Abstract
Cardiac resynchronization therapy is an established treatment modality in heart failure. Though non-response is a serious issue. To address this issue, a good understanding of the electrical activation during underlying intrinsic ventricular activation, biventricular as well as right- and left ventricular pacing is needed. By interpreting the 12-lead electrocardiogram, possible reasons for suboptimal treatment can be identified and addressed. This article reviews the literature on QRS morphology in cardiac resynchronization therapy and its role in optimization of therapy.
Introduction
Cardiac resynchronization therapy (CRT) is a useful treatment modality in patients with left ventricular (LV) dysfunction and ventricular conduction disturbances. The delayed ventricular electrical activation results in a dyssynchronous ventricular contraction. CRT aims to restore the dyssynchronous contraction and has shown to result in improved quality of life, exercise tolerance, cardiac function, and survival.[1-5]
As a significant amount of patients does not respond to CRT, a lot of research has deservedly focused on optimization, and better patient selection. Various techniques have been studied to identify the proper CRT candidate. Interestingly, of all the techniques studied, the most trustful method to identify the presence of ventricular dyssynchrony is the use of the “simple” 12 lead electrocardiogram (ECG).[6,7] This is the reason why current guidelines only include ECG parameters as measurement of ventricular dyssynchrony, which are QRS duration and the presence of left bundle branch block (LBBB) QRS morphology.[8]
Although many studies focus on the ECG to select patients for CRT, only a limited number of studies focus on the ECG during CRT. This seems remarkable as the ECG during CRT can provide important information on LV lead location, presence of scar at LV pacing site, and fusion of intrinsic activation or RV pacing with LV pacing. In this manuscript we review literature on QRS patterns during CRT.
QRS Morphology Patterns
The QRS pattern in CRT is usually composed of two merging activation wave fronts, which makes interpretation more difficult. CRT is mostly achieved by a combination of RV and LV pacing (biventricular pacing) or LV pacing fused with intrinsic ventricular activation. Therefore, it is important to understand the timing and direction of the activation wave fronts during a) underlying intrinsic ventricular activation, b) RV only pacing, and c) LV only pacing, before the QRS patterns in d) biventricular pacing can be understood.
QRS Pattern Of Underlying Ventricular Activation
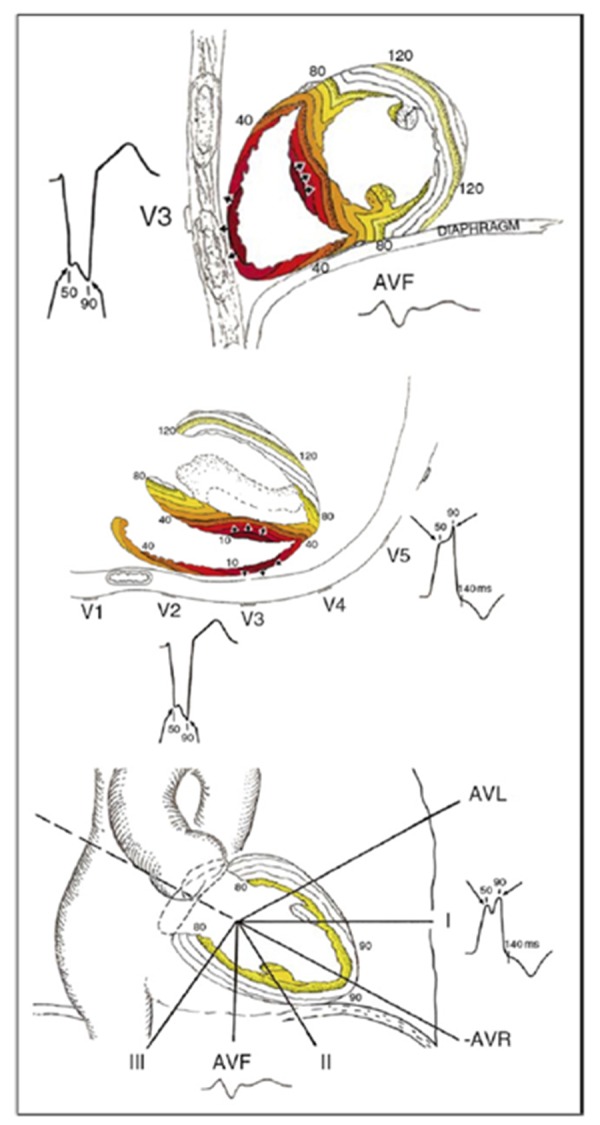
In several studies it has been shown that the ideal patient, who responds to CRT, is the patient with underlying LBBB. In patients with LBBB, conduction through the right bundle branch is not affected and the ventricular activation begins in the right ventricle, before it proceeds to the LV endocardium. The LV endocardium is reached through the septum, which takes 40 to 50 ms. This transseptal conduction time can however be prolonged in the presence of heart failure.[9] It then requires another 50 ms to propagate to the endocardium of the posterolateral wall and takes an additional 50 ms to activate the myocardium at this side of the LV. Producing a total QRS duration of 140 to 150 ms (Figure 1).
Figure 1. QRS morphology in complete left bundle branche block. The LBBB activation sequence and representative QRS-T wave forms are depicted in their anatomic locations for the sagittal, transverse, and frontal planes. Figure used from Strauss et al.[10].
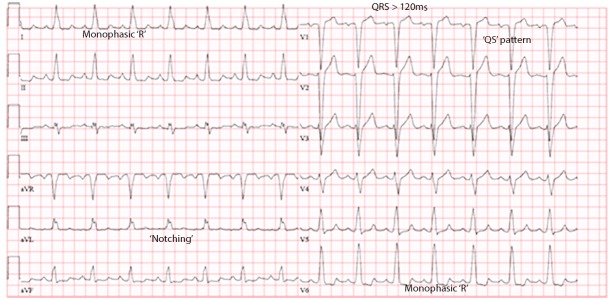
Conventional ECG criteria to describe LBBB include a QRS duration ≥120 ms, QS or rS in lead V1, and a monophasic R wave with no Q waves in leads V6 and I (Figure 2). Strauss et al.[10] strongly supported that notched or slurred R waves should also be present in lead I, aVL, V5, or V6, as demonstrated in figure 1. The first notch, which occurs approximately 50 ms after onset of the QRS, represents the electrical depolarization of the septal endocardium. The second notch occurs when the depolarization wave front begins to reach the epicardium of LV free wall and endocardium of the LV lateral wall. The reason there is slurring with little change in QRS amplitude between the 2 notches is that the magnitude and direction of the mean electrical vector remains constant once depolarization reaches the endocardium of the LV because it has to proceed outward in the septum and around the LV to the lateral free wall. These notches are best seen in leads I, aVL, V1, V2, V5, and V6.
Figure 2. Conventional ECG criteria for left bundle branche block. (1) QRS duration >120 ms, (2) QS in lead V1, (3) monophasic R wave with no Q waves in leads V6 and I. Also included ‘notching’ as described by Strauss et al.[10].
Eventhough studies show LBBB patients to have higher responserates, the large randomized clinical trial such as REVERSE, MADIT-CRT and RAFT have used LBBB criteria that are nonspecific. (QRS≥120ms, rS/QS in V1). Therefore, an important part of patients included in these large trials would have had a non-LBBB QRS morphology when more specific (ESC, AHA or Strauss) criteria would have been used. Studies of activation mapping in non-LBBB patients however, are less common. A recent study using epicardial activation mapping showed that intraventricular conduction delay (IVCD) was associated with a significantly more heterogeneous ventricular activation than in LBBB. Though mean total LV activation time was significantly shorter than in LBBB patients. Possibly because of lesser and more favorable ‘lines of block’ as visualized by ECG mapping. This results in significantly less ventricular electrical uncoupling (VUE) and interventricular dyssynchrony.[11] As a consequence more studies are needed to
identify the LBBB criteria best correlated to outcomes in CRT, and
to identify which patients with a non-LBBB QRS morphology could still benefit from CRT.
QRS Pattern During Rv Pacing
Different RV lead locations have been studied in CRT. RV apex, RV septal and RV outflow tract region are the locations used to target RV pacing. Studies up till now have not shown any differences in outcomes of CRT for the different RV pacing locations.[12-14] In clinical practice the RV pacing site that is mostly used in CRT is the RV apex.
RV pacing results in a LBBB like QRS pattern in the precordial leads with a negative QRS complex in lead V1 recorded at the 4th intercostal space. RV apex pacing usually produces a left superior paced QRS axis in the frontal plane as the activation spreads from right to left and superiorly away from the apex. Occasionally a right superior QRS axis is found in RV apex pacing, especially with enlarged and leftward displaced hearts.
Pacing from a septal RV lead position results in a more horizontal to left inferior heart axis, as in normal intrinsic ventricular activation.[15,16] Positioning of the RV pacing lead at the RV outflow tract shifts the paced QRS axis further to left inferior or even right inferior, as the pacing site shifts more superior, close to the pulmonary valve.
A dominant R wave in lead V1 is considered to be right bundle branch block (RBBB) pattern and associated with a pacing site on the LV free wall. Therefore, a positive QRS complex during RV pacing should prompt evaluation of a RV pacing lead. RV pacing lead can for example be accidently placed in the mid cardiac vein. Firstly however, the position of precordial leads V1 and V2 should be checked as a dominant R wave can sometimes be recorded with ‘misplacement’ at the third intercostal space.[17] Nevertheless, an initial small “r” in lead V1 is also often seen in uncomplicated RV pacing. To our experience this initial “r” in lead V1 is most often seen with an infero-septal RV lead position resulting in a superior spread of activation with late activation of the RV outflow tract. As a consequence, an initial “r” wave in lead V1 during biventricular pacing does not necessarily indicates contribution from LV pacing.
Differences between RV Pacing and LBBB
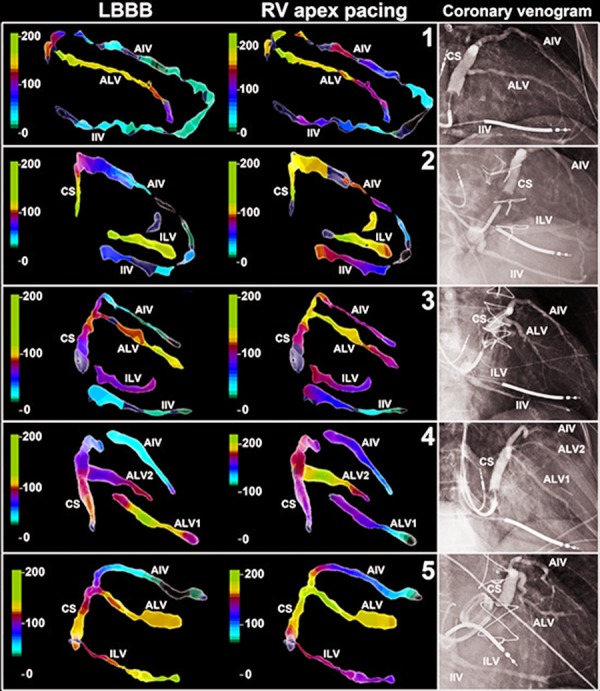
RV apex pacing QRS pattern is often considered to be very similar to LBBB QRS pattern. Therefore in animal experimental studies RV apex pacing is often performed as surrogate for endogenous LBBB.[18] Detailed analysis of the ventricular activation patterns of RV pacing and LBBB however, revealed some clear differences. In general, QRS duration is usually more prolonged in RV pacing as compared to LBBB. Left ventricle activation is affected in several ways. In RV pacing, transseptal activation time is decreased in most patients, but because of an increase in LV free wall activation time, a mild net increase in total LV activation time is the result.19 RV activation itself is prolonged in RV pacing, as compared to intrinsic activation by the his-purkinje system in LBBB.[20,21] In LBBB the activation wave front spreads in a circumferential direction whereas the activation wave front has a more apico-basal direction in RV apex pacing.[21-23] As a consequence the area of latest activation is located more basally in the lateral wall as compared to LBBB (Figure 3).[23,24] Moreover, due to a delayed RV activation and LV activation in an apico-basal direction the interventricular dyssynchrony is often less pronounced as compared to LBBB.[21] Whether less interventricular dyssynchrony during RV pacing as compared to LBBB is associated with decreased CRT response, still needs to be investigated.
Figure 3. Increased delay in LV electrical activation during RV apex pacing as compared to intrinsic LBBB. Local electrical activation time has been projected on the coronary venous electro-anatomic maps using the same color coding for both intrinsic LBBB and RV apex pacing. AIV = anterior inter-ventricular vein, ALV-1= first anterolateral vein, ALV-2 = second antero-lateral vein, CS = coronary sinus, LBBB = left bundle branch block, LEAT = local electrical activation time, LV = left ventricle/ventricular, RV = right ventricle/ ventricular, RAO = right anterior oblique view. Figure adapted from Mafi Rad et al.[23].
QRS Pattern During LV Pacing
Since the beginning of CRT the target location of the LV lead is the LV free wall in a mid-lateral segment.[25] There is increasing evidence that LV lead placement in the region of the latest activation, defined either electrically or mechanically, results in a better response to CRT.[26-28] This region can be reached epicardially via a tributary of the coronary sinus, or surgical placement. Also, endocardial LV lead placement has been described by using an atrial transseptal, ventricular transseptal or transapical approach. However clinical implementation of these approaches awaits more experience and studies on long-term results.[29-32] Therefore information on myocardial activation in pacing using these approaches remains scarce.
For analyzing the ECG during LV pacing it is important to program a very short AV delay or, even better, to stimulate in VVI mode to avoid fusion with intrinsic activation via the right bundle. Fusion produces electrical resynchronization of the two wave fronts coming from the paced LV and intrinsic activated RV (see below). Pacing from the coronary venous system usually results in stimulation of the LV free wall. Therefore resulting in a right bundle branch block QRS pattern with a dominant R wave in lead V1. Exceptions exist with LV lead pacing positioned in the mid cardiac vein with preferential exit to the RV or a LV lead advanced deep in the great cardiac vein resulting in stimulation of the RV outflow tract rather than the LV anterior wall.
Therefore, as a first step in the evaluation of the LV pacing a RBBB QRS pattern should be present in lead V1 (Figure 4, step 1). Next, the frontal plane axis during LV pacing should be used to identify the pacing site in the circumferential direction.[33] A paced QRS with either a left or right superior axis is associated with an inferior or infero-lateral LV lead position. On the other hand, a left inferior or right inferior paced QRS is associated with anterior or antero-lateral LV lead position (Figure 4, step 2). It should be noted that LV pacing does not necessarily results in a negative QRS in lead I. When LV pacing is performed from the basis of the LV, especially in dilated and leftward displaced hearts, the activation spreads from basis to apex and from left to right, resulting a positive QRS in lead I (Figure 5). As a consequence, the QRS axis in the frontal plane can shift from left inferior during LV pacing proximal in the antero-lateral vein to right inferior during LV pacing more distally in the anterolateral vein. It has recently been suggested that the paced QRS axis could not be used for identification of the LV pacing site.34 It was however not taken into account that the QRS-axis shifts depending on the apex-to-base level and that it can change between patients depending on left-ward displacement of the heart.
Figure 4. Protocol for determination of the LV lead position using the LV-paced QRS morphology, based on ECGs from LV originating ventricular tachycardia. Step 1: A positive QRS complex in V1 indicates an LV lead position at the LV free wall. Step 2: Lead aVF differentiates the LV lead position in the circumferential direction with a negative QRS complex indicating a more inferolateral position. Step 3: Trace the transition from positive to negative QRS complexes in the precordial leads to determine the apico-basal direction. Figure used from Van Deursen et al.[33].
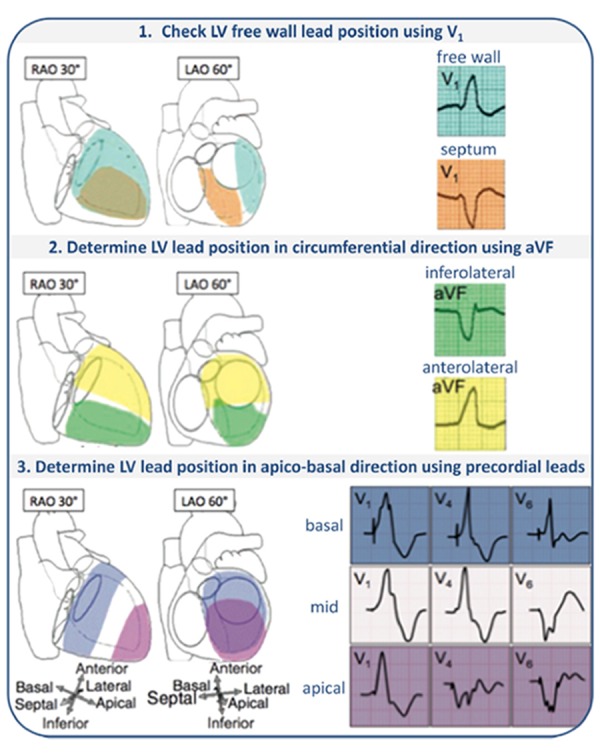
Figure 5. A) ECG obtained from a patient during LV pacing. Note the positive QRS complex in lead I during LV pacing due to B) a very basal LV lateral lead position as can be seen on the chest X-ray.
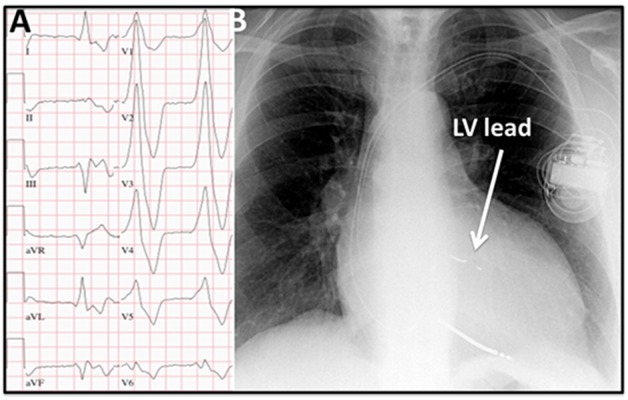
The QRS transition pattern in the precordial leads during LV pacing can be used to differentiate between basal, mid, or apical LV lead position.[33] This transition pattern seems independent of the circumferential orientation.[34] LV pacing from the true LV apex has an RBBB pattern in lead V1 with an early transition to a predominantly negative QRS complex in V2. Positive QRS concordance during LV pacing or late transition (later than V5) suggests a basal LV lead position, a QRS transition pattern in V4–V5 suggests of a midlevel LV lead position, and a QRS transition pattern earlier than V4 indicates a more apical LV lead position (figure 4, step 3).
Also important to analyze during LV pacing is pacing latency. The LV pacing latency is defined as the interval from the pacemaker stimulus to the onset of the earliest paced QRS complex. Assessment of pacing latency requires a 12-lead ECG since an initial isoelectric QRS complex can mimic latency. Endocardial RV pacing usually results in minimal pacing latency (<40ms), but this phenomenon is much more frequent in epicardial LV pacing from the coronary veins.[35-36] This difference in prevalence can be explained by the longer distance from the electrode to the subendocardial His-Purkinje system. Where the impuls has to travel through venous tissue and epicardial fat, the naturally slower epicardial propagation, especially in diseased myocardium, and additional antiarrhythmic drug effects on the myocardium. Prolonged LV pacing latency during simultaneous biventricular pacing can consequently produce an ECG pattern dominated by RV pacing, thus resulting in inadequate CRT.[37]
Also important to realize is that the QRS morphology evaluation during LV pacing is of predictive value for response to CRT. A relatively narrow QRS complex during LV pacing is associated with a better response to CRT.[22-38] Both avoidance of fractionation and large QRS width can be indirect tools to prevent LV pacing in a region of poor conduction due to scar or fibrosis.[39,40]
ECG Pattern During Biventricular Pacing
The ECG during biventricular pacing is often not easy to analyze because of merging wave fronts. CRT is most commonly achieved by using biventricular pacing, resulting in merging wave fronts of RV and LV pacing. The CRT device allows programming of the atrioventricular and interventricular delay in order to optimize the positive effects of CRT. Echocardiography, ECG or hemodynamic measurements are often used to accomplish optimization of CRT programming. However, these forms of optimization are often resource-intensive and haven’t been shown to be beneficial in any large multi-center randomized clinical trial so far. As a consequence, only a minority of physicians routinely optimize AV- and VV-delays in their patients.
Various studies have shown that LV pacing alone can be as effective as biventricular pacing.[41-45] Especially in patients with normal atrioventricular conduction, when LV only pacing is adequately timed with intrinsic activation, response in cardiac function improvement can be even superior to that in biventricular pacing.[42,43] However, adequately timed fusion at rest can be lost as atrioventricular conduction changes during exercise. Algorithms which promote intrinsic activation-based LV pacing should automatically adapt the atrio-left-ventricular pacing delay by periodical evaluation of the intrinsic atrio-right-ventricular conduction time.[44] Studies validating these algorithms are needed to be able to use this pacing mode in daily practice. The QRS axis in the frontal plane during biventricular pacing is most often directed towards the right superior quadrant resulting in a dominant R wave in lead aVR. Sometimes, with a more posterior LV lead position, the QRS axis is directed towards a left superior quadrant. However, a QRS axis in the other quadrants does not necessarily indicate inappropriate programming or LV lead position. Especially with fusion of LV pacing with intrinsic activation and/or a basal LV lead position a normal QRS axis can be found in CRT sometimes.
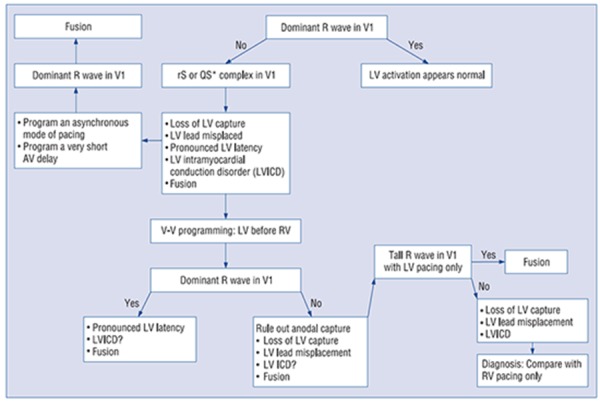
To assess whether there is contribution of LV pacing, QRS morphology has to be evaluated in the precordial leads. The QRS complex during biventricular pacing most often has a dominant R wave in lead V1-2, suggestive of contribution from LV pacing. However, a dominant R wave in lead V1 is not diagnostic of biventricular pacing as RV only pacing occasionally produces athe same pattern, as described earlier. Therefore, adequate assessment of biventricular pacing should include evaluation of RV only and LV only pacing. A negative QRS complex in lead V1 should warrant further evaluation, although it does not necessarilyy indicate inadequate CRT, as this may occur in incorrect lead V1 placement, marked LV pacing latency or slow activation from the LV pacing site, LV lead dislodgement or inappropriate LV lead placement (middle or anterior cardiac vein) (Figure 6).[45]
Figure 6. Algorithm to evaluate the configuration of the paced ECG in lead V1 in CRT. Figure used from Barold and Herweg.[37].
In a large group of patients response to CRT in relation to the biventricular paced ECG was studied. This analysis revealed expression of biventricular fusion on the ECG by new or increasing R wave in lead V1 and V2 to be significantly related to the probability of reverse remodeling after adjusting for the degree of myocardial scar.[46] More recently, an additional, extensive QRS analysis was performed on the biventricular paced QRS morphology in lead V1- 2.[45] Three different V1-V2 QRS complex patterns during CRT were identified (Figure 7):
Figure 7. Wave interference for QRS fusion analysis. BV=biventricular; LBBB=left bundle branch block; LV=left ventricular; QRSBV=biventricular-paced QRS; QRSLBBB=LBBB QRS duration (ms); RV=right ventricular. Figure used from Sweeney et al.[45].
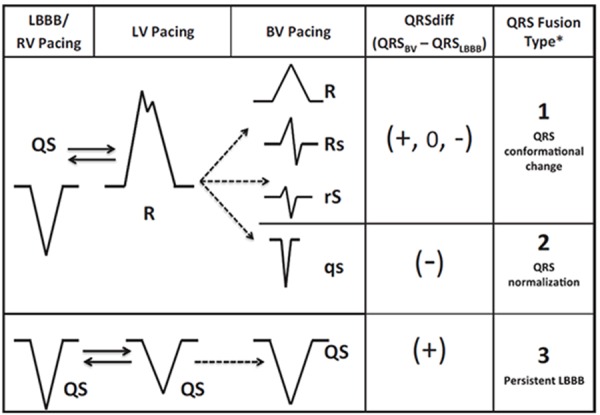
fusion complex with increased or dominant R wave, independent of QRS duration,
QS pattern with QRS duration normalization, and
QS pattern with increased QRS duration. Although QRS type 2 was a relative small group of patients in this study, these patients had the largest benefit in reverse remodeling.
Probably, in this group, RV and LV pacing wave fronts are opposite, generating QRS normalization by activation wave cancellation. On the other hand, another study did not find the presence of a dominant R wave in lead V1, but the activation reversal to dominant negativity in leads I and aVL, to correlate best with acute hemodynamic improvement after AV- and VV- optimisation.[47] Though promising, these analyses should be confirmed in prospective trials.
Literature on the prognostic value of the QRS duration during CRT is controversial. This can partly be explained by the aforementioned studies, where the biventricular paced QRS duration is not of importance with a type 1 QRS pattern in lead V1 but is related to improved outcome in patients with a QS pattern in lead V1 with QRS duration normalization. On the other hand it seems that at least for patients who are upgraded from RV pacing to CRT, QRS narrowing is associated with improved outcome.[48]
Clinical Implications
The 12 lead ECG provides important information for clinical follow-up in CRT, but needs to be carefully evaluated. Understanding the electrophysiology of LBBB, RV, LV, and biventricular pacing with or without fusion helps to evaluate CRT in heart failure patients. However, as the QRS pattern is complex due to merging wave fronts careful analysis of the ECG during underlying rhythm as well as during RV only and LV only pacing should be performed for better understanding of the ECG during CRT. Especially in CRT nonresponders a simple analysis of the QRS pattern in CRT can show whether biventricular pacing is adequately performed. When the QRS duration is not decreasing and no contribution from LV pacing is seen, further analysis of the ECG during intrinsic rhythm, RV only, and LV only pacing can reveal inadequate CRT programming and LV lead positioning
Disclosures
None.
References
- Goldenberg Ilan, Kutyifa Valentina, Klein Helmut U, Cannom David S, Brown Mary W, Dan Ariela, Daubert James P, Estes N A Mark, Foster Elyse, Greenberg Henry, Kautzner Josef, Klempfner Robert, Kuniss Malte, Merkely Bela, Pfeffer Marc A, Quesada Aurelio, Viskin Sami, McNitt Scott, Polonsky Bronislava, Ghanem Ali, Solomon Scott D, Wilber David, Zareba Wojciech, Moss Arthur J. Survival with cardiac-resynchronization therapy in mild heart failure. N. Engl. J. Med. 2014 May 1;370 (18):1694–701. doi: 10.1056/NEJMoa1401426. [DOI] [PubMed] [Google Scholar]
- Tang Anthony S L, Wells George A, Talajic Mario, Arnold Malcolm O, Sheldon Robert, Connolly Stuart, Hohnloser Stefan H, Nichol Graham, Birnie David H, Sapp John L, Yee Raymond, Healey Jeffrey S, Rouleau Jean L. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N. Engl. J. Med. 2010 Dec 16;363 (25):2385–95. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- Bristow Michael R, Saxon Leslie A, Boehmer John, Krueger Steven, Kass David A, De Marco Teresa, Carson Peter, DiCarlo Lorenzo, DeMets David, White Bill G, DeVries Dale W, Feldman Arthur M. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 2004 May 20;350 (21):2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- Cleland John G F, Daubert Jean-Claude, Erdmann Erland, Freemantle Nick, Gras Daniel, Kappenberger Lukas, Tavazzi Luigi. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 2005 Apr 14;352 (15):1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- Linde Cecilia, Abraham William T, Gold Michael R, St John Sutton Martin, Ghio Stefano, Daubert Claude. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J. Am. Coll. Cardiol. 2008 Dec 2;52 (23):1834–43. doi: 10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- Chung Eugene S, Leon Angel R, Tavazzi Luigi, Sun Jing-Ping, Nihoyannopoulos Petros, Merlino John, Abraham William T, Ghio Stefano, Leclercq Christophe, Bax Jeroen J, Yu Cheuk-Man, Gorcsan John, St John Sutton Martin, De Sutter Johan, Murillo Jaime. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008 May 20;117 (20):2608–16. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- Seo Yoshihiro, Ito Hiroshi, Nakatani Satoshi, Takami Mitsuaki, Naito Shigeto, Shiga Tsuyoshi, Ando Kenji, Wakayama Yuji, Aonuma Kazutaka. The role of echocardiography in predicting responders to cardiac resynchronization therapy. Circ. J. 2011;75 (5):1156–63. doi: 10.1253/circj.cj-10-0861. [DOI] [PubMed] [Google Scholar]
- Brignole Michele, Auricchio Angelo, Baron-Esquivias Gonzalo, Bordachar Pierre, Boriani Giuseppe, Breithardt Ole-A, Cleland John, Deharo Jean-Claude, Delgado Victoria, Elliott Perry M, Gorenek Bulent, Israel Carsten W, Leclercq Christophe, Linde Cecilia, Mont Lluís, Padeletti Luigi, Sutton Richard, Vardas Panos E, Zamorano Jose Luis, Achenbach Stephan, Baumgartner Helmut, Bax Jeroen J, Bueno Héctor, Dean Veronica, Deaton Christi, Erol Cetin, Fagard Robert, Ferrari Roberto, Hasdai David, Hoes Arno W, Kirchhof Paulus, Knuuti Juhani, Kolh Philippe, Lancellotti Patrizio, Linhart Ales, Nihoyannopoulos Petros, Piepoli Massimo F, Ponikowski Piotr, Sirnes Per Anton, Tamargo Juan Luis, Tendera Michal, Torbicki Adam, Wijns William, Windecker Stephan, Kirchhof Paulus, Blomstrom-Lundqvist Carina, Badano Luigi P, Aliyev Farid, Bänsch Dietmar, Baumgartner Helmut, Bsata Walid, Buser Peter, Charron Philippe, Daubert Jean-Claude, Dobreanu Dan, Faerestrand Svein, Hasdai David, Hoes Arno W, Le Heuzey Jean-Yves, Mavrakis Hercules, McDonagh Theresa, Merino Jose Luis, Nawar Mostapha M, Nielsen Jens Cosedis, Pieske Burkert, Poposka Lidija, Ruschitzka Frank, Tendera Michal, Van Gelder Isabelle C, Wilson Carol M. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur. Heart J. 2013 Aug;34 (29):2281–329. doi: 10.1093/eurheartj/eht150. [DOI] [PubMed] [Google Scholar]
- Strik Marc, van Deursen Caroline J M, van Middendorp Lars B, van Hunnik Arne, Kuiper Marion, Auricchio Angelo, Prinzen Frits W. Transseptal conduction as an important determinant for cardiac resynchronization therapy, as revealed by extensive electrical mapping in the dyssynchronous canine heart. Circ Arrhythm Electrophysiol. 2013 Aug;6 (4):682–9. doi: 10.1161/CIRCEP.111.000028. [DOI] [PubMed] [Google Scholar]
- Strauss David G, Selvester Ronald H, Wagner Galen S. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am. J. Cardiol. 2011 Mar 15;107 (6):927–34. doi: 10.1016/j.amjcard.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Ploux Sylvain, Lumens Joost, Whinnett Zachary, Montaudon Michel, Strom Maria, Ramanathan Charu, Derval Nicolas, Zemmoura Adlane, Denis Arnaud, De Guillebon Maxime, Shah Ashok, Hocini Mélèze, Jaïs Pierre, Ritter Philippe, Haïssaguerre Michel, Wilkoff Bruce L, Bordachar Pierre. Noninvasive electrocardiographic mapping to improve patient selection for cardiac resynchronization therapy: beyond QRS duration and left bundle branch block morphology. J. Am. Coll. Cardiol. 2013 Jun 18;61 (24):2435–43. doi: 10.1016/j.jacc.2013.01.093. [DOI] [PubMed] [Google Scholar]
- Kutyifa Valentina, Bloch Thomsen Poul Erik, Huang David T, Rosero Spencer, Tompkins Christine, Jons Christian, McNitt Scott, Polonsky Bronislava, Shah Amil, Merkely Bela, Solomon Scott D, Moss Arthur J, Zareba Wojciech, Klein Helmut U. Impact of the right ventricular lead position on clinical outcome and on the incidence of ventricular tachyarrhythmias in patients with CRT-D. Heart Rhythm. 2013 Dec;10 (12):1770–7. doi: 10.1016/j.hrthm.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Bulava Alan, Lukl Jan. Similar long-term benefits conferred by apical versus mid-septal implantation of the right ventricular lead in recipients of cardiac resynchronization therapy systems. Pacing Clin Electrophysiol. 2009 Mar;32 Suppl 1 ():S32–7. doi: 10.1111/j.1540-8159.2008.02224.x. [DOI] [PubMed] [Google Scholar]
- Kristiansen H M, Vollan G, Hovstad T, Keilegavlen H, Faerestrand S. A randomized study of haemodynamic effects and left ventricular dyssynchrony in right ventricular apical vs. high posterior septal pacing in cardiac resynchronization therapy. Eur. J. Heart Fail. 2012 May;14 (5):506–16. doi: 10.1093/eurjhf/hfr162. [DOI] [PubMed] [Google Scholar]
- Molina Luis, Sutton Richard, Gandoy William, Reyes Nicolás, Lara Susano, Limón Froylán, Gómez Susana, Orihuela Consuelo, Salame Latife, Moreno Gabriela. Medium-term effects of septal and apical pacing in pacemaker-dependent patients: a double-blind prospective randomized study. Pacing Clin Electrophysiol. 2014 Feb;37 (2):207–14. doi: 10.1111/pace.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto Yoshio, Hasebe Hideyuki, Osaka Toshiyuki, Yokoyama Eriko, Kushiyama Yasunori, Suzuki Tomoyuki, Kuroda Yusuke, Ichikawa Chizuko, Kamiya Kaichiro, Kodama Itsuo. Right ventricular septal pacing preserves long-term left ventricular function via minimizing pacing-induced left ventricular dyssynchrony in patients with normal baseline QRS duration. Circ. J. 2009 Oct;73 (10):1829–35. doi: 10.1253/circj.cj-09-0256. [DOI] [PubMed] [Google Scholar]
- Barold S Serge, Herweg Bengt, Giudici Michael. Electrocardiographic follow-up of biventricular pacemakers. Ann Noninvasive Electrocardiol. 2005 Apr;10 (2):231–55. doi: 10.1111/j.1542-474X.2005.10201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow Grant V, Silverman Michael G, Tunin Richard S, Lardo Albert C, Nazarian Saman, Kass David A. Efficacy of cardiac resynchronization in acutely infarcted canine hearts with electromechanical dyssynchrony. Heart Rhythm. 2014 Oct;11 (10):1819–26. doi: 10.1016/j.hrthm.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma Niraj. Left ventricular electrical activation during right ventricular pacing in heart failure patients with LBBB: visualization by electrocardiographic imaging and implications for cardiac resynchronization therapy. J Electrocardiol. 2014 Oct 11;48 (1):53–61. doi: 10.1016/j.jelectrocard.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Sharma Parikshit S, Dandamudi Gopi, Naperkowski Angela, Oren Jess W, Storm Randle H, Ellenbogen Kenneth A, Vijayaraman Pugazhendhi. Permanent His-bundle pacing is feasible, safe, and superior to right ventricular pacing in routine clinical practice. Heart Rhythm. 2015 Feb;12 (2):305–12. doi: 10.1016/j.hrthm.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Eschalier Romain, Ploux Sylvain, Lumens Joost, Whinnett Zachary, Varma Niraj, Meillet Valentin, Ritter Philippe, Jaïs Pierre, Haïssaguerre Michel, Bordachar Pierre. Detailed analysis of ventricular activation sequences during right ventricular apical pacing and left bundle branch block and the potential implications for cardiac resynchronization therapy. Heart Rhythm. 2015 Jan;12 (1):137–43. doi: 10.1016/j.hrthm.2014.09.059. [DOI] [PubMed] [Google Scholar]
- Derval Nicolas, Bordachar Pierre, Lim Han S, Sacher Frederic, Ploux Sylvain, Laborderie Julien, Steendijk Paul, Deplagne Antoine, Ritter Philippe, Garrigue Stephane, Denis Arnaud, Hocini Mélèze, Haissaguerre Michel, Clementy Jacques, Jaïs Pierre. Impact of pacing site on QRS duration and its relationship to hemodynamic response in cardiac resynchronization therapy for congestive heart failure. J. Cardiovasc. Electrophysiol. 2014 Sep;25 (9):1012–20. doi: 10.1111/jce.12464. [DOI] [PubMed] [Google Scholar]
- Mafi Rad Masih, Blaauw Yuri, Dinh Trang, Pison Laurent, Crijns Harry J, Prinzen Frits W, Vernooy Kevin. Different regions of latest electrical activation during left bundle-branch block and right ventricular pacing in cardiac resynchronization therapy patients determined by coronary venous electro-anatomic mapping. Eur. J. Heart Fail. 2014 Nov;16 (11):1214–22. doi: 10.1002/ejhf.178. [DOI] [PubMed] [Google Scholar]
- Ludwig Daniel R, Tanaka Hidekazu, Friehling Mati, Gorcsan John, Schwartzman David. Further deterioration of LV ejection fraction and mechanical synchrony during RV apical pacing in patients with heart failure and LBBB. J Cardiovasc Transl Res. 2013 Jun;6 (3):425–9. doi: 10.1007/s12265-013-9457-0. [DOI] [PubMed] [Google Scholar]
- Auricchio A, Stellbrink C, Sack S, Block M, Vogt J, Bakker P, Mortensen P, Klein H. The Pacing Therapies for Congestive Heart Failure (PATH-CHF) study: rationale, design, and endpoints of a prospective randomized multicenter study. Am. J. Cardiol. 1999 Mar 11;83 (5B):130D–135D. doi: 10.1016/s0002-9149(98)01014-5. [DOI] [PubMed] [Google Scholar]
- Adelstein Evan, Alam Mian Bilal, Schwartzman David, Jain Sandeep, Marek Josef, Gorcsan John, Saba Samir. Effect of echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy on mortality and risk of defibrillator therapy for ventricular arrhythmias in heart failure patients (from the Speckle Tracking Assisted Resynchronization Therapy for Electrode Region [STARTER] trial). Am. J. Cardiol. 2014 May 1;113 (9):1518–22. doi: 10.1016/j.amjcard.2014.01.431. [DOI] [PubMed] [Google Scholar]
- Kydd Anna C, Khan Fakhar Z, Watson William D, Pugh Peter J, Virdee Munmohan S, Dutka David P. Prognostic benefit of optimum left ventricular lead position in cardiac resynchronization therapy: follow-up of the TARGET Study Cohort (Targeted Left Ventricular Lead Placement to guide Cardiac Resynchronization Therapy). JACC Heart Fail. 2014 Jun;2 (3):205–12. doi: 10.1016/j.jchf.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Gold Michael R, Birgersdotter-Green Ulrika, Singh Jagmeet P, Ellenbogen Kenneth A, Yu Yinghong, Meyer Timothy E, Seth Milan, Tchou Patrick J. The relationship between ventricular electrical delay and left ventricular remodelling with cardiac resynchronization therapy. Eur. Heart J. 2011 Oct;32 (20):2516–24. doi: 10.1093/eurheartj/ehr329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers Leonard M, van Gelder Berry M, Scheffer Mike G, Bracke Frank A. Mid-term follow up of thromboembolic complications in left ventricular endocardial cardiac resynchronization therapy. Heart Rhythm. 2014 Apr;11 (4):609–13. doi: 10.1016/j.hrthm.2014.01.031. [DOI] [PubMed] [Google Scholar]
- Scheffer Mike G, Ramanna Hemanth, van Gelder Berry M. Left Ventricular endocardial pacing by the interventricular septum route. Europace. 2014 Oct;16(10): p:1520. doi: 10.1093/europace/eut437. [DOI] [PubMed] [Google Scholar]
- van Gelder Berry M, Scheffer Mike G, Meijer Albert, Bracke Frank A. Transseptal endocardial left ventricular pacing: an alternative technique for coronary sinus lead placement in cardiac resynchronization therapy. Heart Rhythm. 2007 Apr;4 (4):454–60. doi: 10.1016/j.hrthm.2006.11.023. [DOI] [PubMed] [Google Scholar]
- van Gelder Berry M, Houthuizen Patrick, Bracke Frank A. Transseptal left ventricular endocardial pacing: preliminary experience from a femoral approach with subclavian pull-through. Europace. 2011 Oct;13 (10):1454–8. doi: 10.1093/europace/eur136. [DOI] [PubMed] [Google Scholar]
- van Deursen Caroline J M, Blaauw Yuri, Witjens Maryvonne I, Debie Luuk, Wecke Liliane, Crijns Harry J G M, Prinzen Frits W, Vernooy Kevin. The value of the 12-lead ECG for evaluation and optimization of cardiac resynchronization therapy in daily clinical practice. J Electrocardiol. 2014 Jan 22;47(2): p:202–11. doi: 10.1016/j.jelectrocard.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Sommer Anders, Kronborg Mads Brix, Witt Christoffer Tobias, Nørgaard Bjarne Linde, Nielsen Jens Cosedis. The paced electrocardiogram cannot be used to identify left and right ventricular pacing sites in cardiac resynchronization therapy: validation by cardiac computed tomography. Europace. 2015 Mar;17 (3):432–8. doi: 10.1093/europace/euu323. [DOI] [PubMed] [Google Scholar]
- Herweg Bengt, Ilercil Arzu, Madramootoo Chris, Krishnan Sendhil, Rinde-Hoffman Debbie, Weston Mark, Curtis Anne B, Barold S Serge. Latency during left ventricular pacing from the lateral cardiac veins: a cause of ineffectual biventricular pacing. Pacing Clin Electrophysiol. 2006 Jun;29 (6):574–81. doi: 10.1111/j.1540-8159.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- Herweg Bengt, Ali Rias, Ilercil Arzu, Madramootoo Chris, Cutro Ray, Weston Mark W, Barold S Serge. Site-specific differences in latency intervals during biventricular pacing: impact on paced QRS morphology and echo-optimized V-V interval. Pacing Clin Electrophysiol. 2010 Nov;33 (11):1382–91. doi: 10.1111/j.1540-8159.2010.02882.x. [DOI] [PubMed] [Google Scholar]
- Barold S Serge, Herweg Bengt. Usefulness of the 12-lead electrocardiogram in the follow-up of patients with cardiac resynchronization devices. Part II. Cardiol J. 2011;18 (6):610–24. doi: 10.5603/cj.2011.0024. [DOI] [PubMed] [Google Scholar]
- Köbe Julia, Dechering Dirk G, Rath Benjamin, Reinke Florian, Mönnig Gerold, Wasmer Kristina, Eckardt Lars. Prospective evaluation of electrocardiographic parameters in cardiac resynchronization therapy: detecting nonresponders by left ventricular pacing. Heart Rhythm. 2012 Apr;9 (4):499–504. doi: 10.1016/j.hrthm.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Chalil S, Stegemann B, Muhyaldeen S A, Khadjooi K, Foley P W, Smith R E A, Leyva F. Effect of posterolateral left ventricular scar on mortality and morbidity following cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2007 Oct;30 (10):1201–9. doi: 10.1111/j.1540-8159.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- Rademakers Leonard M, van Kerckhoven Roeland, van Deursen Caroline J M, Strik Marc, van Hunnik Arne, Kuiper Marion, Lampert Anniek, Klersy Catherine, Leyva Francisco, Auricchio Angelo, Maessen Jos G, Prinzen Frits W. Myocardial infarction does not preclude electrical and hemodynamic benefits of cardiac resynchronization therapy in dyssynchronous canine hearts. Circ Arrhythm Electrophysiol. 2010 Aug;3 (4):361–8. doi: 10.1161/CIRCEP.109.931865. [DOI] [PubMed] [Google Scholar]
- Thibault Bernard, Ducharme Anique, Harel François, White Michel, O'Meara Eileen, Guertin Marie-Claude, Lavoie Joel, Frasure-Smith Nancy, Dubuc Marc, Guerra Peter, Macle Laurent, Rivard Léna, Roy Denis, Talajic Mario, Khairy Paul. Left ventricular versus simultaneous biventricular pacing in patients with heart failure and a QRS complex ≥120 milliseconds. Circulation. 2011 Dec 20;124 (25):2874–81. doi: 10.1161/CIRCULATIONAHA.111.032904. [DOI] [PubMed] [Google Scholar]
- Kass D A, Chen C H, Curry C, Talbot M, Berger R, Fetics B, Nevo E. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999 Mar 30;99 (12):1567–73. doi: 10.1161/01.cir.99.12.1567. [DOI] [PubMed] [Google Scholar]
- van Gelder Berry M, Bracke Frank A, Meijer Albert, Pijls Nico H J. The hemodynamic effect of intrinsic conduction during left ventricular pacing as compared to biventricular pacing. J. Am. Coll. Cardiol. 2005 Dec 20;46 (12):2305–10. doi: 10.1016/j.jacc.2005.02.098. [DOI] [PubMed] [Google Scholar]
- Singh Jagmeet P, Abraham William T, Chung Eugene S, Rogers Tyson, Sambelashvili Alex, Coles James A, Martin David O. Clinical response with adaptive CRT algorithm compared with CRT with echocardiography-optimized atrioventricular delay: a retrospective analysis of multicentre trials. Europace. 2013 Nov;15 (11):1622–8. doi: 10.1093/europace/eut107. [DOI] [PubMed] [Google Scholar]
- Sweeney Michael O, Hellkamp Anne S, van Bommel Rutger J, Schalij Martin J, Borleffs C Jan Willem, Bax Jeroen J. QRS fusion complex analysis using wave interference to predict reverse remodeling during cardiac resynchronization therapy. Heart Rhythm. 2014 May;11 (5):806–13. doi: 10.1016/j.hrthm.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Sweeney Michael O, van Bommel Rutger J, Schalij Martin J, Borleffs C Jan Willem, Hellkamp Anne S, Bax Jeroen J. Analysis of ventricular activation using surface electrocardiography to predict left ventricular reverse volumetric remodeling during cardiac resynchronization therapy. Circulation. 2010 Feb 9;121 (5):626–34. doi: 10.1161/CIRCULATIONAHA.109.894774. [DOI] [PubMed] [Google Scholar]
- Bogaard Margot D, Hesselink Tim, Meine Mathias, Loh Peter, Hauer Richard N, Cramer Maarten J, Doevendans Pieter A, Tuinenburg Anton E. The ECG in cardiac resynchronization therapy: influence of left and right ventricular preactivation and relation to acute response. J. Cardiovasc. Electrophysiol. 2012 Nov;23 (11):1237–45. doi: 10.1111/j.1540-8167.2012.02388.x. [DOI] [PubMed] [Google Scholar]
- Rickard John, Brennan Danielle M, Martin David O, Hsich Eileen, Tang W H Wilson, Lindsay Bruce D, Starling Randall C, Wilkoff Bruce L, Grimm Richard A. The impact of left ventricular size on response to cardiac resynchronization therapy. Am. Heart J. 2011 Oct;162 (4):646–53. doi: 10.1016/j.ahj.2011.07.008. [DOI] [PubMed] [Google Scholar]