Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a common psychological diagnosis in children. This disorder impacts children and adolescents in all areas of life, including academic performance, extracurricular activities, and social interactions. ADHD can continue into adulthood where unemployment and substance abuse has been described. Although behavioral therapy is recommended for all patients with ADHD, medication management typically is initiated soon after diagnosis. Psychostimulants remain the primary medication of choice. This review focuses on the clinical use of psychostimulant medication in children and adolescents. The pharmacodynamic and pharmacokinetic differences between the newest long-acting formulations as well as commonly encountered adverse drug reactions, with suggested management strategies, will be highlighted. Non-stimulant therapy with atomoxetine or alpha2-adrenergic agonists is also reviewed. These agents may be warranted for patients who cannot tolerate psychostimulant therapy or have a comorbid condition. Finally, the 8-year multimodal treatment study results are also discussed.
INDEX TERMS: adolescent, alpha2-agonist, amphetamine, atomoxetine, attention deficit disorder with hyperactivity, central nervous system stimulants, child, clonidine, methylphenidate, pediatric
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common neurobehavioral disorders in childhood. ADHD can impact not only school performance, but also social interaction. If untreated, this could lead to poorer grades and isolation from friends, and in adolescence there is increased drug abuse and delinquent behavior. Pharmacological treatment is highly effective, and ongoing, long-term studies are starting to reveal more evidence to help providers discern proper therapy duration, potential side effects, and more specific outcomes to monitor. Many agents are available and clinicians need to be aware of the pharmacokinetic and pharmacodynamic differences so that an appropriate, patient-specific regimen can be offered. This review will discuss the common medication therapy used for ADHD patients along with strategies to help manage common side effects. This article is not meant to encompass all comorbidities associated with ADHD but rather to highlight the common regimens and challenges seen within the community.
Prevalence
The prevalence of ADHD in any specific population is difficult to determine owing to differences in diagnostic criteria and access to health care. In the United States, it is estimated that 5% to 9% of children aged 5 to 12 years have ADHD, which is similar to the estimated worldwide prevalence of 5.29%.1–3 Male children are twice as likely to be diagnosed as similarly aged females; however, this may be due to males expressing more hyperactive symptoms, while females demonstrate inattentiveness often, which may be more difficult to identify.4,5 ADHD impacts patients of all ethnic backgrounds, but there is a slightly higher prevalence noted in non-Hispanic Caucasians.4 Approximately 70% of patients diagnosed with ADHD as a child will continue to have significant symptoms during adolescence and more than half of them will have impairment into adulthood.6
Etiology
The exact cause of ADHD is unknown, but many potential risk factors have been identified, including biological and environmental risks. Both norepinephrine and dopamine play a critical role in modulating attention in ADHD. Norepinephrine may have more effect on executive functioning (deficits in executive functioning manifest as having difficulty solving problems, remembering tasks, and planning for a future task),7 whereas dopamine may be more important in maintaining attention.8 Genomic studies have identified a variety of dopamine and serotonin receptors (i.e., dopamine 4 and 5, serotonin 1B) having significant association with ADHD.
A genetic component to ADHD has mounting evidence, too, stemming from a twins study completed in Europe, Australia, Scandinavia, and the United States, showing a mean heritability of 76%. This means that 76% of the etiology of ADHD can be explained by genes.9 Some environmental factors that have been associated with ADHD include low birth weight and maternal smoking.1 Thus, there is a multitude of factors that need to be taken into account when considering a diagnosis of ADHD.
Symptoms and Diagnosis
There are 3 hallmark symptoms of ADHD: inattention, hyperactivity, and impulsivity. Inattention presents as difficulty paying close attention to details in schoolwork or other activities, lack of attention to partners in verbal communication, and inability to follow through on tasks. In contrast, impulsivity is seen as the inability to remain seated in situations where it would be expected (e.g., school, work), blurting out responses to questions, completing other people's sentences, or acting without thinking through the consequences first. Hyperactivity often presents as fidgeting when attempting to remain seated or excessive movement throughout the day. The presence of any of these symptoms in a patient justifies being evaluated for ADHD.
The Diagnostic and Statistical Manual of Mental Disorders (DSM-5)10 is used to officially diagnose a patient with ADHD. The DSM-5 separates the hallmark symptoms into 2 categories: inattention and hyperactivity/impulsivity. Within each category there is a list of symptoms, and a pediatric patient (younger than 17 years) must have 6 or more of these symptoms in either category to be diagnosed with ADHD. Patients 17 years of age or older only require 5 symptoms for the diagnosis. Additionally, to be diagnosed, the patient must have expressed the symptoms of ADHD for a minimum of 6 months and have a history of developing several of the symptoms before the age of 12 years. Symptoms also must be present in 2 or more settings, such as home and school, and there must be clear evidence that the symptoms interfere with functioning. In addition to the diagnosis of ADHD, a presentation subtype is described for each patient, based on the number of symptoms found within each category. If the patient has 6 or greater symptoms in both the inattention and the hyperactivity/impulsivity categories, they are designated as having combined presentation ADHD. If the patient has 6 or greater symptoms in the inattentive category only, they are designated as having predominantly inattentive presentation ADHD, whereas if they have 6 or greater symptoms in only the hyperactive/impulsive category, they are considered to have predominantly hyperactive/impulsive presentation ADHD.
A number of rating scales have been created to assist the clinician in the diagnosis of ADHD and in the monitoring of therapy. The Conners Rating Scales and the Vanderbilt ADHD Diagnostic Scales are the 2 most commonly used in clinical practice. The Conners Parent Rating Scale–Revised asks the parent or adolescent patient questions to elucidate information that helps determine the relative degree of presence or absence of the hallmark ADHD symptoms. The Conners Teacher Rating Scale–Revised is similar in nature but evaluates the symptoms in a separate setting and is used if the parents allow information to be obtained from the patient's school. The Vanderbilt ADHD Diagnostic Parent and Teacher Scales evaluate for ADHD symptoms and additionally assess for common comorbid conditions.1 Further detailed information about each rating scale can be found in the article by Pliszka et al.1
PHARMACOTHERAPY OF ADHD
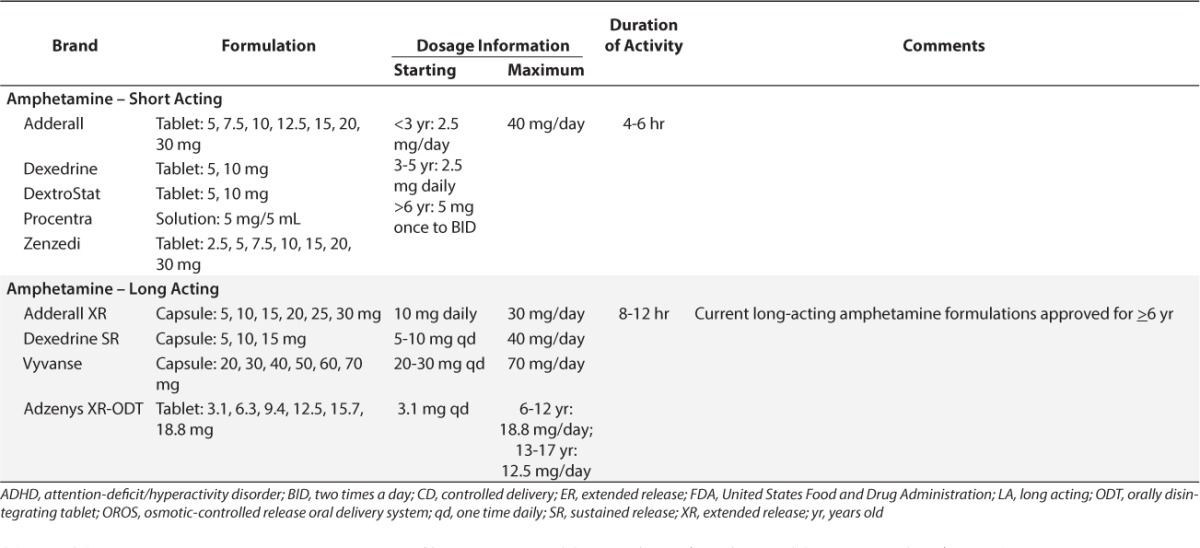
Psychostimulants have been the medications of choice for treating ADHD for more than 60 years. About 75% to 80% of children with ADHD will benefit from the use of psychostimulants. Methylphenidate, dexmethylphenidate, dextroamphetamine, lisdexamfetamine, and the mixed amphetamine salts (dextro- and levo-amphetamine) constitute the psychostimulants currently on the market. They have similar clinical benefits; however, the pharmacokinetic and pharmacodynamic profiles vary greatly. Tables 1 and 2 list the common ADHD medications available as of January 2016.
Table 1.
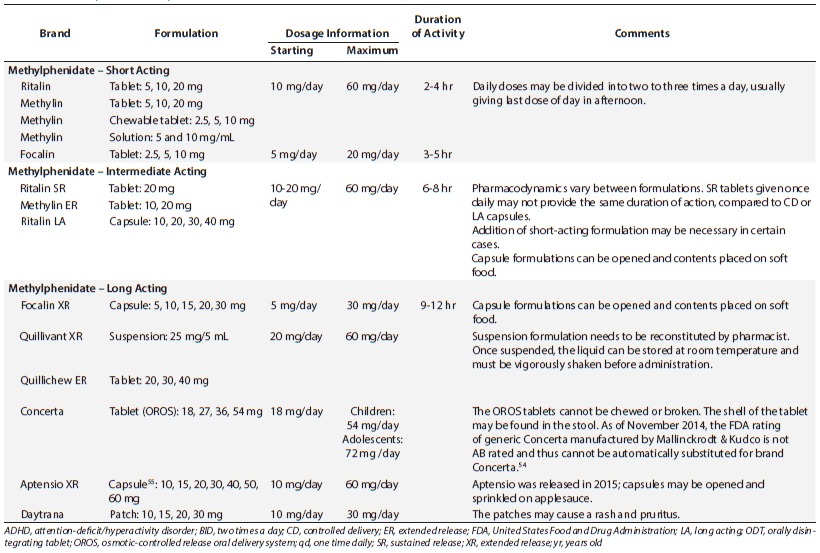
Table 2.
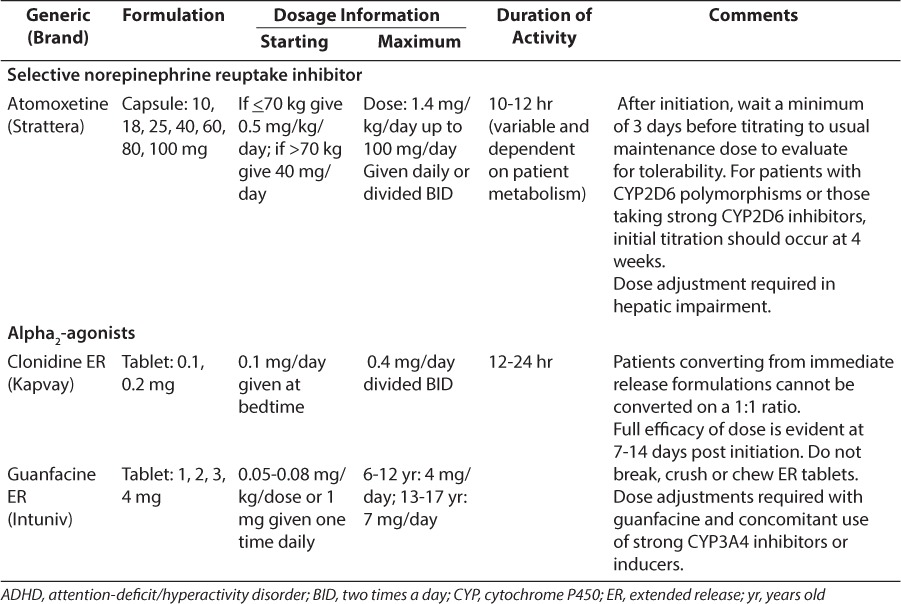
Table 1.
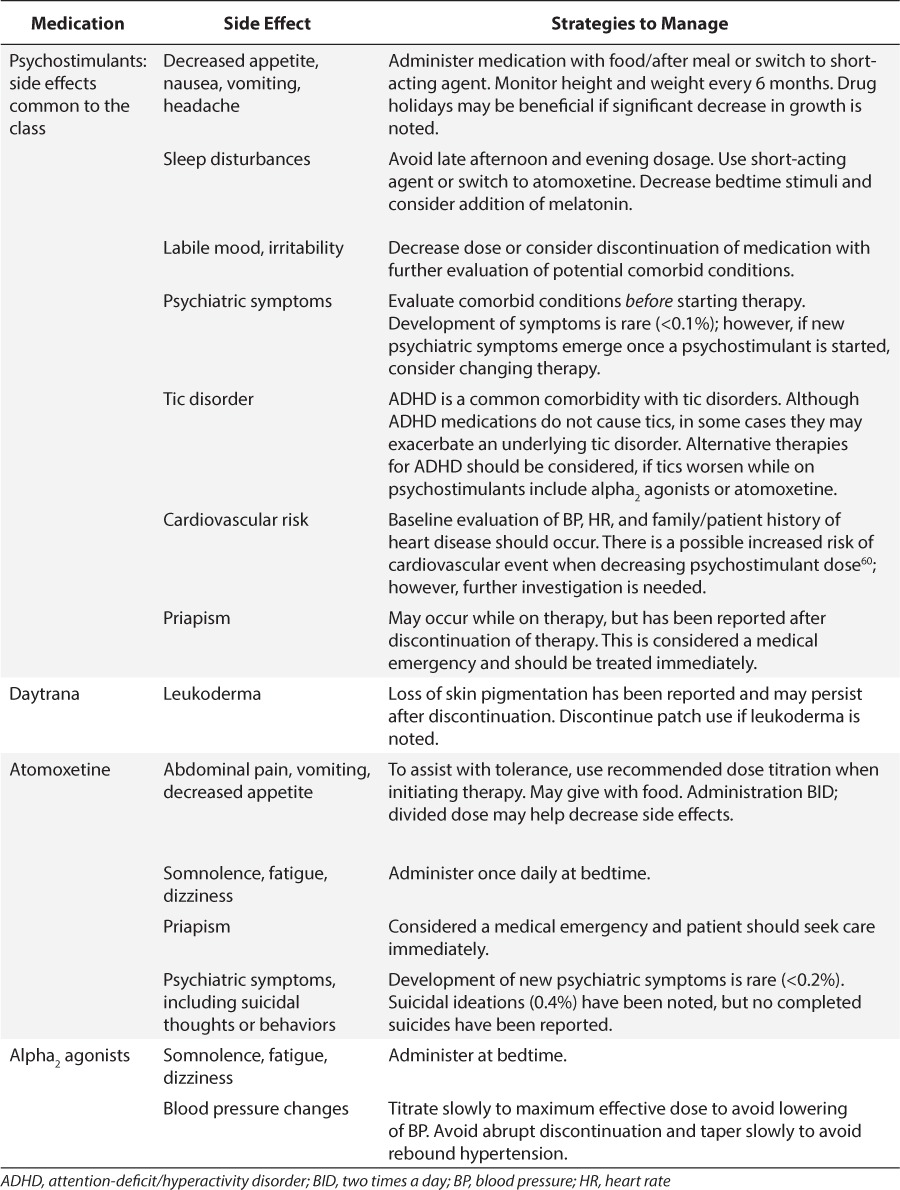
In general, the psychostimulants have similar common adverse reactions including decreased appetite, stomach pain, sleep disturbances, and headaches. Less common adverse reactions include labile mood and growth suppression. Data are conflicting regarding the effect of psychostimulants on growth. There are long-term data available discussing height loss of 1 to 2 cm that is sustained but dependent upon dose and duration of therapy.11 However, a more recent longitudinal study12 was published that disputes this variation in growth. Patients born between 1976 and 1982 were retrospectively evaluated to assess final adult height and peak height velocity and the association between psychostimulant use and duration. There was no significant change in height between those patients treated for ADHD versus non-treated controls. Results also did not show any difference even in patients treated for ≥3 years.12 New warnings of sudden cardiac death are described within the psychostimulants' product information. Table 3 discusses more recent primary literature highlighting strategies for managing potential cardiovascular risk. There is no universal requirement about obtaining a baseline electrocardiogram; however, the American Academy of Pediatrics and the American Heart Association state that an electrocardiogram can be considered in children with risk factors, such as a family history of heart disease or an individual history of chest pain or dizziness.6,13
Table 3.
Drug-drug interactions are not common with the psychostimulants; however, amphetamine products have more potential for interactions than methylphenidate, since amphetamines are metabolized through cytochrome P450 2D6. However, caution is to be taken if methylphenidate is administered with tricyclic antidepressants, phenobarbital, phenytoin, or warfarin, since increased concentrations of these agents has been shown. The exact mechanism of this interaction is unknown.14 Methylphenidate and amphetamine products are contraindicated with monoamine oxidase inhibitors, since the psychostimulants inhibit monoamine oxidase and thus coadministration may cause a hypertensive crisis.14,15
Methylphenidate
Methylphenidate is the medication most widely used as first line treatment for ADHD. Methylphenidate exerts its action on both dopamine and norepinephrine transporters, thus it is believed to increase dopamine and norepinephrine in the prefrontal cortex.16 There are various formulations available. The immediate-release formulations are usually started for younger and smaller children (i.e., <16 kg) who are naïve to psychostimulants.1 The dose is usually given twice daily, about 4 hours apart (i.e., before school and at lunchtime) and can be given up to three times a day. There are liquid formulations available if the child cannot swallow the tablets whole, as is recommended. Food does not change the overall benefit of methylphenidate administration; however, with the long-acting, solid dosage formulations, a high-fat meal eaten at the same time of administration may delay the onset of action.16 Titration to maximum effective dose can occur quite quickly, since the clinical benefit can be seen within a few days to 1 week. Thus, clinicians can titrate doses every 7 days. Once patients are titrated to an appropriate dose, clinicians may consider once-daily formulations.
Methylphenidate once-daily formulations vary in pharmacodynamic effect even though, pharmacokinetically, the dose is listed as once daily for all sustained-release formulations. Concerta (McNeil Pediatrics, Titusville, NJ), Focalin XR (Novartis Pharmaceutics Corporation, East Hanover, NJ), Metadate CD (UCB Inc, Smyrna, GA), and Ritalin LA (Novartis Pharmaceutics Corporation, East Hanover, NJ) are the newest long-acting formulations available. Ritalin SR (Novartis), Metadate ER (UCB), and Methylin ER (Mallinckrodt Inc, Hazelwood, MO) are intermediate-acting formulations and may require either twice-daily dosing or an addition of an immediate-release preparation midday to achieve adequate benefit. When considering once-daily dosing, the clinician needs to take into account when the symptoms occur and the duration of effect that is warranted. The timing of peak concentrations and overall “coverage” vary greatly from product to product. With Ritalin LA and Focalin XR, the spheroidal oral drug absorption system (SODAS; Elan Corporation, Allegan, MI) formulation is used, which provides 2 peaks in concentration, mimicking the administration of twice-daily immediate-release preparations. Fifty percent of the drug is immediately released and 50% is released 4 hours later.17,18 The Metadate CD drug delivery system is a timed release bead formulation (Diffucaps, Adare Pharmaceuticals, Lawrenceville, NJ) that releases 30% of the dose as immediate release and 70% of the dose as extended release.19 Focalin XR, Metadate CD, and Ritalin LA are capsules that can be opened and sprinkled onto soft food, which aids administration, although the beads should not be chewed. The longest acting of the formulations is Concerta, which is the osmotic-controlled release oral delivery system (OROS; ALZA Corporation, Mountain View, CA) formulation that delivers 22% of the drug as immediate release and then by osmotic pressure, the remaining drug is released at a controlled rate.20 This tablet needs to be swallowed whole and not crushed or broken and the outer shell may be seen in the stool. Quillivant XR (Tris Pharma Inc, Monmouth Junction, NJ) is a once-daily suspension, available for patients who cannot take the solid dosage formulations.21 The suspension delivers methylphenidate as 20% immediate release and 80% extended release. Similar to the other extended-release formulations already discussed, a high-fat meal will delay the peak concentration by up to 1 hour. Specific instructions for reconstitution of the oral powder formulation by the pharmacist before dispensing are available in the product information.21
Dexmethylphenidate is the isomeric form of methylphenidate and is available as an immediate-release Focalin (Novartis), and long-acting Focalin XR formulation.22 The dose is about one-half of the racemic methylphenidate formulations. The long-acting formulation is the bimodal release system described above (SODAS).
Methylphenidate also is available as a transdermal patch, Daytrana (Noven Pharmaceuticals Inc, Miami, FL).23 The patch is applied to the hip area every day, typically for 9 hours. The family should apply the patch onto the child in the morning, 2 hours before the desired time of effect, and remove it after 9 hours; however, it should be noted that the drug's effect may continue for up to 2 to 3 hours after taking off the patch. The patch may be removed earlier than 9 hours if a shorter duration of effect is desired or if the patient is experiencing side effects later in the day. The patch is known to produce a rash and irritation at the application site in upwards of 40% of patients.24 The application site should be rotated every day to try and limit the potential for rash; however, if irritation appears, the patient may need a short course of topical corticosteroids and possibly a change to oral methylphenidate. The used patch should be disposed of properly, by folding it in half with the sticky sides stuck together to avoid abuse potential or accidental ingestion by a small child or pet.
Amphetamine
Even though the mechanism of action is believed to be similar for methylphenidate and amphetamine products, the amphetamine products also increase dopamine release. The amphetamine products also have more potential for decreased appetite.25 There are immediate-release tablets, extended-release capsules, and liquid available. The capsule formulations of Adderall XR (Shire US Inc, Wayne, PA), Dexedrine Spansules (Catalent Pharma Solutions, Winchester, KY), and Vyvanse (Shire US) can be opened and placed on soft foods.15,22,26 As with methylphenidate, food does not affect the overall benefit of amphetamines; however, there is a delay in absorption of about 2.5 hours if a high-fat meal is eaten with the mixed amphetamine salts.15 When comparing the immediate-release preparations of amphetamine/dextroamphetamine and methylphenidate, Pelham et al25 reported that amphetamine/dextroamphetamine seemed to have a longer, sustained effect at about half the dose of methylphenidate. However, dosing needs to be individualized by symptom response. Vyvanse (lisdexamfetamine dimesylate) is a prodrug that is converted to the active metabolite dextroamphetamine in the gut, which may reduce the potential for abuse.
Clinical Use of Psychostimulants
Selection of psychostimulants depends upon duration of coverage desired, formulation required, and cost. As mentioned earlier, the pharmacodynamic profile differs among the various agents. If a child or adolescent only needs help during school, the product chosen should provide about 6 to 8 hours of action, whereas if a child or adolescent needs benefit to maintain after-school activities, including homework and driving, a 12-hour product should be considered.16,27 The usual initial regimen starts with an immediate-release psychostimulant administered twice daily (e.g., 5 mg two times daily) for those that are smaller in weight (i.e., <16 kg),1 and then the dose is titrated to a maximum effective dose producing minimal side effects. In children who are older and larger, and adolescents, the smallest effective dose should be initiated and titrated upwards. Many of the sustained-release formulations are available in lower strengths and may be used as initial therapy. In clinical practice, with the newer once-daily formulations now available, most providers will start with a once-daily, long-acting formulation (e.g., Concerta, Ritalin LA, or Metadate CD). If sustained-release formulations are not providing adequate coverage, an immediate-release formulation is added in the afternoon. Unlike other medications used in pediatrics, weight-based dosage is not used routinely with these agents. These medications should be taken daily to gain complete clinical benefit. At least monthly follow-up is recommended with face-to-face interviews and completion of teacher and parent evaluations to determine effectiveness of the regimen.
Drug holidays may be used in a variety of situations. For example, in patients experiencing weight loss, a period off of drug may provide time for a child to gain some weight. Another common drug holiday that families request is time off of medication during the summertime. If a child ends a school year on medication and the family chooses to not administer medication during the summer, it is highly recommended to restart the medication at the end of summer break so that the child can start the new school year with medication. Restarting of the medication should be at least 2 weeks before the start of school in order to achieve maximum benefit. In any case, however, these “drug holidays” are less often implemented, owing to the chronic nature of ADHD. The medication may be discontinued temporarily during the school year once the student is established within his/her class to determine whether or not the medication is at the proper dose or if it is necessary to continue the medication. During any change in dose or trial off of medication, evaluations should be completed so as to discern if medication continues to be needed. If a child reaches the maximum daily dose recommended without clinical benefit, consider changing to a different psychostimulant, especially if no adverse reactions occurred. An example might be in a child who has been on Concerta at maximum daily doses with minimal clinical benefit, but has had no adverse reactions. A clinician may consider switching to an amphetamine-based product, e.g., Adderall (Teva Select Brands, Horsham, PA). However, if adverse drug reactions occur (i.e., decreased appetite, headache), the isomeric formulation of methylphenidate, dexmethylphenidate, could be considered. If side effects persist or if psychostimulants have not provided optimal benefit, non-psychostimulant therapy should be considered.
Atomoxetine
As noted above, psychostimulants are considered to be the first-line medications for the treatment of ADHD. However, some patients may not be able to take agents from this class owing to their inability to tolerate the side effect profile or the presence of a contraindicating factor, such as an allergy to a psychostimulant product or a comorbid tic condition (e.g., Tourette's disorder). Additionally, anywhere from 10% to 30% of patients will not respond to psychostimulants.28 Atomoxetine is an alternative agent for these patients. Atomoxetine is theorized to be a selective norepinephrine reuptake inhibitor.29 As a result of the inhibition, concentrations of norepinephrine and dopamine are increased in the prefrontal cortex of the brain, which is believed to control behaviors. The localization of this effect limits the impact of the medication on the remaining areas of the brain and may explain why atomoxetine lacks abuse potential.30 Therefore, unlike the psychostimulants, atomoxetine is not a controlled substance.
Unlike psychostimulants, the initial dosage for atomoxetine in younger children is based on weight and is then titrated after a minimum of 3 days to allow the patient time to adjust to the medication's impact and adverse drug reactions.29 For young children and adolescent patients weighing less than or equal to 70 kg, the initial dose is 0.5 mg/kg/day given once daily in the morning. After the recommended 3-day adjustment period to assess for tolerability, the dose can be increased to 1.2 mg/kg/day, if needed for efficacy. Doses larger than 1.4 mg/kg/day or 100 mg/day have not been found to improve results and should be avoided owing to increased risks for adverse reactions. Children 6 years of age or older, adolescent patients weighing greater than 70 kg, and adults are not dosed by weight, but instead have a standard initial dose of 40 mg daily. The dose can be titrated to 80 mg after the recommended 3-day adjustment period if needed. If this dose fails to control the patient's ADHD symptoms, it can be titrated to 100 mg after 2 to 4 weeks.31 In both age groups, total daily doses can be given as a single dose in the morning or can be split evenly between a morning dose and an early evening dose.29 Splitting the daily dose into 2 doses does not impact daytime results of the agent, but may decrease side effects experienced by the patient and improve symptoms in the home setting after school.32 This is most likely due to the product's duration of action, which is limited to near 9 hours, as noted in research and review articles by Wigal.31 The exact duration of action is variable between patients and may be due to alterations in metabolism, which will be discussed in the next paragraph. In contrast to the near immediate effects of the psychostimulants, atomoxetine may take 1 to 2 weeks before manifesting an initial response; after reaching the recommended maximum daily dose for his/her weight, a patient's symptoms may continue to improve for up to 2 months.32 If the patient fails to respond to atomoxetine or develops intolerable adverse reactions, the medication can be stopped abruptly without tapering.29
Atomoxetine is metabolized in the liver by the cytochrome P450 2D6 pathway.28,29 Approximately 7% of the Caucasian population are poor metabolizers within this pathway and may require dose adjustments. Similarly, patients using other medications that are known inhibitors of the 2D6 pathway will need to be followed up closely.33
Atomoxetine is relatively well tolerated by patients.34 Somnolence, dry mouth, nausea, constipation, dizziness, and decreased appetite are often seen early in the treatment course and tend to subside significantly over time.29 Insomnia may develop over time and may be worse in those patients with evening doses. Atomoxetine may mildly increase both heart rate and blood pressure, and may produce orthostatic hypotension, but does not impact growth.29,35
Two potentially fatal issues can be seen in patients taking atomoxetine, and pharmacists should counsel the patients and their families about monitoring for these reactions.29,33 As with other medications that impact the central nervous system neurotransmitters, atomoxetine has the potential to increase suicidal ideation in patients and this is listed as a Black Box warning in the product information. However, it is not considered a contraindication. The risk for this reaction is greatest when initially starting the medication or when dose adjustments are made. The patients and their families should be warned to watch for these types of thoughts or significant changes in behavior or mood and to report them immediately to a health care provider for close management. Similarly, patients and families will need to monitor for any evidence of liver failure, such as change in urine color or jaundice. If these symptoms appear, the medication should be stopped immediately and liver function tests performed.
Atomoxetine has been proven effective when compared to placebo in a number of double-blind, placebo controlled trials.36,37 Slightly less clear is the comparative efficacy of atomoxetine and psychostimulants. In 2010, May and Kratochvil38 published an evaluation comparing all psychostimulants to atomoxetine and indicated that in most trials the psychostimulants performed better than atomoxetine. However, when looking at individual agents, the results can differ. For example, immediate-release methylphenidate demonstrates similar efficacy to atomoxetine, but when comparing Concerta to atomoxetine, a clear advantage is observed with Concerta.39 As of 2013, only 1 small pilot study formally evaluated using atomoxetine with methylphenidate. Although the pilot study had promising results, this combination cannot be recommended at this time owing to the limited amount of data available.40
Alpha2-Agonists
Before the release of atomoxetine in 2002,29 there were no Food and Drug Administration (FDA) approved alternatives to psychostimulants for the management of ADHD symptoms. Immediate-release clonidine and guanfacine, alpha2-agonists used for blood pressure control, were used off-label as second-line agents for patients failing 2 psychostimulants or as adjunctive therapy for patients with suboptimal results with a psychostimulant alone.1,33,34 These agents were also used if the patient had a comorbid condition resulting in tics, such as Tourette's disorder, which could be exacerbated by psychostimulant therapy.35 However, these agents could be difficult for the patient to tolerate owing to their side effect profile and they may place the patient at a significant risk for rebound hypertension if abruptly discontinued. Recently, extended-release versions of each drug have become available, which minimize the initial drop in blood pressure and are better tolerated than their immediate-release counterparts.34 These agents should be considered for patients that have failed psychostimulant monotherapy or atomoxetine or require treatment for comorbid psychiatric conditions. Additionally, patients exhibiting aggression may benefit from alpha2-agonist therapy owing to its subtle sedative effect.41 The alpha2-agonists can be used as monotherapy or as adjunctive therapy in patients with suboptimal response to psychostimulants.42,43
The exact mechanism of action for the alpha2-agonists in ADHD is not known.34 The predominant theory is that these agents work by directly mimicking norepinephrine effects at the alpha2A adrenoreceptors in the prefrontal cortex. Guanfacine is selective for the alpha2A adrenoreceptors, while clonidine is active at the alpha2A,B, and C adrenoreceptors. Theoretically, this implies that guanfacine and clonidine will have similar efficacy via stimulation of the alpha2A adrenoreceptors, but guanfacine may have less sedation and impact on blood pressure owing to its lack of effect on the alpha2B and C adrenoreceptors.34
Each product has its own unique dosing regimen. Extended-release guanfacine, Intuniv (Shire US), is approved for patients older than 6 years and is initiated at a dose of 1 mg per day. At weekly intervals the daily dose can be increased by 1 mg until a response is seen or the daily dose reaches 4 mg. Doses larger than 4 mg have not been found to be more effective than smaller doses and increases the side effect potential of the drug. Doses can be taken in the morning or at bedtime, but should consistently be given at the same time each day.42 Improvement in symptoms is typically observed when the dose reaches 0.05 to 0.08 mg/kg/day.42
Extended-release clonidine, Kapvay (Shiongi Inc, Florham Park, NJ), is initiated at 0.1 mg at bedtime. At weekly intervals the daily dose can be titrated by 0.1 mg. Doses larger than 0.1 mg should be split into equal morning and evening doses. If equal doses are not possible, the larger dose should be at night.
The full impact of these agents on ADHD symptoms may not be seen until 7 to 14 days have elapsed.44 If it becomes necessary to stop either of these agents, it is important to taper the medication slowly to avoid rebound hypertension. Doses of extended-release guanfacine can be decreased by 1 mg every 3 to 7 days. Doses of extended-release clonidine are decreased by 0.1 mg every 3 to 7 days.42,43
Extended-release clonidine is metabolized in the liver to inactive metabolites. In terms of drug interactions, this metabolism is beneficial, since serum concentrations are not impacted. However, health care providers should remember that this agent will still exhibit drug interactions by potentiating adverse reactions, such as sedation or hypotension, or antagonizing the effects of other therapies. In contrast, extended-release guanfacine is metabolized by the cytochrome P450 3A4 pathway, and dose adjustments will be needed when using guanfacine with strong inducers or inhibitors of this pathway.42,43
In the long term, the alpha2-agonists are generally well tolerated. Upon initiation of either agent, the patient may experience some transient adverse drug reactions. The most common of these reactions are dizziness, drowsiness, fatigue, and headache. It should be noted that the alpha2-agonists may exacerbate depression and they should not be used in patients with pre-existing depression.42,43 These agents pose a risk for hypotension, orthostatic hypotension, and bradycardia; therefore, patients should be monitored closely at initiation, at subsequent evaluations, and with each dose adjustment. Over time, the patient will develop tolerance to the blood pressure change.34,42,43 Guanfacine has been shown to be less sedating and less potent in decreasing blood pressure and may be tolerated better by patients sensitive to these issues.38 When comparing the alpha2-agonists to the psychostimulants as monotherapy for ADHD, the alpha2-agonists do not perform nearly as well. Assessments indicate that Intuniv and Kapvay are approximately 40% to 75% as effective as the psychostimulants in managing the primary symptoms of ADHD.45,46 However, these agents can be used along with a psychostimulant to provide better results if the patient has suboptimal response to the psychostimulant alone.38,47,48
Non-Standard Therapies
The American Academy of Pediatrics guideline for the diagnosis, evaluation, and treatment of ADHD in children and adolescents recognizes the role of the psychostimulants, atomoxetine, and the alpha2-adrenergic agonists in the management of patients with ADHD.44 Other agents (e.g., venlafaxine, monoamine oxidase inhibitors) have been studied for the potential management of ADHD symptoms; however, these options are not currently FDA-indicated for the condition or suggested for use within the major guidelines.
BEHAVIORAL-BASED THERAPY
There is still a lot of discussion as to where behavioral therapy fits into the armamentarium for ADHD treatment. Should behavior therapy be used as first-line therapy for all patients or only those who are younger or with milder symptoms? Is medication plus behavior therapy the best plan for all patients? Many studies have looked at these questions, and guidelines and recommendations are available from the American Psychiatric Association, the American Medical Association, and the American Academy of Child and Adolescent Psychiatry. Unfortunately, all 3 organizations have slightly different opinions. With more recent studies (e.g., the multimodal treatment study of children with ADHD [MTA]), there is evidence suggesting that researchers have not been identifying and monitoring for the correct outcomes.49 Looking more at functional outcomes versus the DSM listing of symptoms may be more beneficial in the long term. Some of these functional outcomes include activities of daily living, peer and parent relationships, and academic achievement.50–51
Multimodal Treatment Study of Children With Attention-Deficit/Hyperactivity Disorder
The MTA study was started in 1992 and is following up 579 children long term with ADHD. There is now a published 8-year follow-up study in which school-aged children (7–9 years old) have been evaluated after initial intensive randomized therapy with either behavioral-based treatment alone, medication alone, a combination of behavior therapy plus medication or community care.49 The initial intensive treatment phase was for 14 months. Thereafter, the children were followed up “naturally” and without randomization. In a 14-month interim analysis, there was more clinical improvement in the combination therapy group (behavior plus medication therapy). However, it was noted that the children in the MTA medication treatment arms received larger doses of medication for a longer period of time and medication management by a clinical pharmacist occurred monthly, compared to the community-treated group where this did not occur.11,52,53 In the 8-year follow-up study, there appears to be a loss of sustained effects of medication after 2 years. Since the long-term follow-up was done “naturally” and without randomization, it is very difficult to know if sustained clinical benefit may have been maintained with intensive medication management plus behavioral therapy. The one outcome that does seem to hold true, though, is that patient's initial symptoms and severity of illness better predict future outcome than type and intensity of therapy initiated. Children diagnosed with ADHD do poorer overall than their peers without ADHD.49
SUMMARY
Despite the multitude of pharmacological agents for the treatment of ADHD, there are many questions remaining regarding the treatment algorithm that will provide the most clinical benefit with the least side effects. In particular, it is important to consider long-term efficacy of psychostimulant use and protection or lack thereof for delinquent behavior and substance abuse. Despite small studies showing some “protection” against these adolescent behaviors, it is still unknown if ADHD medications will decrease substance use. The MTA study looked at substance use and overall concluded that nicotine and marijuana use is increased in children with an ADHD diagnosis, but current standard pharmacological treatment has not conclusively demonstrated decreases or “protection” against this behavior.61 Psychostimulants remain the agents of choice, with atomoxetine, a non-controlled substance, as an alternative option for those patients unable to tolerate or having failed psychostimulant therapy. Alpha2-agonists offer another option for those patients with comorbid conditions such as tics or aggression. Non-standard therapies, including antidepressants and monoamine oxidase inhibitors, have been studied, but their place in therapy has yet to be determined. Questions still remain as to the exact duration of therapy, but trials off of medication, i.e., drug holidays, may be warranted to determine continued need for medication. Thus, choice of medication, dose, monitoring, and drug holiday options need to be individually tailored.
Abbreviations:
- ADHD
attention-deficit/hyperactivity disorder
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders, fifth edition
- FDA
The United States Food and Drug Administration
- MTA
multimodal treatment study of children with attention-deficit/hyperactivity disorder
- OROS
osmotic-controlled release oral delivery system
- SODAS
spheroidal oral drug absorption system
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Pliszka SR, Bernet W, Bukstein O et al. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit-hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894–921. doi: 10.1097/chi.0b013e318054e724. for the American Academy of Child and Adolescent Psychiatry Work Group on Quality Issues. [DOI] [PubMed] [Google Scholar]
- 2.Willcut EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9(3):490–499. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polanczyk G, de Lima MS, Horta BL et al. The world-wide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 4.Frochlich TE, Lamphear BP, Epstein JN et al. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of U.S. children. Arch Pediatr Adolesc Med. 2007;161(9):857–864. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- 5.Ramtekkar UP, Reiersen AM, Todorov AA, Todd RD. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnosis. J Am Acad Child Adolesc Psychiatry. 2010;49(3):217–228. [PMC free article] [PubMed] [Google Scholar]
- 6.Dopheide JA, Pliszka SR. Attention-deficit-hyperactivity disorder: an update. Pharmacotherapy. 2009;29(6):656–679. doi: 10.1592/phco.29.6.656. [DOI] [PubMed] [Google Scholar]
- 7.Willcutt EG, Doyle AE, Nigg JT et al. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Hunt RD. Functional roles of norepinephrine and dopamine in ADHD. Medscape Psychiatry & Mental Health. March 2006;11(1) http://www.medscape.org/viewarticle/523887. Accessed April 19, 2016. [Google Scholar]
- 9.Faraone SV, Perlis RH, Doyle AE et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 11.MTA Cooperative Group. National Institute of Mental Health multimodal treatment study of ADHD follow-up: changes in effectiveness and growth after the end of treatment. Pediatrics. 2004;113(4):762–769. doi: 10.1542/peds.113.4.762. [DOI] [PubMed] [Google Scholar]
- 12.Harstad EB, Weaver AL, Katusic SK et al. ADHD, stimulant treatment, and growth: a longitudinal study. Pediatrics. 2014;134:e935–e944. doi: 10.1542/peds.2014-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrin JM, Friedman RA, Knilans TK. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122:451–453. doi: 10.1542/peds.2008-1573. [DOI] [PubMed] [Google Scholar]
- 14.Ritalin, Ritalin SR [product information] East Hanover, NJ: Novartis Pharmaceuticals Corporation; April 2015. http://pharma.us.novartis.com/product/pi/pdf/ritalin_ritalin-sr.pdf. Accessed April 1, 2016. [Google Scholar]
- 15.Adderall XR [product information] Wayne, PA: Shire US Inc.; April 2015. http://pi.shirecontent.com/PI/PDFs/Adderal-lXR_USA_ENG.PDF. Accessed April 1, 2016. [Google Scholar]
- 16.Markowitz JS, Straughn AB, Patrick KS. Advances in the pharmacotherapy of attention-deficit-hyperactivity disorder: focus on methylphenidate formulations. Pharmacotherapy. 2003;23(10):1281–1299. doi: 10.1592/phco.23.12.1281.32697. [DOI] [PubMed] [Google Scholar]
- 17.Ritalin LA [product information] East Hanover, NJ: Novartis Pharmaceuticals Corporation; July 2015. http://www.pharma.us.novartis.com/product/pi/pdf/ritalin_la.pdf. Accessed April 1, 2016. [Google Scholar]
- 18.Focalin XR [product information] East Hanover, NJ: Novartis Pharmaceuticals Corporation; April 2015. http://www.pharma.us.novartis.com/product/pi/pdf/focalinXR.pdf. Accessed April 1, 2016. [Google Scholar]
- 19.Metadate CD [product information] Smyrna, GA: UCB Inc.; June 2009. http://www.ucb.com/_up/ucb_com_products/documents/Metadate%20CD%2011E%2004-2012.pdf. Accessed April 1, 2016. [Google Scholar]
- 20.Concerta [product information] Titusville, NJ: McNeil Pediatrics – a division of Ortho-McNeil-Janssen Pharmaceuticals Inc; November 2010. http://www.concerta.net/prescribing-information.html. Accessed April 1, 2016. [Google Scholar]
- 21.Quillivant XR [product information] Monmouth Junction, NJ: Tris Pharma Inc; February 2016. http://labeling.pfizer.com/ShowLabeling.aspx?id=965. Accessed April 1, 2016. [Google Scholar]
- 22.Dexedrine [product information] Winchester, KY: Catalent Pharma Solutions; February 2015. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a37b6ef9-78b4-4b18-8797-ecb583502500. Accessed April 1, 2016. [Google Scholar]
- 23.Daytrana [product information] Miami, FL: Noven Pharmaceuticals Inc; August 2015. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2c312c31-3198-4775-91ab-294e0b4b9e7f. Accessed April 1, 2016. [Google Scholar]
- 24.Anderson VR, Scott LJ. Methylphenidate transdermal system in attention-deficit hyperactivity disorder in children. Drugs. 2006;66(8):1117–1126. doi: 10.2165/00003495-200666080-00007. [DOI] [PubMed] [Google Scholar]
- 25.Pelham WE, Aronoff HR, Midlam JK et al. A comparison of Ritalin and Adderall: efficacy and time-course in children with attention-deficit/hyperactivity disorder. Pediatrics. 1999;103:1–14. doi: 10.1542/peds.103.4.e43. [DOI] [PubMed] [Google Scholar]
- 26.Vyvanse [product information] Wayne, PA: Shire US Inc; January 2015. http://pi.shirecontent.com/PI/PDFs/Vyvanse_USA_ENG.pdf. Accessed April 1, 2016. [Google Scholar]
- 27.Swanson JM, Wigal SB, Wigal T et al. A comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (The Comacs Study) Pediatrics. 2004;113(3):e206–e216. doi: 10.1542/peds.113.3.e206. [DOI] [PubMed] [Google Scholar]
- 28.Mohammadi MR, Akhondzadeh S. Pharmacotherapy of attention-deficit/hyperactivity disorder: nonstimulant medication approaches. Expert Rev Neurother. 2007;7(2):195–201. doi: 10.1586/14737175.7.2.195. [DOI] [PubMed] [Google Scholar]
- 29.Straterra [product information] Indianapolis, IN: Lilly USA LLC; April 2015. http://pi.lilly.com/us/strattera-pi.pdf. Accessed March 31, 2016. [Google Scholar]
- 30.Banaschewski T, Roessner V, Dittmann RW et al. Non-stimulant medications in the treatment of ADHD. Eur Child Adolesc Psychiatry. 2004;13:102–116. doi: 10.1007/s00787-004-1010-x. [DOI] [PubMed] [Google Scholar]
- 31.Wigal SB. Efficacy and safety limitations of attention-deficit hyperactivity disorder pharmacotherapy in children and adults. CNS Drugs. 2009;23(suppl 1):21–31. doi: 10.2165/00023210-200923000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Waxmonsky JG, Waschbusch DA, Akinnusi O, Pelham WE. A comparison of atomoxetine administered as once versus twice daily dosing on the school and home functioning of children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21(1):21–32. doi: 10.1089/cap.2010.0042. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan G, Newcorn JH. Pharmacotherapy for child and adolescent attention-deficit hyperactivity disorder. Pediatr Clin North Am. 2011;58:99–120. doi: 10.1016/j.pcl.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Raga J, Knect C, Szerman N, Martinez MI. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27:15–30. doi: 10.1007/s40263-012-0019-9. [DOI] [PubMed] [Google Scholar]
- 35.Spencer TJ, Kratochvil CJ, Sangal RB et al. Effects of atomoxetine on growth in children with attention-deficit/hyperactivity disorder following up to 5 years of treatment. J Child Adolesc Psychopharmacol. 2007;17(5):689–699. doi: 10.1089/cap.2006.0100. [DOI] [PubMed] [Google Scholar]
- 36.Cheng JY, Chen RY, Ko JS et al. Efficacy and safety of atomoxetine for attention-deficit/hyperactivity disorder in children and adolescents: meta-analysis and meta-regression analysis. Psychopharmacology. 2007;194(2):197–209. doi: 10.1007/s00213-007-0840-x. [DOI] [PubMed] [Google Scholar]
- 37.Kratochvil CJ, Mitlon DR, Vaughan BS, Greenhill LL. Acute atomoxetine treatment of younger and older children with ADHD: a meta-analysis of tolerability and efficacy. Child Adolesc Psychiatry Ment Health. 2008;2:25–34. doi: 10.1186/1753-2000-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.May DE, Kratochvil CJ. Attention-deficit hyperactivity disorder. Drugs. 2010;70(1):15. doi: 10.2165/11530540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Pediatr Drugs. 2009;11(3):203–226. doi: 10.2165/00148581-200911030-00005. [DOI] [PubMed] [Google Scholar]
- 40.Carlson GA, Dunn D, Kelsey D et al. A pilot study for augmenting atomoxetine with methylphenidate: safety of concomitant therapy in children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiatry Ment Health. 2007;1:10–17. doi: 10.1186/1753-2000-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunt RD, Capper L, O'Connell P. Clonidine in child and adolescent psychiatry. J Child Adolesc Psychopharmacol. 1990;1(1):87–102. doi: 10.1089/cap.1990.1.87. [DOI] [PubMed] [Google Scholar]
- 42.Intuniv [product information] Wayne, PA: Shire US Inc; February 2013. http://pi.shirecontent.com/PI/PDFs/Intuniv_USA_ENG.pdf. Accessed January 31, 2016. [Google Scholar]
- 43.Kapvay [product information] Florham Park, NJ: Shiongi Inc; February 2013. http://kapvay.com/pdf/kapvay-conc-v1-USPI.pdf. Accessed July 9, 2015. [Google Scholar]
- 44.American Academy of Pediatrics. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007–1022. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sallee FR, Eaton K. Guanfacine extended-release for attention deficit/hyperactivity disorder. Expert Opin Pharmacother. 2010;11(15):2549–2556. doi: 10.1517/14656566.2010.517523. [DOI] [PubMed] [Google Scholar]
- 46.Jain R, Segal S, Kollin SH, Khayrallah M. Clonidine extended-release tablets for pediatric patients with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(2):171–179. doi: 10.1016/j.jaac.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Burkstein OG, Head J. Guanfacine ER for the treatment of adolescent attention-deficit/hyperactivity disorder. Expert Opin Pharmacother. 2012;13(15):2207–2213. doi: 10.1517/14656566.2012.721778. [DOI] [PubMed] [Google Scholar]
- 48.Sallee FR. The role of alpha2-adrenergic agonists in attention-deficit/hyperactivity disorder. Postgrad Med. 2010;122(5):78–87. doi: 10.3810/pgm.2010.09.2204. [DOI] [PubMed] [Google Scholar]
- 49.Molina BSG, Hinshaw SP, Swanson JM et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pelham WE, Fabiano GA. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2008;37(1):184–214. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- 51.Creating a daily report card from home; 2002. How to establish a daily report card (school-home note); cited 2013 April 24 [treatment materials] New York: University of Buffalo Center for Children and Families; http://ccf.buffalo.edu/resources_downloads.php#PT. Accessed May 19, 2016. [Google Scholar]
- 52.Jensen PS, Arnold LE, Swanson JM et al. 3-year follow-up of the HIMH MTA study. J Am Acad Child Adolesc Psychiatry. 2007;46(8):989–1002. doi: 10.1097/CHI.0b013e3180686d48. [DOI] [PubMed] [Google Scholar]
- 53.Murray DW, Arnold E, Swanson J et al. A clinical review of outcomes of the multimodal treatment study of children with attention-deficit/hyperactivity disorder (MTA) Curr Psychiatry Rep. 2008;10:424–431. doi: 10.1007/s11920-008-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.United States Department of Health and Human Services. US Food and Drug Administration. Questions and answers regarding methylphenidate hydrochloride extended release tablets (generic Concerta) made by Mallinckrodt and Kudco. http://www.fda.gov/drugs/drugsafety/ucm422569.htm. Accessed April 1, 2016.
- 55.Aptensio XR [product information] Greenville, NC: Patheon Manufacturing Services LLC; April 2015. http://aptensioxr.com/resources/full-prescribing-information.pdf. Accessed April 1, 2016. [Google Scholar]
- 56.Zenzedi [product information] Atlanta, GA: Arbor Pharmaceuticals LLC; January 2014. http://zenzedi.com/docs/PIand-MedicationGuide.pdf. Accessed February 2, 2016. [Google Scholar]
- 57.Quillichew ER [product information] Monmouth Junction, NJ: Tris Pharma Inc; December 2015. http://labeling.pfizer.com/ShowLabeling.aspx?id=2577#page2. Accessed February 2, 2016. [Google Scholar]
- 58.Adzenys XR-ODT [product information] Grand Prairie, TX: Neos Therapeutics LP; January 2016. http://www.adzenysxrodt.com/files/PI-Adzenys-XR-ODT-FDA.pdf. Accessed February 2, 2016. [Google Scholar]
- 59.Cortese S, Holtmann M, Banaschewski T et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013;54(3):227–246. doi: 10.1111/jcpp.12036. [DOI] [PubMed] [Google Scholar]
- 60.Dalsgaard S, Kvist AP, Leckman JF et al. Cardiovascular safety of stimulants in children with attention-deficit/hyperactivity disorder: a nationwide prospective cohort study. J Child Adolesc Psychopharmacol. 2014;24(6):302–310. doi: 10.1089/cap.2014.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Molina BSG, Hinshaw SP, Arnold LE et al. Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adolesc Psychiatry. 2013;52(3):250–263. doi: 10.1016/j.jaac.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


