Abstract
Background
Opioids have become the mainstay for treatment of moderate to severe pain and are commonly used to treat surgical pain. While opioid administration has been shown to cause opioid-induced hyperalgesia and tolerance, interactions between opioid administration and surgery with respect to these problematic adaptations have scarcely been addressed. Accumulating evidence suggests opioids and nociceptive signaling may converge on epigenetic mechanisms in spinal cord to enhance or prolong neuroplastic changes. Epigenetic regulation of Bdnf (brain-derived neurotrophic factor) and Pdyn (prodynorphin) genes may be involved.
Results
Four days of ascending doses of morphine treatment caused opioid-induced hyperalgesia and reduced opioid analgesic efficacy in mice. Both opioid-induced hyperalgesia and the reduced opioid analgesic efficacy were enhanced in mice that received hindpaw incisions. The expression of Bdnf and Pdyn (qPCR) was increased after morphine treatment and incision. Chromatin immunoprecipitation assays demonstrated that the Pdyn and Bdnf promoters were more strongly associated with acetylated H3K9 after morphine plus incision than in the morphine or incision alone groups. Selective tropomyosin-related kinase B (ANA-12) and κ-opioid receptor (nor-binaltorphimine) antagonists were administered intrathecally, both reduced hyperalgesia one or three days after surgery. Administration of ANA-12 or nor-binaltorphimine attenuated the decreased morphine analgesic efficacy on day 1, but only nor-binaltorphimine was effective on day 3 after incision in opioid-exposed group. Coadministration of histone acetyltransferase inhibitor anacardic acid daily with morphine blocked the development of opioid-induced hyperalgesia and attenuated incision-enhanced hyperalgesia in morphine-treated mice. Anacardic acid had similar effects on analgesic tolerance, showing the involvement of histone acetylation in the interactions detected.
Conclusions
Spinal epigenetic changes involving Bdnf and Pdyn may contribute to the enhanced postoperative nociceptive sensitization and analgesic tolerance observed after continuous opioid exposure. Treatments blocking the epigenetically mediated up-regulation of these genes or administration of TrkB or κ-opioid receptor antagonists may improve the clinical utility of opioids, particularly after surgery.
Keywords: Epigenetics, incision, opioid-induced hyperalgesia, histone acetylation, BDNF and dynorphin
Background
The use of opioids for the treatment of acute and chronic pain has expanded greatly over the past two decades world wide. Although these medications have tremendous utility as analgesics, particularly for the control of acute pain, maladaptations to chronic use such as analgesic tolerance and opioid-induced hyperalgesia (OIH) limit the utility of opioids and may predispose patients to worsened chronic pain over time or the persistence of pain after trauma including surgeries.1,2 It is remarkable in this regard that the effects of nociceptive signaling on treatment-limiting opioid maladaptations have received little attention. Many spinal signaling molecules including neuropeptides,3 monoxides,4,5 excitatory amino acids,6 neurotrophins,7,8 and endogenous opioids8,9 have been shown to modulate both nociceptive signaling and opioid maladaptations.
The existing literature addressing interactions between nociceptive signaling and opioid maladaptations is both incomplete and inconsistent. Early studies by Kayser and Guilbaud10 demonstrated that tolerance to systemic morphine administration was more complete in arthritic rats that received repeated doses of morphine than pain-free animals undergoing the same treatment. Studies by Colpaert11 using a different opioid and complete Freund’s adjuvant (CFA)-treated rats arrived at the opposite conclusion. Studies in which acute nociception was achieved using formalin have shown either that tolerance to acute morphine administration is reduced or that there is little effect.12,13 Our own studies in which CFA was administered to 12 strains of inbred mice showed that opioid tolerance was enhanced in 9 of the 12 strains tested, though no specific mechanism was identified for the apparent genetic effects.14 On the other hand, it is clearer that opioid pretreatment whether relatively acute15 or more chronic3 causes OIH and exacerbates wound area allodynia after surgical incision. These data parallel clinical observations that patients receiving chronic opioid therapy preoperatively tend to experience higher levels of pain, require higher doses of opioids for pain control, and achieve poorer long-term functional outcomes.16–19 Again, however, a clear mechanism explaining the apparent interaction of opioid exposure and nociceptive signaling has not emerged.
Epigenetic changes are ones involving alterations in the expression of genes that do not rely upon changes in DNA sequence. There is growing consensus that these processes modulate pain severity and duration after injury.20–22 The acetylation of the lysine tails of histone proteins in spinal tissue is an epigenetic mechanism that has been demonstrated to control both nociceptive sensitization after surgical incision and the acquisition of opioid maladaptations such as tolerance and OIH.8,23–25 These observations include the demonstration that agents that block histone acetyltransferase (HAT) reduce post-incision allodynia and hyperalgesic priming, a measure reflecting the propensity to develop chronic pain. Agents that block the deacetylation of histone proteins have opposite effects. Likewise, the blockade of deacetylation exacerbates and prolongs morphine tolerance and OIH in mice. The enhanced expression of brain-derived neurotrophic factor (Bdnf) and prodynorphin (Pdyn) by virtue of the acetylation of histone H3K9 residues was demonstrated in the latter studies. Interestingly, both Bdnf and dynorphin proteins have been shown to support allodynia after hindpaw incision.7,15 Thus, we undertook a series of experiments testing the hypothesis that prior chronic opioid exposure followed by incision converge on the acetylation of H3K9 near the Bdnf and Pdyn promoters to enhance the expression of these genes and exacerbate tolerance, OIH, and mechanical allodynia.
Methods
Animal subjects
Male C57BL/6 J mice (Jackson Laboratory, Bar Harbor, ME) at seven to eight weeks of age were used. Experiments were done after a 7- to 10-day acclimation period subsequent to arrival at animal care facility. Mice were housed four per cage under pathogen-free conditions and were provided food and water ad libitum with a 12:12 light:dark cycle. All animal experimental protocols were approved by the Veterans Affairs Palo Alto Health Care System Institutional Animal Care and Use Committee (Palo Alto, CA) and complied with the Guide for the Care and Use of Laboratory Animals.
Chronic morphine administration
After baseline nociceptive testing, morphine (Sigma Chemical, St. Louis, MO) or 0.9% saline vehicle was subcutaneously administered twice daily to mice on an escalating schedule starting from 10 mg/kg on day 1, 20 mg/kg on day 2, 30 mg/kg day 3, and 40 mg/kg on day 4. Morphine was dissolved in 50–100 ul (micro liter) volumes of 0.9% NaCl as previously described.23
Hindpaw incision
The hindpaw incision model in mice was performed in our laboratory as described previously.3,24 Briefly, mice were anesthetized using isoflurane 2%–3% delivered through a nose cone. After sterile preparation, a 5-mm longitudinal incision was made with a number 11 scalpel on the plantar surface of the right hindpaw. The incision was sufficiently deep to divide deep tissue including the plantaris muscle longitudinally. After controlling bleeding, a single 6-0 nylon suture was used to close the wound and antibiotic ointment was applied.
Behavioral measurement
Mechanical allodynia was assessed using nylon von Frey filaments according to the “up-down” algorithm described by Chaplan et al.26 as previously described.3 Mice were placed on mesh platforms within transparent plastic cylinders and, after acclimation, nylon fibers of sequentially increasing stiffness were applied to the plantar surface of the hindpaw which were left in place for 5 s. Withdrawal of the hindpaw from the fiber was scored as a response. If no response was observed, the next stiffer fiber was applied to the same paw; if a response was observed, a less stiff fiber was applied. Testing continued until four fibers had been applied after the first withdrawal response allowing the estimation of the mechanical withdrawal threshold. Data fitting algorithm allowed the use of parametric statistics for analysis.27
Analgesic efficacy to morphine administration was measured according to previously published methods8 using cumulative morphine dose-response curves or single dose-response measurements. Mice were gently restrained within a cone-shaped cotton tube, and their tail flick latency was measured with 0.1 s precision using a tail flick analgesic apparatus (Columbus Instruments, Columbus, OH). A 10-s cut off time was used to prevent tissue damage or sensitization. The light beam focused on two different points, 1 cm apart, on the tail and the lamp intensity was identical for all animals with baseline tail flick measurements of 3–4 s. Two measurements were made per mouse. For the assessment of tolerance, the cumulative doses of morphine used were 1, 3, and 10 mg/kg. Tail flick latency was determined 25 min after morphine injection. The percent maximal possible effect (MPE) was determined according to the following formula: %MPE = 100 × (measured latency − baseline latency)/(cut off latency − baseline latency).
ANA-12 and nor-binaltorphimine administration
ANA-12, a BDNF receptor (tropomyosin-related kinase B (TrkB)) antagonist, and nor-binaltorphimine (nor-BNI), a selective κ-opioid receptor (KOR) antagonist, were purchased from Sigma Chemical (St. Louis, MO). ANA-12 and nor-BNI were dissolved in 0.9% saline. ANA-12 (100 nmol) or nor-BNI (5 nmol) administered to animals by intrathecal route once (5 µl) and testing done in 30–60 min. The selected drug doses and timing of administration were in accordance with prior publications.28–30
Anacardic acid administration
Anacardic acid was purchased from Sigma Chemical (St. Louis, MO) which was dissolved in Dimethyl sulfoxide (DMSO) and diluted with 0.9% saline (Final DMSO concentration 10%) and given by intra-peritoneal (i.p.) injection (50 mg/kg or vehicle, 100 µl volume). The drug was given once daily concurrently with morning morphine (or saline) treatment during the chronic dosing paradigm and for three days after incision.
Quantification of mRNA
Mice were sacrificed and lumbar spinal cord lumbar segments were quickly dissected in prechilled surface. Tissue was flash frozen in liquid nitrogen and stored at −80℃ until use. The isolation of RNA and subsequent qPCR were carried out as previously described.23 Briefly, after total RNA extraction, RNA concentration was determined by spectrophotometric analysis, and complementary DNA was synthesized from total RNA using random hexamer priming and a first strand synthesis system (Invitrogen, Carlsbad, CA). Expression of genes of interest was determined by quantitative real-time PCR using ABI prism 7900HT system (Applied Biosystems). Β-actin was used as an internal control and the expression level of specific genes was analyzed with ΔΔCt method. Bdnf primer sequences were CCATAAGGACGCGGACTTGTAC (Forward) and AGACATGTTTGCGGCATCCAGG (Reverse). Pdyn primer sequences were CCATCCCAGAATCCAGAGAA (Forward) and CCAGGGTAGGGTGCATAAGA (Reverse). Β-actin primer sequences were AGCCATGTACGTAGCCATCC (Forward) and CTCTCAGCTGTGGTGGTGAA (Reverse).
Chromatin immunoprecipitation assay
Spinal cord tissue was collected and described above. Chromatin immunoprecipitation (ChIP) assay was performed following the protocol adopted and refined previously by our lab.23 Briefly, spinal cord tissue was cross-linked using 1% formaldehyde and sonicated on ice. After clarification by centrifuge, it was aliquoted and snap-frozen in liquid nitrogen. ChIP was performed using chromatin (150 μl) which was diluted 10-fold and purified with specific antibody against acetylated histone (aceH3K9, Upstate Biotechnology, Waltham, MA) or IgG as negative control. Sonicated chromatin 1% was used for input control. The DNA that was released from the bound chromatin after cross-linking reversal and proteinase K treatment was precipitated and diluted in low-TE buffer (1 mM Tris, 0.1 mM EDTA). Quantitative PCR of target gene promoter enrichment in ChIP samples was done by Applied Biosystems (ABI) prism 7900HT system using SYBR Green. Five microliters of input ChIP or IgG sample were used in each reaction in duplicate for three biological samples in each condition. Fold enrichment was calculated as a ratio of the ChIP to mock IgG. An in-plate standard curve determined amplification efficiency, and the 100-fold dilution factor for the input was included. Samples were then normalized to the saline condition. Bdnf PI primer sequences targeted were TGATCATCACTCACGACCACG (Forward) and CAGCCTCTCTGAGCCAGTTACG (Reverse). Bdnf PII primer sequences targeted were TGAGGATAGTGGTGGAGTTG (Forward) and TAACCTTTTCCTCCTCC (Reverse). Bdnf PIV primer sequences targeted were GCGCGGAATTCTGATTCTGGTAAT (Forward) and GAGAGGGCTCCACGCTGCCTTGACG (Reverse). Pdyn primer sequences targeted were CGCTTCCTCTGTGGCACTTC (Forward) and TTGTCCCTGGCAGGCTTCTG (Reverse).
Enzyme immunoassay for BDNF and dynorphin protein levels in spinal cord
Mice were euthanized by carbon dioxide asphyxiation, and spinal cord tissue was harvested by extrusion rapidly. Lumbar spinal cord segments were dissected on a chilled surface, then quick-frozen in liquid nitrogen and stored at −80℃ until required. The lumbar spinal cord segments were homogenized in ice-cold 0.9% saline containing a cocktail of protease inhibitors (Roche Applied Science), centrifuged at 12,000 g for 10 min at 4℃ and then supernatant fractions were frozen at −80℃. An aliquot of the supernatant fraction was subjected to protein assay (Bio-Rad) to normalize protein levels. BDNF concentrations were measured in duplicate by using mouse BDNF ELISA kit (Gen Way Biotech), and the dynorphin levels were assayed in duplicate by using Dynorphin EIA kit (Phoenix Pharmaceuticals) according to the manufacturer’s instructions.
Statistical analysis
All data are expressed as the means ± SEM unless otherwise noted. The data for mechanical sensitivity were analyzed by multiple t tests using the Holm-Sidak method to correct for multiple comparisons. Tolerance data were analyzed by one-way analysis of variance (ANOVA) followed by Sidak post hoc test for multiple comparisons for each dose. The data for qPCR, ChIP, and ELISA experiments were analyzed by one-way ANOVA followed by Fisher Least Significance Difference (LSD) post hoc test for multiple comparisons. For experiments using drug treatments, data were analyzed by one-way ANOVA followed by Sidak post hoc test for multiple comparisons for each timepoint.
Results
Effects of morphine treatment on mechanical hypersensitivity and opioid analgesic efficacy after incision
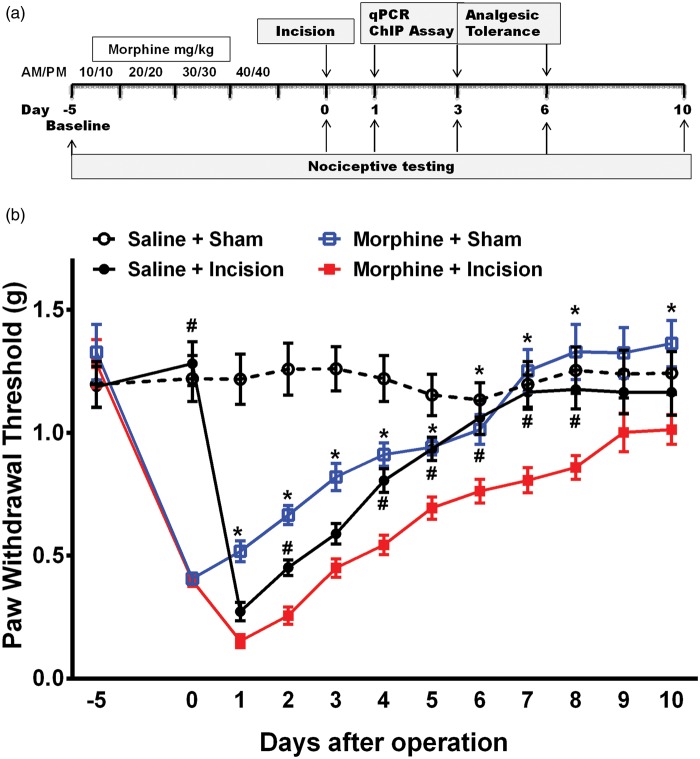
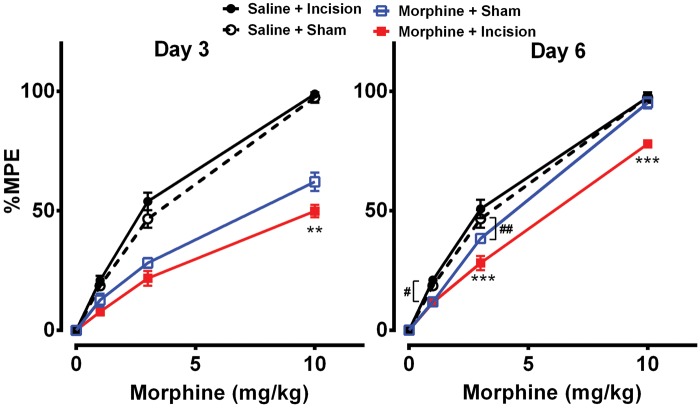
Figure 1(a) shows the experimental timeline and the daily dosing schedule of morphine treatment. Morphine treatment or incision alone resulted in increased mechanical hypersensitivity which resolved by day 7 in both groups (Figure 1(b)). The incision alone group had significantly lower mechanical thresholds than the morphine group on day 1 (p < 0.001), day 2 (p < 0.001), and day 3 (p < 0.001). Mice that had the incision after morphine treatment had significantly lower mechanical thresholds than groups which received either morphine or incision alone from day 2 up to day 8 after operation. Additionally, analgesic efficacy of morphine was reduced on days 3 and 6 after surgery in the morphine plus incision group compared to the other groups (Figure 2).
Figure 1.
Effects of morphine treatment on mechanical hypersensitivity after incision. (a) Schematic representation of experimental timeline showing the daily dosing schedule of morphine treatment and procedures carried out. (b) Morphine treatment or incision alone resulted in increased mechanical hypersensitivity. Mice that had the incision after morphine treatment had significantly lower mechanical thresholds than groups which received either morphine or incision alone. Data were analyzed by multiple t tests using the Holm-Sidak method to correct for multiple comparisons. * indicates significant difference in comparison with morphine, and # indicates significant difference in comparison with incision. Error bars: SEM, n = 8/group.
Figure 2.
Effects of morphine treatment on opioid analgesic efficacy after incision. Mice that had the incision after morphine treatment displayed decreased analgesic efficacy to morphine on days 3 and 6 after surgery compared to morphine treatment alone. Data were analyzed by one-way ANOVA followed by Sidak post hoc test for multiple comparisons for each dose. **p < 0.01, ***p < 0.001 for comparison with morphine and ##p < 0.01 for comparison with controls. Error bars: SEM, n = 6/group.
Epigenetic effects of morphine treatment, incision, and the combination on spinal cord gene expression
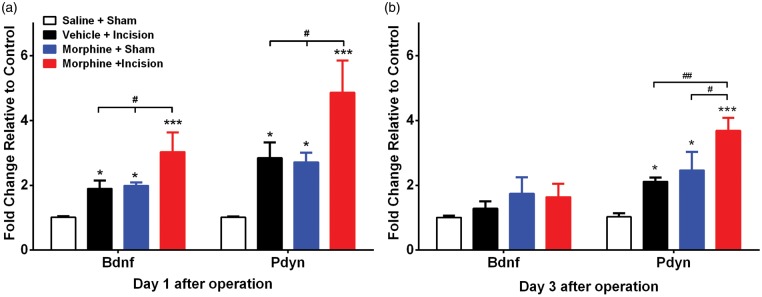
Expression patterns of spinal Bdnf and Pdyn on days 1 and 3 after incision are shown in Figure 3(a) and (b), respectively. Expression of Bdnf was higher in spinal cord tissue of morphine-treated plus incision animals one day after surgery though the trends towards increases were not significant in either the morphine or incision groups alone. At three days after surgery, no changes in Bdnf were identified. The expression changes for Pdyn were more robust with increased levels in spinal tissue observed at both one and three days after incision for all groups and greater enhancement of expression in the morphine plus incision group at both time points.
Figure 3.
Effects of morphine treatment, incision and the combination on spinal cord gene expression. Expression patterns of spinal Bdnf and Pdyn on (a) day 1 and (b) day 3 after incision. Expression of Bdnf was higher in morphine-treated plus incision animals one day after surgery. Increased expression changes for Pdyn were observed at both one and three days after incision for all groups, and greater enhancement of expression in the morphine plus incision group at both time points. Error bars: SEM, n = 5/group; *p < 0.05, ***p < 0.001 for comparison with controls and #p < 0.05 for within groups comparisons. Data were analyzed by two-way analysis of variance (ANOVA) followed by Fisher post hoc test for multiple comparisons within each time point.
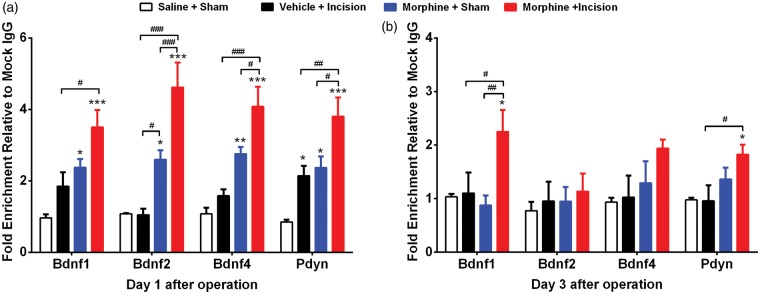
ChIP assays demonstrated that promoter regions of Bdnf and Pdyn were more strongly associated with aceH3K9 on day 1 after incision in morphine-treated group compared with morphine or incision alone groups (Figure 4(a) and (b)). This was true for each of the Bdnf promoters tested. The trends toward elevated aceH3K9 associations were less prominent for Bdnf gene promoters three days after incision, except for the Bdnf1 promoter in the morphine and incision group compared to others. Similarly, elevated aceH3K9 association with the promoter of Pdyn was only significant for the morphine and incision group.
Figure 4.
Epigenetic effects of morphine treatment, incision and the combination on spinal Bdnf and Pdyn expression. Chromatin immunoprecipitation (ChIP) assays done on lumbar spinal tissue (a) day 1 and (b) day 3 after incision. ChIP assays showed that promoter regions of Bdnf and Pdyn were more strongly associated with aceH3K9 on day 1 after incision in morphine-treated group compared with morphine or incision alone groups. Error bars: SEM, n = 5–6/group; *p < 0.05, ***p < 0.001 for comparison with controls and #p < 0.05, ##p < 0.01 for within groups comparisons. Data were analyzed by two-way analysis of variance (ANOVA) followed by Fisher post hoc test for multiple comparisons within each time point.
Effects of chronic morphine treatment and incision on spinal cord BDNF and dynorphin protein levels
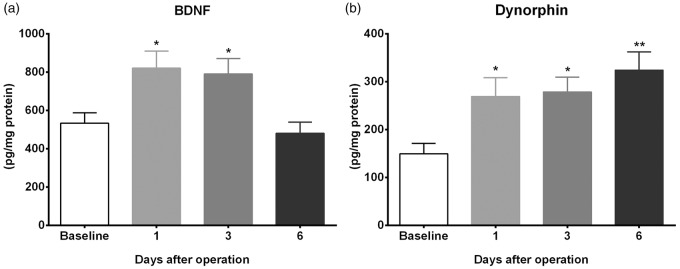
Since greater enhancement of Bdnf and Pdyn expressions was observed in the morphine plus incision group at the time points measured, we went on to determine the time course of protein expression for these genes in the morphine plus incision group. BDNF protein levels are elevated on days 1 and 3 compared to controls but return to baseline values by day 6 after incision (Figure 5(a)). Dynorphin levels follow the same pattern of elevation for days 1 and 3 but remain persistently elevated on day 6 after incision (Figure 5(b)).
Figure 5.
Effects of chronic morphine treatment and incision on spinal cord BDNF and dynorphin protein levels. Enzyme immunoassay (ELISA) for BDNF and dynorphin protein levels in lumbar spinal cord segments were done to determine the time course of the elevated proteins after incision in mice previously exposed to morphine. (a) BDNF levels were elevated on days 1 and 3 but return to baseline values by day 6 after incision. (b) Dynorphin levels remain persistently elevated up to day 6 after incision. Error bars: SEM, n = 6/group, *p < 0.05 and **p < 0.01 for comparison with baseline. Data were analyzed by one-way ANOVA followed by Fisher post hoc test multiple comparisons tests.
Effects of selective inhibition of BDNF and dynorphin signaling
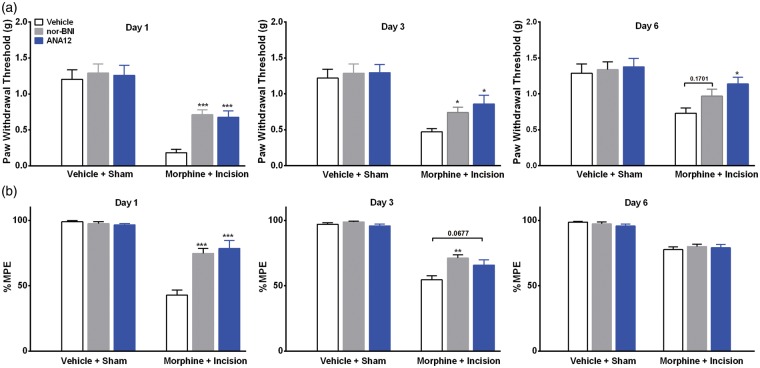
Next, to determine the functional links between Bdnf and Pdyn overexpression and enhanced opioid-induced maladaptations, we used selective antagonists of the TrkB and KORs, ANA-12 and nor-BNI, respectively. TrkB is the primary Central Nervous System (CNS) receptor for BDNF, and dynorphin selectively binds the KOR. Figure 6(a) shows that selective antagonism of the spinal TrkB or the KOR reduced hyperalgesia given on days 1 (p < 0.001) or 3 (p < 0.05) after surgery compared to vehicle treatment. The administration of ANA-12 (p < 0.001) or nor-BNI (p < 0.001) improved the observed reduction of morphine analgesic efficacy on day 1, but only nor-BNI (p < 0.01) was effective on day 3 after surgery in opioid-exposed group (Figure 6(b)).
Figure 6.
Effects of selective inhibition of spinal BDNF and dynorphin signaling. (a) Effects of selective antagonists of the tropomyosin-receptor-kinase (TrkB) and κ-opioid receptors, ANA-12 and nor-BNI, respectively. Both drugs reduced hyperalgesia given on day 1 or 3 after surgery compared to vehicle treatment. (b) The administration of ANA-12 or nor-BNI attenuated the reduced morphine analgesic efficacy on day 1, but only nor-BNI was effective on day 3 after surgery in opioid-exposed group. Error bars: SEM, n = 6/group, *p < 0.05, **p < 0.01 and ***p < 0.001 for comparison between treatments for each time point. Data for each time point were analyzed by one-way ANOVA followed by Sidak multiple comparisons tests.
Effects of HAT inhibition on enhanced hyperalgesia and tolerance after incision following morphine treatment
We went on to determine the effects of HAT inhibition during morphine treatment on increased hyperalgesia and tolerance after incision. Coadministration of anacardic acid daily with morphine not only prevented OIH from occurring (day 0) but also attenuated enhanced hyperalgesia seen on days 1, 3, and 6 (Figure 7(a)). Moreover, the reduced analgesic efficacy of morphine on day 3 after incision was reversed by HAT inhibitor therapy (Figure 7(b)). Additionally, coadministration of anacardic acid daily along with morphine had no effect on morphine analgesic efficacy on day 3 (Figure 6(b)) or paw withdrawal thresholds on days 1, 3, and 6 (data not shown) in controls. Previously, it was shown that anacardic acid administration for several days does not alter baseline or daily nociceptive thresholds in sham groups.25
Figure 7.
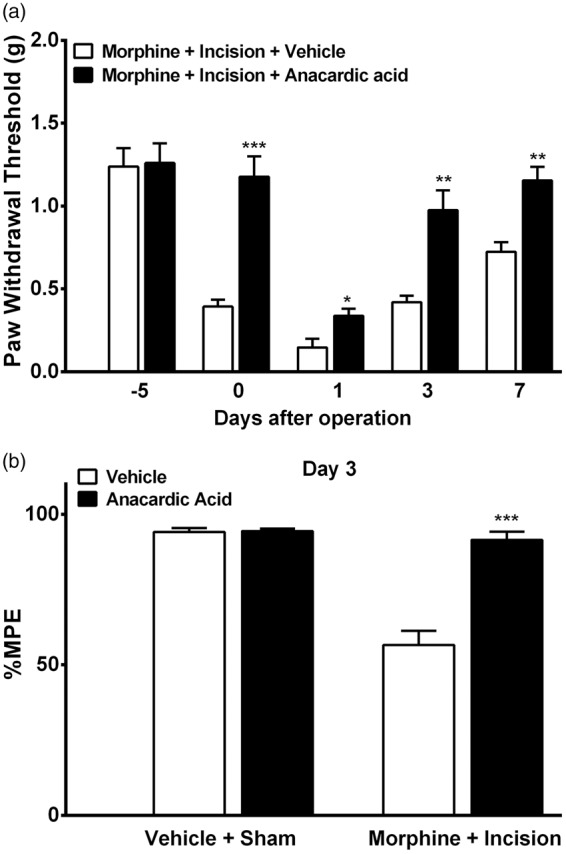
Effects of HAT inhibition on enhanced hyperalgesia and tolerance after incision following morphine treatment. (a) Coadministration of anacardic acid daily with morphine prevented opioid-induced hyperalgesia from occurring. Anancardic acid also attenuated enhanced hyperalgesia seen on days 1, 3, and 6 after incision. (b) Coadministration of anacardic acid daily with morphine attenuated the reduced opioid analgesic efficacy on day 3 after incision, having no effect on morphine analgesic efficacy in the control group. Error bars: SEM, n = 6/group, ns: p > 0.05, *p < 0.05, **p < 0.01, and ***p < 0.00. Data were analyzed by one-way ANOVA followed by Sidak multiple comparisons tests.
Discussion
Opioids have become a mainstay of treatment for many forms of acute and chronic pain. Unfortunately, the chronic use of opioids is problematic in many ways. Important clinical consequences of preoperative or preinjury opioid use include heightened postoperative pain, increased postoperative opioid requirements, an increased likelihood of chronic pain, and poor functional outcome after surgery.17–19,31 In the present series of studies, we evaluated the consequences of surgical incision in mice exposed to chronic opioid administration using a previously established model combining opioid exposure and surgical incision.3,32 The principal findings included the following: (1) chronic opioid exposure followed by incision not only exacerbates OIH but also produces a state of reduced opioid analgesic efficacy after cessation of exposure; (2) opioid exposure and incision interact through spinal epigenetic mechanisms to enhance the expression of two well-established pain related genes, Bdnf and Pdyn; (3) both spinal Bdnf and dynorphin proteins support the observed reduction of opioid analgesic efficacy, as well as, OIH in chronically morphine-treated mice after incision. These experiments are the first to provide a specific epigenetically mediated mechanism for the interaction of opioids and nociceptive signaling. Furthermore, both the BDNF-TrkB and dynorphin-KOR systems may be accessible therapeutic targets.
There is growing interest in epigenetic mechanisms of gene regulation as processes that regulate long-term adaptations to drugs and as processes that regulate the persistence of pain after injuries of various sorts. A wide array of epigenetic processes are being considered with most work currently focused on the methylation of CpG islands in the promoter regions of genes, the covalent modification of histone proteins such as by acetylation, and the synthesis of noncoding RNA species such as micro RNA.20–22 Complementary to our findings, it has been demonstrated acetylation of histone proteins including H3K9 and H4K12 are key epigenetic mechanisms controlling the expression of memory and addiction-related genes in the striatum, nucleus accumbens, and hippocampus.33–35 Importantly, these studies showed that the pattern of histone acetylation is dependent on the specific tissue under study. Additional observations led us to hypothesize that histone acetylation might be involved in the interaction of opioid exposure and nociceptive sensitization after incision. For example, we previously reported that chronic treatment with morphine augmented acetylated H3K9 levels in spinal cord tissue in mice.8 In the same group of studies, it was shown that blocking histone acetylation largely blocked morphine tolerance and OIH. In other experiments, it was observed that hindpaw incision caused the acetylation of H3K9 in spinal tissue, and that histone acetylation controlled the duration of nociceptive sensitization as well as long-term changes in pain signaling such as those involving hyperalgesic priming.25 Thus, anti-nociceptive (opioid) and pro-nociceptive (incision-related) signaling systems appear to converge on a common group of spinal epigenetic mechanisms to affect long-term changes. This would be consistent with the recognized roles of epigenetic systems in causing long lasting changes in tissues ranging from functional differentiation to learning-related neuroplasticity.36
Bdnf is a likely gene for participation in opioid adaptation–nociception interactions for several reasons. This gene was found to be elevated selectively in spinal cord neurons after four days of morphine treatment in mice.23 Furthermore, the systemic administration of the TrkB antagonist ANA-12 was able to prevent OIH if administered along with morphine, or if given as bolus after the four days of morphine treatment. In the same group of studies, it was established that the intrathecal injection of a small amount of BDNF (1 ng) produced transient mechanical allodynia. It should be noted that others have also associated spinal BDNF with OIH, although in these studies, the investigators suggested that spinal microglia are the likely source of the relevant pool of Bdnf.37 These investigators proposed that microglial-derived Bdnf altered chloride ion homeostasis in lamina I neurons leading to OIH (but not tolerance during ongoing morphine treatment). Likewise, the enhanced expression of Bdnf has been noted in spinal cord neurons after hindpaw incision and the intrathecal injection of anti-Bdnf antibodies reduced mechanical allodynia.7 Spinal Bdnf has been shown to contribute to nociceptive sensitization in other models of pain including models of inflammatory and neuropathic pain.38,39 Our findings suggest that morphine treatment and incision together up-regulate Bdnf expression. The Bdnf gene is believed to be under the transcriptional control of several promoters in mice,40 and here, we provide evidence of at least additive interaction between morphine treatment and incision in enhancing acetylated H3K9 association with promoters 1, 2, and 4. Critically, our studies using the intrathecal administration of ANA-12 demonstrate that spinal Bdnf supports both OIH and tolerance (reduced analgesic efficacy) in incised mice with prior morphine exposure.
Our investigations simultaneously pursued the Pdyn gene and spinal dynorphin protein as an additional signaling system supporting both OIH and tolerance in the setting of incision after prior morphine exposure. As for Bdnf, the Pdyn gene is known to be regulated by epigenetic alterations of histone acetylation within the CNS,41 and we have previously demonstrated the up-regulation of Pdyn in spinal cord tissue after chronic morphine administration and enhancement of the upregulation if an inhibitor of histone deacetylation was coadministered with the morphine.8 Although the mature signaling molecule dynorphin may interact with other spinal receptors to support nociceptive signaling, such as spinal bradykinin receptors, it is a well-established agonist of the KOR.42 Similar to observations for Bdnf, we found that morphine treatment or incision alone increase spinal Pdyn expression, and together increase Pdyn to an even greater extent. The elevated expression lasted longer than for Bdnf, at least 72 h after incision. Similarly, persistently elevated dynorphin levels were seen when the BDNF levels had returned to baseline by day 6 after incision in opioid-exposed mice. This may too explain why the intrathecal injection of the KOR antagonist nor-BNI was effective in reversing OIH even six days after incision in the chronically morphine-treated mice. Other reports in the literature are not entirely consistent with respect to dynorphin function after surgical incision with one group reporting spinal up-regulation but no nociception-related function,43 another reporting a pronociceptive function in incised animals after repeated fentanyl administration,15 and a third report demonstrating a long-term anti-hyperalgesic function of spinal dynorphin.44 The disparity amongst the reports emphasizes the need to be specific as to the context in which the measurements are made. Our experimental structure and findings are most similar to the report of Rivat et al.15 in that animals had opioid exposure prior to incision. This may indicate that opioids prime the involved neural tissues to facilitate nociceptive signaling, whereas in the opioid naïve state the balance of the effects of dynorphin is neutral or anti-nociceptive. In this respect, it is interesting to note that continuous opioid exposure enhances descending facilitatory fibers from the Rostral ventromedial medulla (RVM) which are thought to stimulate Pdyn expression and support OIH and tolerance.15,45 We propose that the descending fibers from the RVM activated by opioid exposure and the afferent fibers activated by incision functionally or physically may converge on a common population of spinal neurons to enhance Pdyn expression.
The present study has certain limitations. One limitation is the use of a single type of opioid given according to a single dosing protocol prior to incision. Likewise, surgery is only one type of injury and in the setting of inflammation or nerve injury the types of opioid–nociception interactions that might take place may be different. Additional studies addressing these issues or looking more closely at the mechanisms responsible for activating histone acetylation after incision and opioid exposure would significantly improve our level of understanding. Moreover, alternate epigenetic mechanisms at play have not been probed in the present work. For example, we have recently shown that epigenetic modulation (DNA methylation) of Oprm1 expression is functionally relevant to surgical incision by modifying µ-opioid receptor (MOR) signaling.46 Earlier work had also identified contributions of the MOR to OIH in mice.47 The present work moves our studies to a different opioid receptor system (KOR) where our efforts have been focused.
Conclusions
Prior opioid use intensifies postoperative pain, increases not only opioid requirements but also the likelihood of development of chronic pain and poor functional outcome after surgery. Here, we show spinal epigenetic changes involving Bdnf and Pdyn may contribute to the enhanced postoperative nociceptive sensitization and reduced opioid analgesic efficacy observed after continuous opioid exposure. Our studies when combined with the findings of others suggest that spinal epigenetic mechanisms integrate nociceptive, drug and possibly other types of environmental inputs into long lasting changes in nervous system function. These mechanisms may be very important to the development of chronic pain, particularly with respect to how multiple environmental factors might determine the likelihood of developing chronic pain after injury, and what treatments might be most effective in reducing or preventing chronic pain.
Authors’ contribution
PS helped to draft the submitted manuscript, participated in the design of the study, and performed the behavior assays and statistical analysis. DYL did ChIP, gene expression studies, and analyzed the data. YS and XYS helped perform the ChIP, expression studies, ELISA, and analyzing the data. JDC conceived of the study, participated in its design and coordination, and helped draft the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JDC was supported by U.S. Department of Veterans Affairs merit review award 5I01BX000881.
References
- 1.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006; 104: 570–587. [DOI] [PubMed] [Google Scholar]
- 2.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain 2008; 24: 479–496. [DOI] [PubMed] [Google Scholar]
- 3.Sahbaie P, Shi X, Li X, et al. Preprotachykinin-A gene disruption attenuates nociceptive sensitivity after opioid administration and incision by peripheral and spinal mechanisms in mice. J Pain 2012; 13: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godai K, Hasegawa-Moriyama M, Kurimoto T, et al. Peripheral administration of morphine attenuates postincisional pain by regulating macrophage polarization through COX-2-dependent pathway. Mol Pain 2014; 10: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang DY, Clark JD. Modulation of the NO/CO-cGMP signaling cascade during chronic morphine exposure in mice. Neurosci Lett 2004; 365: 73–77. [DOI] [PubMed] [Google Scholar]
- 6.Mao J, Mayer DJ. Spinal cord neuroplasticity following repeated opioid exposure and its relation to pathological pain. Ann N Y Acad Sci 2001; 933: 175–184. [DOI] [PubMed] [Google Scholar]
- 7.Li CQ, Xu JM, Liu D, et al. Brain derived neurotrophic factor (BDNF) contributes to the pain hypersensitivity following surgical incision in the rats. Mol Pain 2008; 4: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang DY, Li X, Clark JD. Epigenetic regulation of opioid-induced hyperalgesia, dependence, and tolerance in mice. J Pain 2013; 14: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vera-Portocarrero LP, Zhang ET, King T, et al. Spinal NK-1 receptor expressing neurons mediate opioid-induced hyperalgesia and antinociceptive tolerance via activation of descending pathways. Pain 2007; 129: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kayser V, Guilbaud G. Can tolerance to morphine be induced in arthritic rats? Brain Res 1985; 334: 335–338. [DOI] [PubMed] [Google Scholar]
- 11.Colpaert FC. Can chronic pain be suppressed despite purported tolerance to narcotic analgesia? Life Sci 1979; 24: 1201–1209. [DOI] [PubMed] [Google Scholar]
- 12.Rahman AF, Takahashi M, Kaneto H. Development of tolerance to morphine antinociception in mice treated with nociceptive stimulants. Jpn J Pharmacol 1993; 63: 59–64. [DOI] [PubMed] [Google Scholar]
- 13.Vaccarino AL, Couret LC., Jr Formalin-induced pain antagonizes the development of opiate dependence in the rat. Neurosci Lett 1993; 161: 195–198. [DOI] [PubMed] [Google Scholar]
- 14.Liang DY, Guo T, Liao G, et al. Chronic pain and genetic background interact and influence opioid analgesia, tolerance, and physical dependence. Pain 2006; 121: 232–240. [DOI] [PubMed] [Google Scholar]
- 15.Rivat C, Vera-Portocarrero LP, Ibrahim MM, et al. Spinal NK-1 receptor-expressing neurons and descending pathways support fentanyl-induced pain hypersensitivity in a rat model of postoperative pain. Eur J Neurosci 2009; 29: 727–737. [DOI] [PubMed] [Google Scholar]
- 16.Lee D, Armaghani S, Archer KR, et al. Preoperative opioid use as a predictor of adverse postoperative self-reported outcomes in patients undergoing spine surgery. J Bone Joint Surg Am Vol 2014; 96: e89. [DOI] [PubMed] [Google Scholar]
- 17.Patanwala AE, Jarzyna DL, Miller MD, et al. Comparison of opioid requirements and analgesic response in opioid-tolerant versus opioid-naive patients after total knee arthroplasty. Pharmacotherapy 2008; 28: 1453–1460. [DOI] [PubMed] [Google Scholar]
- 18.Rapp SE, Ready LB, Nessly ML. Acute pain management in patients with prior opioid consumption: a case-controlled retrospective review. Pain 1995; 61: 195–201. [DOI] [PubMed] [Google Scholar]
- 19.Zywiel MG, Stroh DA, Lee SY, et al. Chronic opioid use prior to total knee arthroplasty. J Bone Joint Surg Am Vol 2011; 93: 1988–1993. [DOI] [PubMed] [Google Scholar]
- 20.Descalzi G, Ikegami D, Ushijima T, et al. Epigenetic mechanisms of chronic pain. Trends Neurosci 2015; 38: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geranton SM, Tochiki KK. Could targeting epigenetic processes relieve chronic pain states? Curr Opin Support Palliat Care 2015; 9: 138–146. [DOI] [PubMed] [Google Scholar]
- 22.Liang L, Lutz BM, Bekker A, et al. Epigenetic regulation of chronic pain. Epigenomics 2015; 7: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang DY, Sun Y, Shi XY, et al. Epigenetic regulation of spinal cord gene expression controls opioid-induced hyperalgesia. Mol Pain 2014; 10: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahbaie P, Sun Y, Liang DY, et al. Curcumin treatment attenuates pain and enhances functional recovery after incision. Anesth Analg 2014; 118: 1336–1344. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Sahbaie P, Liang DY, et al. Epigenetic regulation of spinal CXCR2 signaling in incisional hypersensitivity in mice. Anesthesiology 2013; 119: 1198–1208. [DOI] [PubMed] [Google Scholar]
- 26.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 27.Poree LR, Guo TZ, Kingery WS, et al. The analgesic potency of dexmedetomidine is enhanced after nerve injury: a possible role for peripheral alpha2-adrenoceptors. Anesth Analg 1998; 87: 941–948. [DOI] [PubMed] [Google Scholar]
- 28.Cazorla M, Premont J, Mann A, et al. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J Clin Invest 2011; 121: 1846–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W, Walwyn W, Ennes HS, et al. BDNF released during neuropathic pain potentiates NMDA receptors in primary afferent terminals. Eur J Neurosci 2014; 39: 1439–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takemori AE, Ho BY, Naeseth JS, et al. Nor-binaltorphimine, a highly selective kappa-opioid antagonist in analgesic and receptor binding assays. J Pharmacol Exp Ther 1988; 246: 255–258. [PubMed] [Google Scholar]
- 31.Rozet I, Nishio I, Robbertze R, et al. Prolonged opioid use after knee arthroscopy in military veterans. Anesth Analg 2014; 119: 454–459. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Sahbaie P, Liang D, et al. Opioids enhance CXCL1 expression and function after incision in mice. J Pain 2014; 15: 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bousiges O, Neidl R, Majchrzak M, et al. Detection of histone acetylation levels in the dorsal hippocampus reveals early tagging on specific residues of H2B and H4 histones in response to learning. PLoS One 2013; 8: e57816. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Kumar A, Choi KH, Renthal W, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 2005; 48: 303–314. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Lv Z, Hu Z, et al. Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIalpha in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology 2010; 35: 913–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guan JS, Xie H, Ding X. The role of epigenetic regulation in learning and memory. Exp Neurol 2015; 268: 30–36. [DOI] [PubMed] [Google Scholar]
- 37.Ferrini F, Trang T, Mattioli TA, et al. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(-) homeostasis. Nat Neurosci 2013; 16: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coull JA, Beggs S, Boudreau D, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005; 438: 1017–1021. [DOI] [PubMed] [Google Scholar]
- 39.Kerr BJ, Bradbury EJ, Bennett DL, et al. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci 1999; 19: 5138–5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Timmusk T, Palm K, Metsis M, et al. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron 1993; 10: 475–489. [DOI] [PubMed] [Google Scholar]
- 41.D’Addario C, Caputi FF, Ekstrom TJ, et al. Ethanol induces epigenetic modulation of prodynorphin and pronociceptin gene expression in the rat amygdala complex. J Mol Neurosci 2013; 49: 312–319. [DOI] [PubMed] [Google Scholar]
- 42.Bannister K, Lee YS, Goncalves L, et al. Neuropathic plasticity in the opioid and non-opioid actions of dynorphin A fragments and their interactions with bradykinin B2 receptors on neuronal activity in the rat spinal cord. Neuropharmacology 2014; 85: 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu X, Vincler MA, Parker R, et al. Spinal cord dynorphin expression increases, but does not drive microglial prostaglandin production or mechanical hypersensitivity after incisional surgery in rats. Pain 2006; 125: 43–52. [DOI] [PubMed] [Google Scholar]
- 44.Campillo A, Cabanero D, Romero A, et al. Delayed postoperative latent pain sensitization revealed by the systemic administration of opioid antagonists in mice. Eur J Pharmacol 2011; 657: 89–96. [DOI] [PubMed] [Google Scholar]
- 45.Ossipov MH, Lai J, King T, et al. Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers 2005; 80: 319–324. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y, Sahbaie P, Liang D, et al. DNA methylation modulates nociceptive sensitization after incision. PLoS One 2015; 10: e0142046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, Angst MS, Clark JD. A murine model of opioid-induced hyperalgesia. Brain Res Mol Brain Res 2001; 86: 56–62. [DOI] [PubMed] [Google Scholar]