Abstract
The creation of a durable radiofrequency (RF) lesion depends on several parameters, including catheter tip electrode size and composition, tip orientation, temperature, RF pulse duration, power, blood flow, and catheter to tissue contact. The development of new contact force (CF) sensor catheters has allowed the measurement of the tip to tissue CF during the RF ablation procedure. Here, we describe the clinical experience obtained using CF catheters for atrial fibrillation ablation, with a specific focus on the impact of CF technology on acute procedural data (procedure and fluoroscopy time).
Introduction
Catheter ablation (CA) has become a well established treatment option for recurrent, symptomatic, drug-resistant atrial fibrillation (AF). Isolating or encircling all accessible pulmonary veins (PVs) is recognized as the cornerstone of any ablation approach.[1] One of the major limitation of CA of AF is the high rate of recurrences, during the short- and long-term follow-up, mainly due to electrical reconnection of the PVs. Therefore, more durable and transmural lesions produced by radiofrequency energy (RF) are desirable to improve the procedural outcome.[2,3] Crucial in the determining of the efficacy of RF lesion is the electrode-tissue contact. The optimization of electrode–tissue contact may have a two potential benefits.[1] First, it allow a more effective RF delivery to tissue with less energy dissipated into the circulating blood pool and creation of more predictable and reliable lesions. This may impact on both the procedure parameters and long-term clinical outcome. Second, monitoring the electrode–tissue contact may help reduce the excessive contact and the complications possibly related to catheter manipulation inside the heart. Here, we describe the clinical experience obtained using CF catheters for AF ablation, with a specific focus on the impact of CF sensing technology on acute procedural data (procedure and fluoroscopy time).
Contact Force Sensing In Catheter Ablation
The efficacy of RF ablation is to a large extent determined by the ability to create durable, transmural lesions. Lesion formation, including durability, is dependent on several interacting factors including catheter tip size, irrigation, stability and orientation to the myocardium, power delivery, ablation duration, and catheter-tissue CF. Yokoyama et al[4] showed a direct correlation between CF and the resulting lesion volume in a canine thigh muscle preparation. Using this catheter at constant RF power (saline irrigation) in the canine thigh muscle preparation, tissue temperature and lesion size increased significantly with increasing CF. The incidence of steam pop and thrombus also increased with increasing CF. The incorporation of real-time CF measurement in an irrigated ablation catheter helped to optimize the selection of RF power and RF application time to maximize RF lesion formation and reduce the risk of steam pop and thrombus in clinical application. Until recently, CF could not be measured directly by ablation catheters. As a result, surrogate measures of CF have been proposed, including electrogram amplitude, pre-ablation impedance and changes during ablation in electrode temperature and impedance.[5] The accuracy of these surrogate measures has not been extensively validated.
In this setting steerable sheaths have been introduced to improve CF during AF ablation.[6] Ullah et al[7] recently demonstrated that steerable-sheaths increased ablation CF, however, there were region-specific heterogeneities in the extent of increment, with some segments where they failed to increase CF.
olved the technology of catheter ablation. Two irrigated CF sensing catheters are now available: the TactiCath™ (St. Jude Medical, USA) (TC) (Figure 1) and the ThermoCool® SmartTouch™ (Biosense Webster, USA) (ST) (Figure 2). The TC catheter measures the CF by micro-deformations of optical fibers, whereas the ST catheter measures micro-deformations of a precision spring connecting the catheter shaft and tip. In bench testing, both systems have a CF resolution of less than 1 gram.
Figure 1. A 3D reconstruction of left atrium by means of the TactiCath™ (St. Jude Medical, USA) catheter. Local contact force values are displayed.
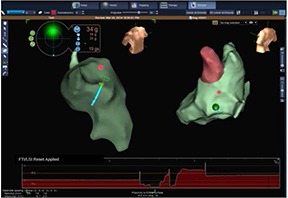
Figure 2. A 3D reconstruction of left atrium by means of the ThermoCool® SmartTouch™ (Biosense Webster, USA) catheter. Local contact force value is displayed.
The theoretically possible benefits of CF sensing technology are numerous.
Safety may be improved by reducing the risk of perforation during catheter manipulation and ablation. Although several initial experiences[8-12] comparing the CF catheters with standard openirrigated tip catheter in AF ablation failed to demonstrate a reduction in complication rate with the use of CF sensing catheters, recently Acka et al[13] evaluated if CF catheters reduce cardiac perforations and other major complications and offer equal safety compared to the non CF catheters and magnetic navigation system, in 1.517 ablation procedures. Complications occurred in 11.3% (n=172) of the procedures. In 2.8% (n=43) a major complication occurred, 0.9% (n=13) had a perforation, 8.5% (n=129) had a minor complication and 2 patients died (0.1%). No cardiac perforation occurred in the CF group, which was significantly different from non CF procedures (0.0% vs. 1.6%; relative risk 0.76, 95% CI 0.74-0.79, P=0.031) and equal to magnetic navigation system (0.0%). This was also observed in the AF subgroup (557 patients) (0.0% vs. 3.3%; RR 0.67, 95% CI 0.63-0.72, P=0.021), and the occurrence of major complications was lower for CF versus non CF procedures (2.1% vs. 7.8%, P=0.010). They concluded that CF-guided CA is superior to non CF catheter with regard to procedural safety and avoidance of cardiac perforation. This difference was due to a reduction of cardiac perforation and major complications in the AF subgroup.
Although clinical practice is suggesting that increasing CF improves RF lesion formation, there are no studies correlating RF lesion size to CF in the beating heart. However CF sensing catheters allowed a lower incidence of acute reconnection, and less need of complementary segmentary RF applications.[14-16]
Impact Of CF On Fluoroscopy Time
Reddy et al[17] were the first to study the relationship between contact force and clinical outcome during RF catheter ablation of atrial fibrillation in the TOCCATA study. Thirty-two patients with paroxysmal AF underwent PV isolation by using a radiofrequency ablation catheter with a CF sensor integrated at its tip (TC). They failed to demonstrate any impact of CF values on procedural and fluoroscopy times, although they observed a trend towards a reduced fluoroscopy time (from 55±32 min vs 32±24 ms, p=0.25) in patients in which the mean CF was > 20 gr as compared with patients in which the mean CF was ≤ 10 gr. Similar results were reported by Wutzler et al[18] They analyzed 143 patients who underwent PV isolation. In 31 patients, PV isolation was performed by monitoring the catheter-tissue contact with a sensing catheter (TC). One hundred and twelve patients in whom conventional PVI was performed without CF information, using an open irrigated ablation catheter (CoolPath, IBI/St. Jude Medical, St. Paul, MN, USA) served as the control group. Circumferential PV isolation was performed with a 3D-Mapping-System (Ensite NavX, St. Jude Medical). A significant reduction in procedure duration was seen in the CF mapping group (128.4 ±29 min vs. 157.7 ±30.8 min, p = 0.001). There were no significant differences observed in ablation time, total ablation energy or fluoroscopy time, although all were reduced in the CF group.
On contrary using the ST catheters several study demonstrated a relevant the impact of CF technology on fluoroscopy and procedure time during AF ablation (Table 1).
Table 1. Impact of CF sensing technology on on procedural and fluoroscopy time.
PAF= paroxysmal atrial fibrillation; OIC= open-irrigated-tip catheter; CF= contact force; PVI= pulmonary vein isolation; FTI= force-time integral; SF= surround flow; PerAF= persistent atrial fibrillation
| Study | Important features | CF sensing technology | Aims and methods | Fluoroscopy time | Procedure time | Key findings |
|---|---|---|---|---|---|---|
| Reddy et al [17] 2012 | 32 PAF | Optical fibers | an OIC with CF mapping capabilities | Higher CF was not associated to changes in fluoroscopy time (32±24 vs 55±32 min, p=0.25) | Higher CF was not associated to changes in procedure time (211±88 vs 188±51 min, p=0.61) | CF did not affect procedural parameters |
| Wutzler et al [18] 2014 | 143 with PAF and PerAF | Optical fibers | an OIC or an OIC with CF mapping capabilities | There were no significant differences observed in fluoroscopy time, although reduced in the contact force group | Procedure duration was significantly shorter in the contact force group (128.4 ±29 min vs. 157.7 ±30.8 min, p = 0.001). | the use of CF information resulted in a shorter procedure time |
| Martinek et al [8] 2012 | 50 PAF | Precision spring | a standard 3.5-mm OIC or a catheter with CF measurement capabilities | 28.6 ±17.4 vs 23.6±13.1 min, p= 0.312 | 185±46 vs 154±39 min p= 0.022 | The use of CF sensing technology was able to significantly reduce ablation and procedure times in PVI. |
| Marion et al [9] 2014 | 60 PAF | Precision spring | a new OIC CF catheter or a non-CF OIC | CF use was associated with significant reductions in fluoroscopy exposure (20.1 ± 4 vs 26.7 ± 5 minutes, p < 0.01) | CF technology was associated with a significant reduction in overall procedure time | the use of CF information resulted in a shorter procedure and fluoroscopy times |
| Stabile et al [19] 2014 | 95 PAF | Precision Spring | a new OIC with CF sensing | Patients in whom the mean CF during ablation was > 20 g required shorter procedural time (92±23 vs.160±67 min, p = 0.01) as compared with patients in whom this value was < 10 g. | value below the median of 543 gs required longer procedural (158.0±74.0 vs. 117.0±52.0 min, p = 0.004) times as compared with those in whom FTI was above this value | CF affected procedural parameters, in particular procedural and fluoroscopy times, without increasing complications |
| Sciarra et al 10 2014 | 63 PAF | Precision spring | impact of a standard OIC, SF OIC and CF catheter | ST and CF catheter obtained a reduction of fluoroscopy time (OIC 34 ± 18 min, CF 20 ± 10 min, p < 0.001; SF 21 ± 13 min, p = 0.02 vs OIC) | STc resulted in a reduction of procedural time (TCc 181 ± 53 min, STc 140 ± 53 min, p < 0.001; SFc 170 ± 51 min, p = NS vs TCc). | Both the CF and the SF OIC catheters significantly reduced radiofrequency and fluoroscopy times, as well as pulmonary veins reconnection rate at 30 min. Moreover, the CF catheter reduced overall duration of the procedure |
| Jarman et al [11] 2014 | 600 with PAF and PerAF | Precision spring | CF and non CF catheters | the use of CF catheters was associated with reduced fluoroscopy time in multivariate analysis (reduction by 7.7 (5.0-10.5) minutes; p<0.001) | Fluoroscopy time was lower when CF technology was employed in all types of AF ablation procedures | |
| Sigmund et al [12] 2015 | 198 with PAF and PerAF | Precision spring | 3.5-mm OIC with CF measurement capabilities and a standard OIC | total fluoroscopy time could be significantly reduced from 28.5 ± 11.0 to 19.9 ± 9.3 minutes (P = 0.0001) | Procedural data showed a significant decline in overall procedure time of 34 minutes (p = 0.0001; 225.8 ± 53.1 vs 191.9 ± 53.3 minutes). | The use of CF technology was able to significantly reduce ablation, procedure, and fluoroscopy times as well as dose in RFCA of AF |
Martinek et a[18] assessed the impact of direct catheter force measurement on acute procedural parameters during RF CA in 50 consecutive patients with paroxysmal AF. Fifty consecutive patients with paroxysmal AF who underwent their first procedure of circumferential PV isolation were assigned to either RF CA using
a standard 3.5-mm open-irrigated-tip catheter (Thermocool®, NavistarTM; Biosense Webster) or
a catheter (ST) with contact force measurement capabilities.
All RFA were performed using a 3-D electroanatomic mapping system with CT integration (Carto3®; Biosense Webster). Procedural data showed a remarkable decline in ablation time (RF time needed for PV isolation) from 50.5 ± 15.9 to 39.0 ± 11.0 minutes (P = 0.007) with a reduction in overall procedure duration from 185 ± 46 to 154 ± 39 minutes (P = 0.022). In parallel, the total energy delivered could be significantly reduced from 70,926 ± 19,470 to 58,511 ± 14,655 Ws (P = 0.019). The number of acute PV reconnections declined from 36% to 12% (P = 0.095).
Marion et al[9] studied 60 patients with paroxysmal AF comparing circular antral CA (guided by Carto 3® System, BiosenseWebster) using either a new open-irrigated CF catheter (ST) or a non-CF open-irrigated catheter (EZ Steer Thermocool, Biosense Webster). Overall, 30 patients were enrolled in each group. Though complete PV isolation was achieved in all cases in both groups, CF use was associated with significant reductions in fluoroscopy exposure (20.1±4 vs 26.7±5 minutes, p < 0.01) and RF time (45.2±18 vs 65.4±22 minutes, p= 0.01).
Stabile et al,[19] in a multicentre prospective study, assessed the effect of direct CF measurement on acute procedural parameters during RF CA of AF. All the patients underwent the first ablation procedure for paroxysmal AF with antral PV isolation, aiming at entry and exit conduction block in all PVs, by means of a open-irrigated tip catheter with CF sensing (ST), guided by Carto 3® System (BiosenseWebster). Ninety-five patients were enrolled in nine centres and successfully underwent ablation. Overall procedure time, fluoroscopy time, and ablation time were 138.0+67.0, 14.3+11.2, and 33.8+19.4 min, respectively. The mean CF value during ablation was 12.2+3.9 g. Force time integral (FTI) analysis showed that patients achieving a value below the median of 543.0 gs required longer procedural (158.0+74.0 vs. 117.0+52.0 min, p= 0.004) and fluoroscopy (17.5+13.0 vs. 11.0+7.7 min, p = 0.007) times as compared with those in whom FTI was above this value. Patients in whom the mean CF during ablation was > 20 g required shorter procedural time (92.0+23.0 vs.160.0+67.0 min, p = 0.01) as compared with patients in whom this value was < 10 g.
Sciarra et al[10] analyzed the impact of the ST catheter and the Surround Flow (BiosenseWebster) catheter (SF) and ThermoCool (BiosenseWebster) catheter, in terms of feasibility and acute efficacy, in 63 patients with paroxysmal AF who underwent PV antral isolation, guided by Carto 3® System (BiosenseWebster). They found that the use of both ST and SF catheters obtained a reduction of fluoroscopy time (ThermoCool 34±18 min, ST 20±10 min, p<0.001; SF 21±13 min, p=0.02 vs ThermoCool) and RF time (ThermoCool 41±13 min, ST 30±14 min, p=0.013; SF 30±9 min, p<0.01 vs ThermoCoolC). The use of ST catheter resulted in a reduction of procedural time (ThermoCool 181±53 min, ST 140±53 min, p<0.001; SF 170±51 min, p=NS vs ThermoCool). The percentage of isolated PVs was comparable between groups (ThermoCool 96 % vs ST 98 % vs SF 96 %; p=NS). The percentage of deconnected PVs at 30 min was lower in ThermoCool (89 %) than in ST (95 %) and in SF (95 %) group (p<0.05).
Jarman et al[11] studied the impact of CF sensing technology on the clinical outcome of ablating AF. A total of 600 AF ablation procedures (200 using CF sensing and 400 using non-CF catheters) performed between 2010 and 2012 (46% paroxysmal, 36% persistent, 18% long-lasting persistent) were analyzed. First time AF ablation procedures employing CF catheter (ST) from 4 centers were matched retrospectively to those without CF catheter (ThermoCool, BiosenseWebster, and SF) in a 1:2 manner by type of AF. Among all cases, the use of CF sensing catheters was associated with reduced fluoroscopy time in multivariate analysis (reduction by 7.7 (5.0-10.5) minutes; p<0.001). Complication rates were similar in both groups.
Sigmund et al[12] assessed the impact of direct CF measurement on acute procedural parameters and outcome of RF CA for paroxysmal and persistent AF. Ninety-nine consecutive patients with paroxysmal (63.6%) or persistent AF underwent left atrial RF CA using a 3.5- mm open-irrigated-tip catheter with CF measurement capabilities (ST). For comparison a case-matched cohort with standard openirrigated- tip catheters (ThermoCool, BiosenseWebster) was used (99 patients). Procedural data showed a significant decline in RF ablation time from 52 ± 20 to 44 ± 16 minutes (P = 0.003) with a remarkable mean reduction in overall procedure time of 34 minutes (P = 0.0001; 225.8 ± 53.1 vs 191.9 ± 53.3 minutes). In parallel, the total fluoroscopy time could be significantly reduced from 28.5 ± 11.0 to 19.9 ± 9.3 minutes (P = 0.0001) as well as fluoroscopy dose from 74.1 ± 58.0 to 56.7 ± 38.9 Gy/cm2 (P = 0.016). Periprocedural complications were similar in both groups.
Many factors justify the reduction in procedural and fluoroscopy time observed with CF sensing catheters. It may relate to increased operator’s confidence during navigation, related to the confidence in the validity of geometry produced by CF sensing feedback,[20] or to the reduction in time required to complete contiguous lesions. Olson et al[21] demonstrated that the decreased lesion size due to intermittent contact can be overcome by increasing duration of ablation time. The CF sensing technology giving the feedback on the quality of catheter-tissue contact helps to reduce the number of RF pulses with intermittent or poor contact which require longer ablation times and often fluoroscopy time. Moreover avoiding ablation at sub-optimal CF may reduce late development of gaps within linear lesions, often performed during AF ablation. Finally, the reduced incidence of acute reconnection, and less need of complementary segmentary RF applications[14-16] allow a reduction in the overall procedure time and therefore also in fluoroscopy time.
Limitations
Although the great amount of the study showed a positive impact of CF sensing catheter on acute procedural data and above all procedural and fluoroscopy time, several issue remain to be clarified. The overall quality of the studies which evaluated the impact of the new CF catheters in AF ablation is poor: none of them is a randomized one, some are retrospectively, only few are multicenter studies. Second, whereas the studies using the ST catheter showed a significant reduction in the fluoroscopy time, this was not observed in the study[18] using the TC catheter. However only few patients were enrolled. Further studies using this technology are warranted to assess this issue. Third, the ST catheter has usually been compared with the Thermocool®, NavistarTM (BiosenseWEbster) catheter. Only one study[10] compared the ST with the SF, and in this case no difference on procedural and fluoroscopy times were founded.
Conclusions
CF sensing technology appears to significantly impact on short term results with shorter procedural and fluoroscopy times, lower incidence of acute reconnection, and less need of complementary segmentary radiofrequency applications. Further randomized studies are warranted to confirm these preliminary data and to compare the CF with other technologies aiming to improve AF CA.
Disclosures
None.
References
- 1.Calkins Hugh, Kuck Karl Heinz, Cappato Riccardo, Brugada Josep, Camm A John, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, DiMarco John, Edgerton James, Ellenbogen Kenneth, Ezekowitz Michael D, Haines David E, Haissaguerre Michel, Hindricks Gerhard, Iesaka Yoshito, Jackman Warren, Jalife José, Jais Pierre, Kalman Jonathan, Keane David, Kim Young-Hoon, Kirchhof Paulus, Klein George, Kottkamp Hans, Kumagai Koichiro, Lindsay Bruce D, Mansour Moussa, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Packer Douglas L, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Reddy Vivek, Ruskin Jeremy N, Shemin Richard J, Tsao Hsuan-Ming, Wilber David. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012 Apr;9 (4):632–696.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Matsuo Seiichiro, Yamane Teiichi, Date Taro, Inada Keiichi, Kanzaki Yasuko, Tokuda Michifumi, Shibayama Kenri, Miyanaga Satoru, Miyazaki Hidekazu, Sugimoto Kenichi, Mochizuki Seibu. Reduction of AF recurrence after pulmonary vein isolation by eliminating ATP-induced transient venous re-conduction. J. Cardiovasc. Electrophysiol. 2007 Jul;18 (7):704–8. doi: 10.1111/j.1540-8167.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- 3.Bertaglia Emanuele, Fassini Gaetano, Anselmino Matteo, Stabile Giuseppe, Grandinetti Giuseppe, De Simone Antonio, Calò Leonardo, Pandozi Claudio, Pratola Claudio, Zoppo Franco, Tondo Claudio, Iuliano Assunta, Gaita Fiorenzo. Comparison of ThermoCool® Surround Flow catheter versus ThermoCool® catheter in achieving persistent electrical isolation of pulmonary veins: a pilot study. J. Cardiovasc. Electrophysiol. 2013 Mar;24 (3):269–73. doi: 10.1111/jce.12031. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama Katsuaki, Nakagawa Hiroshi, Shah Dipen C, Lambert Hendrik, Leo Giovanni, Aeby Nicolas, Ikeda Atsushi, Pitha Jan V, Sharma Tushar, Lazzara Ralph, Jackman Warren M. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol. 2008 Dec;1 (5):354–62. doi: 10.1161/CIRCEP.108.803650. [DOI] [PubMed] [Google Scholar]
- 5.De Bortoli Alessandro, Sun Li-Zhi, Solheim Eivind, Hoff Per Ivar, Schuster Peter, Ohm Ole-Jørgen, Chen Jian. Ablation effect indicated by impedance fall is correlated with contact force level during ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2013 Nov;24 (11):1210–5. doi: 10.1111/jce.12215. [DOI] [PubMed] [Google Scholar]
- 6.Rajappan Kim, Baker Victoria, Richmond Laura, Kistler Peter M, Thomas Glyn, Redpath Calum, Sporton Simon C, Earley Mark J, Harris Stuart, Schilling Richard J. A randomized trial to compare atrial fibrillation ablation using a steerable vs. a non-steerable sheath. Europace. 2009 May;11 (5):571–5. doi: 10.1093/europace/eup069. [DOI] [PubMed] [Google Scholar]
- 7.Ullah Waqas, Hunter Ross J, McLean Ailsa, Dhinoja Mehul, Earley Mark J, Sporton Simon, Schilling Richard J. Impact of steerable sheaths on contact forces and reconnection sites in ablation for persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2015 Mar;26 (3):266–73. doi: 10.1111/jce.12573. [DOI] [PubMed] [Google Scholar]
- 8.Martinek Martin, Lemes Christine, Sigmund Elisabeth, Derndorfer Michael, Aichinger Josef, Winter Siegmund, Nesser Hans-Joachim, Pürerfellner Helmut. Clinical impact of an open-irrigated radiofrequency catheter with direct force measurement on atrial fibrillation ablation. Pacing Clin Electrophysiol. 2012 Nov;35 (11):1312–8. doi: 10.1111/j.1540-8159.2012.03503.x. [DOI] [PubMed] [Google Scholar]
- 9.Marijon Eloi, Fazaa Samia, Narayanan Kumar, Guy-Moyat Benoit, Bouzeman Abdeslam, Providencia Rui, Treguer Frederic, Combes Nicolas, Bortone Agustin, Boveda Serge, Combes Stephane, Albenque Jean-Paul. Real-time contact force sensing for pulmonary vein isolation in the setting of paroxysmal atrial fibrillation: procedural and 1-year results. J. Cardiovasc. Electrophysiol. 2014 Feb;25 (2):130–7. doi: 10.1111/jce.12303. [DOI] [PubMed] [Google Scholar]
- 10.Sciarra Luigi, Golia Paolo, Natalizia Andrea, De Ruvo Ermenegildo, Dottori Serena, Scarà Antonio, Borrelli Alessio, De Luca Lucia, Rebecchi Marco, Fagagnini Alessandro, Bandini Alberto, Guarracini Fabrizio, Galvani Marcello, Calò Leonardo. Which is the best catheter to perform atrial fibrillation ablation? A comparison between standard ThermoCool, SmartTouch, and Surround Flow catheters. J Interv Card Electrophysiol. 2014 Apr;39 (3):193–200. doi: 10.1007/s10840-014-9874-2. [DOI] [PubMed] [Google Scholar]
- 11.Jarman Julian W E, Panikker Sandeep, DAS Moloy, Wynn Gareth J, Ullah Waqas, Kontogeorgis Andrianos, Haldar Shouvik K, Patel Preya J, Hussain Wajid, Markides Vias, Gupta Dhiraj, Schilling Richard J, Wong Tom. Relationship between contact force sensing technology and medium-term outcome of atrial fibrillation ablation: a multicenter study of 600 patients. J. Cardiovasc. Electrophysiol. 2015 Apr;26 (4):378–84. doi: 10.1111/jce.12606. [DOI] [PubMed] [Google Scholar]
- 12.Sigmund Elisabeth, Puererfellner Helmut, Derndorfer Michael, Kollias Georgios, Winter Siegmund, Aichinger Josef, Nesser Hans-Joachim, Martinek Martin. Optimizing radiofrequency ablation of paroxysmal and persistent atrial fibrillation by direct catheter force measurement-a case-matched comparison in 198 patients. Pacing Clin Electrophysiol. 2015 Feb;38 (2):201–8. doi: 10.1111/pace.12549. [DOI] [PubMed] [Google Scholar]
- 13.Akca Ferdi, Janse Petter, Theuns Dominic A M J, Szili-Torok Tamas. A prospective study on safety of catheter ablation procedures: contact force guided ablation could reduce the risk of cardiac perforation. Int. J. Cardiol. 2015 Jan 20;179 ():441–8. doi: 10.1016/j.ijcard.2014.11.105. [DOI] [PubMed] [Google Scholar]
- 14.Kumar Saurabh, Morton Joseph B, Lee Justin, Halloran Karen, Spence Steven J, Gorelik Alexandra, Hepworth Graham, Kistler Peter M, Kalman Jonathan M. Prospective characterization of catheter-tissue contact force at different anatomic sites during antral pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2012 Dec;5 (6):1124–9. doi: 10.1161/CIRCEP.112.972208. [DOI] [PubMed] [Google Scholar]
- 15.Neuzil Petr, Reddy Vivek Y, Kautzner Josef, Petru Jan, Wichterle Dan, Shah Dipen, Lambert Hendrik, Yulzari Aude, Wissner Erik, Kuck Karl-Heinz. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol. 2013 Apr;6 (2):327–33. doi: 10.1161/CIRCEP.113.000374. [DOI] [PubMed] [Google Scholar]
- 16.Haldar Shouvik, Jarman Julian W E, Panikker Sandeep, Jones David G, Salukhe Tushar, Gupta Dhiraj, Wynn Gareth, Hussain Wajid, Markides Vias, Wong Tom. Contact force sensing technology identifies sites of inadequate contact and reduces acute pulmonary vein reconnection: a prospective case control study. Int. J. Cardiol. 2013 Sep 30;168 (2):1160–6. doi: 10.1016/j.ijcard.2012.11.072. [DOI] [PubMed] [Google Scholar]
- 17.Reddy Vivek Y, Shah Dipen, Kautzner Josef, Schmidt Boris, Saoudi Nadir, Herrera Claudia, Jaïs Pierre, Hindricks Gerhard, Peichl Petr, Yulzari Aude, Lambert Hendrik, Neuzil Petr, Natale Andrea, Kuck Karl-Heinz. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm. 2012 Nov;9 (11):1789–95. doi: 10.1016/j.hrthm.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Wutzler Alexander, Huemer Martin, Parwani Abdul Shokor, Blaschke Florian, Haverkamp Wilhelm, Boldt Leif-Hendrik. Contact force mapping during catheter ablation for atrial fibrillation: procedural data and one-year follow-up. Arch Med Sci. 2014 May 12;10 (2):266–72. doi: 10.5114/aoms.2014.42578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stabile Giuseppe, Solimene Francesco, Calò Leonardo, Anselmino Matteo, Castro Antonello, Pratola Claudio, Golia Paolo, Bottoni Nicola, Grandinetti Giuseppe, De Simone Antonio, De Ponti Roberto, Dottori Serena, Bertaglia Emanuele. Catheter-tissue contact force for pulmonary veins isolation: a pilot multicentre study on effect on procedure and fluoroscopy time. Europace. 2014 Mar;16 (3):335–40. doi: 10.1093/europace/eut262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapa Suraj, Asirvatham Samuel J. Maintaining contact for effective mapping and ablation. Circ Arrhythm Electrophysiol. 2014 Oct;7 (5):781–4. doi: 10.1161/CIRCEP.114.002204. [DOI] [PubMed] [Google Scholar]
- 21.Olson Matthew D, Phreaner Nicholas, Schuller Joseph L, Nguyen Duy T, Katz David F, Aleong Ryan G, Tzou Wendy S, Sung Raphael, Varosy Paul D, Sauer William H. Effect of catheter movement and contact during application of radiofrequency energy on ablation lesion characteristics. J Interv Card Electrophysiol. 2013 Nov;38 (2):123–9. doi: 10.1007/s10840-013-9824-4. [DOI] [PubMed] [Google Scholar]


