Abstract
Social monogamy is a mating strategy rarely employed by mammalian species. Laboratory studies in socially monogamous prairie voles (Microtus ochrogaster) demonstrate that oxytocin and vasopressin act within the mesolimbic dopamine pathway to facilitate pair-bond formation. Species differences in oxytocin receptor (OTR) and vasopressin 1a receptor (V1aR) distribution in this pathway are associated with species differences in mating strategy. Here we characterize the neuroanatomical distribution of OTR and V1aR binding sites in naturally occurring populations of Taiwan voles (M. kikuchii), which purportedly display social monogamy. Live trapping was conducted at two sites in 2009–2010 and receptor autoradiography for OTR and V1aR was performed on brains from 24 animals. OTR binding in two brain regions where OTR signaling regulates pair-bonding were directly compared with that of prairie voles. Our results show that like prairie voles, Taiwan voles exhibit OTR in the prefrontal cortex, insular cortex, claustrum, nucleus accumbens, caudate-putamen, dorsal lateral septal nucleus, central amygdala, and ventromedial hypothalamus. Unlike prairie voles, Taiwan voles exhibit OTR binding in the CA3 pathway of the hippocampus, as well as the indusium griseum, which has only previously been documented in tuco-tucos (Ctenomys haigi, C. sociabilis), Syrian hamsters (Mesocricetus auratus) and naked mole-rats (Heterocephalus glaber). V1aR binding was present in the ventral pallidum, lateral septum, nucleus basalis, bed nucleus of the stria terminalis, hippocampus, medial amygdala, and anterior, ventromedial and dorsomedial hypothalamus. Marked individual differences in V1aR binding were noted in the cingulate cortex and several thalamic nuclei, remarkably similar to prairie voles. While pharmacological studies are needed to determine whether oxytocin and vasopressin are involved in pair-bond formation in this species, our results lay a foundation for future investigations into the role of these neuropeptides in Taiwan vole social behavior.
Keywords: Oxytocin, vasopressin, neuropeptide distribution, mating systems, social monogamy, vole, pairing, social behaviour
INTRODUCTION
Social mating systems vary widely across vertebrate species. Social monogamy refers to the condition where one male lives in association with only one female. The prevalence of social monogamy across vertebrates varies considerably, with approximately 90% of bird species forming partnerships that endure at least one breeding season (Lack, 1968) and 400 of over 3000 African cichlid species exhibiting biparental brood care (an indicator of social monogamy; Perrone, 1978). In contrast, monogamy is rare in reptiles (While, Uller & Wapstra, 2009) and amphibians (Gillette, Jaeger, & Peterson, 2000). Estimates suggest only 5% of mammalian species exhibit social monogamy, with genetic monogamy being even less common (Lukas & Clutton-Brock, 2012). The neuropeptides oxytocin (OT) and vasopressin (AVP) play important roles in several aspects of species-typical social behaviors, including those related to monogamy (Young & Wang, 2004; Oldfield & Hofmann, 2011; Johnson & Young, 2015). OT, AVP, and their nonmammalian homologues modulate a variety of social behaviors relevant to mating strategies, including parental and alloparental care (Olazábal & Young, 2006; Rilling & Young, 2014), territorial behavior and aggression (Albers, 2012), vocal communications (Goodson, Evans & Bass, 2003), flocking (Goodson et al., 2009) and pair-bonding (Young & Wang, 2004).
Much of our understanding of neural control of mammalian social mating systems comes from laboratory work on voles (Lim et al., 2004b; Young & Wang, 2004; Johnson & Young, 2015). Socially monogamous prairie voles (Microtus ochrogaster) have relatively high concentrations of oxytocin receptors (OTR) and vasopressin 1a receptors (V1aR) in the reward and reinforcement regions of the brain, namely OTR in the nucleus accumbens (NAcc) and prefrontal cortex (PFC) and V1aR in the ventral pallidum (VP). In contrast, non-monogamous voles, such as montane (M. montanus) and meadow voles (M. pennsylvanicus), have low OTR in the NAcc and low V1aR in the VP. Administration of an OTR antagonist into the PFC or NAcc or infusion of a V1aR antagonist into the VP prevent mating-induced partner-preference formation, a proxy of the pair-bond (Young et al., 2001; Lim & Young, 2004). Thus, species differences in OTR and V1aR in the NAcc and VP, respectively, may underlie differences in pair-bonding between prairie and meadow voles.
Increasing V1aR density in the VP of male meadow vole brains resulted in the display of partner-preferences, providing evidence for a causal link between receptor expression and pair-bonding (Lim et al., 2004b). Receptor density can also vary between individuals, contributing to individual differences in social behavior and providing a potential mechanism for creating different sociosexual phenotypes, which can then shape species’ mating systems through natural selection (Olazábal & Young, 2006; Ross et al., 2009; Barrett, Arambula & Young, 2015). Individual variation in V1aR and OTR density in prairie voles have been linked to genetic polymorphisms in the respective genes (Hammock & Young, 2005; King et al., 2016). These observations suggest intraspecific variation in mating systems, as seen in some mammals (Cushing et al., 2001; Bales et al., 2007), may be influenced by the distribution of neuropeptide receptors in the brain (Solomon et al., 2009; Ophir et al., 2012). However, male prairie voles from Illinois and Kansas vary in their display of monogamous behaviors: Illinois males are more behaviorally sensitive to AVP, yet these populations do not differ in V1aR binding in the brain (Cushing et al., 2001). Thus, while neuropeptide receptor expression in particular brain regions may be associated with variation in mating strategy, receptor density alone cannot predict behavioral patterns across populations.
The objective of this study was to characterize OTR and V1aR distributions in the Taiwan vole (M. kikuchii) brain, a rodent endemic to high elevation (3000m) meadows and forests in central Taiwan (Yu, 1994); sites which may be immediately adjacent to one another, but are characterized by different vegetative composition and structure. While little is known about behavioral differences exhibited between habitats in this species, ecology is an important factor in predicting mating system variation across taxa, both interspecifically (Emlen & Oring, 1977; Schamel et al., 2004) and intraspecifically (Cushing et al., 2001; Brashares & Arcese, 2002; Streatfeild et al., 2011). Thus, we hypothesized that intraspecific ecological variation could also select for differences in mating strategy which could be reflected in OTR and V1aR distributions in Taiwan voles from different habitats. Specifically, we hypothesized that when female dispersion is high, as would be predicted in forest habitats, favoring social monogamy, OTR binding in NAcc and V1aR binding in VP will be high. When female aggregation is high, as would be predicted in meadow habitats, favoring polygyny, OTR binding in NAcc and V1aR binding in VP will be low, paralleling the species differences found between prairie and meadow voles.
In Taiwan voles, field data conducted on meadow sites from three theses (written in Chinese) show females have small litters (2.1±0.5) with high birth weights (3.9±0.9g) and slow postnatal development (0.36g/day for first week) in relation to other Microtus species (Lu, 1991). They are also not sexually dimorphic, have a 1:1 sex ratio with no seasonal changes and show no significant difference in home range (HR) size between sexes. In addition, there is extensive HR overlap only between male-female pairs, and microsatellite analyses of litters reveal no extra pair paternity (Wu, 1998; Wu, 2007; Wu, Chiang & Lin, 2012). Combined, these ecological indicators suggest this species may be socially monogamous (Emlen & Oring, 1977). Thus, Taiwan voles provide an additional vole model to determine whether the neural systems regulating pair-bonding are generalizable to other Microtus species. As a first step towards exploring these issues, we characterized the neuroanatomical distribution of OTR and V1aR in Taiwan voles captured from two ecologically distinct habitats.
MATERIALS AND METHODS
Study site
This study was conducted at two naturally occurring populations 2.26km apart near the Endemic Species Research Institute Alpine Research Station, Hehuanshan, Nantou County, Taiwan (meadow: 24°08′19″N, 121°17′17″E, altitude: 3050m; forest: 24°09′41″N, 121°17′09″E, altitude: 3050m). The meadow site was comprised mainly of dwarf-bamboo (Yushania niitakayamensis), along with various grasses (e.g. Miscanthus sinensis and Carex spp.). The forest site was comprised mainly of conifer trees (Abies kawakamii and Tsuga chinensis) in the canopy, along with dwarf-bamboo and mosses in the understory. The two sites differed significantly in the amount of dwarf-bamboo ground cover, bare ground, and mean and maximum bamboo heights, which could potentially influence the social mating strategies employed by the Taiwan vole.
Sample collection
Voles were live-trapped in non-contiguous meadow (0.36ha, 60×60m) and forest (0.25ha, 50×50m) sites in June 2009 and 2010, during the reproductive season. Trap stations were placed at 10 m spacing with two traps at each station. During both years of study, 98 Ugglan Special traps (Type #3; Grahnab AB, Hillerstorp, Sweden; dimensions: 24×8×6cm) in the meadow and 72 traps in the forest were baited with peanut butter-covered oats and sweet potatoes and monitored twice daily (0700–1000h and 1530–1730h) for five consecutive days. Crumpled paper was provided in each trap for warmth. Captured voles were housed individually in small rat cages and provided ad lib food and water. At the end of the five-day trapping, voles were transported to National Taiwan University, where they were euthanized by rapid decapitation. Whole brains were collected, frozen on powdered dry ice and shipped to the Yerkes National Primate Research Center at Emory University in Atlanta, GA. We collected 11 male and 13 female brains between 2009 and 2010. The brains of two males and two females captured at each site in 2009 (n=8) were sectioned from the anterior prefrontal cortex through the hippocampus, to provide a more comprehensive analysis of neuropeptide binding in Taiwan voles. The brains of the remaining animals (n=16) were sectioned from prefrontal cortex through the NAcc and VP, so that we could make quantitative comparisons between animals from forest and meadow sites. The number of samples collected by year, sex and location is shown in Table 1. In order to make direct species comparisons in OTR, brains were also collected from prairie voles (n=11; 8 male, 3 female) from the Emory University breeding colony, originally derived from field captured voles in Illinois, USA. Housing was as described previously (Barrett et al., 2015) under conditions in which prairie voles are also reproductively active. Brains were frozen on powdered dry ice, and sectioned from the anterior prefrontal cortex through the hippocampus. All brains were sectioned to 20μm thickness on a cryostat and placed on Fisher Super Frost-plus slides (Fisher Scientific, Pittsburgh, PA). Slides were stored at −80ºC until used for receptor autoradiography. This research protocol was approved by IACUC committees at University of Louisiana at Monroe and Emory University and conformed to the laws of both the United States and Taiwan.
Table 1.
Number of whole Taiwan vole brain samples collected by year, location and sex, Hehuanshan, Taiwan.
| 2009 | 2010 | Totals | |||
|---|---|---|---|---|---|
| Females | Males | Females | Males | ||
| Meadow | 3 | 2 | 3 | 3 | 11 |
| Forest | 4 | 3 | 3 | 3 | 13 |
| Totals | 7 | 5 | 6 | 6 | 24 |
Receptor autoradiography
OTR and V1aR receptor autoradiography was performed at Emory University as described previously (Ahern & Young, 2009). Two sets of Taiwan vole brain sections were used to demonstrate specificity of binding by co-incubating the radioligand with a high concentration of an OTR selective competitor ligand. To do this, one set of tissue was co-incubated with both 50pM 125I-OVTA and 1μM of an unlabeled OTR-specific agonist ([Thr4Gly7]OT); the other set was co-incubated with 50pM 125I-LVA and 1μM of an unlabeled V1aR-specific antagonist d(CH2)5[Tyr(Me)2]AVP. After the autoradiography procedure, slides were exposed to BioMax MR film (Kodak) for 72h and then developed. Since OTR binding in the NAcc of Taiwan voles appeared consistently low compared to the highly variable OTR density in prairie voles, we processed prairie vole brains for OTR autoradiography and conducted a second assay that included brains from both species to allow for direct quantitative comparisons. In contrast, V1aR binding was robust in the VP of Taiwan voles, similar to previous observations in prairie voles. Our primary goal was to determine the receptor distributions in two Taiwan vole populations as a foundation for future behavioral pharmacology studies, not to determine mating strategy by direct comparisons of receptor density with monogamous species. Anatomical locations were defined using a rat brain atlas as well as previous receptor mapping studies in prairie voles (Paxinos & Watson, 2006).
Quantification of radioligand binding
Optical density, an indicator of radioligand binding, was quantified using AIS software (Imaging Research, Inc.) in brain regions in which the respective receptor has been shown to be critically involved in pair-bonding: for OTR in the PFC and NAcc and for V1aR in the VP and lateral septum (LS). Optical density values from a set of 125I autoradiographic standards (American Radiolabeled Chemicals, St. Louis, MO) were used to generate a standard curve, from which binding density values for brain regions of interest were extrapolated. Three separate 30×30 pixel circular punches from each brain region were used to calculate the average binding density for each region of interest. For each brain, an average background level was taken in the same way from an adjacent region not displaying binding and this value was subtracted from the average for each brain region in order to account for any individual differences in nonspecific binding. Binding density results for each region were analyzed using two-way fixed effect ANOVAs in SPSS with habitat and sex as factors. An α ≤ 0.05 was used in all statistical tests. Species comparisons in OTR binding in the NAcc and PFC between Taiwan and prairie vole were analyzed using a Student’s t-test (PFC) and t-test for unequal variances (NAcc). In order to determine if there were interactions between sex and species, data was further analyzed using two-way fixed effect ANOVAs in SPSS with sex and species as factors. We analyzed log10 transformed data for NAcc (but not PFC), because raw data did not meet assumption of equality of variances (Levene’s test P < 0.05). All of the Taiwan and prairie vole brains that were collected were used in this comparison.
RESULTS
Specificity of radioligand binding in Taiwan voles
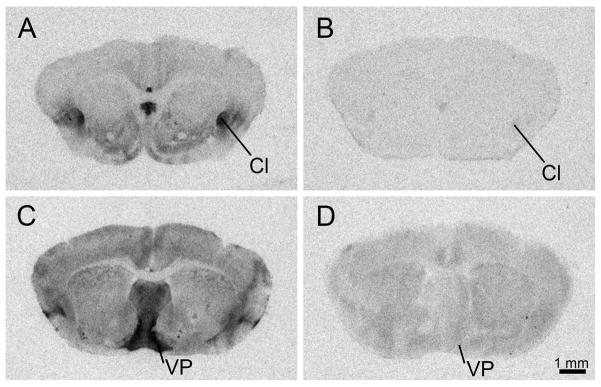
Binding of 125I-OVTA and 125I-LVA was eliminated across the forebrain of the Taiwan vole when co-incubated with a [Thr4Gly7]OT or d(CH2)5[Tyr(Me)2]AVP, respectively (Fig. 1), suggesting that the ligands were selectively binding to OTR and V1aR.
Figure 1.
Representative film autoradiographs showing binding specificity of oxytocin receptor (OTR) and vasopressin 1a receptor (V1aR) radioligands in Taiwan voles. 125I-OVTA alone (A); 125I-OVTA plus 1μM of the OTR antagonist, [Thr4Gly7]OT (B); 125I-LVA alone (C); 125I-LVA plus 1μM of the V1aR antagonist, d(CH2)5[Tyr(Me)2]AVP (D). Cl- claustrum; VP- ventral pallidum.
Distribution of OTR in Taiwan voles and comparison to prairie voles
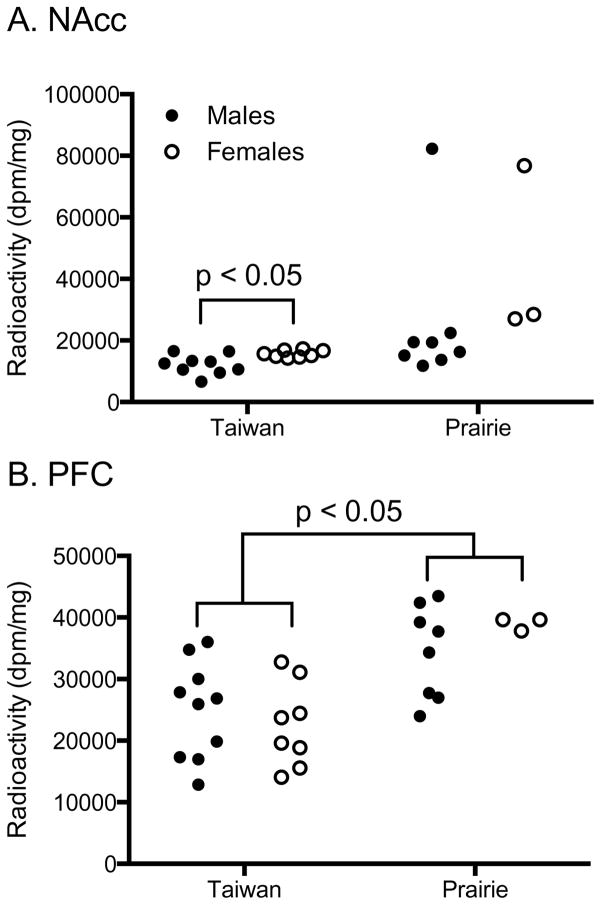
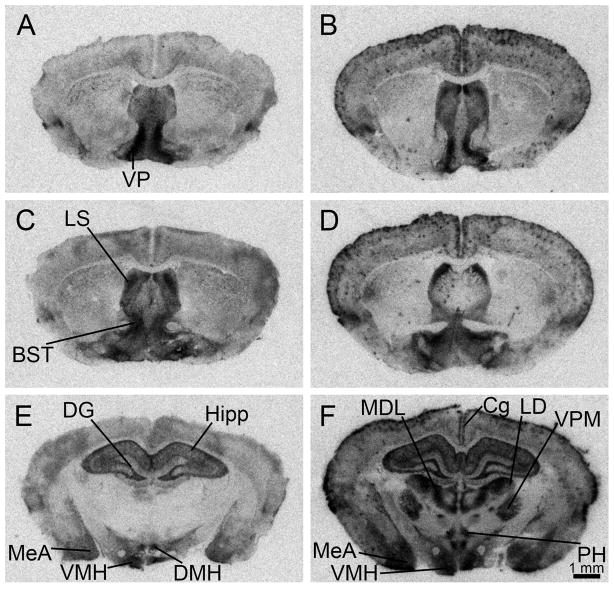
OTR binding in Taiwan voles (Fig. 2 A,C,E,G) was found in the PFC, NAcc, caudate-putamen (CP), insular cortex (IC), claustrum (Cl), indusium griseum (IG), dorsal lateral septal nucleus (LSd), CA3 pathway of the hippocampus (CA3), central amygdala (CeA), and ventromedial nucleus of the hypothalamus (VMH). When compared with prairie voles (Fig. 2 B,D,F,H), quantification and analysis of two brain areas in which OTR has previously been shown to be necessary for pair-bonding showed slightly lower but statistically significant species differences in the PFC (Student’s t-test, t26=4.23, P=<0.0001), but not the NAcc (t-test for unequal variances, t10.19=2.72, P=0.054, mean difference=7.40; Fig. 3). Further analysis to determine potential sex*species interactions revealed 125I-OVTA binding in the PFC was greater in prairie voles than Taiwan voles (F1,29=19.723, P=<0.0001). 125I-OVTA binding in the PFC did not differ by sex (F1,29=0.142, P=0.710). There was no statistically significant sex*species interaction (F1,29=1.368, P=0.253). 125I-OVTA binding in the NAcc did not differ by species (F1,29=2.521, P=0.125) or sex between species (F1,29=1.561, P=0.223). There was no statistically significant sex*species interaction (F1,29=0.143, P=0.709).
Figure 2.
Representative film autoradiographs illustrating the distribution of oxytocin receptor binding at rostrocaudal levels in a female Taiwan vole (A,C,E,G) as compared to a male prairie vole (B,D,F,H). BST- bed nucleus of the stria terminalis; CA3- CA3 pathway of the hippocampus; CeA- central amygdala; Cg- cingulate cortex; Cl- claustrum; CP- caudate-putamen; IC- insular cortex; IG- indusium griseum; LS- lateral septum; NAcc- nucleus accumbens; PFC- prefrontal cortex; VMH- ventromedial nucleus of the hypothalamus.
Figure 3.
Quantification of oxytocin receptor (OTR) binding in the nucleus accumbens (NAcc; A) and prefrontal cortex (PFC; B) shows that prairie voles have significantly greater OTR binding in the PFC, but not the NAcc as compared to Taiwan voles, and that OTR levels in Taiwan voles in these regions overlap with the levels found in prairie voles. OTR binding in the NAcc was also significantly greater in female as compared to male Taiwan voles.
Distribution of V1aR in Taiwan voles
Taiwan voles show V1aR binding in the VP, LS, nucleus basalis (NB; not shown in figure), anteroventral periventricular nucleus (APVn; not shown in figure), bed nucleus of the stria terminalis (BST), the dentate gyrus (DG), the CA1, CA2 and CA3 fields of the hippocampus (Hipp), medial amygdala (MeA), anterior hypothalamus (AH; not shown in figure), VMH and dorsomedial nucleus of the hypothalamus (DMH; Fig. 4). Marked individual differences in V1aR binding were noted in the cingulate cortex (Cg), the posterior hypothalamic nucleus (PH), and several thalamic nuclei, including the lateral division of the mediodorsal nucleus of the thalamus (MDL), the laterodorsal nucleus of the thalamus (LD) and the ventral posteromedial nucleus of the thalamus (VPM; Fig. 4E,F). This marked individual variation was not associated with individual variation in the VP or LS.
Figure 4.
Representative film autoradiographs illustrating the distribution of vasopressin 1a receptor (V1aR) binding at rostrocaudal levels in a male Taiwan vole (A,C,E), as compared to a second male Taiwan vole (B,D,F), in order to show individual differences in V1aR binding in several brain regions. BST- bed nucleus of the stria terminalis; Cg- cingulate cortex; DG- dentate gyrus; DMH- dorsomedial hypothalamus; Hipp- CA1, CA2, and CA3 fields of the hippocampus; LD- laterodorsal nucleus of the thalamus; LS- lateral septum; MDL- lateral division of the mediodorsal nucleus of the thalamus; MeA- medial amygdala; PH- posterior hypothalamic nucleus; VMH- ventromedial nucleus of the hypothalamus; VP- ventral pallidum; VPM- ventral posteromedial nucleus of the thalamus.
Quantitative differences in receptor binding of Taiwan voles from two habitats in brain regions where OTR and V1aR signaling have been shown to be involved in partner-preference formation
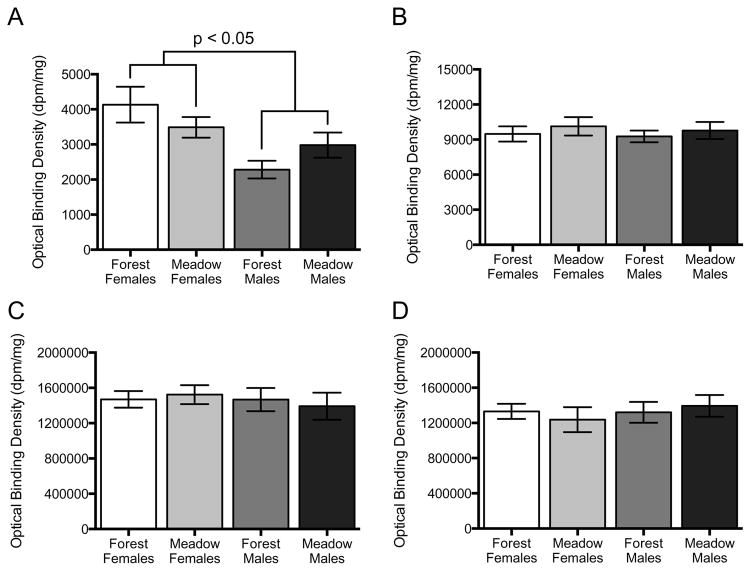
125I-OVTA binding in the NAcc was greater in females than males (F1,19=8.69, P=0.008; Fig. 3A; Fig. 5A). 125I-OVTA binding in the NAcc did not differ by habitat (F1,19=0.005, P=0.946). There was no statistically significant sex*habitat interaction (F1,19=2.82, P=0.110).125I-OVTA binding in the PFC did not differ by sex (F1,19=0.187, P=0.670) or habitat (F1,19=0.719, P=0.407; Fig. 5B). There was no statistically significant sex*habitat interaction (F1,19=0.014, P=0.908).125I-LVA binding in the VP did not differ by sex (F1,20=0.384, P=0.543) or habitat (F1,20=0.008, P=0.931; Fig. 5C). There was no statistically significant sex*habitat interaction (F1,20=0.503, P=0.487). 125I-LVA binding in the LS did not differ by sex (F1,20=0.304, P=0.587) or habitat (F1,20=0.007, P=0.932; Fig. 5D). There was no statistically significant sex*habitat interaction (F1,20=0.288, P=0.597).
Figure 5.
Mean optical densities (a measure of receptor binding level) and standard errors of 125I-OVTA binding in the nucleus accumbens (A) and prefrontal cortex (B) and 125I-LVA binding in the ventral pallidum (C) and lateral septum (D) for forest females (white), meadow females (light gray), forest males (dark gray) and meadow males (black) in 2009 and 2010. OTR binding in the NAcc was significantly greater in female as compared to male Taiwan voles. All other statistical comparisons were not significant.
DISCUSSION
Binding of 125I-OVTA and 125I-LVA radioligands was highly specific in Taiwan voles, as was expected since the ligands have been used previously in other Microtus species (Ahern and Young, 2009). Specific OTR binding was detected in the PFC, NAcc, CP, IC, Cl, LSd, CeA, and VMH, similar to prairie voles (Olazábal & Young, 2006; Beery, Lacey & Francis, 2008; Ophir et al., 2012). In contrast, Taiwan voles exhibited OTR binding in the IG and CA3, where prairie voles do not (Fig. 2). Of particular interest is OTR in the IG, a trait previously thought to be unique to tuco-tucos (Ctenomys haigi, C. sociabilis; Beery et al., 2008), Syrian hamsters (Mesocricetus auratus; Dubois-Dauphin et al., 1992) and naked mole-rats (Heterocephalus glaber; Kalamatianos et al., 2010). While the function of OTR in the IG remains unclear, eusocial naked mole-rats were marked by high OTR binding in the IG not present in solitary Cape mole-rats (Georychus capensis). In contrast, tuco-tucos showed no difference in OTR binding in the IG between social and solitary species (Berry et al. 2008; Kalamatianos et al., 2010).
Taiwan voles had specific V1aR binding in the VP, BST, LS, NB, PA, DG and VMH, which is consistent with prairie vole binding in other studies (Phelps & Young, 2003; Lim, Murphy & Young, 2004a; Beery et al., 2008). Marked individual variation in V1aR binding was observed in Taiwan voles in the Cg and thalamic regions, including the MDL, LD and VPM. This is remarkably similar to prairie voles, where V1aR binding shows marked individual variation in the Cg and thalamus, but not in the VP (Phelps & Young, 2003; Ophir, Wolff & Phelps, 2008). This variation could be associated with mating strategies and reproductive success, as male prairie vole wanderers with lower V1aR binding in the Cg and LD were more reproductively successful than wanderers with high V1aR binding in these areas (Ophir et al., 2008). Interestingly, Taiwan voles display V1aR binding in various hypothalamic structures, including the AH (which is associated with selective aggression in prairie voles; Gobrogge et al., 2009) and the DMH and PH, which are largely absent in prairie voles (Phelps & Young, 2003; Beery et al., 2008).
The Taiwan vole exhibited high levels of V1aR in both the VP and LS, regions in which AVP is known to facilitate pair-bonding. The high binding in the VP is consistent with other monogamous species, including prairie voles, woodland voles (M. pinetorum), California mice (Peromyscus californicus) and common marmosets (Callithrix jacchus), which all have high levels of V1aR in this region compared to related non-monogamous species, including montane voles, meadow voles, white footed mice (Peromyscus leucopus) and rhesus macaques (Macaca mulatta; Insel, Gelhard & Shapiro, 1991; Phelps & Young, 2003; Freeman et al., 2014a). However, V1aR expression does not always predict mating strategy, as there was no relationship between V1aR distributions and mating strategy in a wide survey of Peromyscus (Turner et al., 2010).
Binding of OTR in the PFC and the NAcc was also present in Taiwan voles. OTR signaling in both of these regions is necessary for partner-preference formation in prairie voles, while females in non-monogamous vole species display very low OTR binding in the NAcc (Johnson & Young 2015). Although Taiwan voles displayed significantly less OTR in the PFC when compared to prairie voles, the density of OTR in the NAcc was only marginally lower in Taiwan voles than prairie voles (P=0.054; Fig. 3). While a trend for Taiwan voles to have lower OTR binding in the NAcc is apparent, prairie voles display remarkable variation in OTR binding in the NAcc, with some individuals showing high density of binding. In our sample, Taiwan vole binding was within the range of binding found in prairie voles. Although it is possible that larger sample sizes could reveal species differences on NAcc OTR, we must stress that receptor expression patterns cannot be used to infer mating strategy, or whether OT or AVP can facilitate pair-bonding in particular species. For example, while monogamous common marmosets have high OTR in the NAcc (Schorscher-Petcu, Dupré & Tribollet, 2009; Smith et al., 2010), monogamous titi monkeys (Callicebus cupreus) do not, but instead have V1aR in the NAcc (Freeman et al., 2014b). In addition, monogamous California mice lack OTR binding in the NAcc (Insel et al., 1991). Nevertheless, eusocial naked mole-rats have high levels of OTR in the NAcc, while social Cape mole-rats do not (Kalamatianos et al., 2010). These various findings indicate that although OTR binding in the NAcc is associated with monogamous behaviors in certain species, it is also associated with sociality between non-reproductive conspecifics and alloparental behaviors, as exemplified by naked mole-rats (Kalamatianos et al., 2010).
There was no difference in OTR and V1aR binding between sex and habitat for all the brain regions analyzed, except for OTR binding in the NAcc. While there was no sex or sex*species differences observed between Taiwan and prairie voles (likely due to high variability in the latter), the analysis of Taiwan voles alone revealed females had greater OTR binding in the NAcc than males (P=0.008). The sex difference observed is novel and has not been reported in any other Microtus species (Lim et al., 2004a). Future studies are needed to replicate this finding and elucidate how sex differences in OTR signaling influence this species’ social behavior. Since there was no difference in OTR or V1aR binding between habitats, our hypothesis that intraspecific ecological variation may influence neuropeptide binding was not supported, potentially due to individual movement between habitat types.
Our data demonstrate similar neurochemical pathways known to be involved in pair-bonding are present in Taiwan voles, although the present data cannot be used to infer a monogamous mating strategy, as noted above. However, microsatellite analyses that found no evidence of extra pair paternity suggest that, unlike prairie voles (Solomon et al., 2004), Taiwan voles may be genetically monogamous (Wu et al., 2012), like three other species: P. polionotus (Foltz, 1981), P. californicus (Ribble, 1991) and woodland voles (Marfori et al., 1997). The mechanisms controlling for social versus genetic monogamy are largely unknown. We are currently developing laboratory partner-preference tests which can determine whether OTR or V1aR play a role in Taiwan vole pair-bond formation as occurs in prairie voles. Further research on how mechanisms controlling for social organization differ between social monogamy versus genetic monogamy would be beneficial. By describing the neuroanatomical distribution of OTR and V1aR in the Taiwan vole, we have laid the foundation for future behavioral pharmacological studies to explore any role of OT and AVP in the modulation of sociosexual behavior in this intriguing model organism that could greatly complement research performed in other members of the genus, including prairie, meadow, woodland and more recently, Mandarin voles (Microtus mandarinus; Zhao et al., 2002, Jia et al., 2008; Wu et al., 2011).
Acknowledgments
We thank the Endemic Species Alpine Research Station on Hehuanshan, Taiwan for providing facilities and equipment to conduct our research, Phebe Quan for field assistance, and Joydeep Bhattacharjee and David Tao for statistical advice. This project was funded by National Science Foundation (NSF) Office of International Science and Engineering (0901056 to LDH; 0836799 to LJY), Louisiana Board of Regents Pfund (NSF 0836776 to LDH), NSF Eastern Asia and Pacific Summer Institutes (1015256 to ARC), National Institutes of Health (P51OD011132 to Yerkes National Primate Research Center) and American Society of Mammalogists Grant-in-Aid (to ARC).
References
- Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster) Front Behav Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE. The regulation of social recognition, social communication and aggression: Vasopressin in the social behavior neural network. Horm Behav. 2012;61:283–292. doi: 10.1016/j.yhbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP. Neural correlates of pair-bonding in a monogamous primate. Brain Res. 2007;1184:245–253. doi: 10.1016/j.brainres.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Arambula SE, Young LJ. The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl Psychiatry. 2015;5:e606. doi: 10.1038/tp.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Lacey EA, Francis DD. Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis) J Comp Neurol. 2008;507:1847–1859. doi: 10.1002/cne.21638. [DOI] [PubMed] [Google Scholar]
- Brashares JS, Arcese P. Role of forage, habitat and predation in the behavioural plasticity of a small African antelope. J Anim Ecol. 2002;71:626–638. [Google Scholar]
- Cushing BC, Martin JO, Young LJ, Carter CS. The effects of peptides on partner-preference formation are predicted by habitat in prairie voles. Horm Behav. 2001;39:48–58. doi: 10.1006/hbeh.2000.1633. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Pévet P, Barberis C, Tribollet E, Dreifuss JJ. Localization of binding sites for oxytocin in the brain of the golden hamster. Neuroreport. 1992;3:797–800. doi: 10.1097/00001756-199209000-00019. [DOI] [PubMed] [Google Scholar]
- Emlen ST, Oring LW. Ecology, sexual selection and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- Foltz DW. Genetic evidence for long-term monogamy in a small rodent, Peromyscus polionotus. Am Nat. 1981;117:665–675. [Google Scholar]
- Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrino. 2014a;45:128–41. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, Young LJ. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neurosci. 2014b;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette JR, Jaeger RG, Peterson MG. Social monogamy in a territorial salamander. Anim Behav. 2000;59:1241–1250. doi: 10.1006/anbe.2000.1437. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci. 2009;106:19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Bass AH. Putative isotocin distributions in sonic fish: Relation to vasotocin and vocal-acoustic circuitry. J of Comp Neurol. 2003;462:1–14. doi: 10.1002/cne.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science. 2009;325:862–866. doi: 10.1126/science.1174929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science. 2005;308:1630–1634. doi: 10.1126/science.1111427. [DOI] [PubMed] [Google Scholar]
- Insel TR, Gelhard R, Shapiro LE. The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neurosci. 1991;43:623–630. doi: 10.1016/0306-4522(91)90321-e. [DOI] [PubMed] [Google Scholar]
- Jia R, Tai F, An S, Broders H, Sun R. Neonatal manipulation of oxytocin influences the partner-preference in mandarin voles (Microtus mandarinus) Neuropeptides. 2008;42:525–533. doi: 10.1016/j.npep.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ. Neurobiological mechanisms of social attachment and pair-bonding. Curr Opin Behav Sci. 2015;3:38–44. doi: 10.1016/j.cobeha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamatianos T, Faulkes CG, Oosthuizen MK, Poorun R, Bennett NC, Coen CW. Telencephalic binding sites for oxytocin and social organization: A comparative study of eusocial naked mole-rats and solitary cape mole-rats. J Comp Neurol. 2010;518:1792–1813. doi: 10.1002/cne.22302. [DOI] [PubMed] [Google Scholar]
- King LB, Walum H, Inoue K, Young LJ. Polymorphisms in the oxytocin receptor robustly predict brain expression patterns and social attachment. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2015.12.008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack D. Ecological adaptations for breeding in birds. London: Methuen; 1968. [Google Scholar]
- Lim MM, Murphy A, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) J Comp Neurol. 2004a;468:555–570. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazábal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner-preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004b;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair-bond formation in the monogamous prairie vole. Neurosci. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Lukas D, Clutton-Brock T. Cooperative breeding and monogamy in mammalian societies. Proc R Soc B. 2012;279:2151–2156. doi: 10.1098/rspb.2011.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfori MA, Parker PG, Gregg TG, Vandenbergh JG, Solomon NG. Using DNA fingerprinting to estimate relatedness within social groups of pine voles. J Mammal. 1997;78:715–724. [Google Scholar]
- Olazábal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm Behav. 2006;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Oldfield RG, Hofmann HA. Neuropeptide regulation of social behavior in a monogamous cichlid fish. Physiol Behav. 2011;102:296–303. doi: 10.1016/j.physbeh.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Gessel A, Zheng DJ, Phelps SM. Oxytocin receptor density is associated with male mating tactics and social monogamy. Horm Behav. 2012;61:445–453. doi: 10.1016/j.yhbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Wolff JO, Phelps SM. Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. PNAS. 2008;105:1249–1254. doi: 10.1073/pnas.0709116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. New York: Academic Press; 2006. [Google Scholar]
- Perrone M., Jr Mate size and breeding success in a monogamous cichlid fish. Environ Biol Fishes. 1978;3:193–201. [Google Scholar]
- Phelps SM, Young LJ. Extraordinary Diversity in Vasopressin (V1a) Receptor Distributions among Wild Prairie Voles (Microtus ochrogaster): Patterns of variation and covariation. J Comp Neurol. 2003;466:564–576. doi: 10.1002/cne.10902. [DOI] [PubMed] [Google Scholar]
- Ribble DO. The monogamous mating system of Peromyscus californicus as revealed by DNA fingerprinting. Behav Ecol Sociobiol. 1991;29:161–166. [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its impact on offspring social development. Science. 2014;345:771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schamel D, Tracey DM, Lank DB, Westneat DF. Mate guarding, copulation strategies and paternity in the sex-role reversed, socially polyandrous red-necked phalarope Phalaropus lobatus. Behav Ecol Sociobiol. 2004;57:110–118. [Google Scholar]
- Schorscher-Petcu A, Dupré A, Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neurosci Lett. 2009;461:217–222. doi: 10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Smith AS, Ågmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm Behav. 2010;57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon NG, Keane B, Knoch LR, Hogan PJ. Multiple paternity in socially monogamous prairie voles (Microtus ochrogaster) Can J Zool. 2004;82:1667–1671. [Google Scholar]
- Solomon NG, Richmond AR, Harding PA, Fries A, Jacquemin S, Schaefer RL, Lucia KE, Keane B. Polymorphism at the avpr1a locus in male prairie voles correlated with genetic but not social monogamy in field populations. Mol Ecol. 2009;18:4680–4695. doi: 10.1111/j.1365-294X.2009.04361.x. [DOI] [PubMed] [Google Scholar]
- Streatfeild CA, Mabry KE, Keane B, Crist TO, Solomon NG. Intraspecific variability in the social and genetic mating systems of prairie voles, Microtus ochrogaster. Anim Behav. 2011;82:1387–1398. [Google Scholar]
- Turner LM, Young AR, Rompler H, Schoneberg T, Phelps SM, Hoekstra HE. Monogamy evolves through multiple mechanisms: Evidence from V1aR in deer mice. Mol Biol Evol. 2010;27:1269–1278. doi: 10.1093/molbev/msq013. [DOI] [PubMed] [Google Scholar]
- While GM, Uller T, Wapstra E. Family conflict and the evolution of sociality in reptiles. Behav Ecol. 2009;20:245–250. [Google Scholar]
- Wu JS. Master’s thesis. Tunghai Univ; Taichung, Taiwan: 2007. Mating system of Taiwan vole (Microtus kikuchii): Evidence from field data and microsatellite DNA. in Chinese with English abstract. [Google Scholar]
- Wu JS, Chiang PJ, Lin LK. Monogamous system in the Taiwan Vole (Microtus kikuchii) inferred from microsatellite DNA and home ranges. Zool Stud. 2012;51:204–212. [Google Scholar]
- Wu PJ. Master’s thesis. Tunghai Univ; Taichung, Taiwan: 1998. Population ecology and activity pattern of Kikuchii’s field vole (Microtus kikuchii) in Hohuanshan area. in Chinese with English abstract. [Google Scholar]
- Wu R, Yuan A, Yuan Q, Guo R, Tai F, Song Z, Yu C. Comparison of sociability, parental care and central estrogen receptor alpha expression between two populations of mandarin voles (Microtus mandarinus) J Comp Physiol A. 2011;197:267–277. doi: 10.1007/s00359-010-0609-2. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair-bonding. Nature. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Yu HT. Distribution and abundance of small mammals along a subtropical elevational gradient in central Taiwan. J Zool. 1994;234:577–600. [Google Scholar]
- Zhao Y, Tai F, Wang T, Zhao X, Li BM. Effects of the familiarity on mate choice and mate recognition in Mictrous mandarinus and M. oeconomus. Curr Zool. 2002;48:167–174. [Google Scholar]