Abstract
Background
Pain hypoalgesia has been reported in Rett syndrome patients, a severe neurodevelopmental disorder which can be attributed to mutations in the methyl-CpG binding protein 2 (MeCP2). Here, we examined the role of MeCP2 signaling in tongue heat sensitivity in the normal and inflamed state using Mecp2 heterozygous (Mecp2+/−) mice.
Results
Heat hypoalgesia of the tongue occurred in Mecp2+/− mice and submucosal injection of complete Freund’s adjuvant into the tongue produced a long-lasting heat hyperalgesia at the inflamed site in wild-type mice but not in Mecp2+/− mice. Transient receptor potential vanilloid 1 was expressed in a large number of MeCP2-immunoreactive trigeminal ganglion neurons innervating the tongue in both wild-type and Mecp2+/− mice (70.9% in wild type; 72.1% in Mecp2+/−). The number of transient receptor potential vanilloid 1-immunoreactive trigeminal ganglion neurons innervating the tongue was smaller in Mecp2+/− mice relative to wild-type mice (30.5% in wild type; 20.2% in Mecp2+/−). Following complete Freund’s adjuvant injection, the number of transient receptor potential vanilloid 1- and MeCP2-immunoreactive trigeminal ganglion neurons innervating the tongue, as well as MeCP2 protein expression in trigeminal ganglion, was significantly increased in wild-type mice but not in Mecp2+/− mice. Additionally, tongue heat hyperalgesia following complete Freund’s adjuvant injection was completely suppressed by the administration of SB366791, a transient receptor potential vanilloid 1 antagonist, in the tongue.
Conclusions
These findings indicate that tongue heat sensitivity and hypersensitivity are dependent on the expression of transient receptor potential vanilloid 1 which is regulated via MeCP2 signaling in trigeminal ganglion neurons innervating the tongue.
Keywords: Trigeminal ganglion, transient receptor potential vanilloid 1, tongue inflammation, Rett syndrome
Background
Mutations in methyl-CpG binding protein 2 (MeCP2) are causally related to Rett syndrome, an X-linked dominant neurodevelopmental disorder characterized by a severe deficit in the peripheral and central nervous system affecting mostly females.1 The most serious symptoms in Rett syndrome patients are respiratory abnormalities, various motor deficits, absence of speech, and a general growth deficit.2 MeCP2 is expressed predominantly in mature neurons and has been shown to be able to change the expression levels of target genes.3,4 MeCP2 is considered to be a transcriptional repressor acting through its methyl-CpG binding domain and transcriptional repressor domain, and the binding of MeCP2 to DNA acts by altering the chromatin structure.5–8 MeCP2 represses transcription by recruiting the nuclear receptor corepressor-silencing mediator of retinoic acid and thyroid hormone receptor corepressor complex. Further, MeCP2 regulates alternative splicing via an interaction with Y-box transcription factor, which modulates microRNA processing.9 Pain hypoalgesia in skin has also been reported in Rett syndrome patients, suggesting that MeCP2 plays an important role in setting pain sensitivity.10 It is also reported that heat and mechanical sensitivity of the oral mucosa is different from that of skin.11,12 Moreover, different interaction between nociceptors in primary afferent neurons contributes difference of characteristic feature between skin pain and oral mucosa pain after the incision.13 However, it is not known how MeCP2 in the primary afferent neurons is involved in tongue pain sensitivity and the pain modulation under pathological conditions.
Transient receptor potential vanilloid 1 (TRPV1) is mainly expressed in nociceptive primary afferent neurons and is one of the key molecules involved in pain modulation.14,15 TRPV1 is activated by various noxious stimuli such as high temperature, low pH, and irritant and pungent compounds released during inflammatory processes.16,17 In previous studies, an increase in the number of TRPV1-positive trigeminal ganglion (TG) neurons and increased TRPV1 expression in primary afferent terminals were induced by injection of turpentine oil into the facial skin,18 injection of complete Freund’s adjuvant (CFA) into the masseter muscle,19 or dentinal application of lipopolysaccharide,20 resulting in consequent orofacial heat hyperalgesia. Taken together, we hypothesized that MeCP2 is involved in regulation of TRPV1 expression in TG neurons, in association with orofacial inflammation. In the present study, we examined the role of MeCP2 in tongue heat sensory transduction and hypersensitivity following tongue inflammation produced by CFA injection into the tongue.
Methods
Animals
Female Mecp2+/− mice (20–30 g) and female wild-type littermates (20–30 g) used in this study were obtained by breeding heterozygous females of C57BL/6 background (B6. 129P2(C)-Mecp2 tm1.1Bird/J, Jackson Laboratory, Bar Harbor, ME) with wild-type C57BL/6 male mice (Jackson Laboratory). The genetic status of the mice was established by polymerase chain reaction for the Mecp2 gene at postnatal day 10 according to Jackson Laboratory protocols. Mice were exposed to a 12 h light/dark cycle (lights on at 07:00) and kept in a temperature- and humidity-controlled room (24 ± 1℃, 55 ± 5%) with food and water ad libitum. All procedures were conducted in accordance with the animal experimentation committee at Nihon University. The number of mice used was based on the minimum required for statistical analysis.
Estrous cycle
To confirm the estrous cycle in each mouse, the electrical impedance of the vagina (EIV) was measured with an impedance reader MK-10B (Muromachi, Tokyo, Japan). The probe was left in the vagina under light anesthesia with 1.5% isoflurane (Mylan). Measuring the EIV allows differentiation between each phase of estrous cycle, with high EIV values (over 3000 omega) occurring only at proestrus and the lowest values (under 3000 omega) at metestrus21
Induction of inflammation in the tongue
Under deep anesthesia via intraperitoneal (i.p.) injection of sodium pentobarbital (50 mg/kg, Schering Plough, Whitehouse Station, NJ), 2 µL of CFA or isotonic saline was submucosally injected into the left side of the anterior dorsolateral two thirds of the tongue with a 30-gauge needle attached to a Hamilton syringe (Hamilton, Reno, NV). The location of the needle was limited to the superficial mucosal layer of the tongue without entering the tongue muscle during each injection. Mice were closely monitored for evidence of distress or pain and weight gain following injections.
Assessment of heat sensitivity
Under light anesthesia with 1.5% isoflurane (Mylan, Canonsburg, PA), graded heat stimulation (35–60℃, 1℃/s, cutoff: 60℃) was applied to the tongue using a contact heat probe (9 mm2; Intercross, Tokyo, Japan) followed by pulling the tongue forward as previously described.13 Mice were applied graded heat stimulation after cutting off the supply of 1.5% isoflurane, once noxious pressure applied to the hind paw induced an identical weak flexion reflex of the hind limb to ensure that an adequate level of anesthesia was maintained. The lowest temperature for evoking nocifensive reflex (head withdrawal or avoidance) by heat stimulation of the tongue was defined as heat head-withdrawal reflex threshold (HHWT). The graded heat stimulation was applied at 1 min intervals for each stimulus, and measurements were performed three times and averaged at each time point for each animal. All behavioral tests were performed under blinded conditions.
Immunohistochemistry
To label TG neurons innervating the tongue, 5 µL of 5% hydroxystilbamidine (Fluoro-Gold [FG]; fluorochrome, Denver, CO) dissolved in isotonic saline was injected into the superficial mucosal layer of the tongue using a 30-gauge needle 5 days before CFA injection in wild-type and Mecp2+/− mice. On day 3, mice were anesthetized with i.p. sodium pentobarbital (50 mg/kg) and transcardially perfused with saline followed by a fixative containing 4% paraformaldehyde in 0.1 M phosphate buffer ; pH 7.4). TGs ipsilateral to CFA injection were dissected out, placed in the same fixative overnight at 4℃, and transferred to 0.01 M phosphate-buffered saline (PBS) containing 20% sucrose for 12 h. The TGs were embedded in Tissue Tek (Sakura Finetek, Tokyo, Japan) and cut in the horizontal plane at a thickness of 16 µm. TG sections were thaw mounted onto MAS-coated Superfrost Plus microscope slides (Matsunami, Tokyo, Japan). Every 10th TG section (4 sections/TG) was collected for immunohistochemical analyses. After rinsing with 0.01 M PBS, TG sections were incubated with rabbit anti-MeCP2 polyclonal antibody (1:1000, Millipore; cat. 07–013, Billerica, MA) and guinea pig anti-TRPV1 polyclonal antibody (1:1000, Millipore; cat. AB5566), anti-transient receptor potential melastatin 3 (TRPM3) polyclonal antibody (1:200, Alomone; ACC-050, Jerusalem, Israel), anti-transmembrane protein 16A (TMEM16A) antibody (1:1, Abcam; ab53213, Cambridge, UK), or anti-acetyl-histone H3 antibody (1:400, Cell Signaling; #9649, Beverly, MA) diluted in 0.01 M PBS containing 4% normal goat serum and 0.3% Triton X-100 (Sigma-Aldrich, St. Louis, MO) for 48 h at 4℃. TG sections were then incubated with Alexa Fluor 488 goat anti-rabbit IgG (1:200 in 0.01 M PBS; Thermo Fisher Scientific, Waltham, MA) and Alexa Fluor 568 goat anti-guinea pig IgG (1:200 in 0.01 M PBS; Thermo Fisher Scientific, Waltham, MA) or Alexa Fluor 488 goat anti-rabbit IgG (1:200 in 0.01 M PBS; Thermo Fisher Scientific, Waltham, MA) alone for 120 min. TG sections were coverslipped with PermaFluor (Sigma-Aldrich, St. Louis, MO) and examined under a fluorescence microscope. Using appropriate filters, Alexa Fluor 488-labeled and/or Alexa Fluor 568-labeled neurons, which were labeled with FG, were identified and analyzed using a BZ-9000 system (Keyence, Osaka, Japan). Neurons with an intensity twofold or more than the background average were considered positive. No specific labeling was observed in the absence of primary antibody. The ratio of the number of FG-labeled MeCP2-immunoreactive (IR) and/or TRPV1-IR neurons, the number of TRPV1-IR neurons in FG-labeled MeCP2-IR neurons, and the number of FG-labeled TRPM3-IR or TMEM16A-IR neurons were calculated in four sections of the TG in each group. Furthermore, the ratio of the number of FG-labeled MeCP2-IR, TRPV1-IR, TRPM3-IR, or TMEM16A-IR neurons in each group was calculated by the following formula: 100 × total number of FG-labeled TRPV1-IR or MeCP2-IR, TRPM3-IR or TMEM16A-IR neurons in 4 sections of TG/total number of FG-labeled neurons in 4 sections of TG. The calculation was performed on FG-labeled MeCP2-IR or TRPV1-IR TG neurons classified according to the area of the cells.
Western blotting
On day 3 after CFA or saline injection into the tongue of wild-type mice, mice were perfused transcardially with saline under deep anesthesia using sodium pentobarbital (50 mg/kg, i.p.). TG was removed and homogenized immediately in 50 µL of ice-cold lysis buffer (137 mM NaCl, 20 mM Tris-HCl, pH 8.0, 1% NP-40, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 µg/mL aprotinin, 1 g/mL leupeptin, and 0.5 mM sodium vanadate) using a tube pestle (Thermo Fisher Scientific, Waltham, MA). Following centrifugation of samples at 15,000 rpm for 10 min at 4℃, the supernatants were collected and the protein concentrations were determined using a protein assay kit (Bio-Rad, Hercules, CA). The supernatants were heat denatured in Laemmli sample buffer (Bio-Rad, Hercules, CA), and 20 µL of the sample was subjected to electrophoresis on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto polyvinylidene fluoride membranes (Trans-Blot Turbo Transfer pack, Bio-Rad, Hercules, CA) using Trans-Blot Turbo (Bio-Rad, Hercules, CA). The membrane was rinsed with Tris-buffered saline containing 0.1% Tween 20 (TBST) and incubated with 3% bovine serum albumin (BSA, Bovogen, Essendon, Australia). Then, the membrane was incubated overnight at 4℃ with anti-MeCP2 rabbit antibody (1:1000, Millipore) diluted in TBST with 5% BSA. Bound antibody was visualized using a horseradish peroxidase-conjugated donkey anti-rabbit antibody (Cell Signaling, Beverly, MA) and Western Lightning ELC Pro (Perikin Elmer, Waltham, MA). Band intensity was quantified using a ChemiDoc MP system (Bio-Rad, Hercules, CA) and normalized to β-actin immunoreactivity on blots reprobed with anti-β-actin antibody (1:200, Santa Cruz, Dallas, TX) following removal of bound antibody using a stripping reagent (Thermo Fisher Scientific, Waltham, MA).
Effects of TRPM3 and Anoctamin 1 inhibitors on heat hyperalgesia
We assessed the involvement of TRPM3 and anoctamin 1 (Ano1) in heat hyperalgesia of the tongue induced by CFA injection. The TRPM3 inhibitor (1 µL, 1 mM; Liquiritigenin, Sigma-Aldrich, St. Louis, MO) and the Ano1 inhibitor (1 µL, 1 mM; T16Ainh-A01, Tocris Bioscience, Bristol, UK) were dissolved in 50% dimethylsulfoxide in physiological saline. On day 3 after CFA injection into the tongue, HHWTs were measured before and 30, 60, 90 and 120 min after tongue administration of Liquiritigenin or T16Ainh-A01 as described earlier. Before CFA injection into the tongue, prevalue of HHWTs was measured in advance.
Statistical analysis
Data were expressed as means ± SEM. Statistical analyses were performed by Student’s t-test, one-way repeated-measures analysis of variance (ANOVA) followed by Bonferroni’s multiple-comparison tests or two-way repeated-measures ANOVA followed by Dunnett’s multiple-comparison tests where appropriate. A value of p < 0.05 was considered significant.
Results
Changes in heat sensitivity
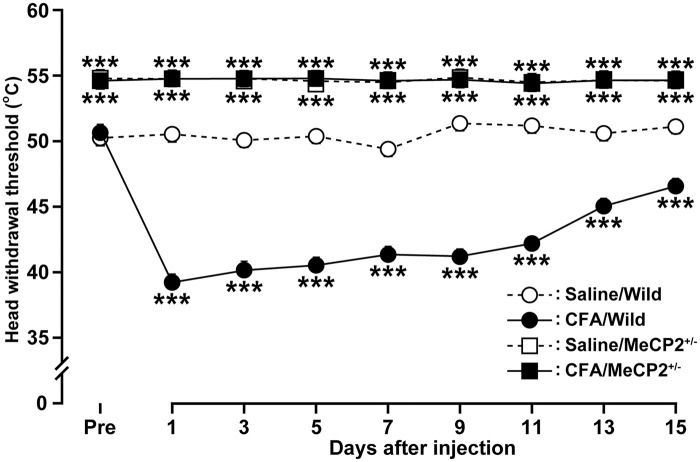
As female mice were used in the present study, we tested whether tongue pain threshold is modulated by the estrous cycle. We did not observe any significant differences in the HHWT of the tongue in female mice at different stage of the estrous cycle (data not shown). Following submucosal CFA injection into the tongue, tongue inflammation was induced accompanied by redness and swelling of the injection site, which lasted for at least 2 weeks (data not shown). In wild-type mice, the HHWT of the tongue significantly decreased on day 1 following CFA injection when compared with that of the saline group (CFA: 39.2 ± 0.5℃; saline: 50.5 ± 0.1℃; Figure 1) and remained significantly lower until day 15 (p < 0.05). In contrast, the HHWT in Mecp2+/− (heterozygous) mice was significantly higher than that of wild-type littermates, indicating decreased heat sensitivity in these genetically altered mice. Unlike the wild-type mice, the HHWT in Mecp2+/− mice did not change following CFA injection into the tongue during the experimental period. All mice did not exhibit motor and/or cognitive deficits.
Figure 1.
Time course of HHWT in the tongue after CFA or saline injection in wild-type or Mecp2+/− mice (n = 5 in each group). ***p < .001 vs. saline-injected wild type; by two-way repeated-measures ANOVA followed by Dunnett’s multiple-comparison tests. HHWT: heat head-withdrawal reflex threshold; CFA: complete Freund’s adjuvant; ANOVA: analysis of variance.
TRPV1-immunoreactive and MeCP2-IR TG neurons innervating the tongue
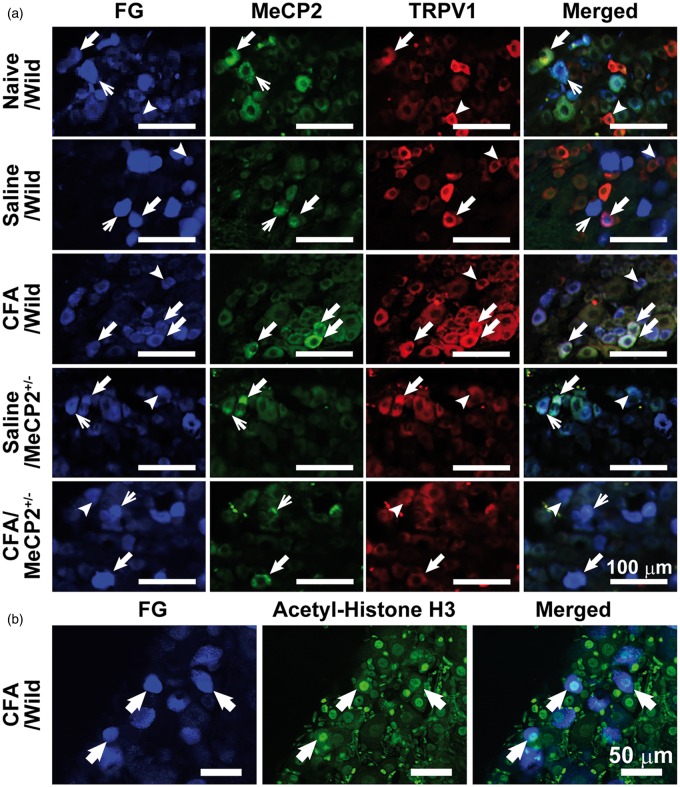
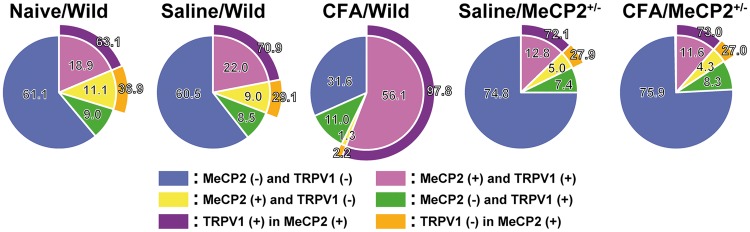
The FG-labeled TRPV1-IR and MeCP2-IR TG neurons innervating the tongue were observed in naive mice and on day 3 after CFA or saline injection in wild-type or Mecp2+/− mice (Figure 2(a)). The FG-labeled acetyl-histone H3-IR TG neurons innervating the tongue were observed in wild-type mice on day 3 after CFA injection (Figure 2(b)). There was no significant difference in the number of FG-labeled neurons on day 3 after CFA or saline injection in wild-type or Mecp2+/− mice (data not shown). TRPV1 was expressed in a large number of MeCP2-IR TG neurons innervating the tongue in each group (naive/wild: 63.1%, saline/wild: 70.9%, Saline/Mecp2+/−: 72.1%, CFA/Mecp2+/−: 73.0%). Interestingly, TRPV1 was expressed in almost all of MeCP2-IR TG neurons innervating the tongue on day 3 after CFA injection in wild-type mice (CFA/wild: 97.8%; Figure 3).
Figure 2.
MeCP2-TRPV1-IR and acetyl-histone H3 TG neurons innervating the tongue. (a) FG-labeled MeCP2-IR and TRPV1-IR TG neurons on day 3 after CFA or saline injection in wild-type or Mecp2+/− mice. Thick arrows indicate FG-labeled MeCP2-IR and TRPV1-IR TG neurons. Arrowheads indicate FG-labeled TRPV1-IR TG neurons. Thin arrows indicate FG-labeled MeCP2-IR TG neurons. Scale bars: 100 µm. (b) FG-labeled acetyl-histone H3-IR TG neurons on day 3 after CFA injection in wild-type mice. Arrowheads indicate acetyl-histone H3-IR TG neurons. Scale bars: 50 µm. MeCP2: methyl-CpG binding protein 2; CFA: complete Freund’s adjuvant; TRPV1-IR: transient receptor potential vanilloid 1-immunoreactive; TG: trigeminal ganglion; FG: Fluoro-Gold.
Figure 3.
Ratio of the number of FG-labeled MeCP2-IR and/or TRPV1-IR TG neurons and ratio of the number of TRPV1-IR TG neurons in FG-labeled MeCP2-IR neurons on day 3 after CFA or saline injection in wild-type or Mecp2+/−mice. MeCP2: methyl-CpG binding protein 2; CFA: complete Freund’s adjuvant; TG: trigeminal ganglion; FG: Fluoro-Gold; TRPV1: transient receptor potential vanilloid 1.
Changes in MeCP2 expression in TG neurons
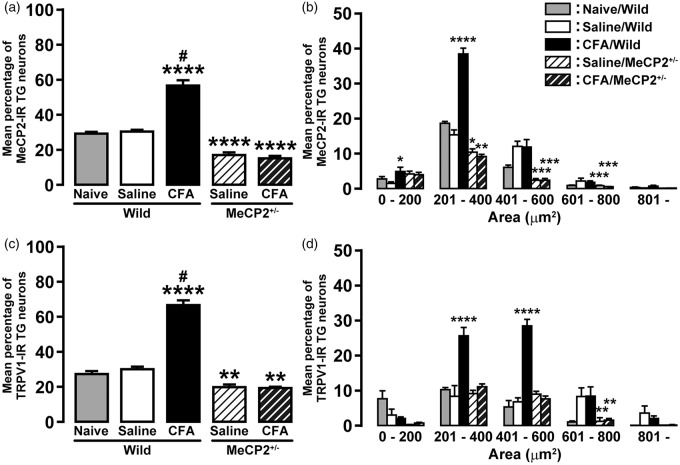
MeCP2 protein expression in TG was significantly increased on day 3 after CFA injection compared with saline-injected mice (saline, 1.7 ± 0.2; CFA, 2.8 ± 0.3; Figure 4). Subsequently, we examined the presence of MeCP2 immunoreactivity in TG neurons innervating the tongue on day 3 after CFA or saline injection. In wild-type mice, the number of MeCP2-IR neurons innervating the tongue was significantly increased after CFA injection compared with that of the saline-injected group or naive mice (naive: 29.9 ± 0.4%; saline: 31.0 ± 0.4%; CFA: 57.4 ± 2.5%, respectively; Figure 5(a)). On the other hand, the number of MeCP2-IR neurons innervating the tongue in saline- and CFA-injected Mecp2+/− mice (saline: 17.7 ± 0.7%; CFA: 15.8 ± 0.7%) was significantly less than that of saline-injected wild-type or naive mice. In wild-type mice, the cell area analysis revealed that the number of MeCP2-IR TG neurons innervating the tongue was markedly increased in cell groups of 0–200 µm2 and 201–400 µm2 in area on day 3 after CFA injection ([0–200 µm2] saline: 1.4 ± 0.4%, CFA: 4.9 ± 1.1%; [201–400 µm2] saline: 15.4 ± 1.3%, CFA: 38.5 ± 1.6%; Figure 5(b)). In all cell groups, there was no significant difference in the number of MeCP2-IR neurons in Mecp2+/− mice on day 3 after CFA injection when compared with saline injection. Moreover, in saline- and CFA-injected Mecp2+/− mice, the numbers of MeCP2-IR neurons in cell groups of 201–400 µm2, 401–600 µm2, and 601–800 µm2 in area were significantly less than that of saline-injected wild-type mice ([201–400 µm2] saline: 10.4 ± 0.9%, CFA: 9.1 ± 0.5%; [401–600 µm2] saline: 2.3 ± 0.4%, CFA: 2.3 ± 0.4%; [601–800 µm2] saline: 0.8 ± 0.2%, CFA: 0.5 ± 0.1%).
Figure 4.
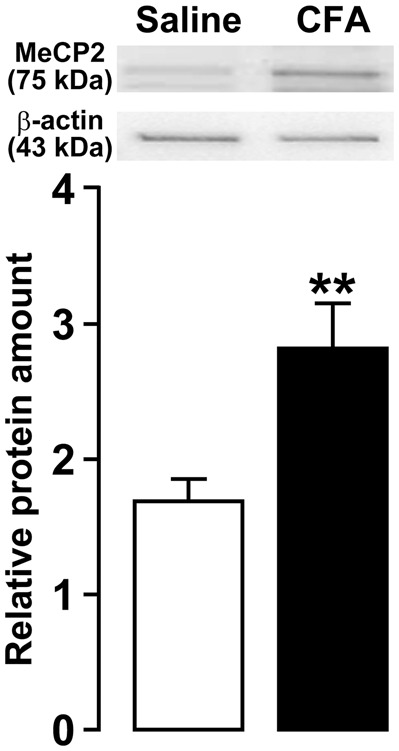
Normalized MeCP2 protein expression in TG on day 3 after saline or CFA injection into the tongue. β-actin was used as a loading control. n = 8 in each group. **p < 0.01 vs. saline, by student’s t tests. MeCP2: methyl-CpG binding protein 2; CFA: complete Freund’s adjuvant; TG: trigeminal ganglion.
Figure 5.
Changes in MeCP2 or TRPV1 expression in TG neurons innervating in tongue after CFA injection. Mean percentage of MeCP2-IR (a) or TRPV1-IR (c) TG neurons, and size-frequency histogram illustrating distribution of FG-labeled MeCP2-IR (b) or TRPV1-IR (d) TG neurons on day 3 after CFA or saline injection in wild-type or Mecp2+/− mice (n = 5 in each). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. saline/wild. #p < 0.0001 vs. CFA/Mecp2+/−, by one-way repeated-measures ANOVA followed by Bonferroni’s multiple-comparison tests. MeCP2: methyl-CpG binding protein 2; CFA: complete Freund’s adjuvant; TG: trigeminal ganglion; FG: Fluoro-Gold; ANOVA: analysis of variance; TRPV1-IR: transient receptor potential vanilloid 1-immunoreactive.
Changes in TRPV1 expression in TG neurons
We also examined the presence of TRPV1 immunoreactivity in TG neurons innervating the tongue on day 3 after CFA or saline injection. In wild-type mice, the number of TRPV1-IR neurons innervating the tongue was significantly increased after CFA injection compared with that of the saline-injected group or naive mice (naive: 27.8 ± 1.1%; saline: 30.5 ± 1.2%; CFA: 67.1 ± 2.3%, respectively; Figure 5(c)). On the other hand, the number of TRPV1-IR neurons innervating the tongue in saline- and CFA-injected Mecp2+/− mice (saline: 20.2 ± 1.2%; CFA: 19.8 ± 0.4%) was significantly less than that of saline-injected wild-type or naive mice. In wild-type mice, the cell area analysis revealed that the number of TRPV1-IR TG neurons innervating the tongue was markedly increased in cell groups of 201–400 µm2 and 401–600 µm2 in area on day 3 after CFA injection ([201–400 µm2] saline: 8.5 ± 2.9%, CFA: 25.7 ± 2.3%; [401–600 µm2] saline: 6.9 ± 1.1%, CFA: 28.6 ± 1.8%; Figure 5(d)). In all cell groups, there was no significant difference in the number of TRPV1-IR neurons in Mecp2+/− mice on day 3 after CFA injection when compared with saline injection. Moreover, in saline- and CFA-injected Mecp2+/− mice, the number of TRPV1-IR neurons in cell group of 601–800 µm2 in area was significantly less than that of saline-injected wild-type mice ([601–800 µm2] saline: 1.3 ± 1.0%, CFA: 1.7 ± 0.4%).
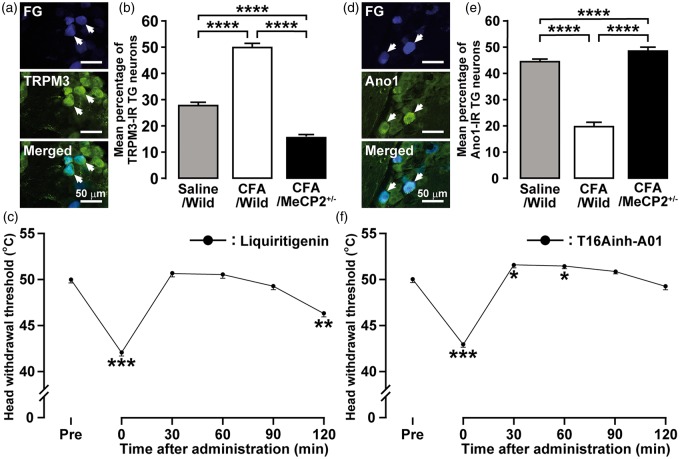
Involvement of TRPM3 and Ano1 in heat hyperalgesia
The TRPM3-IR TG neurons innervating the tongue were observed on day 3 after CFA injection in wild-type mice (Figure 6(a)). The number of TRPM3-IR TG neurons innervating the tongue was significantly increased on day 3 after CFA injection in wild-type but not in Mecp2+/− mice (Figure 6(b)). The decrease of the HHWT was completely recovered 30 min after Liquiritigenin administration into the tongue and the recovery in HHWT persisted until 90 min (Figure 6(c)). The Ano1-IR TG neurons innervating the tongue were observed on day 3 after CFA injection in wild-type mice (Figure 6(d)). The number of Ano1-IR TG neurons innervating the tongue was significantly decreased on day 3 after CFA injection in wild-type but not in Mecp2+/− mice (Figure 6(e)). The decrease of the HHWT was completely recovered and tongue heat hypoalgesia was induced 30 and 60 min after T16Ainh-A01 administration into the tongue (Figure 6(f)).
Figure 6.
Involvement of TRPM3 and Ano1 in heat hyperalgesia following CFA injection into the tongue. (a) FG-labeled TRPM3-IR TG neurons on day 3 after CFA injection in wild-type mice. Arrows indicate FG-labeled TRPM3-IR TG neurons. Scale bars: 50 µm. (b) Mean percentage of TRPM3-IR TG neurons after CFA or saline injection in wild-type or Mecp2+/− mice. Data represent mean ± SEM. Data were analyzed using one-way ANOVA followed by Bonferroni’s multiple-comparison tests, ****p < 0.0001 (n = 5 in each group). (c) HHWTs before and 30, 60, 90, and 120 min after Liquiritigenin administration to the tongue after CFA injection in wild-type mice. Data represent mean ± SEM. Data were analyzed using one-way repeated-measures ANOVA followed by Bonferroni’s multiple-comparison tests, **p < 0.01, ***p < 0.001 (n = 5). (d) FG-labeled Ano1-IR TG neurons on day 3 after CFA injection in wild-type mice. Arrows indicate FG-labeled Ano1-IR TG neurons. Scale bars: 50 µm. (e) Mean percentage of Ano1-IR TG neurons after CFA or saline injection in wild-type or Mecp2+/− mice. Data represent mean ± SEM. Data were analyzed using one-way ANOVA followed by Bonferroni’s multiple-comparison tests, ****p < 0.0001 (n = 5 in each group).(f) HHWTs before and 30, 60, 90 and 120 min after T16Ainh-A01 administration to the tongue after CFA injection in wild-type mice. Data represent mean ± SEM. Data were analyzed using one-way repeated-measures ANOVA followed by Bonferroni’s multiple-comparison tests, *p < 0.05, ***p < 0.001 (n = 5). HHWT: heat head-withdrawal reflex threshold; CFA: complete Freund’s adjuvant; TG: trigeminal ganglion; TRPM3: transient receptor potential melastatin 3; FG: Fluoro-Gold; ANOVA: analysis of variance.
Discussion
Rett syndrome is known to occur predominantly in females and is due to a deficit in MeCP2, which is considered to be a transcriptional factor acting through its methyl CpG binding domain and transcriptional factor domain. Pain hypoalgesia has also been reported in these patients.5,10,22 In animal studies, mechanical allodynia in hind paw induced by the common peroneal and tibial nerve injury was associated with an increase in MeCP2 in dorsal root ganglion (DRG) neurons, indicating the involvement of MeCP2 in modulation of pain transduction under the pathological condition.23
Heat nociception is largely attributed to the TRPV family, which is a critical transducer for noxious heat in primary sensory neurons.16 In previous studies, the number of TRPV1-IR neurons innervating incised tissue was significantly increased and TRPV1 antagonism produced a significant suppression of heat hypersensitivity.13 TRPV1 overexpression in primary afferent neurons induces an inflammatory-mediated heat hyperalgesia.24 Moreover, mice deficient in TRPV1 had prolonged withdrawal latencies to noxious heat in the hind paw and re-expression of TRPV1 in primary afferent neurons restored normal withdrawal latency.25 These studies indicate that cutaneous heat sensitivity is highly dependent on the amount of TRPV1 channels in primary neurons innervating the heat-stimulated site. TRPV1 expressed mainly in primary afferent terminals is known to increase in association with peripheral inflammation, and the increased sensitivity in inflamed tissue was attenuated by intrathecal brain-derived neurotrophic factor (BDNF) neutralizing antibody.26 In the present study, CFA administration into the tongues of wild-type mice enhanced heat sensitivity, while heat hypoalgesia occurred in the inflamed tongues of Mecp2+/− mice. TRPV1 was expressed in a large number of MeCP2-IR TG neurons innervating the tongue in both wild-type and Mecp2+/− mice, and a smaller number of TRPV1-IR TG neurons innervating the tongue were observed in Mecp2+/− mice when compared with the wild type. Together, these results suggest that heat hypoalgesia is induced by inhibition of TRPV1 expression which is highly dependent on MeCP2 signaling.
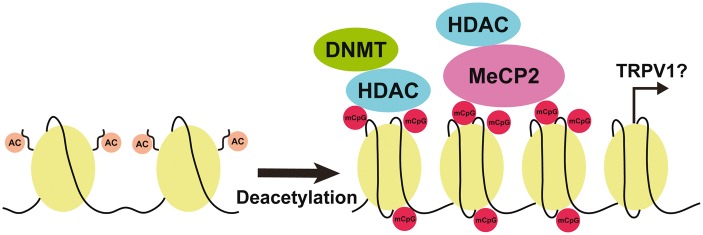
MeCP2 has an ability to selectively bind the methylated DNA and to interact with HDAC-containing complexes, linking DNA methylation and histone deacetylation.27 Further, the MeCP2 signaling is transcriptional repressor that modulates gene expression through the interpretation such as histone deacetylation.28 Thus, many studies indicate that MeCP2 is moderately expressed under the physiological condition and plays an important role in recruiting transcriptional repressors.27 However, recent study indicates that MeCP2 may act not only as a transcriptional repressor but also as an activator.29 We observed that heat sensitivity of the tongue was unchanged in Mecp2+/− mice following CFA injection into the tongue, whereas heat hyperalgesia was induced in the tongue of wild-type mice. TRPV1 was expressed in almost all MeCP2-IR TG neurons innervating the tongue after CFA injection in wild-type mice. There were no significant changes in the number of MeCP2-IR and TRPV1 neurons innervating the tongue after CFA injection in Mecp2+/− mice, whereas the number of MeCP2-IR and TRPV1-IR neurons and MeCP2 protein expression in TG were significantly increased after CFA injection in wild-type mice. Furthermore, most TG neurons innervating the tongue expressed acetyl-histone H3 after CFA injection in wild-type mice. Taken together, these results indicates that MeCP2 upregulation associated with histone H3 deacetylation enhances TRPV1 expression in TG neurons, resulting in heat hyperalgesia in the inflamed tongue (Figure 7).
Figure 7.
Schematic illustration of potential interactions between MeCP2 signaling and TRPV1 expression following CFA injection into the tongue. A complex formed by MeCP2, HDAC, and DNMT binds mCpG and histone deacetylation is accelerated. The histone deacetylation may link with activation of TRPV1 gene transcription. MeCP2: methyl-CpG binding protein 2; TRPV1: transient receptor potential vanilloid 1; CFA: complete Freund’s adjuvant; AC: histone acetylation; DNMT: DNA methyltransferase; HDAC: histone deacetylase; mCpG: methyl-CpG.
TG neurons were divided into <400 µm2, 401–600 µm2 and >601 µm2 in area, predominantly representing neurons that give rise to C-type, Aδ, and Aβ fibers, respectively.30 Although the number of MeCP2-IR TG neurons innervating the tongue was markedly increased only in small-diameter cell groups (<400 µm2 in area), TRPV1-IR TG neurons increased in small- and medium-diameter cell groups (<400 µm2, 401–600 µm2 in area, respectively) following CFA injection. In Mecp2+/− mice, MeCP2-IR neurons in small-, medium- and large-diameter cell groups (<400 µm2, 401–600 µm2, and >601 µm2, respectively) were significantly less than that of the wild-type, though only a small number of TRPV1-IR neurons were observed in large-diameter cell groups. Although further rigorous experiments are needed, our current data suggest that changes in the TRPV1 expression in TG neurons are involved in MeCP2 signaling.
Ano1 is expressed in DRG neurons and activated by heat stimulation over 44℃.31 Furthermore, TRPV1 have high calcium permeability and TRPV1 activation induced Ca2+ influx.32 Most Ano1-IR TG neurons expressed TRPV1, and Ano1 activation was also caused by the entry of calcium through TRPV1.33,34 Inflammatory pain-related behaviors were reduced in Ano1 conditional-knockout mice, suggesting that changes in Ano1 reactivity are involved in the inflammatory pain.35 TRPM3 which is Ca2+ permeable nonselective cation channel is expressed in a large subset of DRG and TG neurons.36 TRPM3-knockout mice exhibit loss of the response to noxious heat stimuli and failed to develop inflammatory heat hyperalgesia.37 In this study, the number of TRPM3-IR TG neurons innervating the tongue was significantly increased and TRPM3 inhibition completely depressed heat hyperalgesia under inflammatory states. The number of Ano1-IR TG neurons innervating the tongue was significantly decreased and Ano1 inhibition induced heat hypoalgesia. The changes in the number of TRPM3-IR and Ano1-IR TG neurons did not occur in Mecp2+/− mice. These findings suggest that the enhancements of TRPM3 and Ano1 activities in TG neurons via MeCP2 signaling are also involved in the tongue heat hyperalgesia in inflammatory states. However, the interaction between the decrease of Ano1 expression and the enhancement of Ano1 activity via MeCP2 signaling is unclear, further studies are needed.
Finally, tongue has multimodal innervations and the main function is the taste detection. In cultured DRG neurons, MeCP2 regulates BDNF expression and downregulation of MeCP2 by microRNAs decreases BDNF expression.38 Further, a subpopulation of taste cells in adulthood persistently expresses BDNF, which suggests that BDNF has a role in mature taste cells. Indeed, BDNF presumably assists to modulate the innervation in a specific subpopulation of taste bud cells.38 These reports including the present study presume that MeCP2 signaling plays a pivotal role in not only changes in heat sensitivity via the alteration of TRPV1 expression but also the taste detection in the tongue.
Conclusion
Heat hypoalgesia of the tongue occurred in Mecp2+/− mice and inflammatory heat hyperalgesia induced in wild-type but not in Mecp2+/− mice. The number of TRPV1- and MeCP2-IR TG neurons innervating the tongue was significantly increased in wild-type but not in Mecp2+/− mice following CFA injection. These findings indicate that tongue heat sensitivity and hypersensitivity are dependent on the expression of TRPV1 which is regulated via MeCP2 signaling in TG neurons innervating the tongue. Further elucidation of MeCP2-mediated pain modulation may shed new light on the pathogenesis of Rett syndrome and provide new therapeutic strategies for pain hypoalgesia in Rett syndrome patients.
Author Contributions
AS, MS, KH, KI: contributed to conception, design, data acquisition, analysis, and interpretation, drafted and wrote the manuscript; KH, KI: contributed to design, data analysis, and interpretation; TS: contributed to design and data analysis and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by a research grant from the Dental Research Center, Nihon University School of Dentistry, Sato and Uemura Funds from Nihon University School of Dentistry, KAKENHI (Grant-in-Aid for Scientific Research [C] 24593064, 26463120) and MEXT-Supported Program for the Strategic Research Foundation at Private Universities 2013-2017.
References
- 1.Amir RE, Van den Veyver IB, Wan M, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 1999; 23: 185–188. [DOI] [PubMed] [Google Scholar]
- 2.Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron 2007; 56: 422–437. [DOI] [PubMed] [Google Scholar]
- 3.Hite KC, Adams VH, Hansen JC. Recent advances in MeCP2 structure and function. Biochem Cell Biol 2009; 87: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci 2004; 27: 306–321. [DOI] [PubMed] [Google Scholar]
- 5.Nan X, Campoy FJ, Bird A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell 1997; 88: 471–481. [DOI] [PubMed] [Google Scholar]
- 6.Theisen JW, Gucwa JS, Yusufzai T, et al. Biochemical analysis of histone deacetylase-independent transcriptional repression by MeCP2. J Biol Chem 2013; 288: 7096–7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bienvenu T, Chelly J. Molecular genetics of Rett syndrome: when DNA methylation goes unrecognized. Nat Rev Genet 2006; 7: 415–426. [DOI] [PubMed] [Google Scholar]
- 8.Lombardi LM, Baker SA, Zoghbi HY. MECP2 disorders: from the clinic to mice and back. J Clin Invest 2015; 125: 2914–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyst MJ, Bird A. Rett syndrome: a complex disorder with simple roots. Nat Rev Genet 2015; 16: 261–275. [DOI] [PubMed] [Google Scholar]
- 10.Downs J, Geranton SM, Bebbington A, et al. Linking MECP2 and pain sensitivity: the example of Rett syndrome. Am J Med Genet A 2010; 152 A: 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green BG, Gelhard B. Perception of temperature on oral and facial skin. Somatosens Res 1987; 4: 191–200. [DOI] [PubMed] [Google Scholar]
- 12.Komiyama O, De Laat A. Tactile and pain thresholds in the intra- and extra-oral regions of symptom-free subjects. Pain 2005; 115: 308–315. [DOI] [PubMed] [Google Scholar]
- 13.Urata K, Shinoda M, Honda K, et al. Involvement of TRPV1 and TRPA1 in incisional intraoral and extraoral pain. J Dent Res 2015; 94: 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997; 389: 816–824. [DOI] [PubMed] [Google Scholar]
- 15.Tominaga M, Caterina MJ, Malmberg AB, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998; 21: 531–543. [DOI] [PubMed] [Google Scholar]
- 16.Tominaga M, Caterina MJ. Thermosensation and pain. J Neurobiol 2004; 61: 3–12. [DOI] [PubMed] [Google Scholar]
- 17.Morales-Lazaro SL, Simon SA, Rosenbaum T. The role of endogenous molecules in modulating pain through transient receptor potential vanilloid 1 (TRPV1). J Physiol 2013; 591(Pt 13): 3109–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pei L, Lin CY, Dai JP, et al. Facial pain induces the alteration of transient receptor potential vanilloid receptor 1 expression in rat trigeminal ganglion. Neurosci Bull 2007; 23: 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonic-Kocijan S, Zhao X, Liu W, et al. TRPV1 channel-mediated bilateral allodynia induced by unilateral masseter muscle inflammation in rats. Mol Pain 2013; 9: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung MK, Lee J, Duraes G, et al. Lipopolysaccharide-induced pulpitis upregulates TRPV1 in trigeminal ganglia. J Dent Res 2011; 90: 1103–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koto M, Miwa M, Togashi M, et al. A method for detecting the optimum day for mating during the 4-day estrous cycle in the rat: measuring the value of electrical impedance of the vagina. Jikken Dobutsu 1987; 36: 195–198. [DOI] [PubMed] [Google Scholar]
- 22.Kudo S. Methyl-CpG-binding protein MeCP2 represses Sp1-activated transcription of the human leukosialin gene when the promoter is methylated. Mol Cell Biol 1998; 18: 5492–5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Liu C, Guo QL, et al. Intrathecal 5-azacytidine inhibits global DNA methylation and methyl- CpG-binding protein 2 expression and alleviates neuropathic pain in rats following chronic constriction injury. Brain Res 2011; 1418: 64–69. [DOI] [PubMed] [Google Scholar]
- 24.Shinoda M, Asano M, Omagari D, et al. Nerve growth factor contribution via transient receptor potential vanilloid 1 to ectopic orofacial pain. J Neurosci 2011; 31: 7145–7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walder RY, Radhakrishnan R, Loo L, et al. TRPV1 is important for mechanical and heat sensitivity in uninjured animals and development of heat hypersensitivity after muscle inflammation. Pain 2012; 153: 1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia CM, Gulick MA, Yu SJ, et al. Up-regulation of brain-derived neurotrophic factor in primary afferent pathway regulates colon-to-bladder cross-sensitization in rat. J Neuroinflammation 2012; 9: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miao CG, Yang YY, He X, et al. New advances of DNA methylation and histone modifications in rheumatoid arthritis, with special emphasis on MeCP2. Cell Signal 2013; 25: 875–882. [DOI] [PubMed] [Google Scholar]
- 28.Zocchi L, Sassone-Corsi P. SIRT1-mediated deacetylation of MeCP2 contributes to BDNF expression. Epigenetics 2012; 7: 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chahrour M, Jung SY, Shaw C, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 2008; 320: 1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurons. J Physiol 1985; 359: 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho H, Yang YD, Lee J, et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci 2012; 15: 1015–1021. [DOI] [PubMed] [Google Scholar]
- 32.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 2001; 413: 203–210. [DOI] [PubMed] [Google Scholar]
- 33.Takayama Y, Uta D, Furue H, et al. Pain-enhancing mechanism through interaction between TRPV1 and anoctamin 1 in sensory neurons. Proc Natl Acad Sci USA 2015; 112: 5213–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanazawa T, Matsumoto S. Expression of transient receptor potential vanilloid 1 and anoctamin 1 in rat trigeminal ganglion neurons innervating the tongue. Brain Res Bull 2014; 106: 17–20. [DOI] [PubMed] [Google Scholar]
- 35.Lee B, Cho H, Jung J, et al. Anoctamin 1 contributes to inflammatory and nerve-injury induced hypersensitivity. Mol Pain 2014; 10: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vriens J, Owsianik G, Hofmann T, et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 2011; 70: 482–494. [DOI] [PubMed] [Google Scholar]
- 37.Manners MT, Tian Y, Zhou Z, et al. MicroRNAs downregulated in neuropathic pain regulate MeCP2 and BDNF related to pain sensitivity. FEBS Open Bio 2015; 5: 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang T, Ma L, Krimm RF. Postnatal reduction of BDNF regulates the developmental remodeling of taste bud innervation. Dev Biol 2015; 405: 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]