Abstract
Introduction: At the present time there is still concern regarding the long-term deleterious effects of right ventricular apical pacing in patients referred for auriculoventricular node ablation (AVNA). Furthermore, scarce information is available regarding differences in the follow up according to the baseline cardiopathy and predictors associated with a worse outcome.
Methods: 104 consecutives patients referred for AVNA were retrospectively analyzed. Patients included were seen in the outpatient clinic at 6, 12 and 24 months post ablation (mean follow-up 24 ± 2 months). An echocardiogram two years after the procedure was obtained in 68 patients. Three categories were done according to the change in the left ventricular function (LVEF) (increase, decrease or absence of change, defined as less than 10% variation in either LVEF).
Results: After two years of follow up there was a decrease in the rate of hospital admission (from 0.9 admission/year to 0.35, p<0.001), an increase in the functional status in at least one NYHA class in 58 patients, and an increase in the global LVEF (from 48.9% to 54,1%; p<0.001). Valvular replacement and LVEF less than 50% were independently associated with a decrease in the LVEF. Regarding safety issues, one patient who presented a polymorphic ventricular tachycardia (Torsade de pointes) 60 minutes after the ablation.
Conclusions: AVNA results in a decrease in hospital admission rates and an improvement in functional status. Baseline LVEF < 50% and mitral valvulopathy were multivariate predictor of LVEF decline, hence, it is our belief that, in this particular population, the “ablate and pace” strategy is not the most suitable option, and or maybe a biventricular pacemaker should be implanted or an AF ablation reconsidered."
Finally, although it is a safe procedure and rate of complications were low, there is a potential risk of fatal complications.
Keywords: Catheter Ablation of the Atrioventricular Node, Right Ventricular Pacing, Biventricular Pacing, Ventricular Tachycardia
Introduction
In the last decade several advances in the field of electrophysiology have been described, as different catheters and modern navigation systems aimed to address complex arrhythmias. However, despite this revolution, the atrioventricular node ablation (AVNA), 30 years after its description by Gallagher[1] and Scheinman,[2] remains a useful and simple tool in selected patients with atrial arrhythmias refractory to medical therapy or ablation.[3]
Conversely, despite the initially reported positive results, there is still concern regarding the long-term deleterious effects of right ventricular apical pacing (RVP), which as it is known, have potentially adverse effects over the left ventricular ejection fraction (LVEF).[4] Moreover, scarce information is available regarding the predictors of worst outcome after AVNA. Finally, there are also concerns regarding the potential complications (failure in the stimulation system, stroke and sudden death[5]).
With this background we aimed to determine the change in LVEF after AVNA and RV apical pacing and determine the clinical predictors of LVEF deterioration.
Methods
Study Design
We retrospectively analyzed 104 consecutives patients referred to the electrophysiology (EP) laboratory for ANAV between January 2003 and January 2011 in three different EP Units (Hospital Clínico Universitario Santiago de Compostela, Spain; Clínica Universidad de Navarra. Pamplona, Spain and Heart Rhythm Management Centre, University Hospital Brussels, Belgium). Patients underwent the procedure for the control of persistent symptoms despite pharmacologic therapy for maintenance of sinus rhythm or ventricular rate control (IIa indication). The variables collected were age, sex, presence of valvular heart disease, ischemic heart disease, type of arrhythmia that led to the indication, medical treatment at the moment of the ablation, pacing mode, and complications during and after the procedure. The functional status (FS) according to the New York Heart Association classification (NYHA), echocardiographic parameters, heart rate and the number of admissions due to heart failure were collected at baseline and two years after the procedure. Special attention was paid to the need of upgrading, the number of deaths from major cardiac events and all causes. The follow up was performed at 6 months, one and two years after the ablation (mean observation period 24 ± 2 months). A transthoracic echocardiogram (TTE) was performed two years after the intervention in 68 patients. Of patients with valvular heart disease, a TTE was obtained in all of them.
Three categories were done according to the change in the left ventricular function two years after the procedure: those who showed an improvement in the LVEF greater than 10%, those in which there was no change in the LVEF (greater than or less than 10%) and finally those in which a decrease in LVEF greater than 10% was detected. This 10% threshold was selected because it has been reported that such a difference is clinically relevant and reproducible by transthoracic echocardiography.[6]
Atrioventricular Junction Ablation and Pacemaker Implantation
Radio frequency ablation of the AV node was performed through the right femoral vein. In those patients were AV block was not achieved through the femoral approach it was performed through the femoral artery. Complete atrioventricular block was achieved in all patients. In those patients without previous pacemaker, a rate-responsive ventricular pacemaker was implanted if the patient was in AF at the time of the procedure and if attempts to restore and maintain sinus rhythm by means of cardioversion were not performed. A dual-chamber, rate-adaptive pacemaker with leads in the right atrium and right ventricle was implanted if the patient was in sinus rhythm at the time of the procedure or if the patient was in AF but restoration and maintenance of sinus rhythm was planned.
Statistical Analysis
Data are presented as percentages or as means ± standard deviation (SD). A two-sided P-value < 0.05 was considered statistically significant. Change in LVEF was assessed as a continuous variable using paired t-tests. Comparisons in the number of hospital admissions were analyzed using Student t tests and chi-square test to assess statistical differences between changes in ejection fraction (LVEF) and the change in functional status. Univariate and multivariate associations of baseline variables with a 10% absolute change in LVEF were assessed using logistic regression analysis. Data were processed using SPSS 15.0 (SPSS Inc, Chicago, IL).
Results
A total of 104 patients were included. Of them, 54,7% were women and 45,3% men. The mean age was 72 years old (Table 1) . Regarding the type of baseline heart disease the most frequent was the valvular origin (32.8%). Main reason for the ablation was fast atrial fibrillation (AF) refractory to medical treatment. With regard to the NYHA functional class, the vast majority of the sample was in NYHA class III (58.6%), the rest were in class II and IV. Mean LVEF was 48.9 + / - 16.2%. Finally, 72,1% of the patients were under beta-blocker treatment, 75% calcium channel antagonists and 62,5% under digoxin.
Table 1. Clinical and demographic baseline characteristics of the study population.
Quantitative variables were expressed as the mean and standard deviation (SD). Abbreviations: ACE: angiotensin-converting enzyme inhibitors. AF: atrial fibrillation. AT: atrial tachycardia. AT1: angiotensin II subtype 1 (AT1) receptor antagonists. AVNA: catheter ablation of the atrioventricular node. FS: functional status. HR: heart rate. ICD: implantable cardioverter defibrillator. LA: left atrial dimension (anteroposterio). LVEF: left ventricular ejection fraction. TDD: Telediastolic diameter. TSD: Telesistolic diameter. TDV: Telediastolic volumeter. NYHA: New York Heart Association functional.
| Age | 72,3 ± 9,64 |
| Men/women (%) | 45,3/54,7 |
| Cardiopathy (%) | |
| Ischemic | 17,2 |
| Valve disease | 32,8 |
| Dilated | 15,6 |
| Tachymyocardiopathy | 12,6 |
| Hypertensive | 3,1 |
| Hypertrophic | 3,1 |
| None | 15,9 |
| FS NYHA (%) | |
| I | 0 |
| II | 20,7 |
| III | 58,6 |
| IV | 20,7 |
| LVEF (%, SD) | 48.9 ±16.22 |
| TDD (mm, SD) | 54,1 ± 9,4 |
| TDV (ml/m2, SD) | 159,9 ± 63,2 |
| LA (AP) (cm) | 5,4 ± 0,9 |
| Reason of the AVNAV (%) | |
| Tachycardic AF | 7,8 |
| Brady-Tachy syndrome | 7,9 |
| AT/left atrial flutter | 12,5 |
| HR basal (lpm) | 110,3 ± 31,6 |
| Admission/year previous to the AVNA | 1,08 ± 1,4 |
| Concomitant medications | |
| Beta-blockers | 72,1% |
| Calcium channel antagonists | 75% |
| Amiodarone | 29,8% |
| Digoxine | 62,5% |
| ACE inhibitors/AT1 | 78,8% |
Concerning outcomes two years after the ablation there was a significant decrease in the number of hospital admissions (0,9 admission / year prior to the procedure to 0.35 admissions / two years later, p <0.001), improvement in functional status (at least one stage of the NYHA) in 58 (55,7%) and an overall improvement in the mean LVEF (n = 48), from 48.9% to 54,1%; p<0.001. Analyzing this variable as the percentage change in LVEF (on those patients with at least one TTE previous to the procedure and another one two years later), an improvement of more than 10% was observed in 14 patients (20,5%), absence of change greater than 10% in 32 (47%) and a decline in the LVEF of more than 10% in 22 patients (32,3%). One patient died within the study period due to progressive heart failure and another one due to a community-acquired pneumonia.
It is noteworthy than 7 patients (6,73%) required and upgrade to biventricular pacing, 3 in patients without valvular heart disease and 4 in those patients with valvular heart disease (n=32, 17 of them with previous mitral valvular replacement). Regarding those patients with valvular heart disease, 6 of them presented LVEF decline (>10%) within the first year, requiring 4 of them upgrading to biventricular pacing (with normalization of LVEF thereafter). One of these patients had clinical and echocardiography deterioration within two months after the ablation, with subsequent improvement two months after the upgrading to BVP.
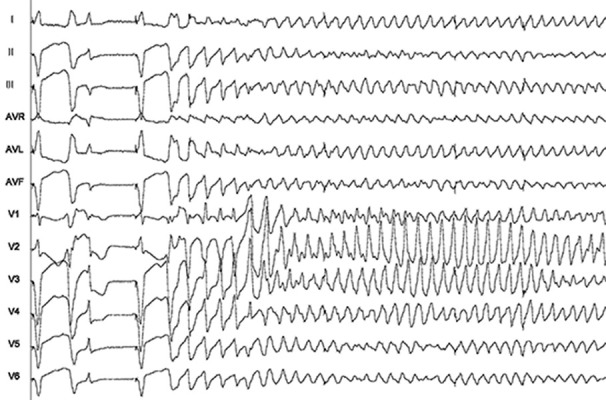
Finally, with regard to the arrhythmic risk, one patient presented a ventricular tachycardia (torsade de pointes) 60 minutes after the ablation, which was preceded by frequents ventricular extrasystoles. It was successfully treated with an external electrical cardioversion (Figure 1) . This patient did not present further events (documented in the pacemaker) after two years of follow up. She had history of mitral valve replacement, and AF with fast ventricular response (around 130-140 bpm) despite combination of BB and ACA. It should be point out that pacemakers were programmed to 80-90 bpm during the first three months after ablation. Afterwards heart rate was decreased to 60 bpm. There were not more remarkable arrhythmias in the rest of the sample (clinical nor recorded in the pacemaker).
Figure 1. Ventricular tachycardia (Torsade de Pointes) recorded 60 minutes after the AVNA in a patient with mitral valve disease and atrial fibrillation with rapid ventricular response.
Univariate and multivariate analyses was performed on those patients who had LVEF impairment within 2 year. Table 2 lists the univariate associations with absolute LVEF deterioration ≥ 10%. Briefly, remark that baseline LVEF < 50% and previous mitral valve replacement were multivariate predictor of LVEF deterioration.
Table 2. Univariate Predictors of left ventricular ejection fraction decline.
| OR | 95% CI | p-value | |
|---|---|---|---|
| History of CHF | 0.50 | 0.15-2.0 | 0.4 |
| Female | 1.1 | 0.9-1.3 | 0.2 |
| Valvular replacement | 4.07 | 3.9-5.1 | 0.04 |
| LVEF < 50% | 5.73 | 1.5-8.9 | 0.03 |
| Use of digoxine | 1.05 | 0.9-2.7 | 0.3 |
| Use of betablokers | 0.88 | 0.7-2.9 | 0.08 |
| Use of CAA | 1.2 | 1.0-1.8 | 0.9 |
| Previous heart rate | 0.90 | 0.88-1.05 | 0.2 |
| QRS duration | 2.1 | 1.9-2.5 | 0.3 |
| Ischemic cardiomiopathy | 2.8 | 2.1-4.3 | 0.1 |
Regarding pacemaker related complications, there were 4 pocket hematomas (3,8%) (defined as palpable mass that protruded more than 2 cm anterior to pulse generator) requiring evacuation in 2 of them due to tense swelling and severe pain. There was one lead fracture that required reimplantation of new lead. No dislocation was seen in this series of patients and in all the patients but one (which required a redo ablation), percentage of the RV pacing was > 90%.
Discussion
This study shows that AVNA results in a decrease in hospital admission rates and also improvement in functional status. Nevertheless, in the present sample, differences in the evolution according to the type of baseline cardiopathy were observed, and patients with mitral replacement and baseline LVEF < than 50% appeared to be especially at risk. Finally, potential complications in the current era are minimal but not negligible.
These conclusions, on the one hand are in line with previous studies, emphasizing its role as a therapeutic option for patients with symptoms refractory to pharmacotherapy, but on the other hand add a potential non-previously described risk factor associated with a more unfavorable outcome, as is the case of those patients with mitral replacement. To the best of our knowledge this is the first study identifying this subgroup of patients and its our opinion that it will deserve further investigation in order to confirm if a CRT a device should be implanted in patients with mitral valvulopathy regardless of LVEF.
Regarding it role as a tool to relieve symptoms and improve quality of life, observational studies reporting changes in exercise duration showed a mean increase of 1.19 minutes (95% CI, 0.52–1.86) after AVNA, at mean follow-up of 8.7 months.[7-9] For instances, in the Multicenter Ablate and Pace Trial8, this intervention was associated with improved quality of life and LVEF. Also in this line, Brignole et al. reported that in patients with paroxysmal AF not controlled by pharmacological therapy, this strategy was effective and superior to drug therapy in controlling symptoms and improving quality of life.[10] Nevertheless, the same group reported one year later that in patients with heart failure and chronic AF, the ablation and pacemaker implantation treatment although was effective and superior to drug therapy in controlling symptoms,[11] its efficacy appears to be less than that observed in uncontrolled studies. As a matter of fact, cardiac performance, evaluated by means of standard echocardiogram and exercise test, did not differ significantly between the 2 groups and remained stable over time. Szili-Torok[12] et al reported significant deterioration after 3 months follow-up. In this study, the main group was composed of patients with long standing AF who all had signs of moderate to severe heart failure. In the present study, there was a global improvement in the functional status, with also a significant decrease in the number or hospitalization.
More controversial and still matter of debate is the effect over the LVEF. In this regard it is worth mentioning the results reported in the meta-analysis performed by Chatterjee et al,[7] where based on observational studies, showed a mean increase of 4.80% (95% CI, 2.01–7.58) after AVNA. Nevertheless, the authors pointed out a significant heterogeneity across studies. When stratified by EF, studies with EF<45%, showed a significant increase in EF after AVNA (+7.44%; 95% CI, 5.4–9.5) with minimal heterogeneity. In contrast, studies with EF > 45% showed no significant change in EF (+1.94%; 95% CI, -2.9% to 6.8%) with substantial heterogeneity. The only retrospective comparison of survival in AF with left ventricular systolic dysfunction (LVSD) found no significant difference in survival between AVNA and pharmacotherapy over a mean follow-up of 3.5 years.[12] Nevertheless, retrospective analysis from the same authors found worse survival with AVNA for patients with LVEF < 40% compared with those with EF > 40%,[14] and others have found the presence of systolic dysfunction and fractional shortening < 20% to be independent predictors of mortality after AVNA.[15] Our results are in this line, being those patients with LVSD population at risk. However, due to the small number if patients with LFEV<40% (n=8), no definitely conclusions can be point out in this regard. Accordingly, there is a general belief that there is a clear need for randomized data assessing the impact of AVNA (RV or BiV pacing) versus pharmacotherapy on survival in the AF population with LVSD. The positive effect of BVP in patients referred to AVNA has been described in the AVAIL CLS / CRTAV trial.[16] In this trial, 108 patients with refractory AF who underwent AVNA were randomized to BVP or RVP. After 6 months of follow up, RVP results in a significant increase in the left atrial volume, LV mass, and worsening of LV contractility compared to patients receiving BVP post AVNA.
Our results also arise for the first time a predictor of deleterious outcome, as is the history of mitral valve replacement. In this regard, a recent study with 13 patients undergoing ANAV stimulation from the RV apex reported that it was associated with reduced LV compliance and increased mitral regurgitation, whereas when it was performed from the outflow tract a decrease in the mitral regurgitation was observed.[17] Therefore, it seems reasonable that this dyssynchrony may have more impact in patients with mitral disease, which as is known, plays an important role in the cardiac mechanics. Such dyssynchrony generated by the RVP could have played an important role in the impairment on the LVEF occurred in these patients with previous mitral valve surgery and normal LVEF, four of them presented with decompensated heart failure, which was solved after upgrading to BVP. No information in this subgroup has been reported in previous studies and it is our belief that it warrants further investigations.
Lastly, malignant ventricular arrhythmias and sudden cardiac death are possible complications after RF ablation of the AVNA. Previous studies have shown that the incidence of these complications ranged from 1,9% to 6.7%.[16,18] This risk is mainly in the 48 hours after the ablation. To the best of our knowledge, we are reporting the first case stated shortly after the procedure. In fact, in a recent meta-analysis, it was recounted than all deaths occurred at least 1 month after AVNA.[7] There have been proposed several independent predictors for sudden death in patients with AF after AVNA and permanent pacing as the presence of diabetes mellitus, NYHA functional class, preablation ventricular arrhythmias, mitral stenosis, aortic stenosis, aortic regurgitation and chronic obstructive pulmonary disease. The patient communicated here had a mitral valve surgery, but interestingly she was in AF with very fast ventricular response before the ablation. Theoretically, exacerbated repolarization abnormalities secondary to abrupt changes in heart rate[19-21] could have play a role and could be an additional risk factor to be taken into account.
In conclusion, AVNA results in a decrease in hospital admission rates and also improvement in functional status in those patients with AF not controlled by pharmacological therapy. Nevertheless, the current evidence in keeping with the observation of this retrospective study, show differences in the long-term evolution according to the type of baseline cardiopathy, in particular in those patients with mitral valvulopathy. Hence, it is our belief that at the present time, in this particular population, the “ablate and pace” strategy is not the most suitable option, and or maybe a biventricular pacemaker should be indicated or an AF ablation reconsidered. Finally, although it is a safe procedure, there is still the potential risk of fatal complications that must be taken into account, even shortly after the ablation, especially when some predictors of sudden death are present.
Limitations
This study has several limitations that must be taken into account. First of all, it should be interpreted in light of the limitations imposed by the retrospective nature of the study design; hence, selection of the study patients was not random. However, the inclusion of consecutive patients minimized selection bias and reflects patients referred for such intervention. Secondly, the study lacks controls in the form of patients with AF who did not undergo AVNA and permanent pacemaker implantation. It also lacks a comparison group comprising patients who are implanted with a CRT device after AVNA for AF. However, our aim is not to directly compare RVA pacing with biventricular pacing, but rather to document the change in LVEF and mitral regurgitation severity with RV apical pacing after AVNA. Thirdly, AF ablation was not considered in these patients mostly due to the presence of failure predictors (there are predominantly older patients with dilated left atrium, mean age 72 and 5,4 cm respectively, in FS III NYHA). However, maybe nowadays, with the improvement if the field of AF ablation, we had give a chance to the ablation over the “ablate and pace strategy”. Hence, it is our belief that this question will need to be addressed also in the near future.
Change in medication after the ablation was not specifically collected; thus difference between groups in terms of the use of medications was not taken into consideration at the time of the analysis.
Index of repeatability of the echocardiograms was not taken into account in the analysis. Although this is an important limitation, due to the fact that the studies were only performed by accredited and expertise doctors in echocardiography, it is our opinion that this have not introduced a bias in the results.
Finally, subgroup analyses are inherently limited by smaller sample sizes, albeit this may be offset by a relative long follow-up.
Conclusions
AVNA results in a decrease in hospital admission rates and an improvement in functional status. Baseline LVEF < 50% and mitral valvulopathy were multivariate predictor of LVEF decline, hence, it is our belief that, in this particular population, the “ablate and pace” strategy is not the most suitable option, and or maybe a biventricular pacemaker should be implanted or an AF ablation reconsidered." Finally, although it is a safe procedure and rate of complications were low, there is a potential risk of fatal complications.
Disclosures
None.
References
- 1.Gallagher J J, Svenson R H, Kasell J H, German L D, Bardy G H, Broughton A, Critelli G. Catheter technique for closed-chest ablation of the atrioventricular conduction system. N. Engl. J. Med. 1982 Jan 28;306 (4):194–200. doi: 10.1056/NEJM198201283060402. [DOI] [PubMed] [Google Scholar]
- 2.Huang S K, Bharati S, Graham A R, Lev M, Marcus F I, Odell R C. Closed chest catheter desiccation of the atrioventricular junction using radiofrequency energy--a new method of catheter ablation. J. Am. Coll. Cardiol. 1987 Feb;9 (2):349–58. doi: 10.1016/s0735-1097(87)80388-1. [DOI] [PubMed] [Google Scholar]
- 3.Díaz-Infante Ernesto, Macías-Gallego Alfonso, García-Bolao Ignacio. Spanish Catheter Ablation Registry. 9th Report of the Spanish Society Of Cardiology Working Group on Electrophysiology and Arrhythmias (2009). Rev Esp Cardiol. 2010 Nov;63 (11):1329–39. doi: 10.1016/s1885-5857(10)70257-7. [DOI] [PubMed] [Google Scholar]
- 4.Wilkoff Bruce L, Cook James R, Epstein Andrew E, Greene H Leon, Hallstrom Alfred P, Hsia Henry, Kutalek Steven P, Sharma Arjun. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002 Dec 25;288 (24):3115–23. doi: 10.1001/jama.288.24.3115. [DOI] [PubMed] [Google Scholar]
- 5.Morady F, Calkins H, Langberg J J, Armstrong W F, de Buitleir M, el-Atassi R, Kalbfleisch S J. A prospective randomized comparison of direct current and radiofrequency ablation of the atrioventricular junction. J. Am. Coll. Cardiol. 1993 Jan;21 (1):102–9. doi: 10.1016/0735-1097(93)90723-e. [DOI] [PubMed] [Google Scholar]
- 6.Chen Lin, Hodge David, Jahangir Arshad, Ozcan Cevher, Trusty Jane, Friedman Paul, Rea Robert, Bradley David, Brady Peter, Hammill Stephen, Hayes David, Shen Win-Kuang. Preserved left ventricular ejection fraction following atrioventricular junction ablation and pacing for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2008 Jan;19 (1):19–27. doi: 10.1111/j.1540-8167.2007.00994.x. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee Neal A, Upadhyay Gaurav A, Ellenbogen Kenneth A, Hayes David L, Singh Jagmeet P. Atrioventricular nodal ablation in atrial fibrillation: a meta-analysis of biventricular vs. right ventricular pacing mode. Eur. J. Heart Fail. 2012 Jun;14 (6):661–7. doi: 10.1093/eurjhf/hfs036. [DOI] [PubMed] [Google Scholar]
- 8.Kay G N, Ellenbogen K A, Giudici M, Redfield M M, Jenkins L S, Mianulli M, Wilkoff B. The Ablate and Pace Trial: a prospective study of catheter ablation of the AV conduction system and permanent pacemaker implantation for treatment of atrial fibrillation. APT Investigators. J Interv Card Electrophysiol. 1998 Jun;2 (2):121–35. doi: 10.1023/a:1009795330454. [DOI] [PubMed] [Google Scholar]
- 9.Natale A, Zimerman L, Tomassoni G, Newby K, Leonelli F, Fanelli R, Beheiry S, Pisano E. AV node ablation and pacemaker implantation after withdrawal of effective rate-control medications for chronic atrial fibrillation: effect on quality of life and exercise performance. Pacing Clin Electrophysiol. 1999 Nov;22 (11):1634–9. doi: 10.1111/j.1540-8159.1999.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 10.Brignole M, Gianfranchi L, Menozzi C, Alboni P, Musso G, Bongiorni M G, Gasparini M, Raviele A, Lolli G, Paparella N, Acquarone S. Assessment of atrioventricular junction ablation and DDDR mode-switching pacemaker versus pharmacological treatment in patients with severely symptomatic paroxysmal atrial fibrillation: a randomized controlled study. Circulation. 1997 Oct 21;96 (8):2617–24. doi: 10.1161/01.cir.96.8.2617. [DOI] [PubMed] [Google Scholar]
- 11.Brignole M, Menozzi C, Gianfranchi L, Musso G, Mureddu R, Bottoni N, Lolli G. Assessment of atrioventricular junction ablation and VVIR pacemaker versus pharmacological treatment in patients with heart failure and chronic atrial fibrillation: a randomized, controlled study. Circulation. 1998 Sep 8;98 (10):953–60. doi: 10.1161/01.cir.98.10.953. [DOI] [PubMed] [Google Scholar]
- 12.Szili-Torok T, Kimman G P, Theuns D, Poldermans D, Roelandt J R T C, Jordaens L J. Deterioration of left ventricular function following atrio-ventricular node ablation and right ventricular apical pacing in patients with permanent atrial fibrillation. Europace. 2002 Jan;4 (1):61–5. doi: 10.1053/eupc.2001.0212. [DOI] [PubMed] [Google Scholar]
- 13.Ozcan C, Jahangir A, Friedman P A, Patel P J, Munger T M, Rea R F, Lloyd M A, Packer D L, Hodge D O, Gersh B J, Hammill S C, Shen W K. Long-term survival after ablation of the atrioventricular node and implantation of a permanent pacemaker in patients with atrial fibrillation. N. Engl. J. Med. 2001 Apr 5;344 (14):1043–51. doi: 10.1056/NEJM200104053441403. [DOI] [PubMed] [Google Scholar]
- 14.Ozcan Cevher, Jahangir Arshad, Friedman Paul A, Munger Thomas M, Packer Douglas L, Hodge David O, Hayes David L, Gersh Bernard J, Hammill Stephen C, Shen Win-Kuang. Significant effects of atrioventricular node ablation and pacemaker implantation on left ventricular function and long-term survival in patients with atrial fibrillation and left ventricular dysfunction. Am. J. Cardiol. 2003 Jul 1;92 (1):33–7. doi: 10.1016/s0002-9149(03)00460-0. [DOI] [PubMed] [Google Scholar]
- 15.Victor Frederic, Mabo Philippe, Mansour Hassan, Pavin Dominique, Kabalu Guillaume, de Place Christian, Leclercq Christophe, Daubert J Claude. A randomized comparison of permanent septal versus apical right ventricular pacing: short-term results. J. Cardiovasc. Electrophysiol. 2006 Mar;17 (3):238–42. doi: 10.1111/j.1540-8167.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- 16.Olgin J E, Scheinman M M. Comparison of high energy direct current and radiofrequency catheter ablation of the atrioventricular junction. J. Am. Coll. Cardiol. 1993 Mar 1;21 (3):557–64. doi: 10.1016/0735-1097(93)90084-e. [DOI] [PubMed] [Google Scholar]
- 17.Twidale N, Manda V, Holliday R, Boler S, Sparks L, Crain J, Carrier S. Mitral regurgitation after atrioventricular node catheter ablation for atrial fibrillation and heart failure: acute hemodynamic features. Am. Heart J. 1999 Dec;138 (6 Pt 1):1166–75. doi: 10.1016/s0002-8703(99)70084-0. [DOI] [PubMed] [Google Scholar]
- 18.Darpö B, Walfridsson H, Aunes M, Bergfeldt L, Edvardsson N, Linde C, Lurje L, van der Linden M, Rosenqvist M. Incidence of sudden death after radiofrequency ablation of the atrioventricular junction for atrial fibrillation. Am. J. Cardiol. 1997 Nov 1;80 (9):1174–7. doi: 10.1016/s0002-9149(97)00635-8. [DOI] [PubMed] [Google Scholar]
- 19.Geelen P, Brugada J, Andries E, Brugada P. Ventricular fibrillation and sudden death after radiofrequency catheter ablation of the atrioventricular junction. Pacing Clin Electrophysiol. 1997 Feb;20 (2 Pt 1):343–8. doi: 10.1111/j.1540-8159.1997.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 20.Brandt R R, Shen W K. Bradycardia-induced polymorphic ventricular tachycardia after atrioventricular junction ablation for sinus tachycardia-induced cardiomyopathy. J. Cardiovasc. Electrophysiol. 1995 Aug;6 (8):630–3. doi: 10.1111/j.1540-8167.1995.tb00440.x. [DOI] [PubMed] [Google Scholar]
- 21.Long QT Syndromes. Curr Treat Options Cardiovasc Med. 2000 Aug;2 (4):317–322. doi: 10.1007/s11936-996-0005-y. [DOI] [PubMed] [Google Scholar]


