Abstract
The genetic lineages of Listeria monocytogenes and other species of the genus Listeria are correlated with pathogenesis in humans. Although matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) has become a prevailing tool for rapid and reliable microbial identification, the precise discrimination of Listeria species and lineages remains a crucial issue in clinical settings and for food safety. In this study, we constructed an accurate and reliable MS database to discriminate the lineages of L. monocytogenes and the species of Listeria (L. monocytogenes, L. innocua, L. welshimeri, L. seeligeri, L. ivanovii, L. grayi, and L. rocourtiae) based on the S10-spc-alpha operon gene encoded ribosomal protein mass spectrum (S10-GERMS) proteotyping method, which relies on both genetic information (genomics) and observed MS peaks in MALDI-TOF MS (proteomics). The specific set of eight biomarkers (ribosomal proteins L24, L6, L18, L15, S11, S9, L31 type B, and S16) yielded characteristic MS patterns for the lineages of L. monocytogenes and the different species of Listeria, and led to the construction of a MS database that was successful in discriminating between these organisms in MALDI-TOF MS fingerprinting analysis followed by advanced proteotyping software Strain Solution analysis. We also confirmed the constructed database on the proteotyping software Strain Solution by using 23 Listeria strains collected from natural sources.
Introduction
The genus Listeria is gram-positive bacterium that can grow in saline and cold environments [1]. At present, the bacterial genus Listeria consists of 17 species, including Listeria monocytogenes, L. innocua, L. welshimeri, L. seeligeri, L. ivanovii, and L. grayi, which have been recognized since the 1960s to 1980s, and other recently emerging species, namely L. marthii, L. recourtiae, L. floridensis, L. aquatica, L. fleischmannii, L. newyorkensis, L. cornellensis, L. weihenstephanensis, L. grandensis, L. riparia, and L. booriae (http://www.bacterio.net/) [2, 3]. Among these, L. monocytogenes and L. ivanovii are known to be pathogenic in warm-blooded hosts. Recently, L. innocua was reported to have pathogenic potential [4]. Most cases of human diseases are associated with L. monocytogenes, which causes severe listeriosis through infections from ready-to-eat foods [5]. L. monocytogenes can be further divided into four evolutionary lineages of 13 serotypes: lineage I (serotypes 1/2b, 3b, 4b, 4d, and 4e), lineage II (serotypes 1/2a, 1/2c, 3a, and 3c), and lineages III (serotypes 4a and 4c) and IV (4a, 4b, 4c) [6, 7]. Serotypes 4b, 1/2a, and 1/2b, which belong to lineages I and II, are commonly isolated from human patients [8], while lineages III and IV from the ruminants [6, 9].
Detection of Listeria species and serotypes of L. monocytogenes is crucial for clinical and food safety. Traditionally, culture-based methods using chromogenic media have been utilized in food laboratories to differentiate L. monocytogenes from other Listeria species [10]. Serotyping of L. monocytogenes is carried out by immunological methods using antisera [11]. Alternative advanced typing methods, such as pulsed-field gel electrophoresis [12], multilocus sequence typing [13, 14], and DNA microarray [15], have been reported. Although reliable typing can be performed using these methods, they are laborious and time-consuming.
Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) has become a standard tool for rapid and reliable microbial identification in clinical and food laboratories. Typing trials of Listeria species and L. monocytogenes have been reported by several research groups so far [16–19]. Barbuddhe et al. reported that L. monocytogenes, L. innocua, L. welshimeri, L. ivanovii and L. seeligeri could be discriminated by a MALDI TOF-MS fingerprinting method that compares obtained profile spectra and registered reference spectral database to calculate a matching score. They also identified that detection of MS peak at mass to ion ratios (m/z) of 5590 and 11179 could differentiate between serotype 4a and 4c of L. monocytogenes [16]. In contrast, a study by Farfour et al. revealed that identification of Listeria spp. could only be achieved at the genus level, except for L. grayi using the MALDI TOF MS Andromas system [17]. Hsueh et al. reported the detected five MS peaks at m/z 5594.85, 6184.39, 11871.31, 5601.21, and 11199.33 could be used to distinguish serotypes 1/2a (lineage II), and 1/2b and 4b (lineage I) of L. monocytogenes [19]. These results from several groups demonstrate that the MS sizes of the detected peaks differ between research groups, and that the proteins were not fully identified, which potentially leads to data inconsistency.
The S10-spc-alpha operon gene encoded ribosomal protein mass spectrum (S10-GERMS) method provides an accurate and reliable m/z database of biomarker proteins encoded in the S10-spc-alpha operon and characteristic genes. The m/z values in the database are confirmed by genomic information and experimentally observed MS peaks in MALDI TOF-MS analysis [20]. The m/z database in this method reflects the subtle m/z differences that occur with even a single amino acid substitution within the selected biomarker proteins of closely related species. Therefore, the S10-GERMS method can realize microbial identification at a higher resolution than the conventional fingerprinting approaches, and has been successfully applied to species, pathovar, or serotype level discrimination of various industrially important bacteria, such as Bacillus spp., Pseudomonas putida, P. syringae, Lactobacillus casei, and the enterohemorrhagic Escherichia coli [21–24]. To realize a fully automatic analysis based on the S10-GERMS method, our group has developed the novel external software ‘Strain Solution’ for MALDI-TOF MS analysis [25], and has made it available from the Shimadzu Corporation (Kyoto, Japan).
Here, we report the construction of an accurate and reliable m/z database of annotated biomarker proteins for Listeria spp. based on the S10-GERMS method. We demonstrate that the selected biomarker proteins from the database can fulfill our aim in discriminating lineages of Listeria monocytogenes, and species of the genus Listeria (L. monocytogenes, L. innocua, L. welshimeri, L. seeligeri, L. ivanovii, L. grayi, and L. rocourtiae) using MALDI-TOF MS analysis.
Materials and Methods
Bacterial strains
For constructing the theoretical MS database, we used publically available L. monocytogenes strains and Listeria spp. listed in Table 1 obtained from the Japan Collection of Microorganisms, RIKEN BRC (Tsukuba, Japan), the American Type Culture Collection (Manassas, VA, USA), and the National BioResource Project GTC Collection (Gifu, Japan). They were aerobically cultivated in the brain heart infusion medium (Becton Dickinson, Franklin Lakes, NJ, USA) at 30°C. L. monocytogenes strains were serotyped using the Agglutinating Sera Listeria Antisera Set (Denka Seiken, Tokyo, Japan) and multiplex PCR as a countercheck [26].
Table 1. Bacterial strains used to construct an MS database.
| No. | Genus | Species | Subspecies | Strain | Serotypea | Lineage | Sourceb |
|---|---|---|---|---|---|---|---|
| 1 | Listeria | monocytogenes | ATCC15313T | 1/2ac | II | ATCC | |
| 2 | Listeria | monocytogenes | JCM2873 | 4d | I | JCM | |
| 3 | Listeria | monocytogenes | JCM7671 | 1/2a | II | JCM | |
| 4 | Listeria | monocytogenes | JCM7672 | 1/2c | II | JCM | |
| 5 | Listeria | monocytogenes | JCM7673 | 3a | II | JCM | |
| 6 | Listeria | monocytogenes | JCM7674 | 4a | III | JCM | |
| 7 | Listeria | monocytogenes | JCM7675 | 4b | I | JCM | |
| 8 | Listeria | monocytogenes | JCM7676 | 1/2b | I | JCM | |
| 9 | Listeria | monocytogenes | JCM7677 | 3b | I | JCM | |
| 10 | Listeria | monocytogenes | JCM7678 | 3c | II | JCM | |
| 11e | Listeria | seeligeri | JCM7679 | JCM | |||
| 12 | Listeria | monocytogenes | JCM7680 | 4d | I | JCM | |
| 13e | Listeria | seeligeri | JCM7682 | JCM | |||
| 14 | Listeria | monocytogenes | JCM7683 | 3b | I | JCM | |
| 15 | Listeria | monocytogenes | ATCC51772 | 1/2a | II | ATCC | |
| 16 | Listeria | monocytogenes | ATCC19115 | 4b | I | ATCC | |
| 17 | Listeria | innocua | ATCC33090T | 6ad | ATCC | ||
| 18 | Listeria | innocua | GTC02960 | NBRP | |||
| 19 | Listeria | ivanovii | ivanovii | JCM7681 | JCM | ||
| 20 | Listeria | ivanovii | londoniensis | ATCC49954 | ATCC | ||
| 21 | Listeria | seeligeli | ATCC35967T | ATCC | |||
| 22 | Listeria | welshimeri | GTC02963 | 6bd | NBRP | ||
| 23 | Listeria | grayi | ATCC19120T | ATCC | |||
| 24 | Listeria | rocourtiae | GTC16429T | NBRP |
aSerotypes of L. monocytogenes were determined using an agglutination test with antisera and PCR.
bATCC: American Type Culture Collection, JCM: Japan Collection of Microorganisms, RIKEN BRC, NBRP: National BioResource Project.
cweak agglutination reaction with H antisera from Denka Seiken.
dSerotype information for strains No. 17 and 22 is provided by the supplier.
eThese strains (JCM7689 and JCM7682) were deposited as L. monocytogenes in JCM but we revealed that they are L. seeligeri.
To evaluate the constructed MS database, environmental isolates of Listeria species (L. monocytogenes, L. innocua, L. ivanovii, L. seeligeri, and L. rocourtiae) listed in Table 2 were used. They were screened from river water in Japan and identified by the conventional method using ALOA media (bioMérieux, Lyon, France) and antisera serotyping kit. Species of Listeria were determined by a physiological biochemical test using the Listeria identification system Api (bioMérieux), and 16S rRNA sequencing [27]. Pathogenicity of L. monocytogenes was confirmed by the CAMP test [28] and multiplex PCR for the inlB, plcA, plcB, and clpE genes [29]. Serotypes of L. innocua were not defined because antisera for serotyping were not available.
Table 2. Bacterial strains used to evaluate the constructed MS database.
| No | Genus | Species | ID code | Serotype (lineage) | Source |
|---|---|---|---|---|---|
| 1 | Listeria | monocytogenes | 2009.6.15–2 | 4b (I) | River water |
| 2 | Listeria | monocytogenes | 2009.6.9–1 | 4b (I) | River water |
| 3 | Listeria | monocytogenes | 2009.6.1–2 | 4b (I) | River water |
| 4 | Listeria | monocytogenes | 12.9.11.1–1 | 4b (I) | River water |
| 5 | Listeria | monocytogenes | 12.9.11.3–1 | 1/2a (I) | River water |
| 6 | Listeria | monocytogenes | 12.10.15.1–1 | 1/2b (I) | River water |
| 7 | Listeria | innocua | 12.9.11.4–4 | River water | |
| 8 | Listeria | innocua | 12.10.15.5–3 | River water | |
| 9 | Listeria | innocua | 12.10.15.2–3 | River water | |
| 10 | Listeria | innocua | 2009.6.9–2 | River water | |
| 11 | Listeria | innocua | 2009.6.5–3 | River water | |
| 12 | Listeria | innocua | 2009.6.3–4 | River water | |
| 13 | Listeria | seeligeri | 2009.6.4–2 | River water | |
| 14 | Listeria | seeligeri | 2009.6.5–4 | River water | |
| 15 | Listeria | seeligeri | 12.9.11.5–3 | River water | |
| 16 | Listeria | seeligeri | 12.9.11.4–3 | River water | |
| 17 | Listeria | monocytogenes | 12.9.11.2–3 | 4b (I) | River water |
| 18 | Listeria | rocourtiae | 12.10.15.4–3 | River water | |
| 19 | Listeria | rocourtiae | 12.9.26.10–3 | River water | |
| 20 | Listeria | rocourtiae | 12.9.26.6–3 | River water | |
| 21 | Listeria | ivanovii | 2009.6.12–3 | River water | |
| 22 | Listeria | ivanovii | 2009.6.8–2 | River water | |
| 23 | Listeria | ivanovii | 2009.6.2 | River water |
Construction of an MS database
The primers for DNA sequence analysis were designed based on the consensus sequence information available from the Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.genome.jp/kegg/) and NCBI (http://www.ncbi.nlm.nih.gov/) (Table 3). DNA fragments for sequence analysis were amplified by PCR using KOD plus DNA polymerase (Toyobo, Osaka, Japan) and extracted genomic DNA as the template as described previously [24]. We used the Big Dye ver. 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) for sequencing, and calculated the theoretical m/z values of biomarker proteins using an in-house macro software programmed based on the workflow of DNA to amino acid conversion, calculating protein MS from each amino acid, and addition of a proton (m/z 1.01) and reducing MS of N-terminal Met (m/z 131.19) when the second amino acid is Ala, Cys, Gly, Pro, Ser, Thr, or Val.
Table 3. DNA Primers used in this study.
| Name | Sequence (5'-3') | Purpose |
|---|---|---|
| Lm-S10-1 | CATGGCGGATGTTCAGGTAA | Amplification of S10 region and sequencing |
| Lm-S10-R | CTCCTTCCAGAATAACGGGT | Amplification of S10 region and sequencing |
| Lm-S10-2 | AGCAGCACAAAACGTGGTAC | Sequencing of S10 region |
| Lm-S10-3 | AAGGAGGACTAACGAATGCC | Sequencing of S10 region |
| Lm-S10-4 | TGCACGCAACTTACAAGGCA | Sequencing of S10 region |
| Lm-S10-5 | CGGACGCAATAACCAAGGTA | Sequencing of S10 region |
| Lm-S10-6 | AATGAACCCGAACGATCACC | Sequencing of S10 region |
| Lm-S10-7 | TACAAGCGCAAAAGCCGTTG | Sequencing of S10 region |
| Lm-S10-8 | GTGCAGCTAACCGTGTGAAT | Sequencing of S10 region |
| Lm-S10-9 | AGGCGGAACTGAAGTTGCAT | Sequencing of S10 region |
| Lm-spc-1 | ACCCGTTATTCTGGAAGGAG | Amplification of spc region and sequencing |
| Lm-spc-R | AAGGCATTACACCCATGGCA | Amplification of spc region and sequencing |
| Lm-spc-F | CTCGTCCATTGTCTGCAACT | Sequencing of spc region |
| Lm-spc-2 | CAAACGTAATGCTAMTTGACCC | Sequencing of spc region |
| Lm-spc-3 | CGTGGTAACTATACGTTGGGT | Sequencing of spc region |
| Lm-spc-4 | GACTGGCGAACGTGTAATCA | Sequencing of spc region |
| Lm-spc-5 | TCCTGCAAACACWCAAGTGATT | Sequencing of spc region |
| Lm-spc-6 | GGAGGGACATATTACATGCCTG | Sequencing of spc region |
| Lm-spc-7 | TTAATCGGACGCCCTCAA | Sequencing of spc region |
| Lm-alpha-F | CTCTACCAAACGCGATGTTC | Amplification of alpha region and sequencing |
| Lm-alpha-R | GGAAACACAGAGCTAGACAAGG | Amplification of alpha region and sequencing |
| Lm-alpha-1 | CCTGACACGCGGAAGAATTA | Sequencing of alpha region |
| Lm-alpha-2 | AAGGCCCGTCCAAAACAGTA | Sequencing of alpha region |
| Lm-alpha-3 | CAGCGATGATGCCAAGTATG | Sequencing of alpha region |
| Lm-alpha-4 | GAAGCAGTTTCACTTGGAGC | Sequencing of alpha region |
| Lm-alpha-5 | AACTGGCTGACCTTGGCTTA | Sequencing of alpha region |
| Lm-L21-F | CCCCTGTGATGGCGAGTCTT | Amplification of L21 and sequencing |
| Lm-L21-R | TCTTCTCGCATAACATCGACTTGAA | Amplification of L21 |
| Lm-S21-F | TGAAGGATTTAAGTGAGTGCATGT | Amplification of S21 and sequencing |
| Lm-S21-R | CGCATCGCTTGTTTCATATCT | Amplification of S21 |
| Lm-S9-F | TTCGGGAGCTAATTTGTTTCAA | Amplification and sequencing of S9 and L13 |
| Lm-S9-R | AACGTTTTCAGAACTGAGGTGC | Amplification and sequencing of S9 and L13 |
| Lm-S9-F2 | CACATATCGACACTGGAGACTTTG | Sequencing of S9 and L13 |
| Lm-L10-F | CTGGAATCAAAGTCGACCCA | Amplification of L10 and sequencing |
| Lm-L10-R | GCAGCAGTTACGCCAAATTCTT | Amplification of S21 |
| Listeria_sp-L31-F | TGTTATAATATYTATACTGTGTGTAAAAGC | Amplification of L31 and sequencing |
| Listeria_sp-L31-R | TGAGACCGTAYTTTTTGTTGAAGC | Amplification of L31 and sequencing |
MALDI-TOF MS analysis
Bacterial cells grown overnight on an agar plate (three colonies) or in 2 mL liquid medium were collected and suspended in 0.5 mL of 70% (v/v) ethanol. Cells were then separated by centrifugation at 10,000 × g for 2 min at 4°C and dehydrated using a centrifugal evaporator (CVE-3100, EYELA, Tokyo, Japan), after which a 10 μL aliquot of 35% (v/v) formic acid and cells were mixed by pipetting. Then, 1.5 μL of this mixture was mixed with 10 μL matrix reagent containing 20 mg/mL sinapinic acid (SA; Wako Pure Chemical Industries, Osaka, Japan) and 1% trifluoroacetic acid (Wako Pure Chemical Industries) in 50% (v/v) acetonitrile; then 1.5 μL was spotted onto the analytical metal plate. Samples were analyzed using an AXIMA Microorganism Identification System (Shimadzu Corporation) with 100 laser shots at a spectrum range of 2000 m/z– 35000 m/z with 500 ppm tolerance. We used α-cyano-4-hydroxycinnamic acid (CHCA) as a matrix for the SARAMIS database searching, as described in a previous report [25]. For calibrating the instrument, Escherichia coli DH5α was used according to the manufacturer’s instructions.
Analysis with Strain Solution software
The datasets of MS and peak intensity in ASCII files were incorporated into the software Strain Solution version 1.0.0. (Shimadzu Corporation) and analyzed according to the instructions. The MS values of the parental database were registered in the software in advance.
Results
Construction of an MS database for discriminating L. monocytogenes lineages
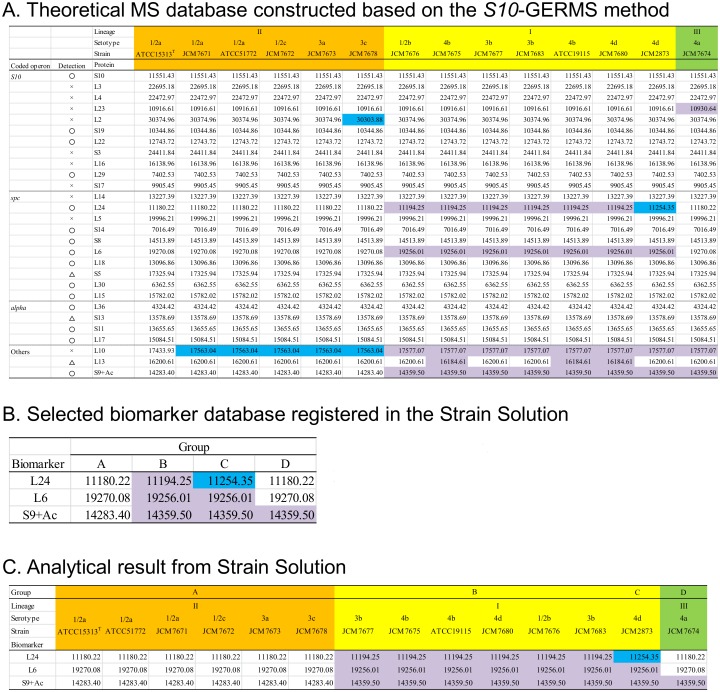
First, we constructed an m/z database of ribosomal proteins encoded in the S10-spc-alpha operon and additional potential biomarkers using publically available L. monocytogenes strains (Fig 1A). The ribosomal proteins L23, L2, L24, and L6 in the S10-spc-alpha operon and the three additional ribosomal proteins L10, L13, and S9 appear capable of differentiating serotypes of L. monocytogenes because their unique MS depending on the strains. From the comparison of the theoretically calculated MS of S9 and its actual observed MS peaks, the mass weight of the observed peak of S9 shifted +43, indicating an acetylated S9. Among these, L23, L2, and L10 were not detected in the MALDI-TOF MS analysis, although they were expected to be powerful biomarkers from their theoretical MS value varieties. We observed the MS peaks of L13 with m/z 16200.61 or 16184.61 in all L. monocytogenes samples and m/z 16187.61 in L. seeligeri strains (data not shown); however, this protein was not suitable as biomarkers because MS differences (shifts) were too small. In contrast, the three ribosomal proteins L24, L6, and S9 were ideal biomarker candidates because they were always detected, regardless of the strain, with the current MS detection tolerance (more than 500 ppm).
Fig 1. Theoretical MS database constructed with publically available L. monocytogenes strains and analysis with Strain Solution software.
A. The theoretical m/z of the ribosomal proteins in S10-spc-alpha operon and an additional three biomarker candidates are shown. Circles in ‘Detection’ means the corresponding peaks are detectable in all strains with default analytical condition (threshold offset: 0.015 mV; threshold response: 1.200) in the AXIMA system. The undetected or weak peaks that we could not always find are marked with an “x”. Triangles indicate that MS differences in each strain or the other protein peaks could not be distinguished from one another with a 500 ppm tolerance, though putative peaks were detected. Characteristic MS values were colored. B. The selected three biomarkers registered as the standard database in Strain Solution. C. The results obtained from the Strain Solution analysis.
Analysis of L. monocytogenes with Strain Solution software
From the results above, we selected three ribosomal proteins (L24, L6, and S9) as biomarker proteins capable of discriminating L. monocytogenes lineages. Four patterns of MS values of these proteins (listed in Fig 1B), which divided L. monocytogenes into four groups (A to D), were preregistered in the analytical software Strain Solution. First, 14 L. monocytogenes strains were identified as ‘L. monocytogenes’ using the conventional fingerprinting analysis software, SARAMIS (data not shown). Next, the MS data obtained with matrix SA was imported into the Strain Solution software. As shown in Fig 1C, the attribution of all biomarkers generated from automated analysis with Strain Solution were correct. The lineages of L. monocytogenes were classified into four groups (A to D) as follows: lineage I [registered group B (serotypes 1/2b, 3b, 4b, 4d, and 4e) and group C (serotype 4d)], lineage II [group A (serotypes 1/2a, 1/2c, 3a, and 3c)] and lineage III [group D (serotype 4a)]. Thus, we confirmed that these three biomarkers, L24 (m/z 11180.22, 11194.25, or 11254.35), L6 (m/z 19270.08 or 19256.01), and acetylated S9 (m/z 14283.40, 14359.50, or 14302.45), are useful to discriminate between lineages or serotypes of L. monocytogenes in MALDI-TOF MS using Strain Solution.
Construction of an MS database for Listeria species
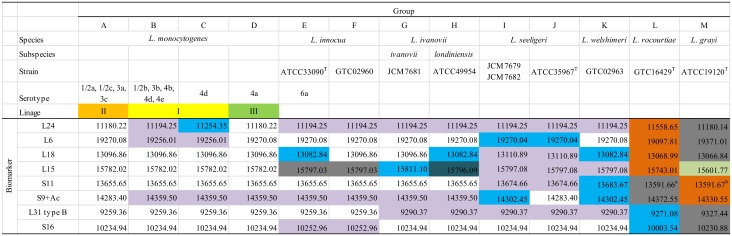
As described above, three promising sets of ribosomal proteins (L24, L6, and S9) were consistently detected by MALDI-TOF MS analysis and were effective for typing of L. monocytogenes. From the analytical MS data obtained from MALDI-TOF MS, these biomarkers were detected in all Listeria spp. as well as L. monocytogenes(S1 Fig). Furthermore, we found that five additional ribosomal proteins, L18 (m/z 13096.86 or 13110.89), L15 (m/z 15782.02, 15797.08, or 15668.86), S11 (m/z 13655.65 or 13674.66), L31 type B (m/z 9259.36, 9290.34, 9327.44, or 9271.3), and S16 (m/z 10234.94, 10252.97, 10230.88, or 10003.54) gave characteristic MS patterns to the species of Listeria. S13 (m/z 13578.69 in L. monocytogenes or 13552.65 in L. seeligeri) was also detected, but one of the peaks overlapped with the peaks of L20 (m/z 13552.08) (data not shown). From these findings, we focused on eight biomarkers (L24, L6, L18, L15, S11, S9, L31 type B, and S16) and constructed a theoretical MS database for Listeria species using publically available strains (Fig 2). It should be noted that S11 in L. rocourtiae and L. grayi was post-translationally modified, while that of the others was not.
Fig 2. Theoretical MS database for Listeria species.
aThe MS [M + H]+ plus m/z 17 of the theoretically calculated value is shown based on the observed MS peak. bThe MS [M + H]+ plus m/z 14 of the theoretically calculated value is shown because it appeared to be methylated.
Comparison of fingerprinting analysis and S10-GERMS method
As mentioned above, the conventional fingerprinting analysis software SARAMIS could properly identify all L. monocytogenes strains to the species level. Here, we analyzed L. innocua, L. ivanovii, L. seeligeri, L. welshimeri, L. rocourtiae, and L. grayi by SARAMIS. As shown in Table 4, most of them were identified as only ‘Listeria spp.’ except for L. grayi, which was correctly identified to the species level. However, L. ivanovii JCM7681, L. seeligeri JCM 7679 and JCM 7682 were misidentified as L. monocytogenes. While L. rocourtiae had a 0% match to the parental database in SARAMIS, resulting in ‘not identified’ at first, it was correctly identified to species level after an additional database was imported according to the manufacturer’s instructions for SuperSpectra™ in SARAMIS. This implies that fingerprinting depends greatly on the reference database quality. The parental database for L. rocourtiae registered here is shown in S1 Table.
Table 4. Results from fingerprinting analysis with SARAMIS.
| Sample | Identity (%) | Family | Genus | Species |
|---|---|---|---|---|
| L. innocua ATCC33090T | 91.8 | Family IV Listeriaceae | Listeria | sp. |
| L. innocua GTC02960 | 99.9 | Family IV Listeriaceae | Listeria | sp. |
| L. ivanovii GTC02961 | 84.4 | Family IV Listeriaceae | Listeria | monocytogenes |
| L. ivanovii ATCC49954T | 99.9 | Family IV Listeriaceae | Listeria | sp. |
| L. seeligeri ATCC35967 | 91.8 | Family IV Listeriaceae | Listeria | sp. |
| L. welshimeri GTC02963T | 97.2 | Family IV Listeriaceae | Listeria | sp. |
| L. rocourtiae GTC16429T | 99.9a | Listeria | rocourtiae | |
| L. grayi ATCC19120 | 99.9 | Family IV Listeriaceae | Listeria | grayi |
| L. seeligeri JCM7679 | 81.1 | Family IV Listeriaceae | Listeria | monocytogenes |
| L. seeligeri JCM7683 | 81.1 | Family IV Listeriaceae | Listeria | monocytogenes |
aThe parental database for SuperSpectra™ in SARAMIS is shown in S1 Table.
Next we tried automated discrimination at the species level using eight biomarkers in the database of Fig 2. First, MS patterns belonging to the group A to K were preregistered in Strain Solution based on Fig 2. The m/z 15797.08, 15797.03, and 15796.09 of L15 were preset as m/z 15797.08 because the MS differences were too small to be detected on a normal MALDI-TOF MS system. Similarly m/z 19270.08 and 19270.04 (specific to L. seeligeri) of L6 were registered as m/z 19270.08 because their MS differences were too small to be recognized. As a result, all Listeria spp. strains listed in Table 1 were assigned to the proper groups by our system. The spectra of the biomarkers obtained in our MALDI-TOF MS analysis are shown in S1 Fig.
Evaluation of the constructed MS database
Strains isolated from environment were used for blind test to evaluate the constructed MS database (Table 2). In fingerprinting analysis with SARAMIS, all L. monocytogenes strains (1–6 and 17) and L. rocourtiae (18–20) were correctly identified as L. monocytogenes and L. rocourtiae at the species-level, respectively. In contrast, the other 13 strains were just identified as Listeria spp., although the SARAMIS database has already included the m/z information of L. innocua and L. ivanovii.
The Strain Solution software further scanned m/z data of a total 20 strains, except for L. rocourtiae. The analytical results of the traditional method and Strain Solution are summarized in Table 5. Using eight biomarkers in Fig 2, all L. monocytogenes (seven strains), L. innocua (six strains), and L. ivanovii (three strains) were correctly identified at the lineage or species level in our system, with a 100% match for each group. However identification accuracy of L. seeligeri was 3/4 (75%) due to the misidentification of No. 13 strain into L. innocua.
Table 5. Identification results by traditional method and Strain Solution.
| Traditional method | Strain Solution | ||
|---|---|---|---|
| Strain No. | Group | ||
| 1, 2, 3, 4, 17 | L. monocytogenes serotype 4b (lineage I) | A | L. monocytogenes lineage I |
| 5 | L. monocytogenes serotype 1/2a (lineage I) | A | L. monocytogenes lineage I |
| 6 | L. monocytogenes serotype 1/2b (lineage I) | A | L. monocytogenes lineage I |
| 7, 12 | L. innocua | F | L. innocua |
| 13 | L. seeligeri | F | L. innocua |
| 8, 9, 10, 11 | L. innocua | E | L. innocua |
| 14, 15, 16 | L. seeligeri | I | L. seeligeri |
| 21, 23 | L. ivanovii | H | L. ivanovii_subsp. londiniensis |
| 22 | L. ivanovii | G | L. ivanovii_subsp. ivanovii |
Accession No
DNA sequences of genes encoding ribosomal proteins of L. monocytogenes in Fig 1, all of which were determined in this study for the first time, were registered in the DNA Data Bank of Japan (Mishima, Japan) as accession numbers LC104817 to LC104928. The sequences of Listeria spp. in Fig 2 were registered as accession numbers LC104929 to LC104975.
Discussion
In this study, we constructed an accurate and reliable MS database to discriminate lineages of L. monocytogenes and species of Listeria in MALDI-TOF MS based on the proteotyping using a S10-GERMS method, which combines both genomics and proteomics. First, we constructed a database of ribosomal proteins encoded in the S10-spc-alpha operon for L. monocytogenes and found that the MS of 15 kinds of proteins varied with serotype (Fig 1). Among these, three potential biomarkers, L24, L6, and S9, whose MS peaks were always detected in MALDI-TOF MS analysis were selected as biomarkers for typing L. monocytogenes. In addition, five potential biomarkers, L18, L15, S11, L31 type B, and S16 with a specific MS value in L. innocua, could be used to differentiate Listeria species, including L. monocytogenes (Fig 2). CHCA is a recommended matrix reagent in fingerprinting methods; therefore, it is difficult to identify high molecular m/z proteins. However, when SA was used as a matrix reagent, the novel biomarkers with high m/z were successfully detected in this study as follows: ribosomal proteins L6 (m/z 19270.08 or 19256.01), L18 (m/z 13096.86 or 13110.89), L15 (m/z 15782.02, 15797.08, or 15668.86), S13 (m/z 13578.69 or 13552.65), S11 (m/z 13655.65 or 13674.66), acetylated S9 (m/z 14283.40, 14359.50, or 14302.45), L31 type B (m/z 9259.36, 9290.34, 9327.44, or 9271.3) and S16 (m/z 10234.94, 10252.97, 10230.88, or 10003.54). Moreover, previously reported MS peaks of m/z 11179, 11871.31, or 11199.33 by other groups [16, 19] were confirmed as solid values of peaks corresponding to the ribosomal protein L24 with m/z 11180.22, 11194.25, or 11254.35, respectively, by proteotyping for the first time. The differences in MS values between previous reports and this study come from the accuracy of the experimental procedure. To realize strain- or serotype-level microbial discrimination at a higher resolution than that of conventional fingerprinting analysis, the accuracy of MS values is important because it relies upon the MS database, which reflects even single amino acid substitutions. In fact, we often observe slight differences in MS peaks derived from the same proteins in closely related microorganisms [23, 25]. Fingerprinting analysis may still greatly be influenced by the culture and/or growing conditions of the target bacteria [30]; therefore, proteotyping data backed up by genetic sequences will be of great importance for correct identification. Our results indicate that ribosomal proteins L24, L6, and S9 are especially important to discriminate lineages of L. monocytogenes and to differentiate L. monocytogenes and L. seeligeri (Figs 1 and 2).
In contrast, the MS peaks of m/z 5590, 5594.85, 5601.21, and 6184.39, identified by previous reports as biomarkers for serotyping L. monocytogenes [16, 19], were observed in this study as m/z 5590 (lineages II and III) and m/z 5595 (lineage I), although serotype 4d strains did not exhibit the corresponding peaks (data not shown). We did not select this unknown protein as a potential biomarker because the peak intensities were insufficient in our analysis, likely due to the use of SA as the matrix.
L. grayi and L. rocourtiae were correctly identified at the species level by SARAMIS and SuperSpectra (Table 3). L. grayi is known to be evolutionarily distant from other Listeria spp. [31], and L. rocourtiae is a recently emerging species isolated from lettuce in Australia [32]. The MS values of the eight biomarkers in these two species differed greatly from those of the other Listeria spp. (Fig 2A). This result is consistent with the concept that ribosomal protein evolution is well associated with bacterial species [33]. However, SARAMIS could not identify them correctly at the species level due to their very similar MS profiles, which may be indistinguishable by conventional fingerprinting (Table 3). Even in such a case, the MS database in Fig 2 with the Strain Solution software plays a significant role in the success of discrimination.
We further analyzed environmental strains for blind test and validated the MS database (Tables 2 and 5). The hemolytic species L. monocytogenes, L. seeligeri, and L. ivanovii are genetically close [34, 35] and sometimes misidentified. In our study, one L. seeligeri strain (No. 13) was identified as L. innocua by Strain Solution. It was most likely to be L. seeligeri by 16S rRNA sequence analysis, but the profiling of physiological biochemical test was L. innocua or L. welshimeri due to the positive signal of arylamidase and d-xylose utilization (data not shown). These results supported by our discrimination result, suggesting that Strain Solution could distinguish such minor differences between very similar strains that might be misidentified or overlooked by traditional methods.
The main aim of this study was the construction of a standardized and reliable database for an important pathogen, the genus Listeria. We successfully demonstrated the capability of the constructed database and Strain Solution software to discriminate L. monocytogenes at serotype level, as well as different species of Listeria that were difficult to identify by conventional fingerprinting methods. Although we assessed this database using naturally isolated strains (Table 2), demonstration using larger scale samples still required for validation. Nevertheless, we believe that proteotyping software, Strain Solution, together with the accurate MS database constructed here can be broadly applied by any laboratory using any MALDI-TOF MS system to perform strain- or serotype-level microbial classification beyond conventional fingerprinting. Therefore, we are willing to evaluate the constructed database in collaboration with institutions possessing Listeria isolates. These investigations will open a new window to discriminate bacteria in clinical and diagnostic laboratories, and also food-related industries.
Supporting Information
Groups A to K correspond to that of Fig 2. Arabic numerals above the graph indicate the number of patterns.
(TIF)
The registration of entries refers to the manual of SuperSpectra.
(DOCX)
Acknowledgments
This work was financially supported by the Aichi Science and Technology Foundation (Japan) as a part of the “Technological Development Project for Food Safety and Security” under Knowledge Hub Aichi. We thank Junko Nakayama (Meijo University) for assistance with DNA sequencing and sample preparation.
Data Availability
All DNA sequence data are available from the DNA Data Bank of Japan (Mishima, Japan) as accession numbers LC104817 to LC104928 and LC104929 to LC104975.
Funding Statement
This work was financially supported by the Aichi Science and Technology Foundation (Japan) as a part of the “Technological Development Project for Food Safety and Security” under Knowledge Hub Aichi (http://www.astf.or.jp/). HT received the funding. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McLauchlin J, Rees C, Genus I. Listeria Pirie 1940a 383AL In: De Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA, et al. , editors. Bergey’s Manual of Systematic Bacteriology, New York: Springer; 2009. 2nd ed, vol. 3, pp. 244–257. [Google Scholar]
- 2.den Bakker HC, Cummings CA, Ferreira V, Vatta P, Orsi RH, Degoricija L, et al. Comparative genomics of the bacterial genus Listeria: Genome evolution is characterized by limited gene acquisition and limited gene loss. BMC Genomics. 2010;11: 688 10.1186/1471-2164-11-688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.den Bakker HC, Warchocki S, Wright EM, Allred AF, Ahlstrom C, Manuel CS, et al. Listeria floridensis sp. nov., Listeria aquatica sp. nov., Listeria cornellensis sp. nov., Listeria riparia sp. nov. and Listeria grandensis sp. nov., from agricultural and natural environments. Int J Syst Evol Microbiol. 2014;64: 1882–1889. 10.1099/ijs.0.052720-0 [DOI] [PubMed] [Google Scholar]
- 4.Favaro M, Sarmati L, Sancesario G, Fontana C. First case of Listeria innocua meningitis in a patient on steroids and eternecept. JMM Case Rep. 2014;1 10.1099/jmmcr.0.003103 [DOI] [Google Scholar]
- 5.Swaminathan B, Gerner-Smidt P. The epidemiology of human listeriosis. Microbes Infect. 2007;9: 1236–1243. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen OF, Skouboe P, Dons L, Rossen L, Olsen JE. Listeria monocytogenes exists in at least three evolutionary lines: evidence from flagellin, invasive associated protein and listeriolysin O genes. Microbiology. 1995;141: 2053–2061. [DOI] [PubMed] [Google Scholar]
- 7.Ward TJ, Ducey TF, Usgaard T, Dunn KA, Bielawski JP. Multilocus genotyping assays for single nucleotide polymorphism-based subtyping of Listeria monocytogenes isolates. Appl Environ Microbiol. 2008;74: 7629–42. 10.1128/AEM.01127-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piffaretti JC, Kressebuch H, Aeschbacher M, Bille J, Bannerman E, Musser JM, et al. Genetic characterization of clones of the bacterium Listeria monocytogenes causing epidemic disease. Proc Natl Acad Sci U S A. 1989;86: 3818–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts AJ, Wiedmann M. Allelic exchange and site-directed mutagenesis probe the contribution of ActA amino-acid variability to phosphorylation and virulence-associated phenotypes among Listeria monocytogenes strains. FEMS Microbiol Lett. 2006;254: 300–307. [DOI] [PubMed] [Google Scholar]
- 10.Ottaviani F, Ottaviani M, Agosti M. Differential agar medium for Listeria monocytogenes. In: Quimper-Froid Symposium Proceedings; 1997. p.6 ADRIA Quimper.
- 11.Seeliger HPR, Höhne K. Serotyping of Listeria monocytogenes and related species. Methods Microbiol. 1979;13: 31–49. [Google Scholar]
- 12.Graves LM, Swaminathan B. PulseNet standardized protocol for subtyping Listeria monocytogenes by macrorestriction and pulsed-field gel electrophoresis. Int J Food Microbiol. 2001;65: 55–62. [DOI] [PubMed] [Google Scholar]
- 13.Salcedo C, Arreaza L, Alcalá B, de la Fuente L, Vázquez JA. Development of a multilocus sequence typing method for analysis of Listeria monocytogenes clones. J Clin Microbiol. 2003;41: 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ragon M, Wirth T, Hollandt F, Lavenir R, Lecuit M, Le Monnier A, et al. A new perspective on Listeria monocytogenes evolution. PLoS Pathog. 2008;4: e1000146 10.1371/journal.ppat.1000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borucki MK, Krug MJ, Muraoka WT, Call DR. Discrimination among Listeria monocytogenes isolates using a mixed genome DNA microarray. Vet Microbiol. 2003;92: 351–362. [DOI] [PubMed] [Google Scholar]
- 16.Barbuddhe SB, Maier T, Schwarz G, Kostrzewa M, Hof H, Domann E, et al. Rapid identification and typing of Listeria species by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl Environ Microbiol. 2008;74: 5402–5407. 10.1128/AEM.02689-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farfour E, Leto J, Barritault M, Barberis C, Meyer J, Dauphin B, et al. Evaluation of the Andromas matrix-assisted laser desorption ionization-time of flight mass spectrometry system for identification of aerobically growing Gram-positive bacilli. J Clin Microbiol. 2012;50: 2702–2807. 10.1128/JCM.00368-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jadhav S, Gulati V, Fox EM, Karpe A, Beale DJ, Sevior D, et al. Rapid identification and source-tracking of Listeria monocytogenes using MALDI-TOF mass spectrometry. Int J Food Microbiol. 2015;202: 1–9. 10.1016/j.ijfoodmicro.2015.01.023 [DOI] [PubMed] [Google Scholar]
- 19.Hsueh PR, Lee TF, Du SH, Teng SH, Liao CH, Sheng WH, et al. Bruker biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometry system for identification of Nocardia, Rhodococcus, Kocuria, Gordonia, Tsukamurella, and Listeria species. J Clin Microbiol. 2014;52: 2371–2379. 10.1128/JCM.00456-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotta Y, Teramoto K, Sato H, Yoshikawa H, Hosoda A, Tamura H. Classification of genus Pseudomonas by MALDI-TOF MS based on ribosomal protein coding in S10-spc-alpha operon at strain level. J Proteome Res. 2010;9: 6722–6728. 10.1021/pr100868d [DOI] [PubMed] [Google Scholar]
- 21.Hotta Y, Sato J, Sato H, Hosoda A, Tamura H. Classification of genus Bacillus based on MALDI-TOF MS analysis of ribosomal protein coded in S10 and spc operons. J Agric Food Chem. 2011;59: 5222–5230. 10.1021/jf2004095 [DOI] [PubMed] [Google Scholar]
- 22.Sato H, Torimura M, Kitahara M, Ohkuma M, Hotta Y, Tamura H. Characterization of the Lactobacillus casei group based on the profiling of ribosomal proteins coded in S10-spc-alpha operons as observed by MALDI-TOF MS. System Appl Microbiol. 2012;35: 447–454. [DOI] [PubMed] [Google Scholar]
- 23.Tamura H, Hotta Y, Sato H. Novel accurate bacterial discrimination by MALDI-time-of-flight MS based on ribosomal proteins coding in S10-spc-alpha operon at strain level S10-GERMS. J Am Soc Mass Spectrom. 2013;24: 1185–1193. 10.1007/s13361-013-0627-8 [DOI] [PubMed] [Google Scholar]
- 24.Ojima-Kato T, Yamamoto N, Suzuki M, Fukunaga T, Tamura H. Discrimination of Escherichia coli O157, O26 and O111 from other serotypes by MALDI-TOF MS based on the S10-GERMS method. PLoS One. 2014;9: e113458 10.1371/journal.pone.0113458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ojima-Kato T, Yamamoto N, Iijima Y, Tamura H. Assessing the performance of novel software Strain Solution on automated discrimination of Escherichia coli serotypes and their mixtures using matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Microbiol Methods. 2015;119: 233–238. 10.1016/j.mimet.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 26.Doumith M, Buchrieser C, Glaser P, Jacquet C, Martin P. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J Clin Microbiol. 2004;42: 3819–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miya S, Takahashi H, Nakagawa M, Kuda T, Igimi S, Kimura B. Genetic Characteristics of Japanese Clinical Listeria monocytogenes Isolates. PLoS One. 2015;10: e0122902 10.1371/journal.pone.0122902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKellar RC. Use of the CAMP test for identification of Listeria monocytogenes. Appl Environ Microbiol. 1994;60: 4219–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volokhov D, Rasooly A, Chumakov K, Chizhikov V. Identification of Listeria species by microarray-based assay. J Clin Microbiol. 2002;40: 4720–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wieme AD, Spitaels F, Aerts M, De Bruyne K, Van Landschoot A, Vandamme P. Effects of growth medium on matrix-assisted laser desorption–ionization time of flight mass spectra: a case study of acetic acid bacteria. Appl Environ Microbiol. 2014;80: 1528–1538. 10.1128/AEM.03708-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stuart S, Welshimer H. Taxonomic reexamination of Listeria Pirie and transfer of Listeria grayi and Listeria murrayi to a new genus, Murraya. Int J Syst Evol Microbiol. 1974;24: 177–185. [Google Scholar]
- 32.Leclercq A, Clermont D, Bizet C, Grimont PA, Le Flèche-Matéos A, Roche SM, et al. Listeria rocourtiae sp. nov. Int J Syst Evol Microbiol. 2010;60: 2210–2214. 10.1099/ijs.0.017376-0 [DOI] [PubMed] [Google Scholar]
- 33.Coenye T, Vandamme P. Organisation of the S10, spc and alpha ribosomal protein gene clusters in prokaryotic genomes. FEMS Microbiol Lett. 2005;242: 117–126. 10.1016/j.femsle.2004.10.050 [DOI] [PubMed] [Google Scholar]
- 34.Liu D, Lawrence ML, Ainsworth AJ, Austin FW. Species-specific PCR determination of Listeria seeligeri. Res Microbiol. 2004;155: 741–746. [DOI] [PubMed] [Google Scholar]
- 35.Graves LM, Helsel LO, Steigerwalt AG, Morey RE, Daneshvar MI, Roof SE, Orsi RH, Fortes ED, Milillo SR, den Bakker HC, Wiedmann M, Swaminathan B, Sauders BD et al. Listeria marthii sp. nov., isolated from the natural environment, Finger Lakes National Forest. Int J Syst Evol Microbiol. 2010;60: 1280–1288. 10.1099/ijs.0.014118-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Groups A to K correspond to that of Fig 2. Arabic numerals above the graph indicate the number of patterns.
(TIF)
The registration of entries refers to the manual of SuperSpectra.
(DOCX)
Data Availability Statement
All DNA sequence data are available from the DNA Data Bank of Japan (Mishima, Japan) as accession numbers LC104817 to LC104928 and LC104929 to LC104975.