Abstract
Background
Fabry disease is an X-linked lysosomal storage disorder due to impaired activity of alpha-galactosidase A with intracellular accumulation of globotriaosylceramide. Associated small fiber pathology leads to characteristic pain in Fabry disease. We systematically assessed sensory system, physical activity, metabolic parameters, and morphology of male and female mice with alpha-galactosidase A deficiency (Fabry ko) from 2 to 27 months of age and compared results with those of age- and gender-matched wild-type littermates of C57Bl/6J background.
Results
From the age of two months, male and female Fabry mice showed mechanical hypersensitivity (p < 0.001 each) compared to wild-type littermates. Young Fabry ko mice of both genders were hypersensitive to heat stimulation (p < 0.01) and developed heat hyposensitivity with aging (p < 0.05), while cold hyposensitivity was present constantly in young (p < 0.01) and old (p < 0.05) Fabry ko mice compared to wild-type littermates. Stride angle increased only in male Fabry ko mice with aging (p < 0.01) in comparison to wild-type littermates. Except for young female mice, male (p < 0.05) and female (p < 0.01) Fabry ko mice had a higher body weight than wild-type littermates. Old male Fabry ko mice were physically less active than their wild-type littermates (p < 0.05), had lower chow intake (p < 0.001), and lost more weight (p < 0.001) in a one-week treadmill experiment than wild-type littermates. Also, Fabry ko mice showed spontaneous pain protective behavior and developed orofacial dysmorphism resembling patients with Fabry disease.
Conclusions
Mice with alpha-galactosidase A deficiency show age-dependent and distinct deficits of the sensory system. alpha-galactosidase A-deficient mice seem to model human Fabry disease and may be helpful when studying the pathophysiology of Fabry-associated pain.
Keywords: Fabry disease, alpha-galactosidase A, pain, mouse model
Background
Fabry disease (FD) is an X-linked lysosomal storage disorder caused by deficient or absent activity of the enzyme alpha-galactosidase A (α-GAL) due to mutations in the encoding α-galactosidase A gene (GLA). The enzymatic defect leads to the accumulation of glycosphingolipids and particularly globotriaosylceramide (Gb3) in a variety of tissues including the nervous system.1 Peripheral neuropathy in patients with FD is mainly of the small fiber type and may be associated with neuropathic pain and impaired temperature perception.2,3 Patients often suffer from severe acral burning pain triggered by fever or physical activity.4 To investigate the underlying disease mechanisms, animal models have been established of which the α-GAL deficient mouse was the first one.5 Using this model, we set out to systematically characterize the sensory, metabolic, and morphologic phenotype of naïve mice with α-GAL deficiency (Fabry ko) in a large mouse cohort stratified for gender and age from 2 to 27 months.
Methods
Ethic statement
All experiments were approved by the Bavarian State authorities (Regierung von Unterfranken, # 54/12). Mice were held at the animal facilities of the Department of Neurology, University of Würzburg, and were fed standard chow (commercially prepared complete diet). Animal use and care were in accordance with the institutional guidelines.
Mice
We investigated 215 naïve Fabry ko mice (89 male, 126 female) with a targeted disruption of the α-GAL gene in male and female mice as described elsewhere5 and 126 (80 male, 46 female) inbred naïve wild-type (WT) littermates of C57Bl/6J background. Mice were held under standard conditions with food and water access ad libitum. Fabry ko breeder pairs were obtained as a kind donation from the animal facilities of the Institute of Virology, University of Würzburg, after permission by Prof. A. Kulkarni (National Institute of Health, Bethesda, USA).5 Male and female Fabry ko and WT mice were investigated monthly from the age of 2 months up to their maximum life span, which was 27 months.
Behavioral tests
Behavioral tests were performed by an experienced investigator (LB) unaware of the study objectives and of genotype. The von-Frey test based on the up-and-down method was used to test for the paw withdrawal thresholds to mechanical stimulation.6 Animals were placed in plexiglass cages on a wire mesh. The plantar surface of the hind paws was touched with a von-Frey monofilament starting at a hair value of 0.69 g. When the animal withdrew its hind paw upon administration of mild pressure, the next finer von-Frey filament was used. If the animal did not react to this stimulation, the next stronger von-Frey filament was applied. Each hind paw was tested three times. The 50% withdrawal threshold (i.e., force of the von-Frey hair to which an animal reacts in 50% of the administrations) was recorded.
Paw withdrawal latencies to cold were measured according to the method of Brenner et al.7 Mice were placed on a glass surface (1/4″), and a dry ice stick was pressed against the glass beneath the plantar side of the hind paw. The time until paw withdrawal was recorded. To avoid tissue damage, the maximum time limit for cold application was 5 s.
Paw withdrawal latencies to heat were measured according to the method of Hargreaves et al.,8 applying a standard Ugo Basile Algesiometer (Comerio, Italy). The animals were placed on a glass surface and a radiant heat source was positioned under one hind paw. The time until paw withdrawal was recorded automatically. To avoid tissue damage, the maximum time limit for heat application was set to 16 s. Each hind paw was tested three times.
Gait analysis was performed as described earlier,9 modified for mice.10 The hind paws of the mice were painted with water-based blue ink and mice were allowed to walk through a cardboard tunnel placed on a strip of two white pages lying one behind the other. Three consecutive footprint pairs were taken to manually measure distinct gait parameters: stride width, stride length on the right and left side, and stride angle. The average values were calculated for each parameter.
Physical activity of the mice was assessed in treadmills (Tecniplast, Hohenpreißenberg, Germany) as described earlier.11 Data were evaluated as rounds per day over a period of six days. During the testing period, mice were restricted to the treadmill, and the treadmill was moved by the mice for one week. Voluntary activity during this period was measured. This test was performed to assess if Fabry ko mice are physically less active similar to Fabry patients who usually avoid physical activity because it may trigger pain.4
Body weight was measured before and after treadmill exercise. Chow intake was assessed by individually weighing the chow in the morning and evening of the test day.
Statistical analysis
For statistical analysis and graph design, SPSS software Version 23 was employed (Ehningen, Germany). Data distribution was tested using histograms and the Kolmogorov–Smirnov test. The parametric Student’s t-test was applied with LED post hoc analysis. Data are expressed as mean and standard error of the mean and are illustrated as bar graphs. Data were stratified for age groups (2, 6–9, 12–15, ≥18 months) and gender. When no intergroup difference was present between data of male and female mice (which was the case in the sensory tests), then an additional assessment was performed pooling data of male and female α-GAL and WT littermates, respectively, and comparing young (2 months) and old (≥18 months) age groups of both genotypes. p values < 0.05 were considered statistically significant.
Results
Young and old Fabry ko mice are hypersensitive to tactile stimulation
Male (Figure 1(a)) and female (Figure 1(b)) Fabry ko mice showed mechanical hypersensitivity in the von-Frey test when compared with WT littermates (p < 0.05 to p < 0.001). This hypersensitivity was independent of gender and age; thus, we pooled data of young (2 months) and old (≥18 months) male and female Fabry ko and WT mice, respectively (Figure 1(c)). Comparison of pooled data showed mechanical hypersensitivity in both age groups of Fabry ko mice compared with WT littermates (p < 0.001; Figure 1(c)).
Figure 1.
Paw withdrawal thresholds to mechanical stimulation. Bar graphs show the results of the von-Frey test in naïve 2, 6–9, 12–15, and ≥18 months old α-GAL deficient (Fabry ko) and wild-type (WT) male (a) and female (b) mice. Male and female Fabry ko mice show reduced mechanical withdrawal thresholds when compared with their WT littermates starting at the age of two months and lasting up to ≥18 months of age. In (c) results of male and female naïve 2 and ≥18 months old α-GAL deficient Fabry ko and WT mice are averaged each. Young and old Fabry ko mice show reduced mechanical withdrawal thresholds when compared with their WT littermates. Fabry ko: 2 months (9 male, 17 female), 6–9 months (29 male, 44 female), 12–15 months (22 male, 10 female), ≥18 months (29 male, 55 female). WT: 2 months (6 male, 6 female), 6–9 months (13 male, 8 female), 12–15 months (36 male, 16 female), ≥18 months (25 male, 16 female). F = female; M = male; mo = months; *p < 0.05, ***p < 0.001.
Fabry ko have age-dependent heat hyper- and hyposensitivity and constant hyposensitivity to cold
Two months old male (Figure 2(a)) and female (Figure 2(b)) Fabry ko mice had lower heat withdrawal latencies, i.e., increased sensitivity to heat, compared to WT littermates (male: p < 0.01, female: n.s.). In both genders, heat withdrawal latencies increased with aging and were longer in ko mice ≥18 months of age compared to WT mice (male: n.s., female: p < 0.01). When pooling data of young and old Fabry ko and WT mice, respectively (Figure 2(c)), young Fabry ko mice showed heat hypersensitivity (p < 0.01) while old Fabry ko mice were heat hyposensitive when compared with WT littermates (p < 0.05).
Figure 2.
Paw withdrawal latencies to heat stimulation. Bar graphs show the results of the heat test in naïve 2, 6–9, 12–15, and ≥18 months old α-GAL deficient (Fabry ko) and wild-type (WT) male (a) and female (b) mice. Two months old male and female Fabry ko mice show shorter withdrawal latencies when compared with their WT littermates. In both genders, heat withdrawal latencies increased with aging and were longer in ≥18 months old Fabry KO mice compared to WT littermates. In (c) results of male and female naïve 2 and ≥18 months old α-GAL deficient Fabry ko and WT mice are averaged each. While young Fabry ko mice show heat hypersensitivity, old Fabry ko mice develop heat hyposensitivity with increased paw withdrawal latencies to heat stimulation when compared with their WT littermates. Fabry ko: 2 months (7 male, 17 female), 6–9 months (31 male, 35 female), 12–15 months (23 male, 22 female), ≥18 months (24 male, 44 female). WT: 2 months (6 male, 6 female), 6–9 months (35 male, 33 female), 12–15 months (34 male, 13 female), ≥18 months (25 male, 20 female). F = female; M = male; mo = months; n.s. = not significant; *p < 0.05, **p < 0.01.
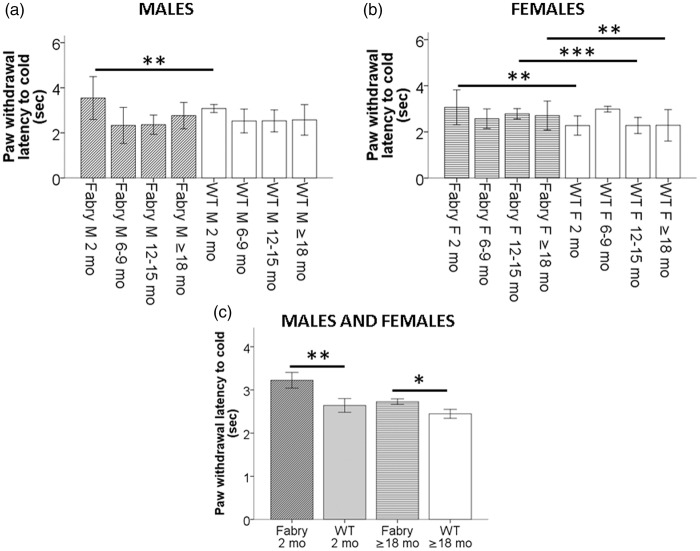
Two months old male (Figure 3(a)) and young and old female (Figure 3(b)) Fabry ko mice were hyposensitive to cold stimulation (p < 0.05 to p < 0.001) when compared with age-matched WT littermates. This was also true for young (p < 0.01) and old (p < 0.05; Figure 3(c)) Fabry ko mice compared to WT littermates when pooling data of the genders, respectively.
Figure 3.
Paw withdrawal latencies to cold stimulation. Bar graphs show the results of the cold test in naïve 2, 6–9, 12–15, and ≥18 months old α-GAL deficient (Fabry ko) and wild-type (WT) male (a) and female (b) mice. Young male Fabry ko mice of two months of age had longer paw withdrawal latencies to cold stimulation than WT mice. Female Fabry ko mice (2 months, 12–15 months, ≥18 months) had longer cold withdrawal latencies compared to their WT littermates. In (c) results of male and female naïve 2 and ≥18 months old α-GAL deficient Fabry ko and WT mice are averaged each. Young and old Fabry ko mice show cold hyposensitivity with increased paw withdrawal latencies to cold stimulation when compared with their WT littermates. Fabry ko: 2 months (7 male, 14 female), 6–9 months (31 male, 36 female), 12–15 months (24 male, 20 female), ≥18 months (32 male, 58 female). WT: 2 months (5 male, 6 female), 6–9 months (36 male, 28 female), 12–15 months (32 male, 20 female), ≥18 months (26 male, 21 female). F = female; M = male; mo = months; *p < 0.05, **p < 0.01, ***p < 0.001.
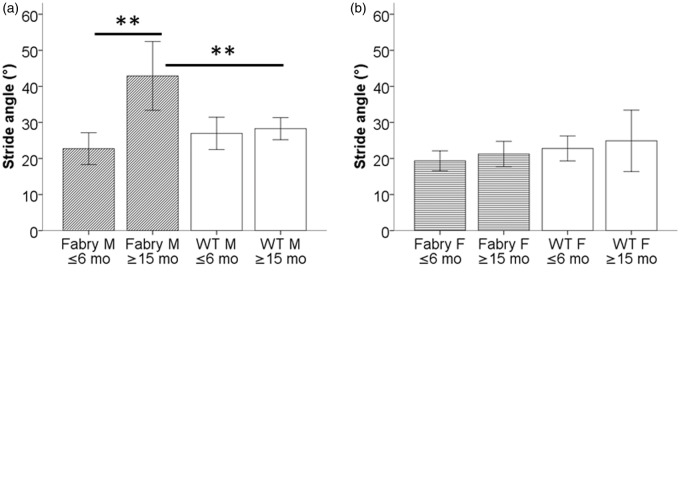
Male Fabry ko mice show impaired gait with aging
Of the determined gait parameters (stride width, stride length on the right and left side, and stride angle), only stride angle showed an intergroup difference (other data not shown). Old male Fabry ko mice had a larger stride angle than young Fabry ko mice and WT littermates (p < 0.01; Figure 4(a)). No such difference was found in female mice (Figure 4(b)).
Figure 4.
Gait analysis. Bar graphs show the results of the analysis of stride angle in α-GAL deficient (Fabry ko) and wild-type (WT) mice. Old male mice (a) had a larger stride angle when ≥15 months of age compared to young mice and to WT littermates. No intergroup difference was found in female mice (b). (c) Schematically illustrates the assessment of two pairs of mouse paw prints for stride angle A°. Fabry ko: ≤6 months (6 male, 6 female), ≥15 months (3 male, 3 female). WT: ≤6 months (6 male, 6 female), ≥15 months (9 male, 3 female). A° = stride angle; F = female; M = male; mo = months; **p < 0.01.
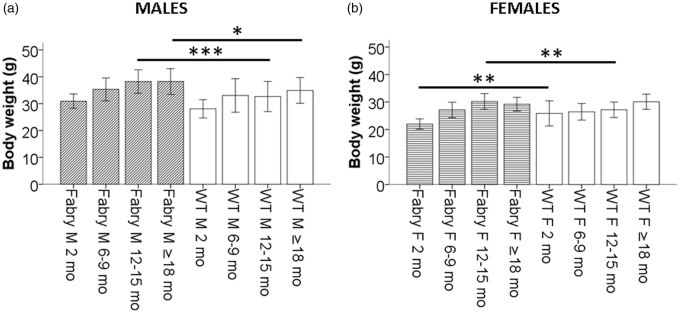
Except for young female mice, Fabry ko mice have a higher body weight than WT littermates
Body weight of male Fabry ko mice was higher compared to WT littermates particularly in older age groups (p < 0.05, p < 0.001; Figure 5(a)). Young female Fabry ko mice had a lower body weight, while adult female Fabry ko mice had a higher body weight than WT littermates (p < 0.01; Figure 5(b)).
Figure 5.
Development of weight. Bar graphs show the development of body weight over time in α-GAL deficient (Fabry ko) and wild-type (WT) male (A) and female (B) mice. Male Fabry ko mice were heavier than their WT littermates particularly in older age groups (12–15 months: p < 0.01; ≥18 months: p < 0.05). Young female Fabry ko mice had a lower body weight than WT littermates (p < 0.01) and a higher body weight when older (p < 0.05). Fabry ko: 2 months (13 male, 18 female), 6–9 months (45 male, 29 female), 12–15 months (31 male, 18 female), ≥18 months (46 male, 48 female). WT: 2 months (10 male, 10 female), 6–9 months (46 male, 22 female), 12–15 months (36 male, 21 female), ≥18 months (29 male, 11 female). F = female; M = male; mo = months; *p < 0.05, **p < 0.01, ***p < 0.001.
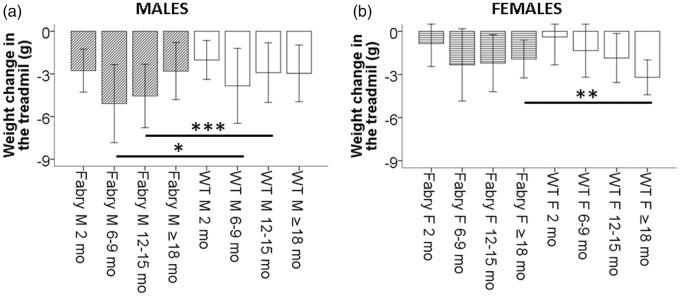
Fabry mice are physically equally to less active but lose more weight during a one-week treadmill experiment
In the one-week treadmill experiment, young male (Figure 6(a)) and female (Figure 6(a)) Fabry ko mice showed similar physical performance as their WT littermates. Male (p < 0.05) and female (n.s.) Fabry ko mice of the age of ≥18 months achieved fewer rounds per day than WT littermates. Chow intake was equal to lower in male Fabry ko mice (Figure 7(a) and (b)), and male Fabry ko mice lost more weight compared to their WT littermates (p < 0.05 and p < 0.001; Figure 8(a)). Weight change in female Fabry ko and WT mice was not different except for less weight loss in female Fabry ko mice at the age of ≥18 months (p < 0.01; Figure 8(b)).
Figure 6.
Physical activity in a one-week treadmill experiment. Bar graphs show the results of the treadmill experiment giving the rotations per day in α-GAL deficient (Fabry ko) and wild-type (WT) male (a) and female (b) mice. While 2–15 months old mice did not differ from their WT littermates, old (≥18 months) male and female Fabry ko mice were physically less active than their WT littermates. Fabry ko: 2 months (13 male, 18 female), 6–9 months (45 male, 29 female), 12–15 months (31 male, 18 female), ≥18 months (46 male, 48 female). WT: 2 months (10 male, 10 female), 6–9 months (46 male, 22 female), 12–15 months (36 male, 21 female), ≥18 months (29 male, 11 female). F = female; M = male; mo = months; n.s. = not significant; *p < 0.05.
Figure 7.
Chow intake in a one-week treadmill experiment. Bar graphs show chow intake during the one-week treadmill experiment in α-GAL deficient (Fabry ko) and wild-type (WT) male (a) and female (b) mice. Except for 12–15 months old male Fabry ko mice that had a lower chow intake compared to their WT littermates, no difference was found for other age groups and also in female Fabry ko mice compared to WT littermates. Fabry ko: 2 months (13 male, 18 female), 6–9 months (45 male, 29 female), 12–15 months (31 male, 18 female), ≥18 months (46 male, 48 female). WT: 2 months (10 male, 10 female), 6–9 months (46 male, 22 female), 12–15 months (36 male, 21 female), ≥18 months (29 male, 11 female). F = female; M = male; mo = months; ***p < 0.001.
Figure 8.
Weight change in a one-week treadmill experiment. Bar graphs show the weight change during the one-week treadmill experiment in α-GAL deficient (Fabry ko) and wild-type (WT) male (a) and female (b) mice. Male Fabry ko mice lost more weight in the treadmill than their WT littermates, while weight loss in female mice was not different between genotypes except for old female Fabry ko mice compared to their WT littermates. Fabry ko: 2 months (13 male, 18 female), 6–9 months (45 male, 29 female), 12–15 months (31 male, 18 female), ≥18 months (46 male, 48 female). WT: 2 months (10 male, 10 female), 6–9 months (46 male, 22 female), 12–15 months (36 male, 21 female), ≥18 months (29 male, 11 female). F = female; M = male; mo = months; *p < 0.05, **p < 0.01, ***p < 0.001.
Fabry ko mice show signs of spontaneous pain behavior
Fabry ko mice showed the following spontaneous behavior: when sitting on a wire mesh and upon stimulation with a von-Frey filament, mice shifted their paws to the inner walls of the covering plexiglass boxes and preferred keeping them on the glass surface (Video 1). Also, mice tended to hold up their paws and toes while seated or while standing on their hind paws during exploratory behavior; the latter leads to backward falls (Video 2).
Fabry ko mice develop orofacial dysmorphism with aging
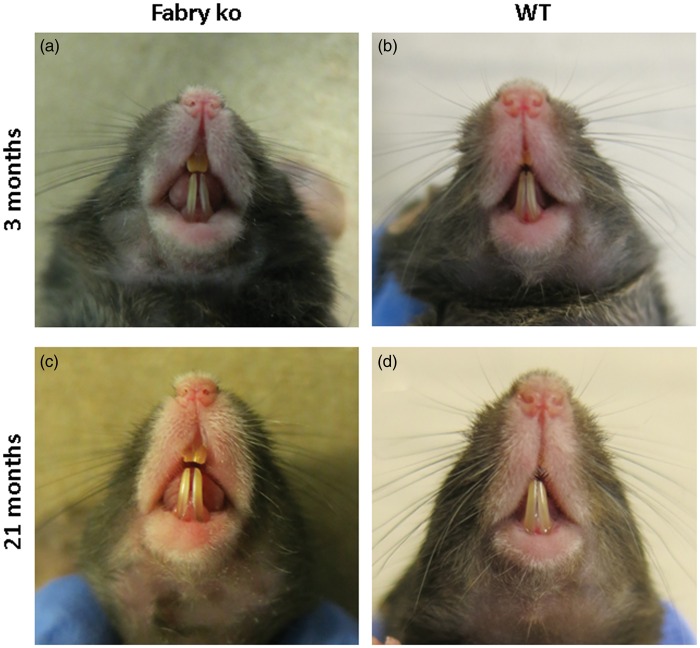
Similar to patients with FD,12 Fabry ko mice developed orofacial dysmorphism with aging. Old Fabry ko mice had a prominent snout with an enlarged tongue and elongated teeth with an widened gap between the front teeth when compared with young ko mice and WT littermates (Figure 9).
Figure 9.
Orofacial dysmorphisms. Photographs show the snout and teeth of 3 (a, c) and 21 months old male α-GAL deficient (Fabry ko) and wild-type (WT) mice. Fabry ko mice have an elongated snout, enlarged tongue and teeth, and a prominent gap between the front teeth.
Discussion
This is the first study systematically investigating the sensory system, physical activity, metabolic parameters, and morphology of a large cohort of male and female α-GAL-deficient mice as a model for FD from the age of 2 months up to 27 months. Our study is also the first in comparing data to WT littermate mice of reported (here C57Bl/6J) background and using an extensive battery of behavioral tests and phenotypical observation overcoming previous drawbacks of studies with low numbers of experimental animals, focus on one gender, few and early age groups, and data comparisons with non-littermate WT mice. We also provide first data on spontaneous pain behavior of α-GAL-deficient mice (Video 2) and on age-dependent orofacial dysmorphism mimicking one characteristic clinical finding in Fabry patients.
Using the same mouse model,5 heat hyposensitivity was described in a previous study in 6 and 12 months old α-GAL deficient male mice compared to “control” mice of unreported background and littermate status using the hot plate test.13 Lakoma et al.14 provided behavioral data of two and three months old male α-GAL-deficient mice compared to WT mice. In these young mice, the authors found mechanical hypersensitivity in the von-Frey test, heat hypersensitivity in the hot plate test, and cold hyposensitivity using the acetone test and the cold plate test,14 which matches our data of young (i.e., two months old) mice. Controversially, heat hyposensitivity was described already in 3 and 11 months old α-GAL deficient male mice5 using the hot plate test; however, here results were compared with non-littermate WT mice of 129S6/SvEvTac origin.15 In a recent study, the same group investigated a different type of α-GAL-deficient mice16 from 3 to 17 months of age and compared heat withdrawal latencies in the hot plate test with non-littermate 129S6/SvEvTac mice and without gender differentiation.17 The authors report increased heat withdrawal latencies in these α-GAL ko mice16 from the age of 3 months with a further increase at 12 to 17 months.17
Following our α-GAL deficient mouse cohort up to 27 months of age until the animals died spontaneously, we additionally show that while mechanical hypersensitivity and cold hyposensitivity persist, heat hypersensitivity turns to hyposensitivity with aging. Although not directly comparable, these findings are in line with sensory findings in FD patients. Using quantitative sensory testing, thermal hyposensitivity has been described in several studies18–20 and evoked pain with mechanically hypersensitive palmar, and plantar skin is frequently reported by FD patients.4 The observed spontaneous behavior of our α-GAL deficient mouse cohort (see Videos 1 and 2) is of particular interest further underlining the sensory hypersensitivity of these mice, even though interpretation can only be made with caution. Physical activity is one of the major pain triggers in FD patients.4 Interestingly, old mice with α-GAL deficiency were physically less active in the treadmill experiment than their WT littermates (Figure 6), but despite also similar chow intake (Figure 7) showed more severe weight loss (Figure 8). Although no direct conclusions can be drawn from this observation, the reduction in physical activity and increased loss of weight may hint toward a reduction in well-being, e.g. due to an increase in painful sensations during the exercise and might, therefore, also parallel patient behavior.
Mice investigated in this study were originally created by targeted disruption of the α-GAL gene in mouse embryonic cells, thus no α-GAL activity is present in male and female mice of this strain.5 This is in contrast to the situation in humans, where X-chromosomal inactivation enables variable expression of α-GAL activity in affected women.21 The mouse model used here allows to asses potential gender differences independent of this variability, which is important, since sex differences in mouse pain behavior and their underlying mechanisms have recently come into attention22–24 and may explain sex-related lack of efficacy of respective treatment strategies.25,26 In the present study, we did not observe any major differences in pain behavior of male and female Fabry ko mice, thus the female gender seems not to induce additional alterations in α-GAL ko mice. In previous studies, this question could not be answered, since either only male mice were investigated13,14 or data were not stratified for gender.15,17
One crucial aspect when comparing different studies on the behavior of α-GAL-deficient mice over time is that the so far published data were obtained in different α-GAL deficient5,16 and non-littermate WT mouse strains (e.g., 129S6/SvEvTac versus C57Bl/6J background). Even when performing the exact same tests, a change in genetic background may lead to diverse results. See, for instance, heat withdrawal latency of 25 s in 12 weeks old WT mice of C57Bl/6J background in Figure 2 of Lakoma et al.14 resulting in heat hypersensitivity of α-GAL-deficient mice versus 15 s in 12 weeks old WT mice of 129S6/SvEvTac background in Figure 5 of Bangari et al.,17 resulting in apparent heat hyposensitivity of α-GAL-deficient mice, although the results of the α-GAL-deficient mice are the same in both studies with approximately 20 s each. Additionally, the use of non-littermate WT control mice makes data comparison difficult. Different reactions in the same tests are known for different WT mouse strains and substrains,27,28 supporting the importance of the use of littermate WT mice to ensure equal conditions.
It is intriguing that orofacial dysmorphic changes develop in Fabry ko mice similar to those in Fabry patients.12 The reasons for these morphological changes that mainly reflect tissue enlargement are not known.
Our data have implications for future studies. First, the α-GAL deficient mouse5 phenotype has similarities with findings in patients with FD; however, disease phenotype in naïve mice is mild. Therefore, new mouse models with an additional increase in Gb3 production29 and thus faster and higher increase in tissue Gb3 load have been developed meanwhile and might open new avenues in future FD pathophysiology research—for the price of a less physiological development. Second, the α-GAL deficient mouse model displays an age-dependent and differential change in sensory and motor functions. This is particularly important and needs to be considered in future studies. Third, no gender difference is present for sensory tests in young and old α-GAL-deficient mice, thus gene disruption leads to a sensory phenotype that is not altered by female gender.
Conclusions
Our study provides the so far largest and most detailed data set on the longest time period of α-GAL-deficient mice sensory, motor, and metabolic development. We show that mice with α-GAL deficiency develop age-dependent and distinct deficits of the sensory system modeling human FD. This data set will help to differentially design and interpret the results of future studies on the pathophysiology of pain in FD.
Acknowledgement
The authors thank Helga Brünner and Katharina Meder for technical assistance.
Authors contributions
NÜ and CS contributed to the study design, data assessment, and manuscript preparation. LB contributed to the behavioral testing. DH contributed to the data assessment and manuscript preparation. LH contributed to the behavioral testing, data assessment, and manuscript preparation.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was financially supported by research funds of the Interdisciplinary Center for Clinical Research (Interdisziplinäres Zentrum für Klinische Forschung, IZKF) of the University of Würzburg (N.Ü.: N-260). N.Ü. and C.S. are funded by the European Union's Seventh Framework Programme 2013–2017 (“ncRNAPain,” grant agreement number 602133).
References
- 1.Zarate YA, Hopkin RJ. Fabry’s disease. Lancet 2008; 372: 1427–1435. [DOI] [PubMed] [Google Scholar]
- 2.Gemignani F, Marbini A, Bragaglia MM, et al. Pathological study of the sural nerve in Fabry’s disease. Eur Neurol 1984; 23: 173–181. [DOI] [PubMed] [Google Scholar]
- 3.Sima AA, Robertson DM. Involvement of peripheral nerve and muscle in Fabry’s disease. Histologic, ultrastructural, and morphometric studies. Arch Neurol 1978; 35: 291–301. [DOI] [PubMed] [Google Scholar]
- 4.Üçeyler N, Ganendiran S, Kramer D, et al. Characterization of pain in Fabry disease. Clin J Pain 2014; 30: 915–920. [DOI] [PubMed] [Google Scholar]
- 5.Ohshima T, Murray GJ, Swaim WD, et al. Alpha-galactosidase A deficient mice: a model of Fabry disease. Proc Natl Acad Sci USA 1997; 94: 2540–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 7.Brenner DS, Golden JP, Gereau RW. A novel behavioral assay for measuring cold sensation in mice. PLoS One 2012; 7: e39765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 9.Kunkel-Bagden E, Dai HN, Bregman BS. Methods to assess the development and recovery of locomotor function after spinal cord injury in rats. Exp Neurol 1993; 119: 153–164. [DOI] [PubMed] [Google Scholar]
- 10.Üçeyler N, Kobsar I, Biko L, et al. Heterozygous P0 deficiency protects mice from vincristine-induced polyneuropathy. J Neurosci Res 2006; 84: 37–46. [DOI] [PubMed] [Google Scholar]
- 11.Üçeyler N, Schütt M, Palm F, et al. Lack of the serotonin transporter in mice reduces locomotor activity and leads to gender-dependent late onset obesity. Int J Obes (Lond) 2010; 34: 701–711. [DOI] [PubMed] [Google Scholar]
- 12.Cox-Brinkman J, Vedder A, Hollak C, et al. Three-dimensional face shape in Fabry disease. Eur J Hum Genet 2007; 15: 535–542. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues LG, Ferraz MJ, Rodrigues D, et al. Neurophysiological, behavioral and morphological abnormalities in the Fabry knockout mice. Neurobiol Dis 2009; 33: 48–56. [DOI] [PubMed] [Google Scholar]
- 14.Lakoma J, Rimondini R, Donadio V, et al. Pain related channels are differentially expressed in neuronal and non-neuronal cells of glabrous skin of Fabry knockout male mice. PLoS One 2014; 9: e108641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall J, Ashe KM, Bangari D, et al. Substrate reduction augments the efficacy of enzyme therapy in a mouse model of Fabry disease. PLoS One 2010; 5: e15033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang AM, Ionnaou YA, Zeidner LM, et al. Generation of a mouse model with agalactosidase A deficiency. Am J Hum Genet 1996; 59: A208 (abstract). [Google Scholar]
- 17.Bangari DS, Ashe KM, Desnick RJ, et al. Alpha-galactosidase A knockout mice: progressive organ pathology resembles the type 2 later-onset phenotype of Fabry disease. Am J Pathol 2015; 185: 651–665. [DOI] [PubMed] [Google Scholar]
- 18.Üçeyler N, He L, Schönfeld D, et al. Small fibers in Fabry disease: baseline and follow-up data under enzyme replacement therapy. J Peripher Nerv Syst 2011; 16: 304–314. [DOI] [PubMed] [Google Scholar]
- 19.Maag R, Binder A, Maier C, et al. Detection of a characteristic painful neuropathy in Fabry disease: a pilot study. Pain Med 2008; 9: 1217–1223. [DOI] [PubMed] [Google Scholar]
- 20.Torvin Moller A, Winther Bach F, Feldt-Rasmussen U, et al. Functional and structural nerve fiber findings in heterozygote patients with Fabry disease. Pain 2009; 145: 237–245. [DOI] [PubMed] [Google Scholar]
- 21.Echevarria L, Benistan K, Toussaint A, et al. X-chromosome inactivation in female patients with Fabry disease. Clin Genet 2016; 89: 44–54. [DOI] [PubMed] [Google Scholar]
- 22.Sorge RE, Mapplebeck JC, Rosen S, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015; 18: 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorge RE, LaCroix-Fralish ML, Tuttle AH, et al. Spinal cord toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci 2011; 31: 15450–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mogil JS, Sorge RE, LaCroix-Fralish ML, et al. Pain sensitivity and vasopressin analgesia are mediated by a gene-sex-environment interaction. Nat Neurosci 2011; 14: 1569–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez V, Szekely B, Lemarie J, et al. The efficacy of a glial inhibitor, minocycline, for preventing persistent pain after lumbar discectomy: a randomized, double-blind, controlled study. Pain 2013; 154: 1197–1203. [DOI] [PubMed] [Google Scholar]
- 26.Ledeboer A, Sloane EM, Milligan ED, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 2005; 115: 71–83. [DOI] [PubMed] [Google Scholar]
- 27.Bryant CD, Zhang NN, Sokoloff G, et al. Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. J Neurogenet 2008; 22: 315–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banik RK, Woo YC, Park SS, et al. Strain and sex influence on pain sensitivity after plantar incision in the mouse. Anesthesiology 2006; 105: 1246–1253. [DOI] [PubMed] [Google Scholar]
- 29.Taguchi A, Maruyama H, Nameta M, et al. A symptomatic Fabry disease mouse model generated by inducing globotriaosylceramide synthesis. Biochem J 2013; 456: 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]