Abstract
Previous studies have shown that ulinastatin, an effective inhibitor of the inflammatory response in clinical applications, can attenuate hyperalgesia in rodents. However, the underlying mechanism remains unclear. In the present study, we first examined the change in the calcineurin level, which plays an important role in regulating cytokine release in the nervous system, following lumbar 5 ventral root transection in the rat. Furthermore, we determined whether intraperitoneal (i.p.) injection of ulinastatin attenuated pain behavior via inhibition of the calcineurin-mediated inflammatory response induced by lumbar 5 ventral root transection. The results showed that the paw withdrawal threshold and paw withdrawal latency were significantly decreased following lumbar 5 ventral root transection compared to the sham group. Neuropathic pain induced by lumbar 5 ventral root transection significantly decreased the expression of calcineurin in the DRG, and calcineurin was mostly located with NF-200-positive cells, IB4-positive cells, and CGRP-positive cells and less with GFAP-positive satellite cells. Furthermore, intrathecal (i.t.) injection of exogenous calcineurin attenuated the pain behavior induced by lumbar 5 ventral root transection. Importantly, intraperitoneal injection of ulinastatin alleviated the pain behavior and calcineurin downregulation induced by lumbar 5 ventral root transection. Lastly, the cytokine IL-10 was significantly decreased following lumbar 5 ventral root transection, and application of calcineurin (intrathecal) or ulinastatin (intraperitoneal) inhibited the IL-10 downregulation induced by lumbar 5 ventral root transection. These results suggested that ulinastatin, by acting on the CN/IL-10 pathway, might be a novel and effective drug for the treatment of neuropathic pain.
Keywords: Calcineurin, ulinastatin, neuropathic pain, cytokine, DRG, IL-10
Introduction
Currently, no common analgesic drugs are available that produce meaningful relief for patients who experience neuropathic pain. Ca2+/calmodulin-dependent protein phosphatase (calcineurin, CN), which is highly expressed in most mammalian tissues, is found in especially high levels in the nervous system. CN is not only a major modulator of immune/inflammatory processes in glial cells but has also been associated with other functions in glial CN signaling that may have a major impact on neurologic function.1 Some evidence implies an important role for CN in diseases of the central nervous system. CN activity is enhanced by pro-inflammatory stimuli, such as TNF-α/LPS, and by neuroprotective signals, such as insulin-like growth factor in astrocytes.2 Phosphatase CN in astrocytes modulates neuronal damage associated with inflammation, which is a common condition in neurodegenerative diseases, such as Alzheimer’s disease or neuropathic pain processes.3 For example, CN activity has been reported to be abnormally reduced in Alzheimer’s disease,4,5 whereas in situ generation of constitutively active CN is neuroprotective during brain ischemia.6 It is believed that activation of the CN signaling pathway via regulated cytokine production unbalances the cytokine microenvironment and mediates central nervous system diseases. Furthermore, it is well established that imbalances between pro-inflammatory and anti-inflammatory cytokines mediate neuropathic pain.7 Studies have shown that CN helps drive the expression of pro-inflammatory cytokines, such as IL-1β, and participates in the neuropathic pain process.3,8 IL-10, an important cytokine, is involved in the chronic pain induced by nerve injury or inflammation.9 However, whether CN mediates neuropathic pain via regulation of the anti-inflammatory cytokine IL-10 remains unclear.
Ulinastatin is widely used as an anti-inflammatory drug. Administration of ulinastatin reduces the levels of TNF-α, IL-1, IL-6, IL-8, myeloperoxidase, and free oxygen radicals and elevates the levels of IL-10 in animal model and human experiments.10–14 Studies have also suggested that ulinastatin, as a serine protease inhibitor, decreases phosphorylation of p38 mitogen-activated protein kinase (p38-MAPK), which in turn attenuates activation of NF-κB and downregulates expression of cytokines such as TNF-α.11 Importantly, recent studies have shown that ulinastatin treatment attenuates the development of neuropathic pain by inhibiting the release of pro-inflammatory IL-6 following spinal nerve ligationin a rat model.15,16 However, whether ulinastatin can regulate CN expression to mediate neuropathic pain is unknown.
Selective injury to motor fibers by lumbar 5 ventral root transection (L5-VRT), without injury to sensory neurons, produces persistent mechanical allodynia and thermal hyperalgesia in rat hindpaws.17 In the present study, we first observed the changes in CN and IL-10 following L5-VRT in the DRG. Next, we determined whether ulinastatin, by acting on the CN/IL-10 pathway, relieved the neuropathic pain induced by L5-VRT. The present study will provide potential support for the use of ulinastatin as a possible therapeutic strategy for neuropathic pain.
Methods and materials
Animals
Male Sprague-Dawley rats (220–250 g) were obtained from the Institute of Experimental Animals of Sun Yat-Sen University, China. The animals were housed on sawdust bedding in plastic cages and provided food and water ad libitum. The room was maintained at 22 ± 1℃ and 50% to 60% humidity with a 12-h light–dark cycle. All of the experimental protocols were approved by the Sun Yat-Sen University Animal Care and Use Committee and were performed in accordance with the guidelines of the National Institutes of Health on the care and ethical treatment of animals.
L5 ventral root transection
L5-VRT model was produced as described previously.18,19 Briefly, surgery was performed on rats under inhalation anesthesia consisting 1% to 3% isoflurane, and a L5 hemilaminectomy was performed to expose the L5 nerve root. The left ventral root was gently exposed with fine forceps and transected 2 to 3 mm proximal to the DRG, and a small portion (2 mm) of the root was dissected. In the sham group, an identical operation was performed to expose the L5 ventral root, but the nerve was not transected. Complete hemostasis was confirmed, and the wound was sutured in two layers.
Drug administration and behavioral evaluation
Drug administration
An intraperitoneal (i.p.) injection of ulinastatin (Techpool Company, Guangdong, China) (10,000, 50,000, 100,000 U/kg) was administered once each day (8 AM) for seven days after L5-VRT. Control animals received an equivalent volume of the vehicle. An intrathecal (i.t.) injection of exogenous CN (BML-SE163-5000, Enzo LifeSciences, USA) (10 U/rat) was administered once each day for seven days.
I.t.injections
Using the method described by Ouyang et al.,20 an i.t. catheter (PE-10, 8.5 cm) was inserted through an opening in the cisternal magna to the lumbar subarachnoid space. Animals showing neurological deficits after implantation were excluded. After surgery, the animals were allowed to recover for one week before the administration of drugs. Animals displaying signs of motor or neurological dysfunction were excluded from the study. The drugs were delivered with a micro syringe in a total volume of 10 µl, followed by 10 µl of saline used to flush the catheter.
Mechanical hyperalgesia
Researchers performing behavioral tests were blinded to the drug treatments. The hindpaw withdrawal threshold was determined by applying mechanical stimuli to the plantar surface of the ipsilateral hindpaw using Von Frey hairs. Mechanical sensitivity was assessed using Von Frey hairs (0.4, 0.6, 1.0, 2.0, 4.0, 6.0, 8.0, 10.0, and 15.0 g) with an up-down method described previously.21 Briefly, the animals were placed under separate transparent plexiglas chambers positioned on a wire mesh floor. Five minutes were allowed for habituation. The 2.0 g stimulus was applied first. If hindpaw withdrawal was absent, the next stronger stimulus was chosen, and if the hindpaw was withdrawn, a weaker stimulus was applied. Each stimulus consisted of a 2 to 3 s application of the Von Frey hair to the lateral surface of the foot with a 5-min interval between stimuli. Quick withdrawal or licking of the hindpaw in response to the stimulus was considered a positive response. Mechanical sensitivity was assessed before and 7,10, and 14 days after L5-VRT.
Thermal hyperalgesia
The rats were placed on the glass floor of an elevated platform. A high intensity, movable radiant heat source was placed underneath the glass and aimed at the plantar surface of the left hindpaw. Care was taken to initiate the test when the animal was at rest (not walking) and the hindpaw was in contact with the glass floor of the testing apparatus. Stimulus onset activated a timer that was controlled by a photocell. The hindpaw withdrawal reflex interrupted the light of the photocell and automatically stopped the timer. Latencies of the reflex were measured from the onset of radiant heat until hindpaw withdrawal to the nearest 0.1 s. Each hindpaw was tested three times at intervals of 5 min. The light intensity was adjusted at the beginning of the experiment to produce latencies of approximately 20 s and held constant thereafter.
Western blot
For western blot analysis, six animals were randomly selected from each group. Rats were anesthetized by inhalation of 1% to 3% isoflurane. The ipsilateral L4 and L5 DRGs were removed and frozen at −80℃ until use. The samples were homogenized on ice in 15 mmol/l Tris buffer containing a cocktail of proteinase inhibitors and phosphatase inhibitors. The total protein concentration in the samples was determined with a Bicinchoninic Acid (BCA) protein assay (Pierce, Rockford IL, USA). The protein samples were separated via gel electrophoresis (SDS-PAGE) and transferred onto a Polyvinylidene Fluoride (PVDF) membrane. The membranes were placed in blocking buffer for 1 h at room temperature and incubated in a primary antibody against Calcineurin A (1:500, CST, USA) overnight at 4℃. Then, the membranes were incubated in horseradish peroxidase-conjugated IgG. An enhanced chemiluminescence solution (Pierce, USA) was used to detect immunocomplexes. Each band was quantified using a computer-assisted imaging analysis system (NIH ImageJ).
Immunochemistry
The rats were anesthetized using 10% chloral hydrate (3–4 ml/kg, i.p.) and perfused with 4% paraformaldehyde through the ascending aorta. The L4 and L5 DRGs were removed and post-fixed in the same fixative overnight and then transferred to 30% sucrose overnight. The DRG tissues were sliced into 16 µm thick sections on a freezing microtome (Leica CM3050S, Germany). All sections were blocked with 3% donkey serum for 1 h at room temperature and incubated for two nights at 4℃ with primary antibodies against CN (1:500, Abcam), GFAP (1:1000, Chemicon), IB4 (1:200, Chemicon), NF-200 (1:200, Chemicon), or CGRP (1:200, Abcam). All sections were treated with a mixture of Alex488 or FITC- and Cy3-conjugated secondary antibodies for 1 h at room temperature. Then, the sections were rinsed and mounted on a gelatin-coated slide. Images of the stained sections were captured with a fluorescence microscope attached to a CCD spot camera (Leica, Germany) and processed with Leica IM50 software (Germany).
Enzyme-linked immunosorbent assays (ELISAs)
IL-10 was measured by an ELISA 7, 10, and 14 days following L5-VRT, and the sham group was also tested. Rats were anesthetized by inhalation of 1% to 3% isoflurane, and the ipsilateral DRGs at L4-L5 were harvested. The tissue was immediately stored in liquid nitrogen at −80℃ until homogenization. The tissue was homogenized in ice–cold phosphate-buffered saline (PBS) followed by centrifugation at 4℃ for 15 min at 13000 × g. The protein concentration in the supernatant was measured using a BCA Protein Assay kit (Pierce, WI, USA) standardized to bovine serum albumin (BSA) according to the manufacturer’s protocol. The IL-10 level was determined using a Quantikine ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. A monoclonal antibody specific for IL-10 was pre-coated on a microplate. The medium collected from all tissues and standards (supplied by R&D) was added to separate wells, allowing the cytokine and immobilized antibody to bond. After washing away any unbound substance, an enzyme-linked polyclonal antibody was added to the wells. This was followed by addition of a substrate and stop solution, which changed the color of the bound substance. The intensity of the color reflected the amount of bound cytokine. Using a curve determined by plotting standard solutions, the cytokine levels were calculated and expressed in pg (cytokine)/mg(tissue)/ml(medium).
Antibodies and drugs used in the present study
The following primary antibodies were used in the present study: rabbit anti-Pan-Calcineurin A (2614, CST, USA), rabbit anti-Calcineurin A (ab3673, Abcam, USA), rabbit anti-β-Actin (4970, CST, USA), mouse anti-NF-200 (MAB5262, Chemicon, USA), mouse anti-GFAP (MAB3402, Chemicon, USA), goat anti-CGRP (ab36001, Abcam, USA), and lectin from Bandeiraea Simplicifolia-BSI-B4 FITC conjugate (L2895, Sigma-Aldrich, USA). The secondary antibodies included fluoresce in (FITC) Affinipure donkey anti-mouse IgG (715-095-150, Jackson ImmunoResearch, USA), Alex Fluor 488 AffiniPure donkey anti-goat IgG (705-545-003, Jackson ImmunoResearch, USA) and Cy3-conjugated donkey anti-rabbit IgG (711-165-152, Jackson ImmunoResearch, USA). The drugs included ulinastatin (Techpool Company, Guangdong, China) and exogenous CN (BML-SE163-5000, Enzo LifeSciences, USA). IL-10 ELISA kits (R1000, R&D Systems Inc, MN, USA) were used to measure IL-10.
Statistical analysis
All data are expressed as the means ± SEM. Differences in changes in values over time were tested using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test or Student’s t test, if only two groups were assessed. For the behavioral test data, two-way ANOVA with Dunnett’s multiple comparisons test and nonparametric tests, including the Kruskal–Wallis H test and the Wilcoxon signed-rank test, were employed. The criterion for statistical significance was P < 0.05. Statistical tests were performed with SPSS 16.0 software (SPSS, USA).
Results
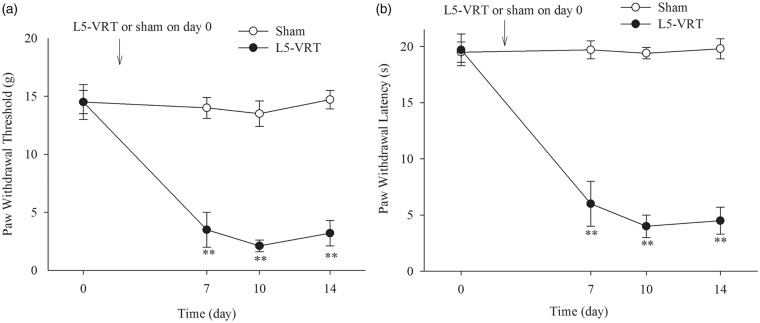
L5-VRT produced significant mechanical and thermal hypersensitivity, as evidenced by a reduction in the ipsilateral hindpaw mechanical withdrawal threshold and hindpaw thermal withdrawal latency from day 7 to day 14 compared to the sham group (Figure 1(a) and (b)).
Figure 1.
(a and b) L5-VRT induces significant decreases in the ipsilateral hindpaw withdrawal threshold and hindpaw withdrawal latency. **P < 0.01 versus the sham group. L5-VRT: lumbar 5 ventral root transection.
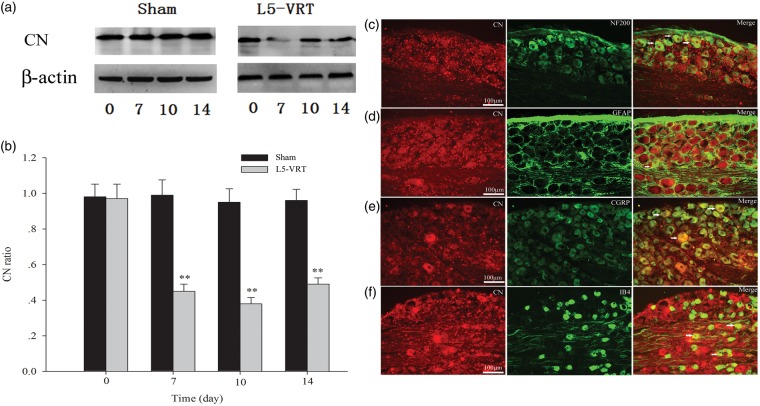
The amount of CN protein was also significantly decreased relative to the sham group on days 7, 10, and 14 following L5-VRT (Figure 2(a) and (b)). Double immunofluorescence staining showed that CN was mostly co-expressed with NF-200 (A fiber neuron marker), IB4 (C fiber neuron marker), and CGRP (peptidergic neuron marker), and less with GFAP (astrocyte marker) (Figure 2(c)–(f)).
Figure 2.
(a and b) L5-VRT induces a significant decrease in CN in the DRG on days 7, 10, and 14 compared to the sham group (**P < 0.01 versus the sham group). (c–f) Double immunofluorescence staining shows CN (red) expression in the DRG neurons in sham rats. CN is mostly co-localized with NF-200-positive cells (c), CGRP-positive cells (e) and IB4-positive cells (f) and less with GFAP-positive satellite cells (d) Scale bars: (c–f) = 100 µm. L5-VRT: lumbar 5 ventral root transection; CN: calcineurin.
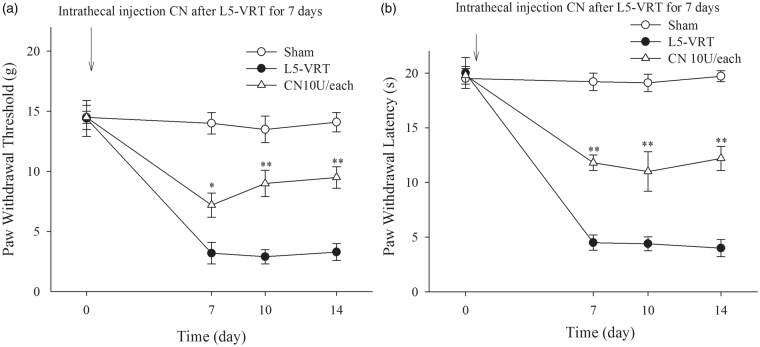
To further determine the role of CN in the neuropathic pain induced by L5-VRT, exogenous CN was continuously i.t. injected at a dose of 10 U/day for seven days, which significantly attenuated the neuropathic pain induced by chronic constriction injury (CCI).22 The results showed that the exogenous CN significantly increased the mechanical and thermal threshold of rats following L5-VRT (Figure 3(a) and (b)).
Figure 3.
(a and b) Intrathecal injection of exogenous CN (10 U) each day for seven days after L5-VRT increases the ipsilateral hindpaw withdrawal threshold and hindpaw withdrawal latency. *P < 0.05, **P < 0.01 versus the L5-VRT group. L5-VRT: lumbar 5 ventral root transection; CN: calcineurin.
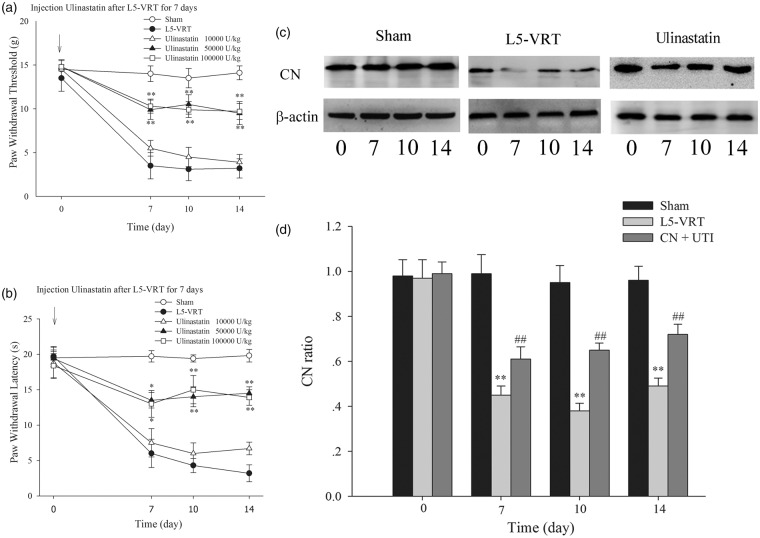
Importantly, continuous i.p. injection of ulinastatin at 50,000 and 100,000 U/kg, but not 10,000 U/kg, for seven days increased the ipsilateral hindpaw mechanical withdrawal threshold and hindpaw thermal withdrawal latency in the L5-VRT rat model. However, no significant difference was found between the 50,000 and the 100,000 U/kg group, indicating that 50,000 U/kg is the optimal dose for continuous i.p. injection (Figure 4(a) and (b)). Furthermore, i.p. injection of ulinastatin at a dose of 50,000 U/kg for seven days prevented the downregulation of CN induced by L5-VRT (Figure 4(c) and (d)). These results suggested that ulinastatin might alleviate the neuropathic pain induced by L5-VRT by preventing downregulation of CN.
Figure 4.
(a and b) Intraperitoneal injection of ulinastatin (10,000, 50,000, or 100,000 U/kg) for seven days before L5-VRT increases the ipsilateral hindpaw withdrawal threshold and hindpaw withdrawal latency; however, no significant difference was obtained between the ulinastatin 10,000U/kg group and the L5-VRT group or between the ulinastatin100,000 U/kg group and the ulinastatin 50,000 U/kg group. *P < 0.05, **P < 0.01 versus the L5-VRT group. (c and d) Intraperitoneal injection of ulinastatin (50,000 U/kg) for seven days prevents the downregulation of CN induced by L5-VRT in the ipsilateral DRG (**P < 0.01 versus the sham group, ##P < 0.01 versus the L5-VRT group). L5-VRT: lumbar 5 ventral root transection; CN: calcineurin.
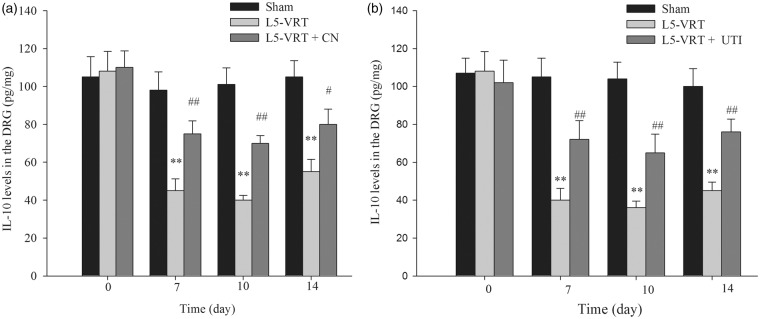
Due to the pivotal role of the anti-inflammatory factor IL-10 in chronic pain induced by nerve injury or inflammation, we further examined IL-10 expression following L5-VRT. The ELISA results showed that IL-10 levels were decreased in the ipsilateral DRG on days 7, 10, and 14 following L5-VRT (Figure 5(a)). The time course of IL-10 downregulation was consistent with that of the decrease in CN. Furthermore, the results showed that i.t. injection of exogenous CN (10 U/day) for seven days prevented IL-10 downregulation in the ipsilateral DRG, suggesting that CN mediates the decrease in IL-10 associated with the neuropathic pain induced by L5-VRT (Figure 5(a)). Lastly, i.p. injection of ulinastatin at a dose of 50,000 U/kg for seven days prevented the downregulation of IL-10 induced by L5-VRT in the ipsilateral DRG (Figure 5(b)).
Figure 5.
L5-VRT induces a significant decrease in the IL-10 protein level in the L4-L5 DRG on days 7, 10, and 14. (a) IL-10 upregulation was induced in the ipsilateral DRG after intrathecal injection of exogenous CN (10 U/day) for seven days. (b) IL-10 was also upregulated after intraperitoneal injection of ulinastatin (50,000 U/kg) for seven days. **P < 0.01 versus the sham group. #P < 0.05, ##P < 0.01 versus the L5-VRT group. The IL-10 levels were determined with an ELISA. L5-VRT: lumbar 5 ventral root transection; CN: calcineurin.
Discussion
In the present study, we first examined the changes in CN and IL-10 following motor fiber injury by L5-VRT. The results showed that the expression levels of CN and IL-10 were significantly downregulated in the DRG by L5-VRT. Application of exogenous CN prevented IL-10 downregulation and attenuated the pain behavior induced by L5-VRT. Importantly, ulinastatin (i.p.) treatment prevented downregulation of the CN/IL-10 pathway and relieved the pain behavior induced by L5-VRT. These results suggested that the CN/IL-10 pathway might be a potential target for ulinastatin therapy for neuropathic pain.
Several studies over the past 10 years have identified neuronal CN as a primary component in dendritic atrophy, synapse loss, neuronal vulnerability, and synaptic dysfunction.23,24 Several reports in the mid- to late 1990s stated that CN can also appear in primary glial cells following inflammatory insult.25–27 Increased CN expression in astrocytes has also been reported to occur in the hippocampus, amygdala, and neocortex during central nervous system diseases, including Alzheimer’s disease, aging, and bilateral carotid artery occlusion, even though the overall tissue levels of CN were reduced.27–31 The presence of many CN-positive astrocytes in the human hippocampus during the early stages of cognitive decline indicates that the upregulation of CN in astrocytes is an antecedent to the dementia that occurs during the later stages of the disease.32 These findings indicate that changes in CN may be unique and may play a critical role in the initiation and maintenance of neurodegenerative diseases. Evidence has shown that CN is not only a global mediator of the immune/inflammatory response and neuroinflammation but is also involved in cytokine production processes and neurologic function.1 In addition to the well-defined role of CN in cytokine production in peripheral immune cells, CN may also be associated with glial cells during neuroimmune/inflammatory signaling in most acute and chronic neurodegenerative diseases. The pro-inflammatory cytokines IL-1β, TNF-α, and interferon γ trigger astrocyte hypertrophy and/or neuroinflammation and activate CN in primary astrocyte cultures.2,32 Many of these factors are increased during injury and neurodegenerative diseases, and astrocytic CN/NFAT activity can propagate from one astrocyte population to another in an autostimulatory manner.33
However, recent studies have also shown that downregulation of CN in the spinal cord participates in CCI-induced neuropathic pain.22 Although the mechanism of nerve injury is different, the present study showed that injury to the motor nerve fibers by L5-VRT also decreased CN expression in the DRG. The immunohistochemistry results showed that CN was mostly co-localized with NF-200-positive cells, IB4-positive cells, and CGRP-positive cells, and less with GFAP-positive satellite cells in the DRG. CN helps to increase the expression of many immune/inflammatory factors, such as NFAT and NF-κB, in astrocytes once it is activated.33,34 CN plays an important role in regulating the inflammatory response,2 and expression of CN was elevated in astrocytes but not in neurons in a mouse aging model.35 Our results showed that CN downregulation following L5-VRT was accompanied by a decrease in IL-10 expression. Furthermore, i.t. injection of exogenous CN alleviated the L5-VRT-induced downregulation of IL-10 and pain behavior. It is possible that the decrease in the CN level inhibited the activity of transcription factors such as NFAT or NF-κB and prevented the expression of the anti-inflammatory factor IL-10. These results suggested that the CN/IL-10 pathway is involved in neuropathic pain induced by motor nerve injury.
Ulinastatin is widely used in clinical applications to induce an anti-inflammatory response. Application of ulinastatin reduces the levels of TNF-α, IL-1, IL-6, IL-8, myeloperoxidase, and free oxygen radicals and elevates IL-10 levels.10–14 Ulinastatin is a putative inhibitor of IL-8 in the brain and can modify acute inflammatory responses to neutrophil infiltration.36 Ulinastatin treatment reduces TNF-α concentrations in rat liver tissue subjected to ischemia-reperfusion and subsequently decreases neutrophil infiltration.37 It is thought that an imbalance in the cytokine micro-environment plays an important role in the development and maintenance of neuropathic pain. Evidence has shown that ulinastatin treatment inhibits the expression of pro-inflammatory cytokine factors such as TNF-α and IL-6.12 In our study, we found that ulinastatin upregulated the expression of the anti-inflammatory cytokine factor IL-10.
IL-10 produces hypoanalgesic responses to inflammatory pain and repeated i.t. injections of plasmid DNA encoding IL-10 reverses allodynia induced by neuropathic pain.38 IL-10 mediated by herpes simplex virus vector reduces neuropathic pain induced by HIV gp120 combined with dideoxycytidine (ddC) in rats.39 IL-10 might be an important immunoregulatory factor that significantly contributes to decreasing the intensity of the inflammatory response by downregulating pro-inflammatory cytokine production at the site of tissue damage. The level of IL-10 protein was increased bilaterally in the lumbar DRG one and three days after CCI rat pain model and its levels declined bilaterally even below baseline level in lumbar DRG seven days from CCI and normalized after 14 days.40 The CCI rats only displayed decreased withdrawal thresholds for mechanical allodynia and thermal hyperalgesia in ipsilateral hind paws,40 while L5-VRT rats produced rapid (one day after transection), robust, and prolonged (56 days) bilateral mechanical allodynia, cold allodynia, and short-term thermal hyperalgesia (14 days).18 Further research is required to estimated relationship between the anti-inflammatory and pro-inflammatory cytokine in contralateral DRG to elucidate the mechanism of L5-VRT pain in future. Ulinastatin may relieve neuropathic pain by rebalancing the cytokine micro-environment. In addition, studies have also suggested that ulinastatin can inhibit MAPKp38 activation and further downregulate NF-κB-mediated TNF-α expression.11
In the present study, ulinastatin prevented a decrease in CN following L5-VRT, and exogenous CN also increased IL-10 expression. These results suggested that ulinastatin acted on CN to upregulate the expression of the anti-inflammatory cytokine IL-10.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the National Natural Science Foundation of China (Grant No. 81300966, 81271246, 81401036).
References
- 1.Furman JL, Norris CM. Calcineurin and glial signaling: neuroinflammation and beyond. J Neuroinflammation 2014; 11: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez AM, Fernandez S, Carrero P, et al. Calcineurin in reactive astrocytes plays a key role in the interplay between proinflammatory and anti-inflammatory signals. J Neurosci 2007; 27: 8745–8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minghetti L. Role of inflammation in neurodegenerative diseases. Curr Opin Neurol 2005; 18: 315–321. [DOI] [PubMed] [Google Scholar]
- 4.Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging 2005; 26: 349–354. [DOI] [PubMed] [Google Scholar]
- 5.Ferri A, Gabbianelli R, Casciati A, et al. Oxidative inactivation of calcineurin by Cu,Zn superoxide dismutase G93A, a mutant typical of familial amyotrophic lateral sclerosis. J Neurochem 2001; 79: 531–538. [DOI] [PubMed] [Google Scholar]
- 6.Shioda N, Moriguchi S, Shirasaki Y, et al. Generation of constitutively active calcineurin by calpain contributes to delayed neuronal death following mouse brain ischemia. J Neurochem 2006; 98: 310–320. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto K, Martin DP, Schmelzer JD, et al. Pro- and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp Neurol 2001; 169: 386–391. [DOI] [PubMed] [Google Scholar]
- 8.Smith HS. Calcineurin as a nociceptor modulator. Pain Physician 2009; 12: E309–E318. [PubMed] [Google Scholar]
- 9.Ledeboer A, Jekich BM, Sloane EM, et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun 2007; 21: 686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang N, Wang F, Wang Y, et al. Ulinastatin improves survival of septic mice by suppressing inflammatory response and lymphocyte apoptosis. J Surg Res 2013; 182: 296–302. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Liu F, Liu H, et al. Urinary trypsin inhibitor attenuates lipopolysaccharide-induced acute lung injury by blocking the activation of p38 mitogen-activated protein kinase. Inflamm Res 2011; 60: 569–575. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka R, Fujita M, Tsuruta R, et al. Urinary trypsin inhibitor suppresses excessive generation of superoxide anion radical, systemic inflammation, oxidative stress, and endothelial injury in endotoxemic rats. Inflamm Res 2010; 59: 597–606. [DOI] [PubMed] [Google Scholar]
- 13.Koga Y, Fujita M, Tsuruta R, et al. Urinary trypsin inhibitor suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Neurol Res 2010; 32: 925–932. [DOI] [PubMed] [Google Scholar]
- 14.Ueki M, Taie S, Chujo K, et al. Urinary trypsin inhibitor reduces inflammatory response in kidney induced by lipopolysaccharide. J Biosci Bioeng 2007; 104: 315–320. [DOI] [PubMed] [Google Scholar]
- 15.Jung KT, Lee HY, Yoon MH, et al. The effect of urinary trypsin inhibitor against neuropathic pain in rat models. Korean J Pain 2013; 26: 356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh SH, So HJ, Lee HY, et al. Urinary trypsin inhibitor attenuates the development of neuropathic pain following spinal nerve ligation. Neurosci Lett 2015; 590: 150–155. [DOI] [PubMed] [Google Scholar]
- 17.Xu JT, Xin WJ, Zang Y, et al. The role of tumor necrosis factor-alpha in the neuropathic pain induced by lumbar 5 ventral root transection in rat. Pain 2006; 123: 306–321. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Xian CJ, Zhong JH, et al. Effect of lumbar 5 ventral root transection on pain behaviors: a novel rat model for neuropathic pain without axotomy of primary sensory neurons. Exp Neurol 2002; 175: 23–34. [DOI] [PubMed] [Google Scholar]
- 19.Zang Y, Chen S-X, Liao G-J, et al. Calpain-2 contributes to neuropathic pain following motor nerve injury via up-regulating interleukin-6 in DRG neurons. Brain Behav Immun 2015; 44: 37–47. [DOI] [PubMed] [Google Scholar]
- 20.Ouyang H, Bai X, Huang W, et al. The antinociceptive activity of intrathecally administered amiloride and its interactions with morphine and clonidine in rats. J Pain 2012; 13: 41–48. [DOI] [PubMed] [Google Scholar]
- 21.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 22.Miletic G, Lippitt JA, Sullivan KM, et al. Loss of calcineurin in the spinal dorsal horn contributes to neuropathic pain, and intrathecal administration of the phosphatase provides prolonged analgesia. Pain 2013; 154: 2024–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reese LC, Taglialatela G. A role for calcineurin in Alzheimer’s disease. Curr Neuropharmacol 2011; 9: 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdul HM, Furman JL, Sama MA, et al. NFATs and Alzheimer’s disease. Mol Cell Pharmacol 2010; 2: 7–14. [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuda T, Takuma K, Asano S, et al. Involvement of calcineurin in Ca2+ paradox-like injury of cultured rat astrocytes. J Neurochem 1998; 70: 2004–2011. [DOI] [PubMed] [Google Scholar]
- 26.Ferrari D, Stroh C, Schulze-Osthoff K. P2X7/P2Z purinoreceptor-mediated activation of transcription factor NFAT in microglial cells. J Biol Chem 1999; 274: 13205–13210. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto T, Kawamata T, Saito N, et al. Isoform-specific redistribution of calcineurin A alpha and A beta in the hippocampal CA1 region of gerbils after transient ischemia. J Neurochem 1998; 70: 1289–1298. [DOI] [PubMed] [Google Scholar]
- 28.Kuno T, Mukai H, Ito A, et al. Distinct cellular expression of calcineurin A alpha and A beta in rat brain. J Neurochem 1992; 58: 1643–1651. [DOI] [PubMed] [Google Scholar]
- 29.Polli JW, Billingsley ML, Kincaid RL. Expression of the calmodulin-dependent protein phosphatase, calcineurin, in rat brain: developmental patterns and the role of nigrostriatal innervation. Brain Res Dev Brain Res 1991; 63: 105–119. [DOI] [PubMed] [Google Scholar]
- 30.Goto S, Matsukado Y, Mihara Y, et al. Calcineurin in human brain and its relation to extrapyramidal system. Immunohistochemical study on postmortem human brains. Acta Neuropathol 1986a; 72: 150–156. [DOI] [PubMed] [Google Scholar]
- 31.Goto S, Matsukado Y, Mihara Y, et al. The distribution of calcineurin in rat brain by light and electron microscopic immunohistochemistry and enzyme-immunoassay. Brain Res 1986b; 397: 161–172. [DOI] [PubMed] [Google Scholar]
- 32.Abdul HM, Sama MA, Furman JL, et al. Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci 2009; 29: 12957–12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sama MA, Mathis DM, Furman JL, et al. Interleukin-1beta-dependent signaling between astrocytes and neurons depends critically on astrocytic calcineurin/NFAT activity. J Biol Chem 2008; 283: 21953–21964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furman JL, Sama DM, Gant JC, et al. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J Neurosci 2012; 32: 16129–16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norris CM, Kadish I, Blalock EM, et al. Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer’s models. J Neurosci 2005; 25: 4649–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okumura Y, Inoue H, Fujiyama Y, et al. Effects of serine protease inhibitors on accumulation of polymorphonuclear leukocytes in the lung induced by acute pancreatitis in rats. J Gastroenterol 1995; 30: 379–386. [DOI] [PubMed] [Google Scholar]
- 37.Aihara T, Shiraishi M, Hiroyasu S, et al. Ulinastatin, a protease inhibitor, attenuates hepatic ischemia/reperfusion injury by downregulating TNF-alpha in the liver. Transplant Proc 1998; 30: 3732–3734. [DOI] [PubMed] [Google Scholar]
- 38.Milligan ED, Sloane EM, Langer SJ, et al. Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain 2006; 126: 294–308. [DOI] [PubMed] [Google Scholar]
- 39.Zheng W, Huang W, Liu S, et al. IL-10 mediated by herpes simplex virus vector reduces neuropathic pain induced by HIV gp120 combined with ddC in rats. Mol Pain 2014; 10: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jancalek R, Dubovy P, Svizenska I, et al. Bilateral changes of TNF-alpha and IL-10 protein in the lumbar and cervical dorsal root ganglia following a unilateral chronic constriction injury of the sciatic nerve. J Neuroinflammation 2010; 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]