] [PubMed] [Google Scholar]
- 3.Hunter HM, Peggs K, Powles R, Rahemtulla A, Mahendra P, Cavenagh J, et al. Analysis of outcome following allogeneic haemopoietic stem cell transplantation for myeloma using myeloablative conditioning--evidence for a superior outcome using melphalan combined with total body irradiation. Br J Haematol. 2005;128:496–502. doi: 10.1111/j.1365-2141.2004.05330.x. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S, Zhang MJ, Li P, Dispenzieri A, Milone GA, Lonial S, et al. Trends in allogeneic stem cell transplantation for multiple myeloma: a CIBMTR analysis. Blood. 2011;118:1979–1988. doi: 10.1182/blood-2011-02-337329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thoma MD, Huneke TJ, DeCook LJ, Johnson ND, Wiegand RA, Litzow MR, et al. Peripheral blood lymphocyte and monocyte recovery and survival in acute leukemia postmyeloablative allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2012;18:600–607. doi: 10.1016/j.bbmt.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 6.DeCook LJ, Thoma M, Huneke T, Johnson ND, Wiegand RA, Patnaik MM, et al. Impact of lymphocyte and monocyte recovery on the outcomes of allogeneic hematopoietic SCT with fludarabine and melphalan conditioning. Bone Marrow Transplant. 2013;48:708–714. doi: 10.1038/bmt.2012.211. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Vidal C, Upton A, Kirby KA, Marr KA. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47:1041–1050. doi: 10.1086/591969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parody R, Martino R, Sanchez F, Subira M, Hidalgo A, Sierra J. Predicting survival in adults with invasive aspergillosis during therapy for hematological malignancies or after hematopoietic stem cell transplantation: Single-center analysis and validation of the Seattle, French, and Strasbourg prognostic indexes. Am J Hematol. 2009;84:571–578. doi: 10.1002/ajh.21488. [DOI] [PubMed] [Google Scholar]
- 9.Porrata LF, Gertz MA, Litzow MR, Lacy MQ, Dispenzieri A, Inwards DJ, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation for patients with primary systemic amyloidosis. Clin Cancer Res. 2005;11:1210–1218. [PubMed] [Google Scholar]
- 10.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eo WK, Jeong da W, Chang HJ, Won KY, Choi SI, Kim SH, et al. Absolute monocyte and lymphocyte count prognostic score for patients with gastric cancer. World J Gastroenterol. 2015;21:2668–2676. doi: 10.3748/wjg.v21.i9.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilcox RA, Ristow K, Habermann TM, Inwards DJ, Micallef IN, Johnston PB, et al. The absolute monocyte and lymphocyte prognostic score predicts survival and identifies high-risk patients in diffuse large-B-cell lymphoma. Leukemia. 2011;25:1502–1509. doi: 10.1038/leu.2011.112. [DOI] [PubMed] [Google Scholar]
- 13.Callander Natalie S, Rathouz Paul J, Asimakopoulos Fotis, Juckett Mark B, Coe Christopher L, Sheerar Dagna, Ersland Karen, Hematti Peiman, Costanzo Erin S. Recovery Of Natural Killer Cells and Monocyte Subsets Following Autologous Peripheral Blood Stem Cell Transplantation Predicts Longer Progression Free Survival Among Multiple Myeloma Patients. ASH. 2013 [Google Scholar]
- 14.Takaba J, Mishima Y, Hatake K, Kasahara T. Role of bone marrow-derived monocytes/macrophages in the repair of mucosal damage caused by irradiation and/or anticancer drugs in colitis model. Mediators Inflamm. 2010;2010:634145. doi: 10.1155/2010/634145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]