Abstract
Chronic itch (pruritus) is an important clinical problem. However, the underlying molecular basis has yet to be understood. The Transient Receptor Potential Vanilloid 1 channel is a heat-sensitive cation channel expressed in primary sensory neurons and involved in both thermosensation and pain, but its role in chronic itch remains elusive. Here, we for the first time revealed an increased innervation density of Transient Receptor Potential Vanilloid 1-expressing sensory fibers in the skin afflicted with chronic itch. Further analysis indicated that this phenomenon is due to an expansion of Transient Receptor Potential Vanilloid 1-expressing sensory neurons under chronic itch conditions. As a functional correlates of this neuronal expansion, we observed an enhanced neuronal responsiveness to capsaicin under the dry skin conditions. Importantly, the neuronal hypersensitivity to capsaicin results in itch, rather than pain sensation, suggesting that the up-regulated Transient Receptor Potential Vanilloid 1 underlies the pain-to-itch switch under chronic itchy conditions. The study shows that there are different mechanisms of chronic pain and itching, and Transient Receptor Potential Vanilloid 1 plays an important role in chronic itch.
Keywords: Chronic itch, pain, Transient Receptor Potential Vanilloid 1, calcium imaging
Introduction
Pain and itch affect billions of people worldwide and cause deleterious effects on both quality of life and productivity. Although both are unpleasant sensations involving sensory discriminative, cognitive, evaluative, affective, and motivational components, they elicit distinct behaviors and have an antagonistic interaction – pain can inhibit itch.1,2 Recent progress suggests that itch and pain are encoded and transmitted by discrete groups of primary sensory neurons (pruriceptive neurons and nociceptive neurons, respectively) in the trigeminal ganglia (TG) and dorsal root ganglia (DRG).1,2
The boundary between pain and itch, however, is blurred under pathological conditions. For instance, iontophoresis of histamine-inducing itch is perceived as painful in chronic pain patients,3,4 while patients with chronic itch perceive noxious mechanical, thermal, and chemical stimuli as itchy.5–7 However, the underlying molecular mechanisms remain unclear.
Transient Receptor Potential Vanilloid 1 (TRPV1) is a heat-sensitive cation channel that is selectively expressed in a population of primary sensory neurons in the TG and DRG. TRPV1 plays an important role in thermal and pain sensations. Furthermore, studies indicate that TRPV1 serves as the downstream transduction channel of histamine H1 receptors and is required for histamine-induced itch.8,9 However, whether TRPV1 is involved in histamine-independent chronic itch and whether it mediates pain-to-itch switch under chronic itch remain unclear. Here, we showed an up-regulation of TRPV1 in dry skin-associated chronic itch, which lead to an enhanced neuronal responsiveness to capsaicin. Interestingly, TRPV1 neuronal hypersensitivity plays an important role in the pain-to-itch switch under chronic itchy conditions, which shed new light on the sensory coding in chronic itch.
Materials and methods
Animals
All experiments were performed in accordance with protocols approved by the Animal Care and Use Committee at the Nanjing University of Chinese Medicine. All mice used in the behavioral tests were 8- to10-week-old males backcrossed to C57Bl/6 background for at least six generations. Pirt-GCaMP3 mice were generated by Dr. Xinzhong Dong. GCaMP3 and neomycin resistance genes were inserted into the Pirt locus using targeted homologous recombination. The inserted genes were knocked-in in frame with the Pirt promoter and replaced the entire coding region of the Pirt gene.10 Pirt-GCaMP3 heterozygotes were used in all Ca2+ imaging experiments.
TRPV1-PLAP transgenic mice were purchased from Jackson Laboratories. PLAP-pGK-Hyg-loxP cassette was knocked in and replaces the entire open reading frame of TRPV1.
Animal model
To experimentally induce dry skin, we treated the cheek or the nape of mice with acetone-ether-water (AEW) as previously reported.11 Animal were shaved at the cheek three days before the start of treatment. A mixture of acetone and diethylether (1:1) was applied to the shaved area for 20 s, followed immediately by distilled water for 30 s. Acetone and ether remove lipid components in the stratum corneum and disrupts the cutaneous barrier. Subsequent water treatment exerts hypotonic stress on the keratinocytes. The animals were treated twice daily.
Behavioral test
Animals were housed in and behavior experiments were performed in a controlled environment of 20℃–24℃, 45%–65% humidity, and with a 12-h day/night cycle. Animals were acclimated to the testing environment for 10 min before the initiation of behavior tests. Animal behavior was analyzed by investigators who were blind to genotype and animal treatment conditions.
Scratching behavior in AEW model animals was observed for 1 h (8:30 a.m. to 9:30 a.m.) before AEW treatments. Scratching and wiping behavior induced by subcutaneously injected capsaicin were observed for 30 min. A bout of scratching was defined as a continuous scratching movement with a hindpaw directed at the treated site or drug injection site. A bout of wiping was defined as a continuous wiping movement with a forepaw directed at the area of the treatment site.
TRPV1-PLAP histochemistry
TRPV1-PLAP heterozygous mice were anesthetized with 1% sodium pentobarbital (50 mg/kg, i.p.), and transcardially perfused with 10–15 mL of 0.1 M phosphate-buffered saline (0.1M PBS, pH 7.4, 4℃) followed by 20–25 mL ice-cold 4% paraformaldehyde (pH 7.4). TGs were isolated from perfused mice, post-fixed in 4% paraformaldehyde (PFA) in PBS for 30 min, and cryoprotected in 30% sucrose at 4℃ for 24 h. TGs were then embedded in optimum cutting temperature compound (OCT, Leica, Wetalar, Germany) and rapidly frozen at −20℃ (CM1950, Leica). Cryoembedded tissues were cut into 20 µm thick slices using sliding microtome (CM1950, Leica).
TRPV1-PLAP staining was performed as previously described.12 Briefly, the skin of TRPV1-PLAP transgenic mouse was dissected after CO2 euthanasia and post-fixed with 1% PFA overnight. For heat inactivation (65℃), skin and TG sections were performed for 2 h. PLAP signal was detected by 5-bromo-4-chloro-3-indolyl phosphate (BCIP)/nitroblue tetrazolium (NBT) in alkaline phosphatase (AP) buffer (0.1 MNaCl, 50 mMMgCl2, 0.1 MTris pH 9.5) on shaker at room temperature overnight.
Tissues were washed with Hank's Balanced Salt Solution (HBSS) after the signal appeared. All imaging was performed with an Olympus fluorescence microscope (BX51, Olympus, Japan).
Real-time PCR
Total RNA from freshly dissected TGs were isolated and purified using a TRIzol/chloroform (Life Technologies, Carlsbad, CA, USA) and isopropanol precipitation procedure, in accordance with the manufacture’s protocol. cDNA was compiled using the Transcript First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland). Real-time PCR was performed using Light Cycler 480 SYBR Green I Master Mix (Roche, Basel, Switzerland) and a Light Cycler 480 II Real-Time PCR instrument (Roche, Basel, Switzerland), according to the manufacturer’s recommended protocol. In short, 1 µL of cDNA was used for each 20 μl reaction. Primers (forward primer: ATCATCAACGAGGACCCAGG, reverse primer: TGCTATGCCTATCTCGAGTGC) were used to amplify TRPV1 of the mouse. Calibrations and normalizations were done using the 2−ΔΔCT method, where ΔΔCT = (CT (target gene) − CT (reference gene)) − (CT (calibrator) − CT (reference gene)). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the reference gene for real-time PCR experiments.
Western blot
Total protein from freshly dissected TGs were isolated and purified using a TRIzol/chloroform (Life Technologies) and isopropanol precipitation procedure after RNA extraction, in accordance with the manufacture’s protocol. The extracted proteins were preserved at −20℃. The protein concentration of the supernatant was determined by Bicinchoninic Acid (BCA) assay. β-actin was selected as an internal control. Polyclonal antibody to the TRPV1 (Neuromics, USA) was used at 1/500 dilution and a monoclonal antibody to the β-actin protein (Santa Cruz Biotechnology, Dallas, TX) was used at a 1/-1000 dilution. Equal quantities of protein (60 µg per lane) were resolved on 12% SDS-polyacrylamide gels. Western blotting was preformed as detailed previously. The intensity of the signal was used to estimate the relative expression of the TRPV1 protein in the tissue extracts.
TG neuron culture
TGs were dissected from 6–8 week old mice and collected in ice cold DH10 medium (90% DMEM/F-12, 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin, Gibco). Dissected TGs were then digested for 40 min at 37℃ in a protease solution (5 mg/ml dispase, 1 mg/ml collagenase type I in HBSS without Ca2+or Mg2+, Gibco). After digestion, TGs were triturated to free neurons and pelleted by centrifugation (1000 r/min, 3 min). Pelleted neurons were then resuspended in DH10 medium and applied onto 15% Bovine Serum Albumin (BSA) column. Neurons were enriched in the bottom of the column after centrifugation (2000 r/min, 3 min). Pelleted neurons were then resuspened in DH10 medium and supplemented with nerve growth factor (NGF) (20 ng/ml) and glial cell line-derived neurotrophic factor (GDNF) (25 ng/ml). Cells were plated onto glass coverslips coated with poly-D-lysine (0.5 mg/ml, Sigma) and laminin (10 mg/ml, Sigma).
Ca2+ imaging
Cultured TG neurons and whole TGs from AEW-treated mice were adopted for Ca2+ imaging. For cultured neurons, dissociated neurons were incubated for 18–24 h (95% O2, 5% CO2) before testing.13 For whole TGs imaging, mice were sacrificed by CO2 asphyxiation, and TG explants were isolated and allowed to recover for 30 min in Ca2+ imaging buffer before testing. All experiments were performed at room temperature (25℃) inside a bath perfusion chamber. Green fluorescent protein (GFP) signals from Pirt-GCaMP3 mice were imaged at 488 nm excitation to detect Ca2+ transients.
Data analysis
All data are presented as mean ± SEM. Statistical comparisons were performed using two-tailed Student’s t tests. Differences were considered statistically significant at p < 0.05.
Results
Adoption of a dry-skin itchy model in mice
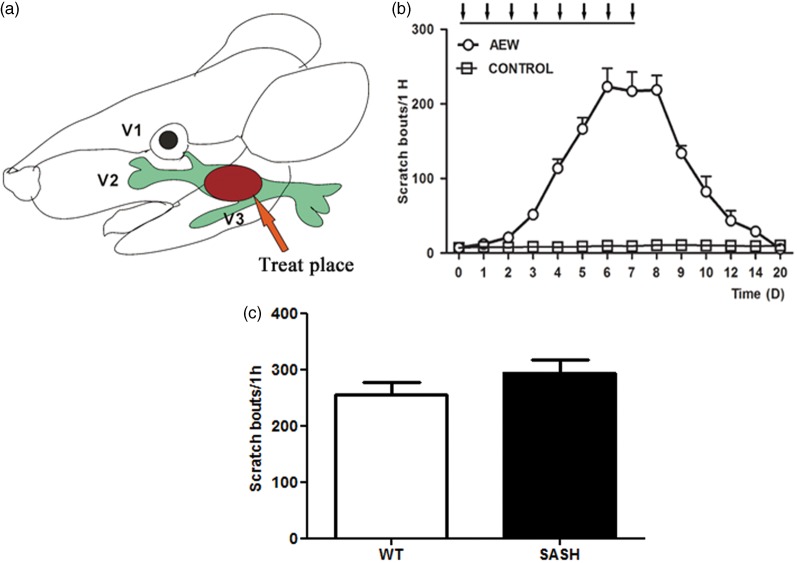
To explore the molecular mechanisms of chronic itch, we utilized a well-established dry skin itch model generated by topical application of an acetone/ether mixture followed by water (AEW, Figure 1(a)).11,14–18 AEW treatment produces severe skin barrier dysfunction, robust scratching, and changes in gene expression in sensory neurons and the skin,11,15,17 which recapitulates the dry skin symptom present in many chronic itchy conditions in humans, including xerodermia, atopic dermatitis, and psoriasis. During AEW treatment in mice, itch behavior follows a clear temporal pattern that includes induction, maintenance, and recovery (Figure 1(b)). Itch-related scratching behaviors increase during the induction phase, plateau during the maintenance phase, and diminish during the recovery phase. However, it remains unclear how the AEW treatment leads to chronic itch. Our pilot studies indicate that mast cell deficiency does not prevent AEW-induced itch in Kit(w-sh) (SASH) mice (Figure 1(c)), arguing against the involvement of mast cells or allergic reactions in dry skin itch. In addition, dexamethasone, a steroid drug with potent anti-inflammatory and immunosuppressant effects, cannot relieve AEW itch (data not shown), despite its wide use in treating chronic skin diseases including psoriasis and atopic dermatitis. These results, together with previous studies,11 strongly suggest that the pathogenesis of AEW itch is independent of allergic immune reactions and provide a unique model for us to study the mechanisms of dry skin-associated chronic itch.
Figure 1.
AEW mice shows significant increase in scratching behavior. (a) Diagram of the AEW treatment location and trigeminal nerve dermatome. (b) Spontaneous scratching behavior in AEW treated wild-type mice. Compared with water-treated mice (n = 6), AEW treatment (from D0 to D7) induces significant scratching behavior, starting from the third day and increases dramatically until the peak on the seventh day (n = 6). The arrows in the figure indicate the treatment of AEW. (c) Mast cell deficiency does not prevent AEW itch in Kit(w-sh) mice.
Increased TRPV1 expression in the dry skin model
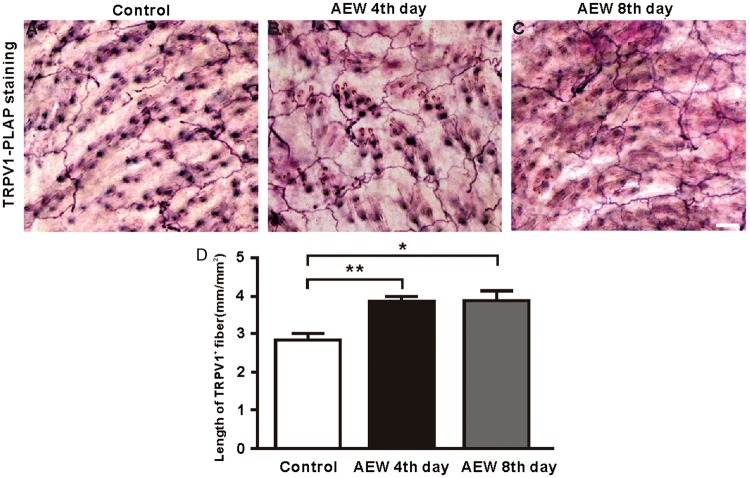
Histological examinations reveal increased cutaneous nerve densities in the patients with atopic dermatitis, which is positively correlated with itch severity. This observation suggests that higher density of sensory fibers may be at least partly responsible for intense itching in the skin. To determine the role of TRPV1 expression in chronic itch induced by AEW, we first examined the changes in their axon innervation in the skin area treated with AEW. Indeed, the innervation density of TRPV1+ sensory fibers ^is significantly increased under dry skin conditions (Figure 2(a)–(c)). To quantify the changes in the innervation density of TRPV1+ sensory fibers, we measured the length of TRPV1+ sensory fibers per square millimeter. The density of TRPV1+ sensory fiber was increased by 35.89% in AEW-treated mice (2.87 ± 0.25 (control) vs. 3.90 ± 0.20 (AEW fourth day); Figure 2(d)), implying a potential involvement of TRPV1+ sensory fibers in chronic itch.
Figure 2.
AEW treatment increases TRPV1+ sensory fibers density in cheek skin. (a) PLAP staining of TRPV1+ sensory fibers that innervate the cheek skin in cheek. (b) TRPV1+ sensory fibers density is increased in cheek skin at the fourth day of AEW treatment. (c) TRPV1+ sensory fibers density in cheek skin at eighth day of AEW treatment. (d) TRPV1+ sensory fibers density is in AEW treated mice. Data are presented as mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001. Scale bar: 50 µm.
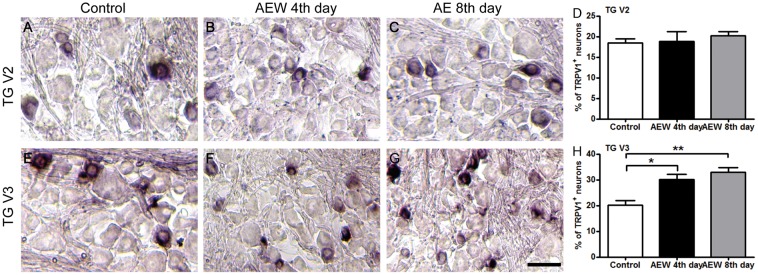
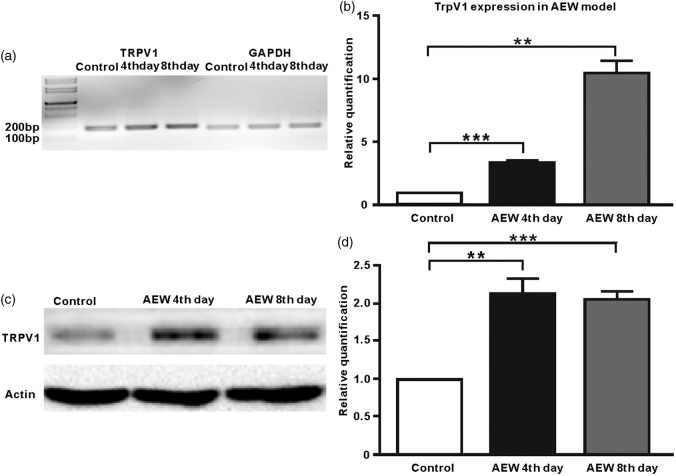
To determine whether the increased innervation density of TRPV1+ sensory fibers is due to an expansion of TRPV1+ sensory neurons under the dry skin condition, we examined the changes in the proportion of TRPV1+ neurons in the TGs after AEW treatments. Using TRPV1-PLAP reporter mice, we examined the TRPV1 expression by conducting PLAP-histachemistry. The trigeminal nerve comprises three branches: the ophthalmic (V1), maxillary (V2), and mandibular (V3), which innervate discrete orofacial regions, respectively (Figure 1(a)). We generated AEW cheek model selectively on the cheek skin innervated by the V3 branch of the trigeminal nerve (Figure 1(a)) and found that the proportion of TRPV1+ neurons significantly increased in V3 (20.2 ± 1.77 (control) vs. 30.2 ± 1.84 (AEW fourth day) vs. 32.9 ± 1.75 (AEW eighth day); Figure 3(e)–(h)), but not in the neighboring V2 branch (18.5 ± 0.99 (control) vs. 18.8 ± 2.38 (AEW fourth day) vs. 20.3 ± 0.91 (AEW eighth day); Figure 3(a)–(d)). Correlating well with this result, real-time PCR and Western blot results confirm that the expression of TRPV1 in the TG was significant increased after AEW treatment (Figure 4). Together, these data indicate that dry skin causes an expansion of TRPV1+ neurons in the TGs and hence increased TRPV1+ axonal innervation in the skin.
Figure 3.
TRPV1+ neuron population is increased in V3 of the Trigeminal ganglion after AEW treatment. PLAP-histochemistry staining of trigeminal ganglia from AEW-treated TRPV1-PLAP reporter line. (a–d) The proportion of TRPV1+ population is unaffected in V2 of the Trigeminal ganglion. (e–h) The fraction TRPV1+ neuron in V3 of TG is significantly increased after AEW treatment. Data are presented as mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001. Scale bar: 100 µm.
Figure 4.
TRPV1 Expression is significantly increased in the trigeminal ganglion after AEW treatment. (a–b) Real-time PCR results indicate that of the TRPV1 expression is increased after AEW treatment. (c–d) Western blot results confirm an enhanced TRPV1 expression after AEW treatment.
Enhanced neuronal responsiveness to capsaicin under the dry skin conditions
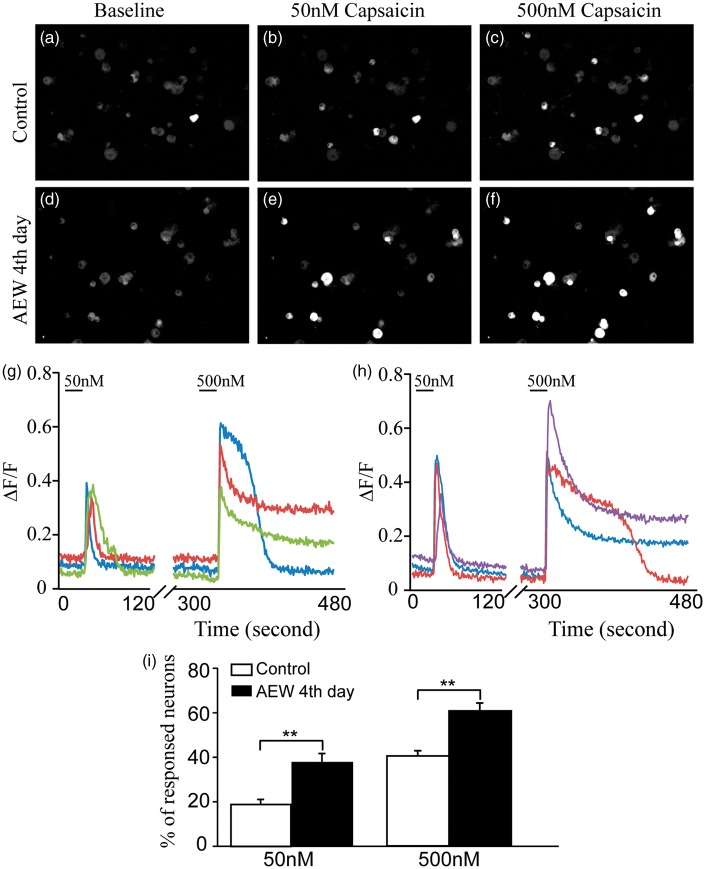
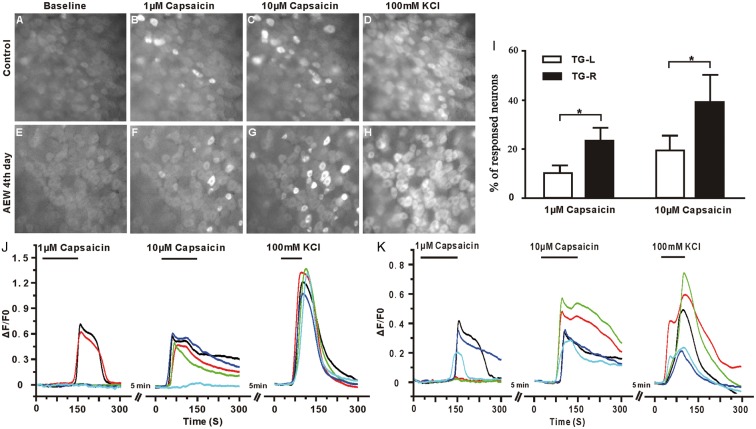
In addition to molecular characterization, we further studied the changes in physiological properties of TRPV1+ neurons under the dry skin condition using two complementary strategies. First, we cultured TG neurons from AEW-treated mice at the fourth day and examined their responses to capsaicin using calcium imaging techniques. The responsiveness to 50 nM capsaicin was significantly increased in TG neurons from AEW-treated lateral (37.41% ± 4.29%), compared with those from the contralateral (18.97% ± 2.26%, p < 0.01; Figure 5). Furthermore, capsaicin of higher concentration (500 nM) causes more responses in sensory neurons in both groups, but sensory neurons from AEW-treated lateral display more responsiveness (61.85% ± 3.61%) than controls from contralateral ganglia (41.30% ± 2.25%, p < 0.01; Figure 5). The advantage of this strategy is that cultured sensory neurons are highly sensitive to chemical stimuli and easy to be imaged. However, because the gene expression profile and physiological properties of sensory neurons likely are altered by dissociation and in vitro culture, we, secondly, used a complementary strategy of recording the calcium activity of sensory neurons in whole TGs from AEW-treated PirtGCaMP3/+ mice at the fourth day, in which the calcium indicator GCaMP3 is expressed in sensory neurons. The proportion of sensory neurons that responded to 1 μM capsaicin was significantly increased in the V3 branch of the TG from the AEW-treated lateral (23.14% ± 9.71%; normalized by 100 mM KCl activation) (Supplemental video. 1) than those from the contralateral (9.98% ± 5.80%, p < 0.05) (Supplemental video. 2). Furthermore, difference between the treated and control TGs was observed upon 10 μM capsaicin challenge (19.47 ± 10.56 (control) vs. 38.93 ± 20.07 (AEW fourth day), p < 0.05; Figure 6).
Figure 5.
The proportion of TG neurons sensitive to capsaicin is significantly increased in AEW treated mice. (a) Representative images of GCaMP3 fluorescence in cultured TG neurons from control mice at baseline and after treatment with (b) 50 nM capsaicin and (c) 500 nM capsaicin. (d) Representative images of GCaMP3 fluorescence in cultured TG neurons from AEW treated mice at baseline and after treatment with (e) 50 nM capsaicin and (f) 500 nM capsaicin. (g–h) Calcium traces of representative TG neurons from control (g) and AEW treated mice (h). (i) Compared to control TG neurons, TG neurons from AEW treated mice show significant increases in the fraction responsive to 50 nM (378 neurons from three mice) and (j) 500 nM capsaicin (656 neurons from three mice). Data are presented as mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 6.
AEW treatments dramatically increase the sensitivity of sensory neurons to capsaicin in the trigeminal ganglia. (a–d) Representative images of GCaMP3 fluorescence in whole TG explants from control mice at baseline (a) and after treatment with 1 μM capsaicin (b), and 10μM capsaicin (c). (d) 100 mM KCl was used as the positive control. (e–h) Representative images of GCaMP3 fluorescence of TG from mice after three days of AEW treatment at baseline (e) and after treatment with 1μM capsaicin (f), 10 μM capsaicin (g), and 100 mM KCl (h). (j–k) Compared with TGs from control mice (j), there is a significant increase in the fraction of neurons responsive to capsaicin in TGs from AEW treated mice (k). (i) Percentage of representative neurons from control (j) and AEW treated TGs (k). Data are presented as mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001.
Pain-to-itch switch under chronic itchy conditions
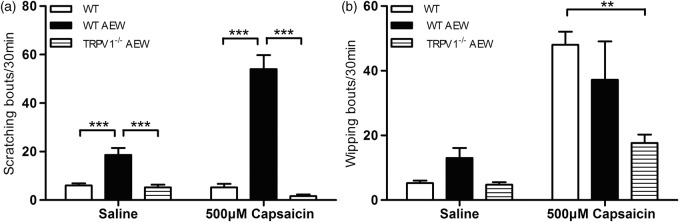
To determine whether this up-regulated TRPV1 expression underlie the commonly observed “pain-to-itch” switch in chronic itch, we did intradermal injection of capsaicine into the cheek skin of AEW-treated mice. Capsaicine usually evokes pain-related wiping behavior, rather than scratching, in WT mice.14 However, capsaicine elicited robust itch-related scratching behavior (54.00 ± 4.86) on Day 4 of AEW treatment (before the onset of excessive spontaneous scratching; Figure 7(a)), compared with water-treated control mice (5.25 ± 1.44, p < 0.01). Importantly, AEW-treated TRPV1-deficient mice do not display scratching response to capsaicin (1.66 ± 0.67), indicating that capsaicin-induced scratching is mediated by TRPV1 under chronic itch conditions. In contrast, no difference was observed between AEW-treated mice and control WT mice in their pain-related wiping behavior (48.0 ± 4.08 vs. 37.2 ± 12.12; Figure 7(b)).
Figure 7.
AEW treated mice show significantly increased scratch behavior after subcutaneous capsaicin injection. (a) Compared with control mice, AEW treated mice show significantly increased scratching behavior after subcutaneous capsaicin injection (n = 5), without detectable changes in wiping behavior (b). Data are presented as mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001.
As a control, saline injection also elicited a small increase in scratching in AEW-treated mice (18.67 ± 7.86), but not in control WT mice (6.00 ± 1.83) and AEW-treated TRPV1-deficient mice (5.25 ± 1.10). In contrast, no significant difference was observed in their wiping behavior (13.00 ± 7.04 vs. 5.25 ± 1.50; Figure 7). These results suggest that injection-related mechanical pain also elicits itch under chronic itchy conditions.
Discussion
It has widely observed that hot shower aggrevates itch in patients living with xerosis (dry skin), atopic dermatitis, and psoriasis. However, the underlying mechanisms are unclear. In this study, we combined mouse genetics, quantitative behavioral assays, sensory fiber anatomy, and GCaMP3-assisted calcium imaging to investigate the role of heat sensitive channel TPRV1 in chronic itch and its involvement in mediating pain-to-itch switch. Our study unravealed an enhanced innervation density of TRPV1+ sensory fibers in the skin of AEW model mice, due to an expansion of TRPV1+ sensory neurons. Moreover, this increased expression of TRPV1 is essential to neuronal hypersensitivity to capsaicine and leads to capsaicine-induced itch under dry skin conditions. Our findings thus provide a plausible mechanism for heat-induced itch in chronic itch and pave the avenue for future development of effective novel therapies for itch management.
Our results suggest that sensitization of TRPV1+ nerve endings underlies pain-to-itch switch (itch hypersensitivity). Interestingly, TRPV1-related peripheral sensitization is also a well-established mechanism of chronic pain. NGF has been found to elevate under inflammatory conditions,19 which directly increases the expression of TRPV1 in sensory neurons. Interestingly, similar NGF increase was found in the skin and serum of itchy patients with atopic dermatitis.20 It is, therefore, reasonable to conceive that the similar mechanism may mediate the up-regulation of TRPV1 in both chronic itch and chronic pain.
Interestingly, the upregulation of TRPV1 leads to distinct behavioral consequences (pain vs. itch) in chronic pain and chronic itch. As NGF indiscriminately up-regulates the expression of TRPV1 in TrkA+ sensory neurons, which include both nociceptive neurons and itch-sensing neurons, the behavioral consequences are likely due to the central sensitization under different conditions. Although capsaicin activates both nociceptive neurons and itch-sensing neurons via TRPV1, the pain pathway inhibits the itch pathway under normal and chronic pain conditions and hence generates pain sensation. In contrast, in chronic itch, the central sensitization of itch-signaling pathway could amplify capsaicin-induced sensory inputs from peripheral itch-sensing neurons, which overpowers the pain pathway and mediates “pain-to-itch” switching in chronic itch. The distinct behavioral consequences of peripheral sensitization in chronic itch and chronic pain offer important information on the abnormal sensory coding under pathological conditions and provide new insights on difference between chronic itch and chronic pain.
Authors’ contributions
GY and NY collected and analyzed the data; FL and CW performed TRPV1-PLAP staining; MC and YY and CZ performed Western blot; CG, ZW, HS, MT, QH, and DH helped breed the animals; GY, QL, and ZT designed the experiment and wrote the manuscript. GY and NY contributed equally to this work. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Natural Science Foundation of Jiangsu Province to GY (BK20151571), the National Natural Science Foundation of China to Z-X T(31271181, 31471007), the Overseas and Hong Kong, Macau Scholars Collaborative Fund to X-Z D(31328012), the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) to GY and Z-X T, Cooperative Innovation Center for Molecular Target New Drug Study to Z-X T.
References
- 1.Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest 2010; 120: 3773–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci 2013; 15: 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron R, Schwarz K, Kleinert A, et al. Histamine-induced itch converts into pain in neuropathic hyperalgesia. Neuroreport 2001; 12: 3475–3478. [DOI] [PubMed] [Google Scholar]
- 4.Birklein F, Claus D, Riedl B, et al. Effects of cutaneous histamine application in patients with sympathetic reflex dystrophy. Muscle Nerve 1997; 20: 1389–1395. [DOI] [PubMed] [Google Scholar]
- 5.Rukwied R, Heyer G. Administration of acetylcholine and vasoactive intestinal polypeptide to atopic eczema patients. Exp Dermatol 1999; 8: 39–45. [DOI] [PubMed] [Google Scholar]
- 6.Hosogi M, Schmelz M, Miyachi Y, et al. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain 2006; 126: 16–23. [DOI] [PubMed] [Google Scholar]
- 7.Ikoma A, Fartasch M, Heyer G, et al. Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch. Neurology 2004; 62: 212–217. [DOI] [PubMed] [Google Scholar]
- 8.Leonardi A. Role of histamine in allergic conjunctivitis. Acta Ophthalmol Scand Suppl 2000; 230: 18–21. [DOI] [PubMed] [Google Scholar]
- 9.Shim WS, Tak MH, Lee MH, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci 2007; 27: 2331–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YS, Chu Y, Han L, et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 2014; 81: 873–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyamoto T, Nojima H, Shinkado T, et al. Itch-associated response induced by experimental dry skin in mice. Jpn J Pharmacol 2002; 88: 285–292. [DOI] [PubMed] [Google Scholar]
- 12.Liu Q, Vrontou S, Rice FL, et al. Molecular genetic visualization of a rare subset of unmyelinated sensory neurons that may detect gentle touch. Nat Neurosci 2007; 10: 946–948. [DOI] [PubMed] [Google Scholar]
- 13.Liu Q, Weng HJ, Patel KN, et al. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal 2011; 4: ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han L, Ma C, Liu Q, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 2013; 16: 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson SR, Nelson AM, Batia L, et al. The ion channel TRPA1 is required for chronic itch. J Neurosci 2013; 33: 9283–9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nojima H, Cuellar JM, Simons CT, et al. Spinal c-fos expression associated with spontaneous biting in a mouse model of dry skin pruritus. Neurosci Lett 2004; 361: 79–82. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain 2010; 151: 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao ZQ, Huo FQ, Jeffry J, et al. Chronic itch development in sensory neurons requires BRAF signaling pathways. J Clin Invest 2013; 123: 4769–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji RR, Samad TA, Jin SX, et al. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002; 36: 57–68. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi J, Aihara M, Kobayashi Y, et al. Quantitative analysis of nerve growth factor (NGF) in the atopic dermatitis and psoriasis horny layer and effect of treatment on NGF in atopic dermatitis. J Dermatol Sci 2009; 53: 48–54. [DOI] [PubMed] [Google Scholar]