Abstract
Streptococcus mutans is a major pathogen of dental caries. Collagen-binding proteins (CBPs) (approximately 120 kDa), termed Cnm and Cbm, are regarded as important cell surface antigens related to the adherence of S. mutans to collagenous tissue. Furthermore, CBP-positive S. mutans strains are associated with various systemic diseases involving bacteremia, such as infective endocarditis. Endodontic infection is considered to be an important cause of bacteremia, but little is known regarding the presence of S. mutans in dental pulp tissue. In the present study, the distribution and virulence of S. mutans in dental pulp tissues were investigated by focusing on CBPs. Adhesion and invasion properties of various S. mutans strains were analyzed using human dental pulp fibroblasts (HDPFs). CBP-positive strains had a significantly higher rate of adhesion to HDPFs compared with CBP-defective isogenic mutant strains (P<0.001). In addition, CBP-positive strains induced HDPF proliferation, which is a possible mechanism related to development of hyperplastic pulpitis. The distribution of S. mutans strains isolated from infected root canal specimens was then analyzed by PCR. We found that approximately 50% of the root canal specimens were positive for S. mutans. Approximately 20% of these strains were Cnm-positive, while no Cbm-positive strains were isolated. The Cnm-positive strains isolated from the specimens showed adhesion to HDPFs. Our results suggest that CBP-positive S. mutans strains exhibit high colonization in dental pulp. This could be a possible virulence factor for various systemic diseases.
Introduction
Streptococcus mutans is a major pathogen of dental caries and is also regarded as a causative agent of infective endocarditis [1]. Collagen-binding proteins (CBPs) of approximately 120 kDa, termed Cnm and Cbm, in S. mutans have been characterized as LPXTG-anchored cell surface proteins [2, 3]. Cnm and Cbm have a frequency of distribution in S. mutans oral strains of approximately 10–20% and 2%, respectively [3, 4]. In our previous study, bacterial DNA of CBP-positive S. mutans strains was detected in cardiovascular specimens at a high frequency [5]. Additionally, in vitro and in vivo findings have demonstrated that these strains are associated with the pathogenesis of infective endocarditis [5]. More recently, we reported that these CBP-positive strains are possible virulence factors associated with cerebral hemorrhage, ulcerative colitis, non-alcoholic steatohepatitis, and IgA nephropathy [6–9].
With progression of dental caries, enamel demineralization occurs, exposing dentin tissue [10]. CBP-positive S. mutans strains have an advantage for attachment to dentin because type I collagen is the main organic component, comprising 85–91% of the organic matrix [2, 11]. In a recent study that used an ex vivo tooth binding model, a Cnm-positive strain showed enhanced binding to collagenous dentin [12]. Bacteria harbored deep in dentin caries are considered to be the primary source of endodontic infection [13]. This is because most species that are detected in carious dentin are the same as those found in infected root canals [14]. These endodontic bacteria are thought to invade the bloodstream, resulting in bacteremia, which is the first step in the pathogenesis of systemic diseases induced by oral bacteria [15]. Dental procedures, and even daily tooth brushing, may cause translocation of microorganisms from the oral cavity into the bloodstream [16]. Additionally, treatment of an infected root canal, which may be a reservoir for oral bacteria, increases the risk of bacteremia [17]. However, few reports have described the pathogenicity of S. mutans in inflamed pulp.
In the present study, we focused on the pathogenicity of CBP-positive S. mutans strains in relation to human dental pulp fibroblast cells (HDPFs). The distribution of CBP-positive S. mutans strains in inflamed pulp specimens, and adhesion and invasion properties of isolated strains were investigated with a primary focus on CBPs.
Materials and Methods
S. mutans strains and growth conditions
This study was conducted in full adherence to the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of the Osaka University Graduate School of Dentistry (approval number: H23-E1). Prior to the collection of specimens, the subjects or their guardian (when the subjects were children) were informed of the study contents by use of written forms, as well as verbal explanations. Written informed consent for participation was obtained. The Cnm-positive S. mutans strain TW295 (serotype k) and its Cnm-defective mutant TW295CND [18], as well as the Cbm-positive strain NN2193-1 (k) and its Cbm-defective mutant NN2193-1CBD [3], were used. In addition, the We also used CBP-negative strains MT8148 (c) and NN2004 (c). Additionally, the Cnm-positive strain TW871 (CBP+), its Cnm-defective mutant TW871CBD [19], and TW871PD, an isogenic mutant of TW871 without protein antigen (PA) expression [18], were used. S. mutans clinical strains isolated from inflamed dental pulp were used (S1 and S2 Tables). All strains were confirmed to be S. mutans based on their biochemical properties and observation of rough colony morphology on Mitis-salivarius agar (Difco Laboratories, Detroit, MI, USA) plates containing bacitracin (0.2 U/mL; Sigma-Aldrich Co., St. Louis, MO, USA) and 15% (wt/vol) sucrose (MSB agar). For growth conditions, all strains were cultured overnight in brain heart infusion broth (Difco Laboratories) from our laboratory stock. Bacterial cells were collected by centrifugation and washed by phosphate-buffered saline (PBS). If necessary, bacterial numbers were adjusted for each assay, as described in the subsections of “Adhesion to and invasion of HDPFs”, “Cell proliferation assay” and “Collagen-binding properties of clinical strains”. Isogenic mutant strains were cultured in brain heart infusion broth with erythromycin added to a final concentration of 10 μg/mL.
Cell cultures
Prior to collection of specimens, the subjects or their guardians were informed of the study contents, and written informed consent was obtained from all of the participants. Normal human dental pulp tissue was obtained from a non-carious primary canine of an 8-year-old boy at the time of an expedient pulpectomy for orthodontic treatment. In addition, a non-carious third molar of a 50-year-old man that was extracted because of pericoronitis was also obtained and HDPFs were established from explant cultures of the pulp tissue, as previously described [20]. The explants were cultured in EBM-2 (Lonza, Walkersville, MD, USA) at 37°C in a water-saturated atmosphere of 95% air and 5% CO2. HDPFs obtained in this manner were maintained and used within five to nine passages.
Adhesion to and invasion of HDPFs
The adhesion and invasion properties of S. mutans strains with HDPFs were evaluated using a previously described method [21, 22], with some modifications. Approximately 1×105 HDPFs were seeded in parallel wells of 24-well tissue culture plates (Costar®, Corning Inc., Corning, NY, USA). Prior to infection, the wells were washed three times with PBS and antibiotic-free medium was added. HDPFs were infected by addition of 1×107 colony-forming units (CFU) of S. mutans strains in antibiotic-free medium to the wells. For adhesion assays, the medium was removed after 1.5 h of aerobic incubation and infected cells were washed three times with PBS, followed by addition of sterile distilled water to disrupt the cells. For invasion assays, the medium was removed after 2 h of aerobic incubation and infected cells were washed three times with PBS. Medium containing penicillin (50 μg/mL) and gentamycin (300 μg/mL) was then added and incubation was performed for 3 h, after which sterile distilled water was added to disrupt the cells. For the adhesion and invasion assays, dilutions of cell lysates infected with S. mutans were plated onto MSB plates and cultured at 37°C for 2 days. The adherence and invasion rates were determined by the ratio of resuspended to infected cells. Data are expressed as the mean ± standard deviation of triplicate experiments.
Fluorescence microscopy
To confirm adhesion and invasion of bacteria visually, a double-fluorescence technique was used for observation with confocal scanning laser microscopy, as previously described [5]. The infected cells were washed three times with PBS, fixed with 3% paraformaldehyde (Wako Pure Chemical Industries, Osaka, Japan) for 10 min, and washed with PBS. They were then incubated with rabbit anti-Cnm serum [3] diluted to 1:500 with PBS–0.5% bovine serum albumin for 60 min at room temperature. Following incubation, culture dishes were washed three times with PBS and incubated with Alexa Fluor 555-conjugated goat anti-rabbit immunoglobulin G (Molecular Probes®, Life Technologies Co., Eugene, OR, USA), which was diluted to 1:500 with PBS–0.5% bovine serum albumin for 30 min at room temperature to visualize attached bacteria. The HDPFs were then permeabilized by adding 0.4% Triton X-100 solution for 5 min and actin filaments were stained with Alexa Fluor 488 conjugated to phalloidin (Molecular Probes®) for 30 min at room temperature to visualize the cellular cytoskeleton. Nuclei were stained with 4′, 6-diamidino-2-phenylindole, dihydrochloride solution (Wako Pure Chemical Industries). Culture dishes were examined by confocal scanning laser microscopy using a TCS-SP5 microscope (Leica Microsystems GmbH, Wetzlar, Germany), as well as a DMI6000 B fluorescence microscope (Leica) and a 63× oil immersion objective.
Cell proliferation assay
Methyl tetrazolium (MTT) assays were performed using a previously described method [23], with some modifications. Briefly, 1×105 HDPF cells were cultured in 24-well culture plates and infected by addition of 1×107 CFU of each tested S. mutans strain in antibiotic-free medium to the wells. The medium was removed after 6 h of aerobic incubation. Infected cells were washed three times with PBS, and then 100 μL of an MTT (Sigma-Aldrich Co.) solution (5 mg/mL) was added to each well with 1 μL of medium, and cells were incubated at 37°C for 4 h. Next, 1 μL of 0.04 N HCl in isopropanol was added and mixed thoroughly to dissolve the dark blue crystals. The plates were allowed to stand at room temperature overnight. Readings were performed using a Benchmark Plus microplate spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA) at a wavelength of 595 nm. Data are shown as the mean of four determinations.
Isolation of S. mutans in inflamed dental pulp specimens
Prior to collection of the specimens, the subjects or their guardians were informed of the study contents and written informed consent was obtained from all of the participants. The subjects were 64 children and adolescents (age range: 1–20 years) who visited Osaka University Dental Hospital from February 2013 to September 2014 to receive root canal treatment under local anesthesia because of severe childhood caries (n = 49), trauma (n = 12), or fracture of the central cusp (n = 3). Root canal specimens were collected using sterile root canal instruments and stored in sterile saline in sterile plastic tubes. Specimens were streaked onto Mitis-salivarius agar (Difco Laboratories) plates containing bacitracin (0.2 U mL-1; Sigma-Aldrich Co.), as well as 15% sucrose (MSB agar). Five colonies from the plates of each subject were selected and grown in brain heart infusion broth (Difco Laboratories).
Molecular methods for determination of the serotype and presence of genes encoding CBPs of S. mutans strains
Bacterial DNA was extracted using a previously described method [24]. Briefly, bacterial cells were collected and incubated with 62.5 μL of lysozyme chloride from chicken egg white (2.0 mg/mL; Sigma-Aldrich Co.) and 0.25 μL of lysozyme hydrochloride from chicken egg white (10 mg/mL; Wako Pure Chemical Industries) for 90 min at 37°C. Genomic DNA was then extracted using 600 μL of Cell Lysis solution (Qiagen, Düsseldorf, Germany) and incubated at 80°C for 5 min, followed by addition of 3 μL of RNase A (10 mg/mL; Qiagen) to the mixture and incubation at 37°C for 30 min. In addition, 200 μL of Protein Precipitation solution (Qiagen) was added and vortexed vigorously for 20 min, and then centrifuged at 10,000 × g for 3 min. The supernatant was combined with 600 μL of isopropanol (Wako Pure Chemical Industries) and centrifuged. The precipitate was then resuspended with 70% ethanol (Wako Pure Chemical Industries), centrifuged, combined with 100 μL of DNA hydration solution (Qiagen), and stored as a DNA extract. Confirmation of S. mutans, serotype determination, and collagen-binding gene detection were carried out by PCR using TaKaRa Ex Taq polymerase (TAKARA BIO, Shiga, Tokyo, Japan) with S. mutans-, serotype-, cnm-, and cbm-specific sets of primers [3, 4, 24–26] (Table 1), template DNA, and 1.5 mM of MgCl2, according to the supplier’s protocols. Amplification was performed using the following parameters. To detect S. mutans, 30 cycles of a denaturing step at 98°C for 10 s, and primer annealing and extension steps at 70°C for 1 min were performed. To detect the cnm and cbm genes, we performed initial denaturation at 95°C for 4 min, and then 30 cycles consisting of 95°C for 30 s, 60°C for 30 s, and 72°C for 2 min, with a final extension at 72°C for 7 min. The PCR products were subjected to electrophoresis in 1.5% or 0.7% agarose gel-Tris-acetate-EDTA buffer. The gel was stained with 0.5 μg of ethidium bromide per mL and photographed under ultraviolet illumination.
Table 1. PCR primers used in the present study.
| Primer set | Purpose | Sequence (5′–3′) | Amplified size | Reference |
|---|---|---|---|---|
| MKD-F | S. mutans detection | GGC ACC ACA ACA TTG GGA AGC TCA GTT | 433 | 25 |
| MKD-R | GGA ATG CCG ATC AGT CAA CAG GAT | |||
| SC-F | Serotype c determination | CGG AGT GCT TTT TAC AAG TGC TGG | 727 | 26 |
| SC-R | AAC CAC GGC CAG CAA ACC CTT TAT | |||
| SE-F | Serotype e determination | CCT GCT TTT CAA GTA CCT TTC GCC | 517 | 26 |
| SE-R | CTG CTT GCC AAG CCC TAC TAG AAA | |||
| SE-F | Serotype f determination | CCC ACA ATT GGC TTC AAG AGG AGA | 316 | 26 |
| SE-R | TGC GAA ACC ATA AGC ATA GCG AGG | |||
| CEFK-F | Serotype k determination | ATT CCC GCC GTT GGA CCA TTC C | 294 | 27 |
| K-R | CCA ATG TGA TTC ATC CCA TAC C | |||
| Cnm-1F | cnm amplification | GAC AAA GAA ATG AAA GAT GT | 1728 | 4 |
| Cnm-1R | GCA AAG ACT CTT GTC CCT GC | |||
| Cbm-1F | cbm amplification | GAC AAA CTA ATG AAA TCT AA | 1814 | 3 |
| Cbm-1R | GCA AAA ACT GTT GTC CCT GC |
Collagen-binding properties of clinical strains
Collagen-binding properties were evaluated according to a previously described method [28], with some modifications. Type I collagen (collagen [type I] in 0.1 N acetic acid; Sigma-Aldrich Co.) was coated onto 96-well plates (Tissue Culture Plate; Beckton Dickinson Ltd., Franklin Lakes, NJ, USA) overnight at 4°C. The plates were then washed three times with PBS and blocked for 1.5 h with 5% bovine serum albumin in PBS. The wells were washed again with PBS containing 0.01% Tween 20. Cells from overnight cultures of S. mutans grown in brain heart infusion broth were collected by centrifugation. Bacterial numbers were adjusted with PBS, and then added to the coated wells (2×109 CFU per well). After a 3-h incubation at 37°C, adherent cells were washed three times with PBS, and then fixed with 200 μL of 25% formaldehyde at room temperature for 30 min. After an additional three washes with PBS, adherent cells were stained with 200 μL of 0.05% crystal violet (Wako Pure Chemical Industries) in water for 1 min. These cells were washed three times with PBS and the dye was dissolved by adding 7% acetic acid (200 μL), after which density measurements were performed at a wavelength of 595 nm. Results for each strain are expressed as the values when the properties of TW871 were defined as 100%. Data are expressed as the mean ± standard deviation of three independent experiments using three wells for each sample.
Statistical analyses
Statistical analyses were performed using the computational software package StatView 5.0 (SAS Institute Inc., Cary, NC, USA). Intergroup differences for adhesion, invasion, and proliferation rates were analyzed using the Student’s t-test. A P value of less than 0.05 was considered to be statistically significant.
Results
S. mutans adhesion to and invasion of human pulp cells
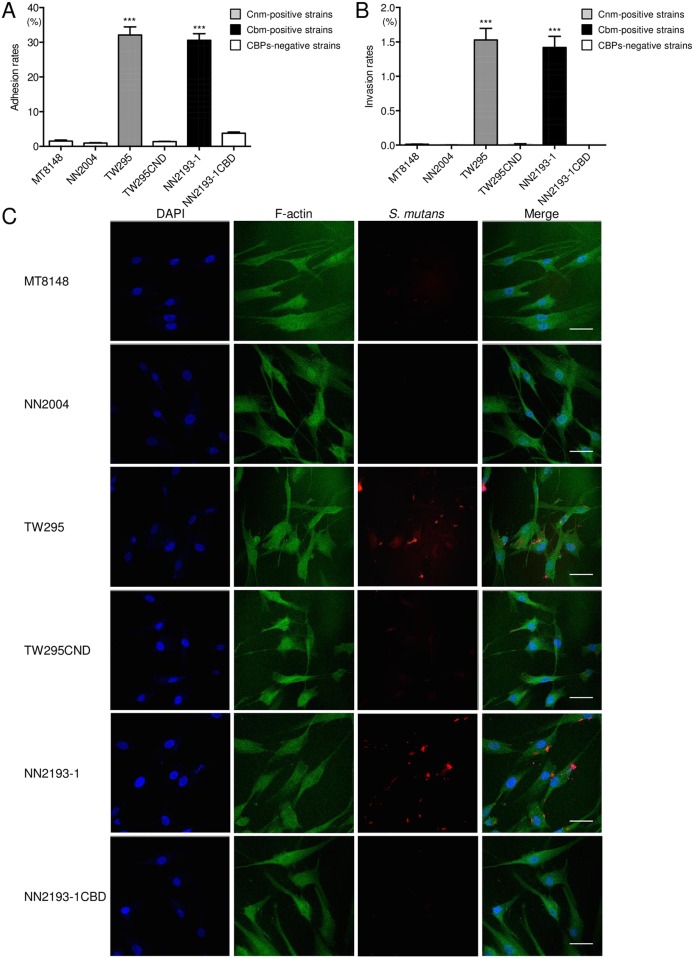
The rates of adhesion to and invasion of HDPFs isolated from primary teeth for the TW295 (Cnm-positive) and NN2193-1 (Cbm-positive) strains were significantly higher compared with their Cnm- and Cbm-defective isogenic mutant strains, respectively, and other CBP-negative strains (P<0.001; Fig 1A and 1B). Observation by confocal scanning laser microscopy showed that the strains TW295 and NN2193-1 had infected the HDPFs (Fig 1C). However, no Cnm- or Cbm-defective isogenic mutant, or other CBP-negative strains had infected the fibroblasts. Higher adhesion and invasion properties of TW295 and NN2193-1 were also observed following examination of HDPFs isolated from the permanent tooth (S1 Fig).
Fig 1. Adhesion and invasion properties of S. mutans strains with HDPFs.
(A) Adhesion to and (B) invasion of HDPFs isolated from a primary tooth. These rates were calculated based on the ratio of recovered infected strains with a multiplicity of infection (MOI) of 100. Data are expressed as the mean ± SD from three independent experiments, with three wells analyzed for each sample. CBP-positive strains showed significant differences compared with CBP-negative strains (***P<0.001). (C) Representative confocal scanning laser microscopic images of HDPFs isolated from primary teeth. Nuclei are stained blue, F-actin is green, and bacterial cells adherent to HDPFs are red. Bars, 40 μm.
Effects of S. mutans infection on HDPF cells
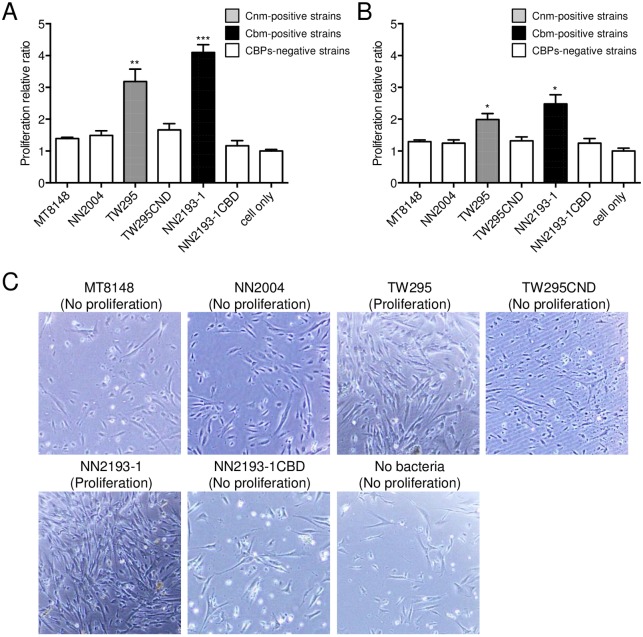
Infection with the strains TW295 and NN2193-1 stimulated proliferation of HDPFs isolated from primary teeth. This amount of infection was significantly greater compared with that stimulated by the CBP-defective isogenic mutant and other CBP-negative strains (P<0.01 and P<0.001, respectively; Fig 2A). CBP-positive S. mutans strains did not cause prominent proliferation of HDPFs isolated from permanent teeth, although there was significantly greater proliferation in CBP-positive strains compared with CBP-negative strains (P<0.05; Fig 2B). When we compared the relative ratios that were obtained in experiments using HDPFs isolated from primary and permanent teeth, those for the TW295 and NN2193-1 strains were significantly higher in cultures with primary teeth specimens than in permanent teeth (P<0.05 and P<0.01). Light microscopy images showed that infection with TW295 and NN2193-1 induced overgrowth of HDPFs isolated from primary teeth (Fig 2C).
Fig 2. Effects of S. mutans infection of HDPFs.
MTT assay results following infection of HDPFs from a (A) primary and (B) permanent tooth with S. mutans strains at an MOI of 100 for 6 h. Data are expressed as the relative ratio standardized to non-infected HDPFs and the mean ± SD, with analysis of three independent experiments. CBP-positive strains showed significant differences compared with CBP-negative strains (Student’s t-test; *P<0.05, **P<0.01, ***P<0.001). (C) Light microscopy images showing morphology of HDPFs from a primary tooth infected with S. mutans at an MOI of 100 for 6 h.
Frequency of distribution of S. mutans and serotype classification in bacteria isolated from inflamed dental pulp
The S. mutans strains isolated from inflamed dental pulp in the present study are shown in S1 Table. S. mutans was isolated from 30 (46.9%) of 64 root canal specimens (Fig 3A), all of which were classified as serotype c or e, with neither serotype f nor k detected (S1 and S2 Tables). In addition, the detection rate of S. mutans in inflamed dental pulp caused by development of dental caries was significantly higher than that in inflamed dental pulp caused by trauma (P<0.001; Fig 3B).
Fig 3. Distribution of S. mutans in inflamed pulp specimens.
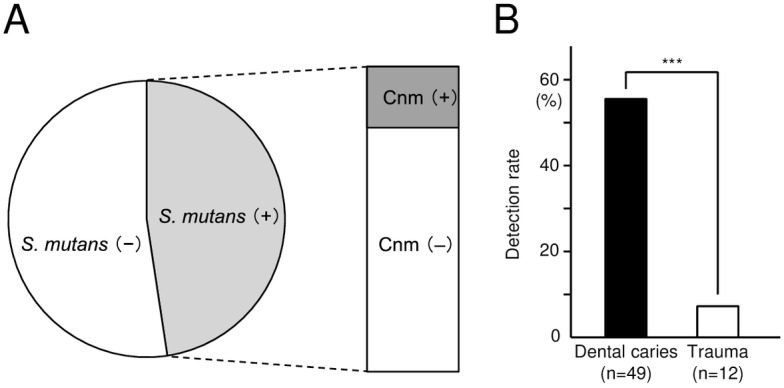
(A) Distribution of Cnm-positive and Cnm-negative S. mutans strains. (B) Detection rates of cnm-positive S. mutans isolated from inflamed pulp induced by dental caries (n = 49) and trauma (n = 12). Significant differences were found (Student’s t test; ***P<0.001).
Distribution and collagen-binding properties of strains with the cnm gene
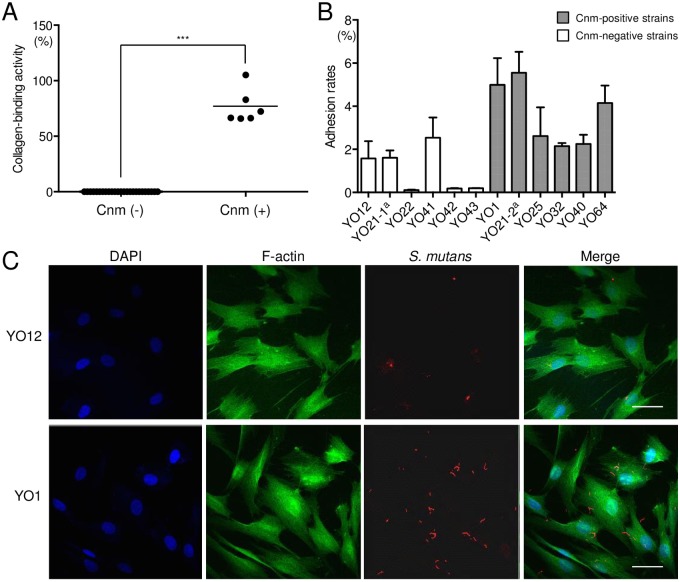
Of the 30 S. mutans strains isolated from dental pulp, six were positive for the cnm gene (20.0%), whereas none were positive for the cbm gene (Fig 3A). One of the six cnm-positive specimens (YO21) harbored cnm-positive and cnm-negative S. mutans strains (S1 Table). All of the cnm-positive S. mutans strains and none of the cnm-negative strains showed collagen-binding properties (Fig 4A).
Fig 4. Virulence properties of S. mutans strains isolated from inflamed pulp specimens.
(A) Binding to type I collagen of S. mutans strains. Significant differences were found (Student’s t test; ***P<0.001). (B) Adhesion properties of S. mutans strains with HDPFs. aYO21-1 and YO21-2 were isolated from the same patient. (C) Representative confocal scanning laser microscopic images of HDPFs isolated from a primary tooth. Nuclei are stained blue, F-actin is green, and bacterial cells adherent to HDPFs are red. Bars, 20 μm.
Adhesion to and invasion of HDPFs by strains isolated from inflamed pulp
All of the cnm-positive S. mutans strains showed properties of adhesion to HDPFs at a rate >2% (Fig 4B), whereas all of those without the cnm gene showed adhesion of <3%. The average rate of adhesion of the cnm-positive strains was significantly higher than that of the cnm-negative strains (P<0.01). Observation by confocal scanning laser microscopy also showed interaction of the cnm-positive S. mutans strain YO1 with HDPFs, whereas few bacterial interactions were observed when the cnm-negative S. mutans strain YO12 was the infecting bacterium (Fig 4C). The average rate of invasion of the cnm-positive strains was 0.3%, whereas most of the cnm-negative strains had negligible invasion properties, with a significant difference between the groups (P<0.01, data not shown).
Discussion
S. mutans is a major pathogen of dental caries in humans. Several studies of the cariogenicity of S. mutans have focused on bacterial cell surface proteins, such as the three types of glucosyltransferases, which synthesize water-insoluble or water -soluble glucans from sucrose [29–31]. Another bacterial cell surface protein is the cell surface 190-kDa protein antigen, known as PA, streptococcal protein antigen P (SpaP), or antigen I/II, and this is correlated with cellular hydrophobicity and sucrose-independent adhesion [32]. Recently, CBPs termed Cnm and Cbm were shown to have a frequency of distribution in oral strains ranging from approximately 10 to 20% [3]. The mechanisms of colonization and pathogenesis in the oral cavity related to CBPs are completely distinct from the other cell surface proteins because CBP-positive strains have an advantage of strong adhesion to collagenous tissue [12]. In the present study, we evaluated the virulence of CBP-positive S. mutans strains toward pulp cells and demonstrated their presence in inflamed root canals.
Some CBP-positive strains have low cellular hydrophobicity because of a lack of expression of the 190-kDa protein antigen [19], which is considered to be a major virulence factor for the onset of dental caries. In addition, most CBP-positive strains lack the glucan-binding protein GbpA, which is important for adherence to tooth surfaces [18]. CBP-positive S. mutans strains missing GbpA have lower levels of sucrose-independent and sucrose-dependent adhesion compared with CBP-negative strains [28]. However, Cnm-positive strains show strong adhesion to collagenous hard tissues, such as those of the dentin and root. This suggests that Cnm-positive strains have greater virulence compared with Cnm-negative strains in deep caries, such as dentin caries and areas with pulpitis. A previous study found that clinical parameters indicating dental caries were significantly increased in patients harboring Cnm-positive S. mutans compared with those with Cnm-negative strains [4].
Among CBP-positive S. mutans strains, TW295 showed large bacterial mass formation, whereas small bacterial cells of YO1 were widely distributed (S2 Fig), when these strains were infected with HDPFs. In our previous study, TW295 and NN2193-1 did not express the 190-kDa cell surface protein antigen PA [18]. We also previously showed that approximately 25% of the CBP-positive strains lacked PA [18]. However, all CBP-positive strains, including YO1, isolated from inflamed dental pulp in the present study expressed PA (data not shown). The CBP+/PA− strain infected with human vein endothelial cells forms a large bacterial mass, resulting in a high level of adhesion and invasion properties [5, 18]. However, CBP+/PA+ S. mutans strains do not show such mass formation, resulting in lower adhesion and invasion properties in human vein endothelial cells compared with the CBP+/PA− strain [18]. Although the mechanism of bacterial mass formation remains to be determined, formation of this mass could be related to high virulence in cardiovascular disease [18]. In the present study, we also observed bacterial mass formation in CBP+/PA− S. mutans strains infecting HDPFs (S2 Fig). To confirm this result, we compared the adhesion properties of S. mutans TW871 (CBP+), its Cnm-defective mutant TW871CBD [21], and TW871PD, an isogenic mutant of TW871 without PA expression [18], to HDPFs. TW871 showed significantly higher adhesion properties to HDPFs compared with TW871CND (S2 Fig). However, TW871PD showed bacterial mass formation with the highest adhesion properties. Further large-scale studies of S. mutans clinical strains isolated from inflamed dental pulp should be performed to clarify the frequency of distribution of CBP+/PA− strains in dental pulp specimens.
Proliferation of fibroblast cells is a response to strong inflammation [33]. With regard to proliferation of fibroblast cells in dental pulp, proliferative pulpitis, also named pulp polyps, is an inflammatory type of hyperplasia [34]. In the present study, we found evidence that infection of HDPFs with CBP-positive S. mutans strains that were isolated from primary and permanent teeth triggered HDPF proliferation. However, such cellular proliferation induced by the CBP-positive S. mutans was not observed in human vein endothelial cells (data not shown). Among the infected HDPFs, proliferation was more prominently observed in those that were isolated from the primary tooth compared with those that were isolated from the permanent tooth. In our study, the adhesion and invasion rates of CBP-positive strains with HDPFs isolated from the primary teeth were not significantly different compared with those with HDPFs isolated from the permanent teeth. This finding suggests that the distinct proliferation rates of the two cell types may have been owing to differential expression of host molecules, such as fibroblast growth factors. Additional studies are needed to clarify the detailed mechanism of proliferation of HDPFs infected by S. mutans.
S. mutans was detected in approximately 30% of cases of dentin caries in direct contact with pulp in a previous study that used molecular analysis [13], similar to the detection rate in our study. In addition, we found that 20% of these bacteria were CBP-positive. All of these bacteria were Cnm-positive, while no Cbm-positive strains were isolated from inflamed pulp, even though the Cbm-positive strain NN2193-1 showed high levels of adhesion to and invasion of HDPFs. A possible explanation for this finding is the relatively low frequency of distribution of Cbm-positive strains in dental plaque or saliva (approximately 2%), whereas Cnm-positive S. mutans strains showed a distribution of approximately 10–20% [3]. Although the overall distribution rate of Cbm-positive S. mutans strains is not high, they are important for development of cardiovascular diseases because of their ability to induce platelet aggregation in the presence of fibrinogen [35]. Large-scale studies of S. mutans clinical isolates obtained from several hundred subjects should be performed to clarify the distribution of Cbm-positive S. mutans in inflamed pulp.
S. mutans is involved in infective endocarditis. We have investigated the relationship of CBP-positive S. mutans strains with the pathogenicity of cardiovascular diseases. We have reported that CBP-positive S. mutans strains may also be an important virulence factor in other systemic diseases, such as hemorrhagic stroke, ulcerative colitis, non-alcoholic fatty liver disease, and IgA nephropathy [6–9]. The initial step in these systemic diseases is invasion of the bloodstream by S. mutans [16]. Bacteremia can be caused by invasive dental treatments, such as tooth extraction, scaling, and root planning, as well as conservative dental procedures [16, 36]. A recent report showed that S. mutans was detected in carious dentin in direct contact with irreversible pulpitis at a high frequency [13]. In the present study, S. mutans organisms were isolated from infected root canals in direct contact with the vascular system, indicating that the canal is a bacterial reservoir, resulting in frequent occurrence of bacteremia. Therefore, deleterious habits, such as increased sucrose consumption or poor oral hygiene leading to severe dental caries, may facilitate infection of CBP-positive S. mutans strains into the bloodstream.
In summary, CBP-positive S. mutans strains show adhesion to and invasion of HDPFs, and this induces proliferation of these cells in primary tooth cultures. Furthermore, S. mutans strains are present in inflamed pulp at a high frequency, of which approximately 20% are Cnm-positive. Our findings indicate that the presence of CBP-positive S. mutans in root canals as an endodontic pathogen may be a risk factor for bacteremia, especially during root canal treatment.
Supporting Information
Rates were calculated based on the ratio of recovered/infected strains with a multiplicity of infection of 100. Data are expressed as the mean ± SD from three independent experiments, with three wells analyzed for each sample. CBP-positive and CBP-negative strains showed significant differences (***P<0.001).
(PDF)
Representative confocal scanning laser microscopic images of HDPFs isolated from primary teeth infected with (A) YO1 and TW295, and (B) TW871 and their isogenic mutant strains. Nuclei are stained blue, F-actin is green, and bacterial cells adherent to HDPFs are red. Bars, 20 μm. (C) Adhesion properties of TW871 and isogenic mutant strains to HDPFs isolated from a primary tooth. The adhesion rates were calculated based on the ratio of recovered infected strains with a multiplicity of infection (MOI) of 100. Data are expressed as the mean ± SD from three independent experiments, with four wells analyzed for each sample. There were significant differences between the parent strains and isogenic mutants (**P<0.01; ***P<0.001).
(PDF)
(DOC)
(DOCX)
Acknowledgments
The authors thank Prof. Howard K. Kuramitsu, State University of New York at Buffalo, for editing the manuscript. This work was supported by the Japan Society for the Promotion of Science KAKENHI (Grant nos. 15K11363 and 15H05049).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by JSPS KAKENHI (grant numbers 15K11363 and 15H05049). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nakano K, Ooshima T. Serotype classification of Streptococcus mutans and its detection outside the oral cavity. Future Microbiol. 2009;4: 891–902. 10.2217/fmb.09.64 [DOI] [PubMed] [Google Scholar]
- 2.Sato Y, Okamoto K, Kagami A, Yamamoto Y, Igarashi T, Kizaki H. Streptococcus mutans strains harboring collagen-binding adhesin. J. Dent. Res. 2004;83: 534–539. [DOI] [PubMed] [Google Scholar]
- 3.Nomura R, Nakano K, Naka S, Nemoto H, Masuda K, Lapirattanakul J, et al. Identification and characterization of a collagen-binding protein, Cbm, in Streptococcus mutans. Mol. Oral Microbiol. 2012;27: 308–323. 10.1111/j.2041-1014.2012.00649.x [DOI] [PubMed] [Google Scholar]
- 4.Nomura R, Nakano K, Taniguchi N, Lapirattanakul J, Nemoto H, Grönroos L, et al. Molecular and clinical analyses of the gene encoding the collagen-binding adhesin of Streptococcus mutans. J Med Microbiol. 2009;58: 469–475. 10.1099/jmm.0.007559-0 [DOI] [PubMed] [Google Scholar]
- 5.Nomura R, Naka S, Nemoto H, Inagaki S, Taniguchi K, Ooshima T, et al. Potential involvement of collagen-binding proteins of Streptococcus mutans in infective endocarditis. Oral Dis. 2013;19: 387–393. 10.1111/odi.12016 [DOI] [PubMed] [Google Scholar]
- 6.Nakano K, Hokamura K, Taniguchi N, Wada K, Kudo C, Nomura R, et al. The collagen-binding protein of Streptococcus mutans is involved in haemorrhagic stroke. Nat. Commun. 2011;2: 485 10.1038/ncomms1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kojima A, Nakano K, Wada K, Takahashi H, Katayama K, Yoneda M, et al. Infection of specific strains of Streptococcus mutans, oral bacteria, confers a risk of ulcerative colitis. Sci. Rep. 2012;2: 332 10.1038/srep00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naka S, Nomura R, Takashima Y, Okawa R, Ooshima T, Nakano K. A specific Streptococcus mutans strain aggravates non-alcoholic fatty liver disease. Oral Dis. 2014;20: 700–706. [DOI] [PubMed] [Google Scholar]
- 9.Misaki T, Naka S, Kuroda K, Nomura R, Shiooka T, Naito Y, et al. Distribution of Streptococcus mutans strains with collagen-binding proteins in the oral cavity of IgA nephropathy patients. Clin. Exp. Nephrol. 2015;In press. [DOI] [PubMed] [Google Scholar]
- 10.Selwitz R, Ismail. Pitts Dental caries. Lancet 2007;369: 51–59. [DOI] [PubMed] [Google Scholar]
- 11.Orsini G, Majorana A, Mazzoni A, Putignano A, Falconi M, Polimeni A, et al. Immunocytochemical detection of dentin matrix proteins in primary teeth from patients with dentinogenesis imperfecta associated with osteogenesis imperfecta. Eur. J. Histochem. 2004;58: 2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller JH, Avilés-Reyes A, Scott-Anne K, Gregoire S, Watson GE, Sampson E, et al. The collagen binding protein Cnm contributes to oral colonization and cariogenicity of Streptococcus mutans OMZ175. Infect. Immune. 2015;83: 2001–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rôças IN, Lima KC, Assunção IV, Gomes PN, Bracks IV, Siqueira JF Jr. Advanced caries microbiota in teeth with irreversible pulpitis. J. Endod. 2015;In press. [DOI] [PubMed] [Google Scholar]
- 14.Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, et al. Bacteria of Dental Caries in Primary and Permanent Teeth in Children and Young Adults. 2008;46: 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goffart J and Gillet P. Endodontic biofirms and secondary infection of total hip arthroplasty. 2007;62: 736–742. [PubMed] [Google Scholar]
- 16.Seymour RA, Lowry R, Whitworth JM, Martin MV. Infective endocarditis, dentistry and antibiotic prophylaxis; time for a rethink? Br Dent J 2000;189: 610–616. [DOI] [PubMed] [Google Scholar]
- 17.Brincat M, Savarrio L, Saunders W. Endodontics and infective endocarditis—is antimicrobial chemoprophylaxis required? Int. Endod. J. 2006;39: 671–682. [DOI] [PubMed] [Google Scholar]
- 18.Nomura R, Naka S, Nemoto H, Otsugu M, Nakamura S, Ooshima T, et al. Potential high virulence for infective endocarditis in Streptococcus mutans strains with collagen-binding proteins but lacking PA expression. Arch. Oral Biol. 2013;58: 1627–1634. 10.1016/j.archoralbio.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 19.Nakano K, Nomura R, Taniguchi N, Lapirattanakul J, Kojima A, Naka S, et al. Molecular characterization of Streptococcus mutans strains containing the cnm gene encoding a collagen-binding adhesin. Arch. Oral Biol. 2010;55: 34–39. 10.1016/j.archoralbio.2009.11.008 [DOI] [PubMed] [Google Scholar]
- 20.Adachi T, Nakanishi T, Yumoto H, Hirao K, Takahashi K, Mukai K, et al. Caries-related bacteria and cytokines induce CXCL 10 in dental pulp. J. Dent. Res. 2007;86: 1217–1222. [DOI] [PubMed] [Google Scholar]
- 21.Nakano K, Nomura R, Matsumoto M, Ooshima T. Roles of oral bacteria in cardiovascular diseases—from molecular mechanisms to clinical cases: Cell-surface structures of novel serotype k Streptococcus mutans strains and their correlation to virulence. J Pharmacol Sci. 2010;113: 120–125. [DOI] [PubMed] [Google Scholar]
- 22.Abranches J, Miller JH, Martinez AR, Simpson-Haidaris PJ, Burne RA, Lemos JA. The collagen-binding protein Cnm is required for Streptococcus mutans adherence to and intracellular invasion of human coronary artery endothelial cells. Infect. Immun. 2011;79: 2277–2284. 10.1128/IAI.00767-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosmann T. Rapid colorimeteric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunolog. Methods. 1983;65: 55–63. [DOI] [PubMed] [Google Scholar]
- 24.Nakano K, Lapirattanakul J, Nomura R, Nemoto H, Alaluusua S, Grönroos L, et al. Streptococcus mutans clonal variation revealed by multilocus sequence typing. J. Clin. Microbiol. 2007;45: 2616–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoshino T, Kawaguchi M, Shimizu N, Hoshino N, Ooshima T, Fujiwara T. PCR detection and identification of oral streptococci in saliva samples using gtf genes. Diagn. Microbiol. Infect. Dis. 2004;48:195–199. [DOI] [PubMed] [Google Scholar]
- 26.Shibata Y, Ozaki K, Seki M, Kawato T, Tanaka H, Nakano Y, et al. Analysis of loci required for determination of serotype antigenicity in Streptococcus mutans and its clinical utilization. J. Clin. Microbiol. 2003;41: 4107–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakano K, Nomura R, Shimizu N, Nakagawa I, Hamada S, Ooshima T. Development of a PCR method for rapid identification of new Streptococcus mutans serotype k strains. J. Clin. Microbiol. 2004;42:4925–4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waterhouse JC, Russell RR. Dispensable genes and foreign DNA in Streptococcus mutans. Microbiol. 2006;152: 1777–1788. [DOI] [PubMed] [Google Scholar]
- 29.Mukasa H, Shimamura A, Tsumori H. Purification and characterization of cell-associated glucosyltransferase synthesizing insoluble glucan from Streptococcus mutans serotype c. J. Gen. Microbiol. 1989;135: 2055–2063. [DOI] [PubMed] [Google Scholar]
- 30.Fukushima T, Ikeda T, Kuramitsu HK. Expression of Streptococcus mutans gtf genes in Streptococcus milleri. Infect. Immun. 1992;60: 2815–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukasa H, Shimamura A, Tsumori H. Purification and characterization of basic glucosyltransferase from Streptococcus mutans serotype c. Biochim. Biophys. Acta. 1982;719: 81–89. [DOI] [PubMed] [Google Scholar]
- 32.Okahashi N, Sasakawa C, Yoshikawa M, Hamada S, Koga T. Molecular characterization of a surface protein antigen gene serotype c Streptococcus mutans implicated in dental caries. Mol. Microbiol. 1989;3: 673–678. [DOI] [PubMed] [Google Scholar]
- 33.Sivapathasundharam B. Oral and maxillofacial pathology: Chapter 10 Disease of the pulp and periapical tissues. Pocket Dent. 2015.
- 34.Kendall RT, Feghali-Bostwick CA. fibroblasts in fibrosis: novel roles and mediators. Front. Pharmacol. 2014;5: 123 10.3389/fphar.2014.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nomura R, Otsugu M, Naka S, Teramoto N, Kojima A, Muranaka Y, et al. Contribution of Streptococcus mutans Serotype k Strains Interaction with Fibrinogen to the Pathogenicity of Infective Endocarditis. Infect. Immun. 2014;82: 5223–5234. 10.1128/IAI.02164-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts GJ, Lucas VS, Omar J. Bacterial endocarpditis and orthodontics. J. R. Coll/ Surg. Edinb. 2000;45: 141–145. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rates were calculated based on the ratio of recovered/infected strains with a multiplicity of infection of 100. Data are expressed as the mean ± SD from three independent experiments, with three wells analyzed for each sample. CBP-positive and CBP-negative strains showed significant differences (***P<0.001).
(PDF)
Representative confocal scanning laser microscopic images of HDPFs isolated from primary teeth infected with (A) YO1 and TW295, and (B) TW871 and their isogenic mutant strains. Nuclei are stained blue, F-actin is green, and bacterial cells adherent to HDPFs are red. Bars, 20 μm. (C) Adhesion properties of TW871 and isogenic mutant strains to HDPFs isolated from a primary tooth. The adhesion rates were calculated based on the ratio of recovered infected strains with a multiplicity of infection (MOI) of 100. Data are expressed as the mean ± SD from three independent experiments, with four wells analyzed for each sample. There were significant differences between the parent strains and isogenic mutants (**P<0.01; ***P<0.001).
(PDF)
(DOC)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.