Abstract
Background
Despite promising role of diuretics to manage fluid overload among chronic kidney disease (CKD) patients, their use is associated with adverse renal outcomes. Current study aimed to determine the extent of renal deterioration with diuretic therapy.
Methods
A total 312 non-dialysis dependent CKD (NDD-CKD) patients were prospectively followed-up for one year. Fluid overload was assessed via bioimpedance spectroscopy. Estimated GFR (eGFR) was calculated from serum creatinine values by using Chronic Kidney Disease- Epidemiology Collaboration (CKD-EPI) equation.
Results
Out of 312 patients, 64 (20.5%) were hypovolemic while euvolemia and hypervolemia were observed in 113 (36.1%) and 135 (43.4%) patients. Overall 144 patients were using diuretics among which 98 (72.6%) were hypervolemic, 35 (30.9%) euvolemic and 11 (17.2%) were hypovolemic. The mean decline in estimated GFR of entire cohort was -2.5 ± 1.4 ml/min/1.73m2 at the end of follow up. The use of diuretics was significantly associated with decline in eGFR. A total of 36 (11.5%) patients initiated renal replacement therapy (RRT) and need of RRT was more profound among diuretic users.
Conclusions
The use of diuretics was associated with adverse renal outcomes indicated by decline in eGFR and increasing risk of RRT initiation in our cohort of NDD-CKD patients. Therefore, it is cautiously suggested to carefully prescribe diuretics by keeping in view benefit versus harm for each patient.
Introduction
Chronic kidney disease (CKD) is a global health concern that substantially increases the risk of mortality and the use of specialized health care [1]. Progressive loss of renal function causes reduced sodium filtration and inappropriate suppression of tubular reabsorption that ultimately lead to volume expansion [2]. Fluid overload frequently manifests in patients with moderate to particularly late stages of CKD and has been associated with hypertension, congestive heart failure (CHF), left ventricular hypertrophy (LVH) as well as edema. In such cases, diuretics are frequently prescribed to control blood pressure and for symptomatic relief of fluid overload [3, 4]. However, the role of diuretics remains quite controversial in CKD patients. Apart from their beneficial effects, these agents also decrease glomerular filtration rate (GFR) and cause metabolic disturbances that in turn increases risk of cardiovascular events [5, 6].
Various guidelines suggest the use of loop (GFR<30ml/min/1.73m2) and thiazide diuretics (GFR >30ml/min/1.73m2) in CKD patients [7]. Unfortunately, randomized controlled trials demonstrating clinical benefits and subsequent harms of diuretic therapy in mild to moderate CKD patients do not exist. Observational studies with small sample size and short duration have shown that diuretics decrease blood pressure (BP) and improve edema in CKD patients but their use, particularly at higher doses, is associated with rise in serum creatinine and several metabolic complications [4,8–9].
The clinical assessment of fluid overload is relatively difficult and diuretics are mostly prescribed in clinical settings on the basis of high blood pressure and physical signs of edema. Although edema can roughly estimate excess extravascular volume but it is of limited value in assessing excess intravascular volume. Moreover, several liters of water should be retained before physical signs of edema become visible [10]. Other techniques to assess fluid status include ultrasonic evaluation of inferior vena cava diameter but it is subjected to interpatient and interoperator variability. Biomarkers such as brain natriuretic peptide (BNP) and N-terminal pro brain natriuretic peptide (NT-pro BNP) can reflect changes in fluid status but both are influenced by presence of cardiovascular disease (CVD) and are also accumulated in CKD patients, rendering these methods inappropriate for evaluation of fluid status in CKD patients [11].
Recently few studies have used bioimpedance spectroscopy i.e. Body Composition Monitoring (BCM) for assessment of fluid status in CKD patients and have shown association of fluid overload with decline in renal function in non-dialysis dependent (NDD) CKD patients [2, 11–13]. However, all except one have not addressed the use of diuretics and its association with both volume overload and decline in renal function [4]. In order to overcome this clinical issue, we conducted a prospective observational study to assess association of diuretics use with severity of fluid overload and loss of renal function/decline in eGFR. The purpose of current study was not to devalue the potential benefits of diuretic therapy among CKD patients. We intended to see the extent of eGFR decline and odds of RRT initiation among NDD-CKD patients receiving diuretics. The findings of the present study could be hypothesis-generating, forming evidence to be considered during future research.
Methodology
Study location and Participants
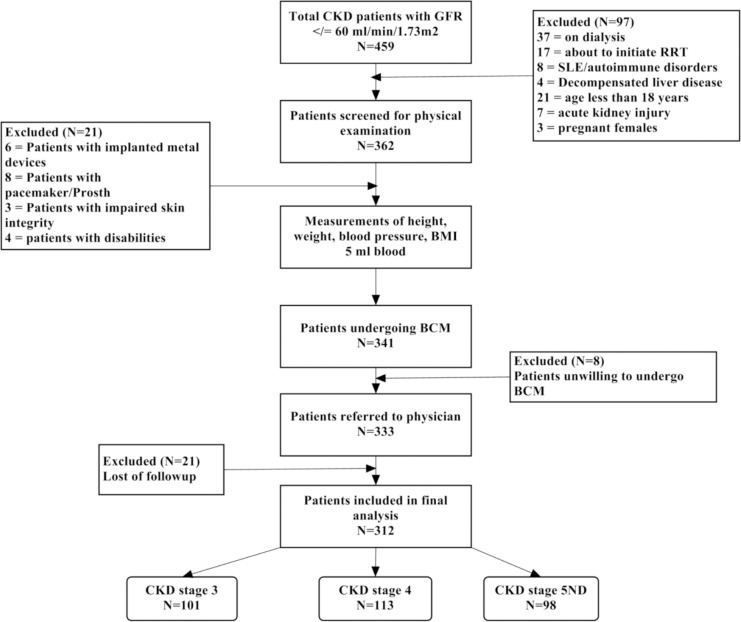
Current study was conducted at a tertiary care hospital in North-Eastern part of Malaysia. All patients visiting outpatient nephrology clinic with confirm diagnosis of CKD (stage 3 to 5ND i.e. non dialysis) according to K/DOQI were invited to participate in the study. Subjects with active infection, decompensated liver disease (liver cirrhosis, ascites), acute kidney injury and autoimmune disorders i.e. systemic lupus erythematous (SLE) were excluded on account of rapid decline in eGFR (>5ml/min/1.73m2). Moreover, participants with pregnancy, malignancies, prosthesis, pacemakers, implanted metal devices (vales, stents or sutures), disabilities and impaired skin integrity were excluded from study due to pre- requirements of BCM (Fig 1). Subjects with uncontrolled hypertension (BP >180/110 mmHg), urinary tract obstruction, unstable CKD (having >15% increase in serum creatinine over last 3 months), congestive heart failure, allergy to sulfa drugs and using diuretics or NSAIDs at the time of beginning of study were excluded at the preliminary stage (before physical examination) of patient recruitment.
Fig 1. Methodological flow chart of study.
Ethical consideration
Current study was approved by ethical committee [Jawatankuasa Etika Penyelidikan-Manusia (JEPeM] of Hospital University Sains Malaysia. All the patients were asked to sign a consent form before participating in study. Identity of each patient was kept confidential and patients were anonymized during data analysis.
Data collection
Patient demographics were recorded from hospital database and by in person interviewing. A physical examination was performed to measure height, weight and blood pressure. Blood pressure was recorded as mean of three consecutive measurements with 5 minutes interval, using one single calibrated manual sphygmomanometer. Comorbidities were defined as follow: diabetes; repeated determination of fasting plasma glucose (>6.4mmol/L), high blood pressure; an average systolic/diastolic blood pressure of ≥140/90mm Hg or prescription of anti-hypertensive medications, hyperlipidemia; low density lipoprotein (LDL)level of >100 mg/dl and cholesterol level >200mg/dl, Cardiovascular disease (CV); CV disease includes coronary artery disease or ischemic heart disease (blockage of coronary artery by artherosclerosis that results in reduced blood supply to heart) and includes stroke, angina and myocardial infarction. Body mass index (BMI) was subsequently calculated as weight/height (kg/m2). Following an overnight fasting, 5 ml of blood was withdrawn to measure full blood count, serum creatinine, calcium, phosphorous, albumin and lipid profile. Estimated GFR (eGFR) was calculated from serum creatinine values by using Chronic Kidney Disease- Epidemiology Collaboration (CKD-EPI) renal function predictive equation [14]. Severity of leg edema was graded on a 4-point scale as: 0 (none), 1 (mild), 2 (moderate), 3 (severe) via assessment chart used in hospital. After routine examination, patient then underwent measurement of fluid status by means of Bioimpedance spectroscopy followed by their scheduled check-up at clinic. Both patients and consulting physicians were unaware of BCM results. Prescription of diuretics was then checked via Hospital online prescription database by entering patient code. All patients were followed up at outpatient nephrology clinic at 3 months interval to ascertain vital status and renal function for a period of one year.
Measurement of Fluid status
A multi frequency (5–1000kHz) bioimpedance spectroscopy device (Body composition Monitor, BCM, Fresenius Medical Care, Germany) was used to assess fluid status. The principle of measuring current flow through body is dependent on applied frequency. At low frequencies, current predominantly passes through extracellular space while at higher frequencies it passes through both intracellular and extracellular water. This device has been intensively validated against different gold standards in general and hemodialysis population [10,15,16]. However few studies have shown its validation in NDD-CKD population [11–13,17]. The measurements were performed after a 5 minute resting period with patient lying in the supine position. Electrodes were attached to one foot and one hand at the ipsilateral side. Metal and electronic devices that might interact with transmission were removed before start of procedure. Furthermore, the procedure was performed after ensuring that patient had not consumed a heavy meal at least within 4 to 5 hour, had not exercised within 12 hours prior to test and had not consumed any beverages including alcohol and caffeine within 24 hours before the test. In the present study, absolute overhydration/fluid overload (OH), intracellular water (ICW), extracellular water (ECW) and total body water (TBW) were calculated based on bioelectrical impedance analysis following the model of Moissl et al [18]. The value of overhydration (OH) as calculated by BCM was used as an indicator of fluid overload. In general population, the 90th percentile of OH is +1.1 L. Accordingly, OH > 1.1L was classified as fluid overload (hypervolemia). Hypovolemia was defined as OH value lower than 10th percentile -1.1 L. An OH value between ± 1 was defined as euvolemia i.e. normal hydration status [19, 20]. Bioimpedance analysis (BIA) was done twice i.e. at the time of beginning of study and at the end of follow-up (1 year).
Statistical analysis
The accordance of quantitative data with normal distribution was examined with the Kolmogorov-Smirnov test. Continuous data were presented as mean (standard deviation). Categorical data were presented as frequency (proportion) for which frequency served as numerator and total number of patients served as denominator. Relevant denominator was stated before proportion, where it varied. Comparison of categorical variables between two groups was done by using Chi-Square test (if at least 80 percent of cells have expected frequencies of 5 or more) or Fisher`s Exact test (if less than 80 percent of cells have expected frequencies of 5 or more). Comparison of continuous variables was done by a Student’s t-test when comparing two groups and by ANOVA when comparing more than two groups. Decline in kidney function was assessed by eGFR slope, defined as regression coefficient between eGFR and time.
Univariate correlation between eGFR decline and potential explanatory variables was assessed by Pearson Correlation analysis. Correlations were analyzed to determine strength of relationship between continuous variables. Significant variables in univariate were then entered into multivariate regression analysis to identify variables that are independently associated with decline in eGFR. By keeping overhydration (OH), blood pressure and baseline eGFR as continuous variable, univariate and multivariate cox regression analysis was done to find hazard ratio (HR) of diuretic users and non-users in terms of adverse renal outcomes (initiation of RRT, decline in eGFR). For all analysis a p-value of <0.05 was considered statistically significant. All categorical data were entered by coding 0 indicating absence/ no while 1 showing presence/yes. Data were analyzed by using SPSS 20.0.0.
Results
Baseline demographics and clinical characteristics of study participants for each category of fluid status are shown in Table 1. After applying exclusion criteria, a total of 312 clinically stable NDD-CKD patients (mean age 64.5 ± 6.43, 57% male) were enrolled in current study. All patients had moderate to severe CKD with mean eGFR 21.4 ± 9.2 ml/min/1.73 m2 (32%, 36% and 31% in CKD stage 3, 4, 5ND respectively). About 81% of study participants were hypertensive and 64% were diabetics. Pre-existing and documented cardiovascular and cerebrovascular disease were noted in 29% and 10% patients, respectively.
Table 1. Clinical characteristics of CKD patients stratified by OH values.
| Demographics | Overall N = 312 | Hypovolemic N = 64 | Euvolemic N = 113 | HypervolemicN = 135 | p-value |
|---|---|---|---|---|---|
| Age (years) | 64.5 ± 6.43 | 67.0 ± 7.68 | 62.3 ± 5.81 | 65.1 ± 6.73 | 0.2 |
| Male gender | 178 (57.0%) | 28 (43.8%) | 57 (50.4)% | 93 (68.9%) | 0.001 |
| Body mass index (kg/m2) | 24.1 ± 5.4 | 24.3 ± 3.8 | 23.7 ± 3.8 | 24.6 ± 3.9 | 0.5 |
| Current Smoker | 96 (30.7%) | 20 (31.2%) | 27 (23.8%) | 49 (36.2%) | 0.1 |
| Current alcohol drinkers | 0 | 0 | 0 | 0 | |
| Comorbidities | |||||
| Hypertension | 252(80.7%) | 44(68.7%) | 89(78.7%) | 119(88.1%) | 0.01 |
| Diabetes mellitus | 200 (64.1%) | 28 (43.7%) | 66 (58.4%) | 106 (78.5%) | <0.001 |
| Hyperlipidemia | 166 (53.2%) | 35 (54.6%) | 55 (48.6%) | 76 (56.2%) | 0.8 |
| Cardiovascular disease | 90 (28.8%) | 14 (21.8%) | 30 (26.5%) | 46 (34.0)% | 0.04 |
| Cerebrovascular disease | 33 (10.6%) | 3.0 (4.6%) | 12 (10.6%) | 20 (14.8%) | 0.003 |
| CKD staging | |||||
| Stage 3 | 101 (32.4%) | 34 (53.1%) | 50 (44.2%) | 17 (12.6%) | <0.001 |
| Stage 4 | 113 (36.2%) | 22 (34.4%) | 42 (37.2%) | 49 (36.3%) | 0.03 |
| Stage 5ND (non-dialysis) | 98 (31.4%) | 8.0 (12.5%) | 21 (18.6%) | 69 (51.1%) | <0.001 |
| eGFR ml/min/1.73m2 | 21.4 ± 9.2 | 27.1 ± 9.9 | 27.0 ± 10.3 | 16.4 ± 9.6 | 0.03 |
| Systolic BP(mmHg) | 140.4 ± 21.3 | 133.5 ± 15.4 | 138.2 ± 18.7 | 147.8 ± 10.1 | <0.001 |
| Diastolic BP(mmHg) | 74.5 ± 10.1 | 75.4 ± 9.1 | 73.3 ± 10.3 | 75.2 ± 11.4 | 0.6 |
| Leg edema score>1 | 72 (23.0%) | 3 (4.6%) | 13 (11.5%) | 56 (41.4%) | <0.001 |
| Body composition parameters | |||||
| Lean tissue index(kg/m2) | 13.9 ± 3.1 | 14.2 ± 2.8 | 13.5 ± 2.5 | 13.8 ± 2.7 | 0.04 |
| Fat tissue index (kg/m2) | 9.6 ± 3.5 | 10.3 ± 4.1 | 9.7 ± 3.8 | 9.4 ± 3.5 | 0.07 |
| Total body water (TBW) L | 34.9 ± 5.4 | 33.0 ± 5.8 | 34.3 ± 5.6 | 37.4 ± 7.6 | 0.001 |
| Intracellular water (ICW) L | 18.2 ± 3.4 | 17.6 ± 3.4 | 18.4 ± 3.3 | 18.5 ± 4.2 | 0.7 |
| Extracellular water(ECW) L | 16.7 ± 3.7 | 15.4 ± 2.4 | 15.9 ± 2.9 | 18.9 ± 4.1 | <0.001 |
| ECW: ICW ratio | 0.91 ± 0.2 | 0.87 ± 0.2 | 0.86 ± 0.2 | 1.02 ± 0.2 | 0.001 |
| Fluid overload (OH) L | 1.0 (0.5–2.3) | -1.6 (-1.2 - -2.3) | 1.0(-0.4–0.9) | 2.9 (2.2–4.2) | <0.001 |
| Laboratory profile | |||||
| Na (mmol/L) | 138.54 ± 3.35 | 138 ± 3.4 | 138.40 ± 3.2 | 140.67 ± 3.0 | 0.4 |
| K (mmol/L) | 4.36 ± 0.53 | 4.36±0.59 | 4.32 ± 0.48 | 4.96 ± 0.45 | 0.3 |
| Urea (mmol/L) | 10.21 ± 5.77 | 8.81 ± 3.69 | 8.84 ± 4.78 | 12.07 ± 6.31 | 0.04 |
| Uric acid (mg/dl) | 7.9 ± 1.5 | 7.6 ± 1.4 | 7.9 ± 1.9 | 8.2 ± 1.8 | 0.02 |
| Ca-phosphate product (mg2/dl2) | 37.3 | 37.7 | 36.5 | 37.9 | 0.6 |
| Albumin (g/dl) | 4.2 ± 0.4 | 4.4 ± 0.5 | 4.3 ± 0.6 | 4.0 ± 0.3 | 0.03 |
| Fasting blood sugar (g/dl) | 101 | 101 | 99 | 106 | 0.4 |
| Glycated hemoglobin (%) | 5.7 | 5.6 | 5.6 | 6.1 | 0.03 |
| Cholesterol (mg/dl) | 182 (155–212) | 188 (164–216) | 183 (160–212) | 175 (141–204) | 0.03 |
| Triglycerides (mg/dl) | 119 (83–169) | 126 (90–167) | 109 (80–172) | 115 (75–161) | 0.425 |
| Urine protein >1+ * | 162 (51.9%) | 25 (39.0%) | 48 (42.5%) | 89 (65.9%) | <0.001 |
| Medication | |||||
| Beta blockers (BB) | 110 (35.3%) | 18 (28.1%) | 38 (33.6%) | 54 (40%) | 0.318 |
| Calcium channel blockers (CCB) | 190 (60.9%) | 26 (40.6%) | 60 (53.1%) | 104 (77%) | 0.02 |
| RAAS blockers | 177 (56.7%) | 24 (37.5%) | 63 (55.8%) | 90 (66.6%) | 0.04 |
| Diuretics Therapy | 144 (46.1%) | 11 (17.2%) | 35 (30.9%) | 98 (72.6%) | |
| Mono Diuretic therapy | 108 (34.6%) | 11 (14%) | 35 (32.7%) | 62 (45.9%) | |
| • Loop diuretics | 69 (22.1%) | 7 (10.9%) | 10 (8.8%) | 52 (38.5%) | |
| • Thiazide Diuretics | 39 (12.5%) | 4 (6.3%) | 25 (22.1%) | 10 (7.4%) | |
| Multiple diuretic therapy | 36 (11.5%) | 0 | 0 | 36 (15.6%) |
Values of categorical variables are presented as percentages (%) whereas continuous variables are shown as mean ± SD or median with interquartile range.
P-value for continuous variables is calculated by one way ANOVA or Kruskal-Wallis H test, as appropriate, p-value for categorical variables is calculated by X2 test
P-value are calculated by comparing three groups (hypovolemic, hypervolemic and euvolemic)
*Assessment of urine protein was done via dipstick test
Mono diuretic therapy: either loop or thiazide diuretic, Multiple diuretic therapy: concomitant use of more than one diuretic
RAAS: renin-angiotensin aldosterone system blockers (include ACEI &ARBS)
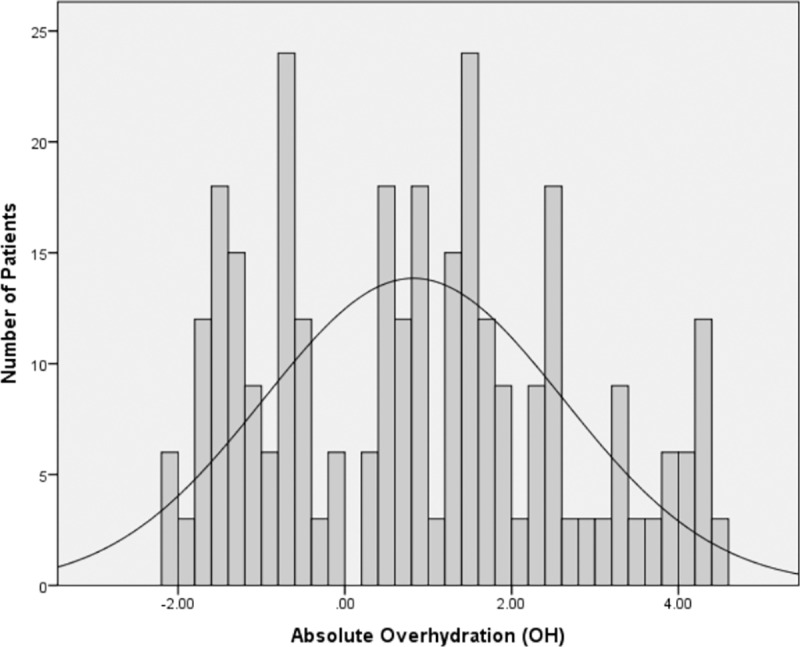
Distribution of absolute overhydration (OH) in entire cohort is shown in Fig 2. On the basis of OH value, 64 (20.5%) patients were hypovolemic while euvolemia and hypervolemia were observed in 113 (36.2%) and 135 (43.3%) patients, respectively. Majority of the hypovolemic and euvolemic patients belonged to CKD stage 3 followed by CKD stage 4 while CKD stage 5 was more prevalent in hypervolemic group. Hyperlipidemia was found to be equally distributed among three categories of fluid status while all other comorbidities were more prevalent among hypervolemic patients. With the exception of β blockers, there was statistically significant difference in medication use of three groups, with hypervolemic patients receiving more medications than the two other groups. Inter-comparison between three groups showed that there was a step-wise increase in levels of extracellular water, total body water, systolic blood pressure, urinary protein excretion, glycated hemoglobin, serum uric acid, blood urea nitrogen (BUN) and leg edema score from hypovolemic to hypervolemic group. On the other hand, a stepwise decline was observed in lean tissue mass, serum hemoglobin, cholesterol and albumin from hypervolemic to hypovolemic group.
Fig 2. Distribution of absolute overhydration (0H) in 312 NDD-CKD patients, ranging between -2.1–4.4 L (82± 1.79).
Diuretics and Fluid Overload
A total of 153 (49.0%) patients received diuretics based on physician assessment of leg edema, blood pressure and cardiovascular complications. During follow-up period, diuretics were discontinued in 9 patients for clinical reasons; therefore these patients were excluded from final analysis leaving 144 (46.1%) patients receiving diuretics until end of follow-up. Baseline eGFR of patients receiving diuretics versus non-users is shown in Table 2. Out of 144 patients prescribed with diuretics, majority of the patients (72.6%) were hypervolemic while euvolemia and hypovolemia were observed in 35 (30.9%) and 11 (17.2%) patients, respectively. Loop diuretics were predominantly prescribed in hypervolemic patients [52 (38.5%)] while thiazide diuretics were received by euvolemic patients [25 (22.1%)]. Multiple diuretic therapies were observed in 36 (15.6%) patients and all of them were hypervolemic.
Table 2. Baseline value of eGFR in different fluid status categories with respect to diuretic use.
| Fluid status category | eGFR ml/min/1.73m2 Diuretics users | eGFR ml/min/1.73m2 Diuretics non-users | P-value |
|---|---|---|---|
| Hypovolemic | 28.9 ± 9.6 | 28.5 ± 10.1 | 0.26 |
| Euvolemic | 28.4 ± 10.8 | 29.8 ± 11.1 | 0.07 |
| Hypervolemic | 19.9 ± 8.2 | 21.5 ± 8.4 | 0.42 |
Clinical Outcomes
In order to assess the impact of diuretics on clinical outcomes of patients, we categorized patients on the basis of diuretic use (Table 3) and assessed outcomes in terms of decline in eGFR, initiation of RRT and death. At the end of follow-up, the mean decline in eGFR of entire cohort was -2.5 ± 1.4 ml/min/1.73m2. Inter-comparison between diuretic users and non-users showed that decline in eGFR and initiation of RRT were more profound among diuretic users compared to non-users (Table 3).
Table 3. Comparison of outcomes between diuretic users and non-users.
| Outcomes | Total cohort N = 312 | Diuretic users N = 144 | Non-users N = 168 | p-value |
|---|---|---|---|---|
| S-cr measurements | 4 (3–7) | 5 (3–7) | 5 (3–7) | |
| eGFR | 23.7±7.1 | 22.3 ± 7.4 | 25.1 ± 6.8 | <0.001 |
| ΔeGFR (ml/min/1.73m2) | -2.5±1.4 | -3.5 ± 1.6 | -1.6 ± 0.77 | 0.02 |
| RRT | 36 (11.5%) | 30 (20.8%) | 6 (3.5%) | <0.001 |
| Death | 2 (0.6%) | 2 (1.4%) | 0 |
p-value calculated by student t-test for continuous variables and chi-square test for categorical variables between diuretic users and non-users
S-cr: serum creatinine, ΔeGFR: change in estimated glomerular filtration rate. RRT: renal replacement therapy
Possible and well documented progression factors of GFR decline along with diuretic use were tested by Pearson`s correlation. Factors that showed significant correlation were subjected to multivariate regression analysis in order to find out independent determinants of GFR decline (Table 4). Systolic blood pressure, Diabetes mellitus, fluid overload, proteinuria, diuretics use and baseline eGFR were found to be significantly associated with GFR decline in current study.
Table 4. Determinants of eGFR decline in entire cohort (n = 312).
| Variables | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| Beta Coefficient (β) | R2 | p-value | Beta Coefficient (β) | R2 | Incremental R2 | p-value | |
| Age | -0.358 | 0.128 | <0.001 | -0.241 | 0.058 | 0.058 | 0.067 |
| Male sex | -0.517 | 0.267 | 0.001 | -0.283 | 0.080 | 0.136 | 0.082 |
| Smoking | 0.224 | 0.050 | 0.081 | 0.137 | 0.018 | 0.006 | 0.253 |
| Systolic Blood pressure | -0.530 | 0.281 | 0.003 | -0.368 | 0.135 | 0.104 | 0.012 |
| Diabetes mellitus | -0.715 | 0.511 | <0.001 | -0.427 | 0.182 | 0.123 | 0.005 |
| Cardiovascular disease | -0.217 | 0.047 | <0.001 | -0.092 | 0.008 | 0.042 | 0.142 |
| Hyperlipidaemia | 0.364 | 0.132 | 0.083 | 0.211 | 0.044 | 0.003 | 0.360 |
| Fluid overload (OH) | -0.792 | 0.627 | 0.001 | -0.497 | 0.247 | 0.122 | 0.003 |
| Proteinuria | -0.699 | 0.489 | <0.001 | -0.562 | 0.315 | 0.041 | <0.001 |
| Diuretics | -0.583 | 0.340 | <0.001 | -0.384 | 0.147 | 0.062 | 0.016 |
| Baseline eGFR | 0.738 | 0.545 | <0.001 | 0.544 | 0.295 | 0.071 | <0.001 |
Multivariate Model R2 = 0.768
Continuous variables: age, systolic blood pressure, fluid overload, baseline eGFR
Dichotomous variables: male sex (0: female/1: male), smoking (0: non-smokers/1: smokers), diabetes mellitus (0: absent/1: present), cardiovascular disease (0: absent/1: present), Hyperlipidemia (0: absent/1: present), Proteinuria (0: absent/1: present), diuretics (0: non-users/1: users)
Multivariate regression analysis was performed by enter method
Renal replacement therapy (RRT) was initiated in 36 (11.5%) patients at the end of follow-up period. It is worthwhile to mention that among patients who initiated RRT, 30 patients were using diuretics. Majority of these patients (n = 28) initiated hemodialysis while peritoneal dialysis was chosen by 8 patients. With respect to CKD staging, out of 36 patients who progressed to RRT, 19 (63.3%) belonged to CKD stage 5 while 11 patients (36.7%) had CKD stage 4. Fortunately, none of the patient in CKD stage 3 initiated RRT. Mortality was observed in two cases and it is interesting to note that these patients belonged to CKD stage 5 hypervolemic group receiving diuretic therapy. Cardiogenic shock was the documented cause of fatality in both cases. We did not observe any death among patients with CKD stage 3 and stage 4.
We further performed multivariate regression analysis to evaluate risk of initiation of dialysis and eGFR decline with respect to diuretic use. The unadjusted and adjusted risk of commencing RRT in diuretic users and non-users is shown in Table 5. Regardless of fluid status category, diuretic users had increased risk of RRT initiation and eGFR decline than non-users.
Table 5. Hazard ratio of diuretic users and non-users in relation to renal outcomes.
| Renal outcomes | Unadjusted univariate HR (95% CI) | p-value | Adjusted multivariate HR (95% CI) | p-value |
|---|---|---|---|---|
| Initiation of RRT | ||||
| Non-users (Reference) | 1 | 1 | ||
| Diuretic users | 3.4 (1.29–2.56) | <0.001 | 2.5 (1.69–3.12) | 0.03 |
| Decline in eGFR | ||||
| Non-users (Reference) | 1 | 1 | ||
| Diuretic users | 5.6 (2.57–4.83) | <0.001 | 3.8 (2.32–4.61) | 0.01 |
Model adjusted for fluid status, systolic BP, baseline eGFR as continuous variable
CV disease, proteinuria, diabetes mellitus were adjusted as categorically
Discussion
Current study evaluated fluid status in a cohort of 312 NDD-CKD patients by using noninvasive technique of bioimpedance spectroscopy (BIS). By using cutoff values of absolute overhydration (OH), study subjects were divided into 3 categories of fluid status i.e. hypovolemia, euvolemia and hypervolemia. A total of 135 (43.2%) patients met the definition criteria of hypervolemia that is less than to what reported in different studies. Reason for low prevalence of hypervolemia in our study might be attributed to difference in cutoff values of fluid overload. On the basis of absolute OH range (0.9–4.1L), maximum prevalence of hypervolemia (65%) was reported by Tsai et al [12]. We did not include 0.9 to 1.1 L in our cutoff range. Similarly in other studies hypervolemia was reported to be 52% and 54.6% based on OH > 7% and OH/ECW ≤ 0.15 respectively [11, 13]. Although the prevalence of hypervolemia reported in our study is less than to what reported in other studies, still 43% of our study subjects were hypervolemic indicating approximately half of NDD-CKD patients are not getting adequate care, despite of their regular visits to nephrology clinic where much attention is paid to volume status. These findings suggest that current clinical and technical tools for aiding physicians to diagnose fluid status and achieve euvolemia are not sufficient. An additional non-invasive tool such as BCM can be useful for routine clinical use in hospitals and would effectively aid diagnosis of fluid overload in NDD-CKD patients.
A total of 144 (46.1%) patients received diuretics at baseline. Apart from management of hypertension and peripheral edema, diuretics are prescribed to control fluid overload [3]. It is interesting to note that diuretics were found to be associated with adverse renal outcomes in our study cohort. Poor renal outcomes among diuretic users might be contributed to several factors. Firstly, maximum diuretic use was observed in hypervolemic patients. As evident from the term “hypervolemia”, these patients were having higher ECW as compared to the other two groups. Hypervolemia itself causes eGFR decline by independently effecting vascular and endothelial cells leading to artheosclerosis and arterial stiffness [21]. Moreover, fluid overloaded patients have increased extravascular volume and decreased intravascular volume leading to decrease blood flow towards kidney [22, 23]. In addition, a recent study has shown strong association between fluid overload and proteinuria. As proteinuria significantly causes decline in eGFR, this might add to another possibility of poor renal outcomes in hypervolemic patients [24]. Secondly, systolic blood pressure (SBP) was significantly higher among these patients causing more use of other classes of anti-hypertensives. Both RAAS and CCB alter intra-glomerular hemodynamics leading to nephrotoxicity [25, 26]. However, the attribution of hypervolemia, proteinuria, higher SBP and use of anti-hypertensives was adjusted in all statistical analysis (Tables 3–5). Despite adjustment of these confounders, diuretics were found to be independently associated with poor renal outcomes indicated by decline in eGFR and initiation of RRT in NDD-CKD patients (Tables 4 and 5).
Previously, a number of evidences have reported that diuretic therapy is either detrimental to renal function or it significantly allows quantifiable renal impairment [4, 9, 27]. Secondary analysis of major hypertensive trials have shown that diuretics cause significant increase in serum creatinine values [28–30]. Data analysis of NHANES III survey reported that increase creatinine level is directly proportional to diuretic prescriptions [31]. Similar results were reported by Hawkins and Houston in their retrospective analysis of United States Renal Data System (USRDS). By using data fusion methodology, authors found that increase diuretic distribution is directly associated with ESRD incidence [32]. Similar few small sample size observational studies conducted in CKD patients have also reported rise in serum creatinine with diuretic use [9].
To the best of our knowledge, our study is first hospital based clinical study demonstrating association of diuretic use with decline in eGFR and initiation of RRT in NDD-CKD patients. The exact mechanism by which diuretics cause renal injury is not clear. Apart from metabolic disturbances, diuretics directly cause apoptosis in distal tubular cells of nephron [33]. Hypokalemia that is the major metabolic disturbance of both thiazide and loop diuretics leads to renal hypertrophy and tubulointerstitial fibrosis. Concomitant administration of more than one diuretic causes massive volume loss that results in renal vasoconstriction with increased tubular uptake of sodium chloride (NaCl) and decreased urine output (UO). Prolonged vasoconstriction then leads to tubular dysfunction and necrosis [34]. Moreover, co-administration of diuretics with other anti-hypertensives especially vasodilators (calcium channel blockers) results in rapid fluctuations of BP causing pre-renal azotemia [33].
The purpose of current study is not to the challenge the potential benefits of diuretics in NDD-CKD patients. There is a well reported evidence for potential benefits of diuretics as not only anti-hypertensive agents but they also significantly reduce risk of cardiovascular and cerebrovascular diseases. However, we made an attempt to highlight the potential risks of diuretics with an intent to underscore clinicians’ attention towards dark side of diuretic therapy that can be potentially fatal for patients in long run. Need of the hour is careful selection of hypervolemic NDD-CKD patients where benefit of using diuretic therapy outweighs subsequent risks and use of other equally rational treatments (CCB, RAAS blockers).
Limitation
Some potential limitations of current study are needed to be addressed. Being an observational study, confounding by indication was the main limitation. Efforts were made to minimize bias by adjusting confounders in all statistical analysis. Fluid status was measured once only at the time of beginning of study, therefore changes in fluid status over time were not considered. Secondly, BCM device has not been validated in CKD patients but previous studies in CKD population has shown that there is a linear relationship between leg edema score and severity of fluid overload as assessed by BCM device. Therefore, it is widely assumed in clinical practice that BCM gives appropriate results of fluid status in CKD patients. Furthermore, fluid overload might lead to underestimation of creatinine levels and therefore gives misleading creatinine values in hypervolemic patients. Use of diuretics was recorded categorically, not as time varying dose of medication. Our study lacks information regarding salt consumption and dietary intake, as both of them affect fluid status and diuretic efficacy; therefore it might affect study results. Lastly, the follow-up period was only 12 months. A longer follow-up period will give better understanding of outcomes of diuretics especially in terms of decline in eGFR and disease progression. Despite mentioned limitations, current study is strengthened by its prospective nature and being first study in Asia that shows association between diuretic use and decline in renal function in clinical settings.
Conclusions
In conclusion, current study demonstrates that diuretic use is an independent predictor of adverse renal outcomes in NDD-CKD patients causing decline in eGFR and increasing the risk of RRT initiation. Unless a contradictory data from randomized controlled trial discourages above findings, it is cautiously concluded that irrespective of fluid overload, diuretics cause adverse renal outcomes. Future interventional studies or double blinded randomized controlled studies with large sample size are needed to rule out the association between diuretic use (type and dose) and renal outcomes in NDD-CKD patients, as this complicated triangle is present in majority of NDD-CKD patients. Such studies should be designed to include time averaged defined daily dose of diuretics.
Data Availability
Individual data points are available upon request due to ethical restrictions by Medical Research Advisory Committee [Jawatankuasa Etika Penyelidikan Manusia USM (JEPeM-USM)] as stated in “Rule Number 3” (Privacy and Confidentiality of data) of “Ethical Considerations” and these rules are available on its official website (www.jepem.kk.usm.my) by downloading “Template for Research Protocol”. Furthermore, confidentiality of medical information of patients is strictly required by ethical board and following statement was also consented to the patients during data collection. However, individual data points can be provided to the researchers who meet the criteria for access to confidential data via approval from the Medical Research Advisory Committee and the Chronic Kidney Disease Resource Center, School of Medical Sciences University Sains Malaysia; contact person (In-charge Data and Records) zamli@usm.my.
Funding Statement
The authors have no support or funding to report.
References
- 1.Khan YH, Sarriff A, Adnan AS, Khan AH, Mallhi TH, Jummaat F. Progression and outcomes of non‐dialysis dependent chronic kidney disease patients: A single center longitudinal follow‐up study. Nephrol. 2015. ( 10.1111/nep.12713). [DOI] [PubMed] [Google Scholar]
- 2.Hung SC, Kuo KL, Peng CH, Wu CH, Lien YC, Wang YC, et al. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. 2014; 85(3): 703–709. 10.1038/ki.2013.336 [DOI] [PubMed] [Google Scholar]
- 3.London GM. Cardiovascular disease in chronic renal failure: pathophysiologic aspects. Semin Dial. 2003; 16(2): 85–94. [DOI] [PubMed] [Google Scholar]
- 4.Vasavada N, Agarwal R. Role of excess volume in the pathophysiology of hypertension in chronic kidney disease. Kidney Int. 2003; 64(5): 1772–1779. [DOI] [PubMed] [Google Scholar]
- 5.Franse LV, Pahor M, Di-Bari M, Shorr RI, Wan JY, Somes GW, et al. Serum uric acid, diuretic treatment and risk of cardiovascular events in the Systolic Hypertension in the Elderly Program (SHEP). J Hypertens. 2000; 18(8): 1149–1154. [DOI] [PubMed] [Google Scholar]
- 6.Duarte JD, Cooper-DeHoff RM. Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics. Expert Rev Cardiovasc Ther. 2010; 8(6):793–802. 10.1586/erc.10.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005; 67(6): 2089–2100. [DOI] [PubMed] [Google Scholar]
- 8.Mehta RL, Pascual MT, Soroko S, Chertow GM, PICARD Study Group. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. ACC Curr J Rev. 2003; 12(2): 68–69. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R, Sinha AD. Thiazide diuretics in advanced chronic kidney disease. J Am Soc Hypertens. 2012; 6(5): 299–308. 10.1016/j.jash.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 10.Devolder I, Verleysen A, Vijt D, Vanholder R, Van Biesen W. Body composition, hydration, and related parameters in hemodialysis versus peritoneal dialysis patients. Perit Dial Int. 2010; 30(2): 208–214. 10.3747/pdi.2008.00284 [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz Z, Yildirim Y, Oto F, Aydin FY, Aydin E, Kadiroglu AK, et al. Evaluation of volume overload by bioelectrical impedance analysis, NT-proBNP and inferior vena cava diameter in patients with stage 3&4 and 5 chronic kidney disease. Ren Fail. 2014; 36(4): 495–501. 10.3109/0886022X.2013.875815 [DOI] [PubMed] [Google Scholar]
- 12.Tsai YC, Tsai JC, Chen SC, Chiu YW, Hwang SJ, Hung CC et. Association of fluid overload with kidney disease progression in advanced CKD: a prospective cohort study. Am J Kidney Dis. 2014; 63(1): 68–75. 10.1053/j.ajkd.2013.06.011 [DOI] [PubMed] [Google Scholar]
- 13.Hung SC, Kuo KL, Peng CH, Wu CH, Wang YC, Tarng DC. Association of fluid retention with anemia and clinical outcomes among patients with chronic kidney disease. J Am Heart Assoc. 2015; 4(1): e001480 10.1161/JAHA.114.001480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150(9): 604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wabel P, Chamney P, Moissl U, Jirka T. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif. 2009; 27(1): 75–80. 10.1159/000167013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wizemann V, Rode C, Wabel P. Whole-body spectroscopy (BCM) in the assessment of normovolemia in hemodialysis patients In: Hemodialysis-From Basic Research to Clinical Trials. Contrib Nephrol Basel, Karger; 2008. pp. 115–118. [DOI] [PubMed] [Google Scholar]
- 17.Tsai YC, Tsai JC, Chiu YW, Kuo HT, Chen SC, Hwang SJ, et al. Is fluid overload more important than diabetes in renal progression in late chronic kidney disease?. PloS One. 2013; 8(12): e82566 10.1371/journal.pone.0082566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moissl UM, Wabel P, Chamney PW, Bosaeus I, Levin NW, Bosy-Westphal A, et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas. 2006; 27(9): 921 [DOI] [PubMed] [Google Scholar]
- 19.Wieskotten S, Heinke S, Wabel P, Moissl U, Becker J, Pirlich M, et al. Bioimpedance-based identification of malnutrition using fuzzy logic. Physiol Meas. 2008; 29(5): 639 10.1088/0967-3334/29/5/009 [DOI] [PubMed] [Google Scholar]
- 20.Tai R, Ohashi Y, Mizuiri S, Aikawa A, Sakai K. Association between ratio of measured extracellular volume to expected body fluid volume and renal outcomes in patients with chronic kidney disease: a retrospective single-center cohort study. BMC Nephrol. 2014; 15(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briet M, Bozec E, Laurent S, Fassot C, London GM, Jacquot C, Froissart M, Houillier P, Boutouyrie P. Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Intern. 2006; 69(2): 350–357. [DOI] [PubMed] [Google Scholar]
- 22.Hur E, Usta M, Toz H, Asci G, Wabel P, Kahvecioglu S, et al. Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: a randomized controlled trial. Am J Kidney Dis. 2013; 61(6): 957–965. 10.1053/j.ajkd.2012.12.017 [DOI] [PubMed] [Google Scholar]
- 23.Alvarez‐Lara MA, Martín‐Malo A, Espinosa M, Rodríguez‐Benot A, Aljama P. Blood pressure and body water distribution in chronic renal failure patients. Nephrol Dial Transplant. 2001; 16(1): 94–97. [DOI] [PubMed] [Google Scholar]
- 24.Schork A, Woern M, Kalbacher H, Voelter W, Nacken R, Bertog M, et al. Association of Plasminuria with Overhydration in Patients with CKD. Clin J Am Soc Nephrol. 2016; 11(5): 761–9. 10.2215/CJN.12261115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onuigbo MA. Can ACE inhibitors and angiotensin receptor blockers be detrimental in CKD patients?. Nephron Clin Pract. 2011; 118(4): c407–419. 10.1159/000324164 [DOI] [PubMed] [Google Scholar]
- 26.Pannu N, Nadim MK. An overview of drug-induced acute kidney injury. Crit Care Med. 2008; 36(4): S216–23. 10.1097/CCM.0b013e318168e375 [DOI] [PubMed] [Google Scholar]
- 27.Dussol B, Moussi‐Frances J, Morange S, Somma‐Delpero C, Mundler O, Berland Y. A pilot study comparing furosemide and hydrochlorothiazide in patients with hypertension and stage 4 or 5 chronic kidney disease. J Clin Hypetens. 2012; 14(1): 32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franse LV, Pahor M, Di Bari M, Somes GW, Cushman WC, Applegate WB. Hypokalemia associated with diuretic use and cardiovascular events in the Systolic Hypertension in the Elderly Program. Hypertens. 2000; 35(5): 1025–30. [DOI] [PubMed] [Google Scholar]
- 29.Brown MJ, Palmer CR, Castaigne A, de Leeuw PW, Mancia G, Rosenthal T, et al. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet. 2000; 356(9227): 366–72. [DOI] [PubMed] [Google Scholar]
- 30.ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J Am Med Assoc. 2002; 288(23): 2981–97. [DOI] [PubMed] [Google Scholar]
- 31.Coresh J, Wei GL, McQuillan G, Brancati FL, Levey AS, Jones C, et. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med. 2001; 161(9): 1207–16. [DOI] [PubMed] [Google Scholar]
- 32.Hawkins RG, Houston MC. Is Population-Wide Diuretic Use Directly Associated With the Incidence of End-Stage Renal Disease in the United States?* A Hypothesis. Am J Hypertens. 2005; 18(6): 744–9. [DOI] [PubMed] [Google Scholar]
- 33.Choudhury D, Ahmed Z. Drug-associated renal dysfunction and injury. Nat Clin Pract Nephrol. 2006; 2(2): 80–91. [DOI] [PubMed] [Google Scholar]
- 34.Cotter G, Metra M, Milo‐Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure—Re‐distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008; 10(2): 165–9. 10.1016/j.ejheart.2008.01.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual data points are available upon request due to ethical restrictions by Medical Research Advisory Committee [Jawatankuasa Etika Penyelidikan Manusia USM (JEPeM-USM)] as stated in “Rule Number 3” (Privacy and Confidentiality of data) of “Ethical Considerations” and these rules are available on its official website (www.jepem.kk.usm.my) by downloading “Template for Research Protocol”. Furthermore, confidentiality of medical information of patients is strictly required by ethical board and following statement was also consented to the patients during data collection. However, individual data points can be provided to the researchers who meet the criteria for access to confidential data via approval from the Medical Research Advisory Committee and the Chronic Kidney Disease Resource Center, School of Medical Sciences University Sains Malaysia; contact person (In-charge Data and Records) zamli@usm.my.