Abstract
Background
Resveratrol, a component of red wine, has been reported to decrease prostaglandin E2 production by inhibiting the cyclooxygenase-2 cascade and to modulate various voltage-dependent ion channels, suggesting that resveratrol could attenuate inflammatory hyperalgesia. However, the effects of resveratrol on inflammation-induced hyperexcitability of nociceptive neurons in vivo remain to be determined. Thus, the aim of the present study was to determine whether daily systemic administration of resveratrol to rats attenuates the inflammation-induced hyperexcitability of spinal trigeminal nucleus caudalis wide-dynamic range neurons associated with hyperalgesia.
Results
Inflammation was induced by injection of complete Freund’s adjuvant into the whisker pad. The threshold of escape from mechanical stimulation applied to whisker pad in inflamed rats was significantly lower than in control rats. The decreased mechanical threshold in inflamed rats was restored to control levels by daily systemic administration of resveratrol (2 mg/kg, i.p.). The mean discharge frequency of spinal trigeminal nucleus caudalis wide-dynamic range neurons to both nonnoxious and noxious mechanical stimuli in inflamed rats was significantly decreased after resveratrol administration. In addition, the increased mean spontaneous discharge of spinal trigeminal nucleus caudalis wide-dynamic range neurons in inflamed rats was significantly decreased after resveratrol administration. Similarly, resveratrol significantly diminished noxious pinch-evoked mean after discharge frequency and occurrence in inflamed rats. Finally, resveratrol restored the expanded mean size of the receptive field in inflamed rats to control levels.
Conclusion
These results suggest that chronic administration of resveratrol attenuates inflammation-induced mechanical inflammatory hyperalgesia and that this effect is due primarily to the suppression of spinal trigeminal nucleus caudalis wide dynamic range neuron hyperexcitability via inhibition of both peripheral and central cyclooxygenase-2 cascade signaling pathways. These findings support the idea of resveratrol as a potential complementary and alternative medicine for the treatment of trigeminal inflammatory hyperalgesia without side effects.
Keywords: Inflammation, resveratrol, trigeminal system, hyperalgesia, single unit recording, cyclooxygenase
Background
Trans-resveratrol (trans-3,4′-5-trihydroxystilbene) is a naturally occurring polyphenol present in red wine and various food products. It is well known that resveratrol has a variety of biological actions, including cardiovascular protective, neuroprotective, anticancer, and anti-inflammatory effects.1,2 However, recent studies have reported that resveratrol modulates neuronal excitability of the peripheral and central nervous systems (CNSs) via various voltage-dependent ion channels3–6 and ligand-gated ion channels,7,8 including neurons in the sensory information processing system. For example, resveratrol inhibits voltage-gated Na+ currents in primary afferent neurons, such as dorsal root ganglion (DRG) neurons,3 and modulates several types of potassium channels5,6 Resveratrol decreases prostaglandin E2 (PGE2) production by inhibiting cyclooxygenase (COX)-2 cascades and is a potent inhibitor of inducible COX-2.9,10 PGE2 is a well-known inflammatory mediator and sensitizer of peripheral nociceptors that can also act on the CNS, including somatosensory neurons in the spinal dorsal horn.11–13 Previous reports indicated that resveratrol inhibits inflammation-induced hyperalgesia by suppressing COX-1 and COX-2 activity.10,14,15 It is well known that the acidic antipyretic analgesic nonsteroidal anti-inflammatory drugs are potent inhibitors of COX-2.16 Together, these observations suggest that resveratrol may be a potential therapeutic agent for the prevention of inflammatory hyperalgesia.
There is an important relay station in the trigeminal spinal nucleus for the transmission of orofacial sensory information that is functionally subdivided into three nuclei (from rostral to caudal): oralis, interpolarlis, and caudalis.17 It is well known that the spinal trigeminal nucleus caudalis (SpVc) and the upper cervical (C1–C2) dorsal horn are important relay stations for trigeminal nociceptive inputs from inflammation and tissue injury.17,18 Chronic pathological conditions such as tissue inflammation can change the properties of somatic sensory pathways, leading to hyperalgesia,19 changes in the excitability of primary afferent neurons (peripheral sensitization), and altered information processing in the trigeminal spinal nucleus or higher centers.20 Rat models of inflammation in the orofacial region have been developed using complete Freund’s adjuvant (CFA) for investigations of trigeminal pathological pain.18,21–23 Previous studies reported that CFA inflammation induced hyperexcitability of SpVc wide-dynamic range (WDR) neurons in response to mechanical stimuli.18,22 There are reports that SpVc and C1–C2 WDR neurons contribute to the mechanism of hyperalgesia and referred pain associated with dental pain.24,25 Recently, we reported that, in the absence of inflammatory or neuropathic pain, acute intravenous administration of resveratrol suppresses SpVc WDR neuron excitability, and so resveratrol has potential as a complementary and alternative medicine (CAM) for the treatment of trigeminal nociceptive pain without side effects.26 Indeed, many recent reports have described the use of CAM, such as herbal medicines and acupuncture, for the treatment of persistent clinical chronic pain.27–29 Because resveratrol has no known toxic side effects,30 it could be a candidate CAM for the therapeutic treatment of pain. In addition, Cady et al.31 reported that dietary grape seed polyphenol extract inhibited temporomandibular joint (TMJ) inflammation-induced pain by suppressing the development of peripheral and central sensitization, including neuron–glial cell interactions in the trigeminal ganglia.31 Together, these studies suggest that resveratrol could attenuate inflammation-induced hyperexcitability of SpVc neurons associated with trigeminal hyperalgesia. However, until now, no studies have addressed this possibility. Therefore, the aim of the present study was to investigate whether chronic resveratrol administration to rats could attenuate the inflammation-induced hyperexcitability of SpVc neurons associated with hyperalgesia in vivo.
Methods
The experiments performed in the present study were approved by the Animal Use and Care Committee of Azabu University and were consistent with the ethical guidelines of the International Association for the Study of Pain.32 Every effort was made to minimize the number of animals used and their suffering.
Induction of cutaneous inflammation and resveratrol administration
Experiments were performed on adult male Wistar rats (250–310 g; n = 25). Rats were divided into three groups as follows: (a) a naïve (control) group (n = 7); (b) an inflamed group (n = 8), in which inflammation was induced using CFA; and (c) inflamed rats treated with resveratrol (2 mg/kg, i.p.; n = 10). All rats were anesthetized with sodium pentobarbital (45 mg/kg, i.p.), after which rats in the inflamed groups were injected with CFA (0.05 mL, 1:1 oil:saline suspension) into the left side of the whisker pad, as described previously,23 whereas rats in the naïve group were injected with vehicle (0.9% NaCl). In some experiments, the CFA-induced inflammation was verified by extravasation of Evan’s blue dye (50 mg/mL, 1 mL/kg, i.v.); postmortem examination of the injected facial region revealed accumulation of blue dye in the skin, indicating that the plasma protein extravasation was due to localized inflammation.18,21
Resveratrol was dissolved in dimethyl sulfoxide. Aliquots (0.2 mL) of the stock solution (20 mmol/L) were stored at −20℃ and were diluted to the desired concentrations in saline prior to use. Because the effects of resveratrol are manifested within 24 h,10 resveratrol was administered to rats daily before behavioral and electrophysiological testing.
Mechanical threshold for escape behavior
The mechanical threshold for escape behavior was determined as described previously.23,33 Briefly, zero, one, and two days after CFA or vehicle injection into the whisker pad, mechanical hyperalgesia was assessed using a set of von Frey hairs (Semmes-Weinstein Monofilaments; North Coast Medical, Gilroy, CA) in the ipsilateral and contralateral whisker pad. To evaluate the escape threshold of rats, von Frey mechanical stimuli were applied to the whisker pad in an ascending series of trials. Each von Frey stimulus was applied three times in each series of trials. Escape threshold intensity was defined as the point at which the rats moved their head away from at least one of the three stimuli.
Extracellular single-unit recording of SpVc WDR neuronal activity
Electrophysiological recordings were made two days after CFA or vehicle injection. Rats were anesthetized with pentobarbital sodium (45 mg/kg, i.p.) and anesthesia was maintained with additional doses of 2–3 mg/kg per hour through a cannula inserted in the jugular vein, as required. The level of anesthesia was confirmed by the absence of the corneal reflex and a lack of response to paw pinching. Rectal temperature was maintained at 37.0 ± 0.5℃ with a homeothermic blanket during recording. Rats were then placed in a stereotaxic apparatus and the activity of a single neuron from the SpVC region was recorded extracellularly. Single neuron activity was recorded using a glass micropipette filled with 2% Pontamine sky blue and 0.5 M sodium acetate according to the stereotaxic coordinates of Paxinos and Watson.34 Neuronal activity was amplified (DAM80 differential amplifier; World Precision Instruments), filtered (0.3–10 kHz), monitored with an oscilloscope (SS-7672; Iwatsu, Tokyo, Japan), and recorded for off-line analysis by Power Lab and Chart 5 software (ADInstruments, UK), as described previously.26
Experimental protocols
Recordings of extracellular WDR unit activity were performed as follows. Mechanical stimulation (with a paint brush) was used as a search stimulus to quickly identify the receptive field and to avoid sensitization of peripheral receptors. Single units that responded on the left-side orofacial skin (whisker pad) were searched for using a brush and a set of von Frey hairs. Noxious pinch stimulation was applied to the whisker pad with calibrated forceps (5 s) that evoked a pain sensation when applied to a human subject. After identification of WDR SpVc neurons in the whisker pad that were responding, we determined whether there was spontaneous discharge from these neurons. We compared discharge rates induced by mechanical stimulation in naïve and inflamed rats. The threshold for mechanical stimulation was determined by using nonnoxious and noxious mechanical stimulation with von Frey hairs (0.16, 0.4, 2, 6, 10, 15, 26, 60 g; 5 s each) applied at 5-s intervals. The mechanical receptive field of neurons was mapped by probing the whisker pad with von Frey hairs and then outlined on a life-sized drawing of a rat on tracing paper.24,26 The WDR neuronal discharges induced by mechanical stimulation were quantified by subtracting background activity from evoked activity. Spontaneous discharge frequencies were determined over 2–5 min. Mean firing rates of SpVc WDR neurons evoked by mechanical stimulation were compared before and after drug administration. Because previous studies have demonstrated that WDR neurons in the SpVc region have an important role in the mechanism underlying hyperalgesia and referred pain associated with orofacial pain,18,24,25,35 the focus of the present study was on the effects of resveratrol on SpVc WDR neuronal activity; we did not examine nociceptive-specific neurons.36 Peristimulus histograms (bin = 100 ms) were generated in response to each stimulus. Spontaneous discharge was subtracted from neuronal responses during the analysis. After discharges were recorded for 60 s after pinching the skin in the receptive field. Mean spontaneous, mechanical stimulation-evoked discharge frequencies, after discharge frequency, and the mean mechanical threshold of SpVc WDR neurons were compared among three groups (naïve, inflamed (CFA, untreated), and inflamed + resveratrol treated).
Identification of recording sites
At the end of recording sessions, rats were deeply anesthetized and anodal DC currents (30 μA, 5 min) were passed through a recording micropipette. Rats were perfused transcardially with saline and 10% formalin. Frozen coronal sections (30 µm) were cut and stained with hematoxylin–eosin. Recording sites were identified from the blue spots, whereas electrode tracks were constructed using the blue spots in combination with micromanipulator readings.
Data analysis
Values are expressed as the mean ± SEM. Statistical analysis was performed using two-way repeated measures analysis of variance followed by Tukey–Kramer or Dunnett’s post hoc tests for behavioral and electrophysiological data. Two-sided p < 0.05 was considered significant.
Results
Inflammation-induced hyperalgesia
After CFA injection, rats were tested for abnormal pain sensation by probing the injected site and the whisker pad with von Frey filaments. The threshold for escape from mechanical stimulation applied to the whisker pad area was significantly reduced in inflamed versus naïve rats (2.4 ± 0.7 vs. 56.2 ± 3.8 g, respectively; p < 0.05; Figure 1) two days after CFA or vehicle injection. In addition, the threshold for escape from mechanical stimulation was significantly reduced in inflamed rats one day after injection (p < 0.05; Figure 1). No significant differences in the threshold for escape were observed in the contralateral whisker pad area between the naïve and inflamed groups (58.1 ± 2.8 vs. 59.3 ± 4.2 g, respectively).
Figure 1.
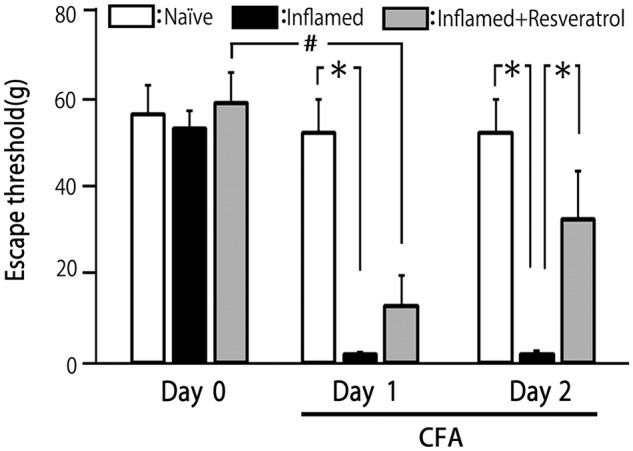
Comparison of changes in the escape threshold among naïve (control), inflamed, and resveratrol-treated inflamed rats. Mechanical stimulation using von Frey hairs was applied to the ipsilateral whisker pad of naïve (saline injected; n = 7), inflamed rats (injection of CFA into the whisker pad; n = 8) and resveratrol-treated inflamed (2 mg/kg, i.p.; n = 10) rats to assess hyperalgesia. Data are the mean ± SEM. *p < 0.05 compared with inflamed rats; #p < 0.05 compared with Day 1. CFA: complete Freund’s adjuvant.
Effects of chronic resveratrol administration on hyperalgesia
Resveratrol administration (2 mg/kg, i.p.) partially reversed the reduced escape threshold from mechanical stimulation in Day 1 inflamed rats, but the escape threshold remained significantly lower than in naïve rats (Figure 1). However, as shown in Figure 1, resveratrol had restored the escape threshold to control levels in Day 2 inflamed rats, with the threshold in untreated and treated inflamed rats on Day 2 being 2.4 ± 0.7 and 34.6 ± 1 2.8 g, respectively (p < 0.05). No significant differences in the threshold for escape between naïve and treated inflamed rats on Day 2 (52.7 ± 7.7 vs. 34.6 ± 12.8 g, NS)
General characteristics of SpVc WDR neurons
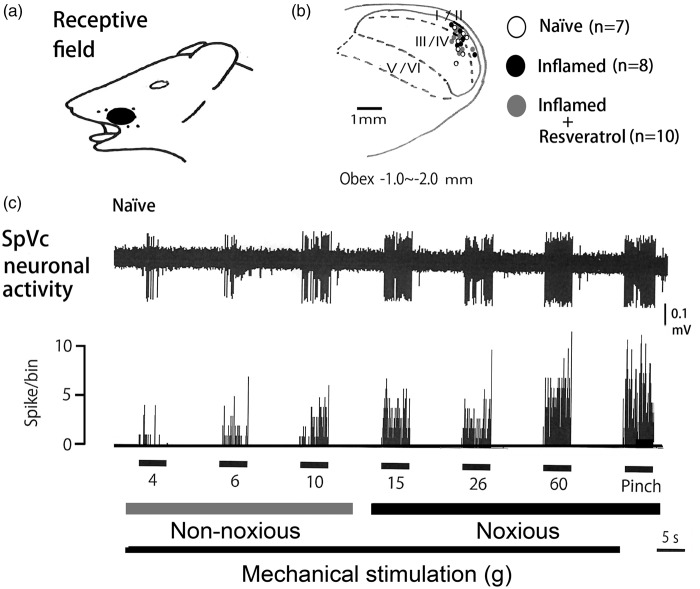
In all, 25 SpVc WDR neurons responding to mechanical stimulation of the whisker pad were analyzed in naïve (n = 7), inflamed (n = 8), and resveratrol-treated inflamed (n = 10) rats. These SpVc neurons that responded to nonnoxious and noxious mechanical stimulation exhibited a somatic receptive field in the orofacial area (mainly the whisker pad; Figure 2(a)). Every neuron recorded belonged to the category of WDR neurons. As shown in Figure (2b), typical recording sites were distributed primarily in the maxillary and mandibular branches, and recording sites were found in Layers I–II (n = 11; 44%) and III–V (n = 14; 56%) in the SpVc (obex between −1.0 and −2.0 mm). There were no obvious differences in recording sites for each of the units among the three groups. As shown in Figure 2(c), graded mechanical stimulation was applied to the most sensitive area of the receptive field, with the increased firing frequency of SpVc neurons being proportional to stimulus intensity.
Figure 2.
General characteristics of SpVc WDR neuronal activity in the whisker pad. (a) Receptive field of the whisker pad in the facial skin. (b) Distribution of SpVc WDR neurons responding to nonnoxious and noxious mechanical stimulation of the facial skin (n = 25). Numbers below each drawing indicate the frontal plane in relation to the obex. (c) Examples of SpVc WDR neuronal firing in response to nonnoxious and noxious mechanical stimuli. SpVc: spinal trigeminal nucleus caudalis; WDR: wide-dynamic range.
Changes in excitability of SpVc WDR neurons following inflammation
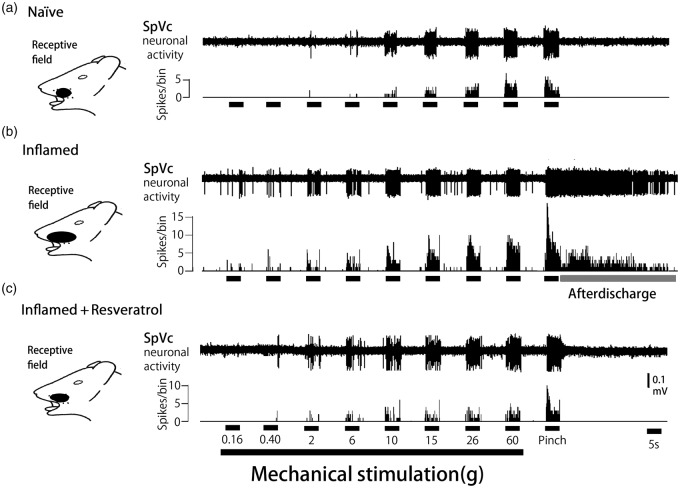
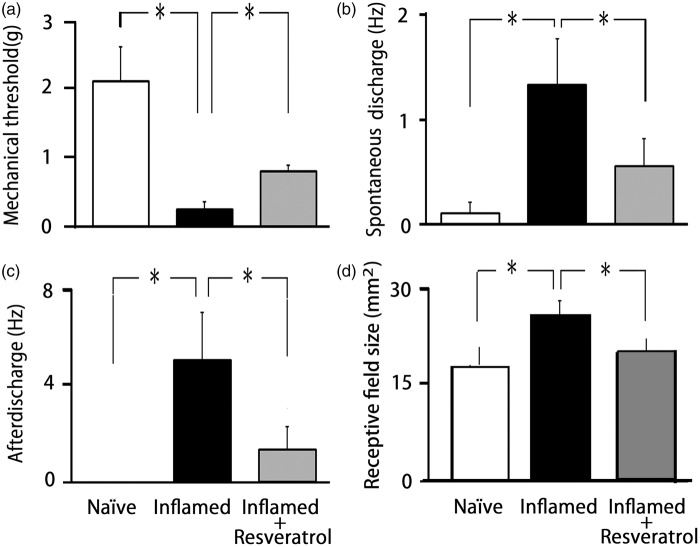
We confirmed in the present study that CFA induced hyperexcitability of SpVc WDR neurons compared with naïve rats (Figure 3(a)), as reported previously.18 In naïve rats, spontaneous discharges were observed in 14.3% (1/7) of SpVc neurons (Figure 3(a)). Most neurons fired at a low frequency, with a mean firing frequency of 0.1 ± 0.2 Hz (n = 7; without mechanical stimulation), whereas approximately 62.5% of WDR neurons (5/8; mean firing frequency 3.5 ± 0.3 Hz) were spontaneously active in inflamed rats (Figure 3(b)). In inflamed rats, SpVc WDR neurons exhibited significantly stronger responses to nonnoxious mechanical stimulation than in naïve rats (Figure 3(b)), as reported previously.18 The mean firing frequencies of SpVc WDR neurons in response to mechanical stimuli (0.16, 2, 10, 60 g, pinch) were significantly greater in inflamed than naïve rats (p < 0.05; Figure 4). The mean mechanical threshold was significantly decreased in inflamed compared with naïve rats (0.5 ± 0.3 vs. 2.1 ± 0.5 g, respectively; p < 0.05; Figure 5(a)), whereas the mean spontaneous discharge was increased significantly in inflamed rats (Figure 5(b)). Although there was no obvious after discharge in response to noxious pinch in naïve rats (0/7; 0%), most SpVc neurons in inflamed rats (5/8; 62.5%) showed after discharges following noxious pinch stimulation (frequency 6.3 ± 2.4 Hz, duration 21.2 ± 6.2 s; Figures 3(b) and 5(c)). The mean size of the receptive field was significantly greater in inflamed than naïve rats (28.2 ± 2.6 vs. 17.1 ± 3.1 mm2, respectively; p < 0.05; Figure 5(d))
Figure 3.
Reversal by chronic resveratrol of SpVc WDR neuronal hyperactivity after induction of orofacial inflammation. Examples of discharges from SpVc WDR neurons in response to nonnoxious and noxious mechanical stimulation in (a) naïve (control), (b) inflamed, and (c) resveratrol-treated inflamed rats (2 mg/kg, i.p.). Note that resveratrol administration restored the inflammation-induced decreases in the mechanical stimulation threshold to evoke neuronal firing, increases in spontaneous discharge and the size of the receptive field, and the occurrence of noxious pinch-evoked discharges in inflamed rats to control levels. SpVc: spinal trigeminal nucleus caudalis; WDR: wide-dynamic range.
Figure 4.
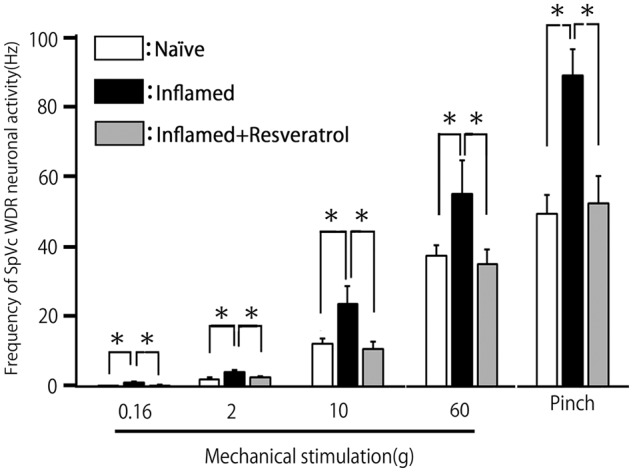
Summary of resveratrol reversal of the augmented discharge frequency of SpVc WDR neurons after induction of orofacial inflammation. (a) Mean discharge frequencies of SpVc WDR neurons evoked by mechanical stimulation (nonnoxious and noxious) of the whisker pad in naïve (control), inflamed, and resveratrol-treated inflamed rats (2 mg/kg, i.p.). *p < 0.05 compared with inflamed rats. SpVc: spinal trigeminal nucleus caudalis; WDR: wide-dynamic range.
Figure 5.
Summary of resveratrol reversal of SpVc WDR neuron hyperexcitability after induction of orofacial inflammation. (a) Mean mechanical threshold, (b) mean spontaneous discharge, (c) mean noxious pinch-evoked after discharge frequency, and (d) mean size of the receptive field of SpVc WDR neurons in naïve (control), inflamed, and resveratrol-treated inflamed rats (2 mg/kg, i.p.). *p < 0.05 compared with inflamed rats. SpVc: spinal trigeminal nucleus caudalis; WDR: wide-dynamic range.
Effects of chronic resveratrol on hyperexcitability of SpVc WDR neurons in inflamed rats
Based on the results of behavioral analyses of escape threshold, the effects of chronic resveratrol (2 mg/kg, i.p.) administration on the hyperexcitability of SpVc WDR neurons were evaluated in inflamed Day 2 rats. Ten SpVc WDR neurons responding to mechanical stimulation of the whisker pad were analyzed; the excitability of 8 of the 10 neurons (80%) had returned to control levels after two days treatment with resveratrol, whereas two neurons remained hyperexcitable. Typical examples of the effects of chronic resveratrol administration on discharge rates of SpVc WDR neurons in inflamed rats in response to nonnoxious (0.16–10 g) and noxious mechanical (15–60 g, pinch) stimulation are shown in Figure 3(c). As shown in Figure 3(c), the CFA-induced reduction in mechanical threshold and augmented spontaneous, as well as noxious and nonnoxious, firing frequency in inflamed rats returned to levels in naïve rats after resveratrol treatment. As shown in Figure 4, the mean discharge frequency of SpVc WDR neurons in inflamed rats in response to nonnoxious and noxious mechanical stimuli was significantly decreased after resveratrol treatment (p < 0.05). Resveratrol also significantly returned the mean mechanical stimulation threshold in inflamed rats to control levels (Figure 5(a): p < 0.05). The mean spontaneous discharges of SpVc WDR neurons in inflamed rats returned to levels as in naïve rats after resveratrol treatment (Figure 5(b); p < 0.05). Similarly, resveratrol treatment of inflamed rats decreased the number of neurons exhibiting after discharges in response to noxious pinch (75% vs. 20% in untreated and treated inflamed rats, respectively), as well as the mean firing frequency of these after discharges, which were significantly restored to control levels after resveratrol treatment (Figure 5(c): p < 0.05). Resveratrol treatment also reduced the mean size of the receptive field in inflamed rats to control levels (p < 0.05; Figure 5(d)). Daily systemic administration of vehicle had no significant on either spontaneous-, nonnoxious-, noxious mechanical-, or pinch-evoked hyperexcitability of SpVc WDR neurons in inflamed rats (data not shown).
Discussion
Resveratrol attenuates trigeminal hyperalgesia
Previous studies indicated that resveratrol attenuates nociceptive behavior in various pain models, including neuropathic and inflammatory pain.10,14,15 In the present study, the behavioral experiments showed that (a) the threshold of escape from mechanical stimulation applied to the orofacial area was significantly lower in inflamed than naïve rats, as reported previously;18 (b) one day after the induction of inflammation, there is tendency for resveratrol (2 mg/kg, i.p.) to return the decreased threshold of escape toward control levels; and (c) by Day 2 after the induction of inflammation, repeated dosing with resveratrol had significantly increased the threshold of escape to control levels. These findings are in agreement with those of a previous study that showed an antinociceptive effect of resveratrol with a dose as low as 2 mg/kg, i.p., and no further increase in effect with higher doses.15 Pham-Marcou et al.10 also demonstrated a prolonged antinociceptive effect of resveratrol that lasted between 3 and 48 h after i.p. injection that was mediated by inhibition of COX-2 mRNA levels of both the DRG and spinal cord. Because resveratrol is considered a potent, nonspecific COX-1/COX-2 inhibitor,9,10,14,15 it can be assumed that suppression of peripheral and central PGE2 production following chronic resveratrol administration may be responsible for the return of the escape threshold from mechanical stimulation to control levels in inflamed rats.
Possible mechanism for resveratrol suppression of hyperalgesia-associated SpVc WDR neuron hyperexcitability
Peripheral tissue injury and inflammation of the innervating trigeminal nerve can alter the properties of trigeminal somatic sensory pathways, causing behavioral hypersensitivity and resulting in increased responses to pain caused by noxious stimuli (e.g. hyperalgesia).19 In the present study, we found that (a) the decreased mean mechanical stimulation threshold in inflamed rats was returned to control levels following daily systemic administration of resveratrol, in accordance with findings of the behavioral study; and (b) the mean discharge frequency of SpVc WDR neurons in inflamed rats in response to both nonnoxious and noxious mechanical stimuli was returned to control levels on Day 2 of resveratrol treatment, suggesting that systemic administration of resveratrol can alter the inflammation-induced hypersensitivity of SpVc WDR neurons.
It has been reported that PGE2 facilitates activation of transient receptor potential vanilloid 1 and tetrodotoxin-resistant (TTX-R) Na+ channels3,19,37 and that resveratrol inhibits both tetrodotoxin-sensitive (TTX-S) and TTX-R Na+ currents in acutely dissociated DRG neurons. TTX-R Na+ channels (e.g. Nav1.8 and Nav1.9) appear to be selectively expressed in small- and medium-sized DRG neurons.38 Compelling evidence indicates that these small DRG neurons are somata, which give rise to thinly and unmyelinated C- and Aδ-fibers that primarily conduct nociceptive stimuli. Modulation of these Na+ channels involves activation of adenylate cyclase and increases in cAMP, possibly leading to protein kinase A-dependent phosphorylation of the channels. By this mechanism, PGE2 produced during an inflammatory response may significantly increase the excitability of nociceptive fibers (peripheral sensitization). Because it has been reported that the excitability of small-diameter trigeminal ganglion neurons seen after PGE2 application is associated with an increase in TTX-R Na+ currents,39 it can be assumed that resveratrol inhibits the excitability of small-diameter trigeminal ganglion neurons by suppressing TTX-R Na+ currents induced by COX-related PGE2 production. It is a reasonable assumption that at least part of the peripheral antinociceptive action of resveratrol arises from the prevention of peripheral sensitization, in addition to its effect as an antipyretic analgesic.
Conversely, PGE2 also acts in the CNS, namely in the spinal dorsal horn and SpVc neurons, to produce hyperalgesia.11 Inflammation-induced increases in COX-2 mRNA and protein levels have been demonstrated in the spinal cord,13,40 where Cox-1 and Cox-2 are expressed constitutively. Recent evidence indicates that a major stimulus for the induction of COX-2 is the proinflammatory cytokine interleukin-1β, which is found in the periphery as well as the CNS and is produced in response to inflammation.13,41 Two possible molecular mechanisms have been proposed for the central actions of PGE2 in producing hyperalgesia: (a) PGE2 reduces inhibitory glycinergic neurotransmission through postsynaptic mechanisms42 or (b) at higher concentrations, PGE2 directly depolarizes deep dorsal horn neurons.12 Thus, the central antinociceptive effects of systemically administered resveratrol are likely due to the suppression of both PGE2-induced reductions in inhibitory glycinergic neurotransmission and PGE2-induced depolarization of SpVc neurons.
Previous studies in the chronic inflamed model have reported the occurrence of after discharge from SpVc WDR neurons following noxious mechanical stimulation and that these changes are associated with neuronal sensitization during persistent pain.43,44 Interestingly, in the present study, we demonstrated that the after discharge following noxious pinch observed in inflamed rats was abolished by resveratrol administration. Although the precise mechanism as to how chronic administration of resveratrol suppresses pinch-evoked after discharges remains to be elucidated, two possible mechanisms can be postulated, as follows. First, it has been reported in cats that antagonists of neurokinin 1 receptor (NK1R), the endogenous receptor for substance P, inhibited pinch-evoked after discharges from WDR neurons in the spinal cord.45 Thus, it can be speculated that chronic administration of resveratrol attenuates NK1R-mediated after discharges of WDR neurons in the SpVc. Second, a previous study suggested that local GABAergic mechanisms exert tonic control of nociceptive mechanoreceptor transmission in SpVc neurons, contributing to mechanical receptive properties.24 Because Higashima et al.46 reported that the GABAA receptor antagonist bicuculline inhibits the generation of after discharges from hippocampal neurons, whereas the GABAB receptor antagonist phaclofen enhances them in slice preparations, it is possible that chronic administration of resveratrol attenuates GABAA receptor-mediated after discharges of WDR neurons in the SpVc.
In addition, in the present study, we found that the significant increase in the mean size of the receptive field in inflamed rats was returned to control levels by resveratrol. Although the precise mechanism as to how chronic administration of resveratrol suppresses expansion of the receptive field in CFA-induced inflammation remains to be determined, it is possible that resveratrol alters the size of the receptive field by modulating local GABAergic mechanisms, which are involved in the tonic control of nociceptive mechanoreceptive transmission. However, further studies are needed to investigate this possibility.
Functional significance of resveratrol suppression of hyperalgesia-associated SpVc neuron hyperexcitability
The CFA inflamed rat model is a widely accepted trigeminal chronic pain model.21,22 Following tissue injury and inflammation of the area innervating the orofacial area, changes in neuronal properties lead to pathological pain, such as hyperalgesia and allodynia.18,22 Indeed, it has been reported previously that SpVc WDR neuron hyperexcitability induced by TMJ inflammation contributes to ectopic mechanical allodynia innervating the whisker pad.18
In the present study, resveratrol was able to return the inflammation-induced increased mean spontaneous discharge frequency of SpVc WDR neurons to control levels. Burstein et al.47 reported that the ongoing activities observed in the SpVc are responsible for ongoing headache (spontaneous pain). The origin of ongoing activity in the central neurons that relay sensory information is of considerable clinical interest because it has been suggested as a determinant of the level of posttraumatic injury and chronic pain.48,49 A more recent study demonstrated that ongoing activity of WDR neurons in the SpVc is driven from the periphery, because microinjection of lidocaine into the trigeminal ganglia causes a significant decrease in ongoing activity.50 Together with these results, the findings of the present study suggest that resveratrol attenuates the increased spontaneous discharge activity of SpVc WDR neurons innervating the whisker pad resulting from peripheral or trigeminal ganglion sensitization51 and so contributing to spontaneous pain.
Although a previous study indicated that dietary grape seed polyphenol extract inhibited TMJ inflammation-induced pain,31 little is known regarding the mechanism underlying the antinociceptive effects polyphenols. More recently, we reported that in the absence of inflammatory or neuropathic pain, acute intravenous administration of resveratrol suppressed SpVc WDR neuronal activity in response to noxious stimulation and that resveratrol may therefore have potential as a CAM without side effects in the treatment of trigeminal nociceptive pain.26 In the present study, under in vivo conditions, daily systemic administration of resveratrol attenuated inflammation-induced hyperexcitability of trigeminal SpVc neurons associated with hyperalgesia in rats. Recently, there has been an increase in the number of CAM for the treatment of persistent chronic pain.29,52 Patients frequently turn to CAM therapies such as herbal medicines and acupuncture for pain control when conventional medical treatments are ineffective.27,28 In recent studies, the potential effects of diet and dietary supplementation on conditions associated with pain have been the focus of considerable research.53–55 The findings of the present study contribute to the search for and development of analgesic drugs with fewer side effects for the treatment of pathological pain, including orofacial pain. The findings from the present in vivo study support the idea that resveratrol could be a potential CAM for the treatment of trigeminal inflammatory hyperalgesia.
Conclusion
The present study provides evidence that chronic administration of resveratrol attenuates inflammation-induced mechanical hyperalgesia and that this effect is due primarily to the suppression of SpVc WDR neuron hyperexcitability via inhibition of both peripheral and central COX cascade signaling pathways. These findings support the notion that resveratrol could be an effective CAM, with fewer side effects, for the treatment of trigeminal inflammatory hyperalgesia.
Acknowledgment
The authors thank Inter-Biotech (http://www.inter-biotech.com) for the English language editing of this paper.
Authors’ Contributions
KS, ST, and ES performed the behavioral and electrophysiological experiments. NM, SH, and YK performed the histochemical experiment. YS, MI, and YK interpreted the data and helped finalize the manuscript. MT participated in the design of the study and wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Fremont L. Biological effects of resveratrol. Life Sci 2000; 66: 663–673. [DOI] [PubMed] [Google Scholar]
- 2.Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB 2003; 17: 1975–1985. [DOI] [PubMed] [Google Scholar]
- 3.Kim HI, Kim TH, Song JH. Resveratrol inhibits Na+ currents in rat dorsal root ganglion neurons. Brain Res 2005; 1045: 134–141. [DOI] [PubMed] [Google Scholar]
- 4.Liew R, Stagg MA, MacLeod KT, et al. The red wine, polyphenol resveratrol, exerts acute direct action on guinea-pig ventricular myocytes. Eur J Pharmacol 2005; 519: 1–8. [DOI] [PubMed] [Google Scholar]
- 5.Gao Z-B, Hu G-Y. Trans-resveratrol, a red wine ingredient, inhibits voltage-activated potassium currents in rat hippocampal neurons. Brain Res 2005; 1056: 68–75. [DOI] [PubMed] [Google Scholar]
- 6.Grannados-Soto V, Argulles CF, Ortiz MI. The peripheral antinociceptive effect of resveratrol is associated with activation of potassium channels. Neuropharmacology 2002; 43: 917–923. [DOI] [PubMed] [Google Scholar]
- 7.Gao Z-B, Chen X-Q, Hu G-Y. Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hyppocampus. Brain Res 2006; 1111: 41–47. [DOI] [PubMed] [Google Scholar]
- 8.Lee B-H, Hwang S-H, Choi S-H, et al. The resveratrol enhances 5-hydroxytryptamine type 3A receptor -mediated ion currents: role of arginine 222 residue in pre-transmembrane domain I. Biol Pharm Bull 2011; 34: 523–527. [DOI] [PubMed] [Google Scholar]
- 9.Subbramaiah K, Chung WJ, Michaluart P, et al. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. Biol Chem 1998; 273: 21875–21882. [DOI] [PubMed] [Google Scholar]
- 10.Pham-Marcou TA, Beloeil H, Sun X, et al. Antinociceptive effect of resveratrol in carageenun-evoked hyperalgesia in rats: prolonged effect related to COX-2 expression impairment. Pain 2008; 140: 274–283. [DOI] [PubMed] [Google Scholar]
- 11.Neugebauer V, Geisslinger G, Rumenapp P, et al. Antinociceptive effects of R(-) and S(+)-flurbiprofen on rat spinal dorsal horn neurons rendered hyperexcitable by an acute knee joint inflammation. JAPET 1995; 275: 618–628. [PubMed] [Google Scholar]
- 12.Baba H, Kohno T, Moore KA, et al. Direct activation of rat spinal dorsal horn neurons by prostaglandin E2. J Neurosci 2001; 218: 1750–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samad TA, Moore KA, Sapirstein A, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 2001; 410: 471–475. [DOI] [PubMed] [Google Scholar]
- 14.Torres-Lopez JE, Ortiz MI, Castaneda-Hernandez G, et al. Comparison of the antinociceptive effect of celecoxib, diclofenac and resveratrol in the formalin test. Life Sci 2002; 70: 1669–1676. [DOI] [PubMed] [Google Scholar]
- 15.Gentilli M, Mazoit JX, Bouaziz H, et al. Resveratrol decreases hyperalgesia induced by carrageenan in the rat hind paw. Life Sci 2001; 68: 1317–1321. [DOI] [PubMed] [Google Scholar]
- 16.Brune K, Zeihofer HU. Antipyretic analgesics: Basic aspects. In: McMahon SB, Kolzenburg M. (eds). Wall and Melzack’s textbook of pain 2006,. 5th ed London: Elsevier Churchill Livingstone; Chapter 29, pp. 459–469. [Google Scholar]
- 17.Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity and their clinical correlates. Crit Rev Oral Biol Med 2000; 11: 57–91. [DOI] [PubMed] [Google Scholar]
- 18.Takeda M, Takahashi M, Mastumoto S. Suppression of neurokinin-1 receptor in trigeminal ganglia attenuates central sensitization following inflammation. J Peri Nerv Syst 2012; 17: 169–181. [DOI] [PubMed] [Google Scholar]
- 19.Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci (Suppl) 2002; 5: 1062–1067. [DOI] [PubMed] [Google Scholar]
- 20.Millan MJ. The induction of pain: an integrative review. Prog Neurobiol 1999; 57: 1–164. [DOI] [PubMed] [Google Scholar]
- 21.Imbe H, Iwata K, Zhou Q-Q, et al. Orofacial deep and cutaneous tissue inflammation and trigeminal neuronal activation. Cells Tissues Organs 2001; 169: 238–247. [DOI] [PubMed] [Google Scholar]
- 22.Iwata K, Tashiro A, Tsuboi Y, et al. Medullary dorsal horn neuronal activity in rats with persistent temporomandibular joint and perioral inflammation. J Neurophysiol 1999; 82: 1244–1253. [DOI] [PubMed] [Google Scholar]
- 23.Takeda M, Takahashi M, Kitagawa J, et al. Brian-derived neurotrophic factor enhances the excitability of small-diameter trigeminal ganglion neurons projecting to the trigeminal nucleus interpolaris/caudalis transition zone following masetter muscle inflammation. Mol Pain 2013; 9: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda M, Tanimoto T, Matsumoto S. Change in mechanical receptive field properties induced by GABAA receptor activation in the trigeminal spinal nucleus caudalis neurons in rats. Exp Brain Res 2000; 134: 409–416. [DOI] [PubMed] [Google Scholar]
- 25.Takeda M, Tanimoto T, Ito M, et al. Role of capsaicin-sensitive afferent inputs from the masseter muscle in the C1 spinal neurons responding to tooth-pulp stimulation in rats. Exp Brain Res 2005; 16: 107–117. [DOI] [PubMed] [Google Scholar]
- 26.Takehana S, Sekiguchi K, Inoue M, et al. Systemic administration of resveratrol suppress the nociceptive neuronal activity of spinal trigeminal nucleus caudalis in rats. Brain Res Bull 2016; 120: 117–122. [DOI] [PubMed] [Google Scholar]
- 27.Rao JK, Mihaliak K, Kroenke K, et al. Use of complementary therapies for arthritis among patients of rheumatologists. Ann Intern Med 1999; 131: 409–416. [DOI] [PubMed] [Google Scholar]
- 28.Konvicka JJ, Meyer TA, McDavid AJ, et al. Complementary/alternative medicine use among chronic pain clinic patients. Perianesth Nurs 2008; 23: 17–23. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg EI, Genao I, Chen I, et al. Complementary and alternative medicine use by primary care patients with chronic pain. Pain Med 2008; 9: 1065–1072. [DOI] [PubMed] [Google Scholar]
- 30.Russo GL. Ins and outs of dietary phytochemical in cancer chemoprevention. Biochem Pharmacol 2007; 74: 533–544. [DOI] [PubMed] [Google Scholar]
- 31.Cady RJ, Hirst JJ, Durham PL. Dietary grape seed polyphenols repress neuron and glia activation in trigeminal ganglion and trigeminal nucleus caudalis. Mol Pain 2010; 6: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 33.Safieh-Garabedian B, Poole S, Allchorne A, et al. Contribution of interleukin-1β to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol 1995; 115: 1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 2nd ed New York: Academic Press, 1986. [Google Scholar]
- 35.Nishikawa T, Takeda M, Tanimoto T, et al. Convergence of nociceptive information from temporomandibular joint and tooth-pulp afferents on C1 spinal neurons in the rat. Life Sci 2004; 75: 1465–1478. [DOI] [PubMed] [Google Scholar]
- 36.Ness TJ, Randich A. Intravenous lidocaine inhibits nociceptive reflex and spinal neurons in the rat. Anesthesiology 2000; 92: 1685–1691. [DOI] [PubMed] [Google Scholar]
- 37.Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997; 389: 816–824. [DOI] [PubMed] [Google Scholar]
- 38.Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 1996; 379: 257–262. [DOI] [PubMed] [Google Scholar]
- 39.Kadoi M, Takeda M, Matsumoto S. Prostaglandin E2 potentiates the excitability of small-diameter trigeminal root ganglion neurons projecting onto the superficial layer of cervical dorsal horn in rats. Exp Brain Res 2007; 176: 227–236. [DOI] [PubMed] [Google Scholar]
- 40.Beiche F, Scheuerer S, Brune K, et al. Up-regulation of cyclooxygenase-2 mRNA in the rat spinal cord following peripheral inflammation. FEBS Lett 1996; 390: 165–169. [DOI] [PubMed] [Google Scholar]
- 41.Ek T, Jarfelt M, Mellander L, et al. Proinflammatory cytokines mediate the systemic inflammatory response associated with high-dose cytarabine treatment in children. Med Pediatr Oncol 2001; 37: 459–464. [DOI] [PubMed] [Google Scholar]
- 42.Ahmadi S, Lippross S, Neuhuber WL, et al. PGE2 selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nat Neurosci 2002; 5: 34–40. [DOI] [PubMed] [Google Scholar]
- 43.Kitagawa J, Kanda K, Sugiura M, et al. Effect of chronic inflammation on dorsal horn nociceptive neurons in aged rats. J Neurophysiol 2005; 93: 3594–3604. [DOI] [PubMed] [Google Scholar]
- 44.Tsuboi Y, Iwata K, Dostrovsky JO, et al. Modulation of astroglial glutamine synthetase activity affects nociceptive behaviour and central sensitization of medullary dorsal horn nociceptive neurons in a rat model of chronic pulpitis. Eur J Neurosci 2011; 34: 292–302. [DOI] [PubMed] [Google Scholar]
- 45.Radhakrishnan V, Henry JL. Antagonism of nociceptive responses of cat spinal dorsal horn neurons in vivo by the NK1-receptor antagonists CP-96,345 and CP-99,994, but not CP-96,344. Neuroscience 1995; 64: 943–958. [DOI] [PubMed] [Google Scholar]
- 46.Higashima M, Ohno K, Kinoshita H. Involvement of GABAA and GABAB receptors in after discharges generation in rat hippocampal slices. Brain Res 2000; 865: 186–193. [DOI] [PubMed] [Google Scholar]
- 47.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack: clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain 2000; 123: 1703–1709. [DOI] [PubMed] [Google Scholar]
- 48.Sorkins LS, Wallace MS. Acute pain mechanisms. Surg Clin North Am 1999; 79: 213–229. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki R, Dickenson AH. Differential pharmacological modulation of the spontaneous stimulus-independent activity in the rat spinal cord following peripheral nerve injury. Exp Neurol 2006; 198: 72–80. [DOI] [PubMed] [Google Scholar]
- 50.Roch M, Messlinger K, Kulchitsky, et al. Ongoing activity in trigeminal wide-dynamic range neurons is driven from the periphery. Neuroscience 2007; 150: 681–691. [DOI] [PubMed] [Google Scholar]
- 51.Takeda M, Takahashi M, Matsumoto S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci Biobehav Rev 2009; 33: 784–792. [DOI] [PubMed] [Google Scholar]
- 52.Kessler RC, Davis RB, Foster DF, et al. Long-term trends in the use of complementary and alternative medical therapies in the United States. Ann Intern Med 2001; 135: 262–268. [DOI] [PubMed] [Google Scholar]
- 53.Shir Y, Raja SN, Weissman CS, et al. Consumption of soy diet before nerve injury preempts the development of neuropathic pain in rats. Anesthesiology 2001; 95: 1238–1244. [DOI] [PubMed] [Google Scholar]
- 54.Tall JM, Raja SN. Dietary constituents as novel therapies for pain. Clin J Pain 2004; 20: 19–26. [DOI] [PubMed] [Google Scholar]
- 55.Rivat C, Richebé P, Laboureyras E, et al. Polyamine deficient diet to relieve pain hypersensitivity. Pain 2008; 137: 125–137. [DOI] [PubMed] [Google Scholar]