Abstract
Background
The function of the Cannabinoid 1 receptor (CB1R) in the development of neuropathic pain is not clear. Mounting evidence suggest that CB1R expression and activation may contribute to pain. Cannabinoid 1 receptor knockout mice (CB1R−/−) generated on a C57Bl/6 background exhibit hypoalgesia in the hotplate assay and formalin test. These findings suggest that Cannabinoid 1 receptor expression mediates the responses to at least some types of painful stimuli. By using this mouse line, we sought to determine if the lack of Cannabinoid 1 receptor unveils a general hypoalgesic phenotype, including protection against the development of neuropathic pain. The acetone test was used to measure cold sensitivity, the electronic von Frey was used to measure mechanical thresholds before and after spared-nerve injury, and analysis of footprint patterns was conducted to determine if motor function is differentially affected after nerve-injury in mice with varying levels of Cannabinoid 1 receptor.
Results
At baseline, CB1R−/− mice were hypersensitive in the acetone test, and this phenotype was maintained after spared-nerve injury. Using calcium imaging of lumbar dorsal root ganglion (DRG) cultures, a higher percentage of neurons isolated from CB1R−/− mice were menthol sensitive relative to DRG isolated from wild-type (CB1R+/+) mice. Baseline mechanical thresholds did not differ among genotypes, and mechanical hypersensitivity developed similarly in the first two weeks following spared-nerve injury (SNI). At two weeks post-SNI, CB1R−/− mice recovered significantly from mechanical hypersensitivity, while the CB1R+/+ mice did not. Heterozygous knockouts (CB1R+/−) transiently developed cold allodynia only after injury, but recovered mechanical thresholds to a similar extent as the CB1R−/− mice. Sciatic functional indices, which reflect overall nerve health, and alternation coefficients, which indicate uniformity of strides, were not significantly different among genotypes.
Conclusion
Cold allodynia and significant recovery from spared-nerve injury-induced mechanical hypersensitivity are two novel phenotypes which characterize the global CB1R−/− mice. An increase in transient receptor potential channel of melastatin 8 channel function in DRG neurons may underlie the cold phenotype. Recovery of mechanical thresholds in the CB1R knockouts was independent of motor function. These results indicate that CB1R expression contributes to the development of persistent mechanical hypersensitivity, protects against the development of robust cold allodynia but is not involved in motor impairment following spared-nerve injury in mice.
Keywords: Cannabinoid 1 receptor, CB1R knockout, dorsal root ganglion, allodynia, spared-nerve injury, neuropathic pain
Background
Despite the plethora of studies examining the potential of cannabinoids for the treatment of pain of various etiologies, relatively little information is known about the spatial and temporal changes of the targets of these compounds within the nervous system. One of these targets is the cannabinoid 1 receptor (CB1R), a G-protein coupled receptor localized on neurons in the peripheral and central nervous systems that modulates neurotransmitter release.1 Because CB1R is one of the most abundant G-protein coupled receptors in the body and is located on key circuits involved in nociception,2,3 perturbations of CB1R expression may affect the experience of pain. After injury and during painful states, there are dynamic changes in CB1R expression; the magnitude and direction of these changes appear to be dependent upon the severity of the insult, the type of tissues affected, and the time points examined relative to the initial injury.4,5 However, it is not clear how CB1R expression is involved in the development of neuropathic pain. For cannabinoids to be used safely and effectively in the treatment of neuropathic pain, it is necessary to understand how expression of CB1R affects the course of sensory and motor impairment after nerve injury.
Cannabinoids have been suggested as potential adjuvants for neuropathic pain treatment, a particularly difficult condition to treat in humans since it is often refractory to any treatments.6 Cannabinoid usage is controversial, partly because of its small therapeutic window before the manifestation of a myriad of side effects associated with stimulation of CB1R in the central nervous system.7 Moreover, there is evidence to suggest that CB1R stimulation may not be effective in relieving neuropathic pain8 and may actually contribute to hyperalgesia in experimental pain models in animals and humans.9–13 Specifically in preclinical neuropathic pain models, the effectiveness of cannabinoids is inconsistent. For example, in the chronic constriction injury (CCI) model, intrathecal injection of the non-specific CBR agonist WIN 55,212-2 is effective in mitigating mechanical allodynia, and it was postulated that CB1R up-regulation in the spinal cord after injury mediates this pain relieving effect.14 In contrast, others have demonstrated that an antagonist of CB1R administered systemically significantly reduces mechanical and thermal hyperalgesia induced by CCI in rats and in mice.15 Several additional studies show that compounds that inhibit CB1R promote pain relief and recovery after peripheral nerve and burn injuries,16–18 suggesting that CB1R expression and activation can be maladaptive.
To date, no one has examined the contribution of CB1R expression in the development of spared-nerve injury (SNI)-induced neuropathic pain.19–21 In contrast to other peripheral nerve injury models, SNI is a particularly well-suited model of treatment-refractory neuropathic pain, since standard analgesics such as morphine and gabapentin are much less effective in rats with SNI.22 The mixed CBR receptor (CB1R/CB2R) agonist WIN 55,212-2 when administered to mice with SNI is similarly ineffective.8 Interestingly, low doses of WIN 55,212-2 are antihyperalgesic in several other pain models,8,23,24 suggesting that cannabinoid receptors are differentially modulated depending on the injury. We sought to determine if the lack of CB1R unveils a general hypoalgesic phenotype. SNI was performed on CB1R wildtype (+/+), homozygous knockout (−/−), and heterozygous knockout mice (+/−) generated by Zimmer et al.25 The electronic von Frey and acetone tests were performed before and after injury to determine whether a global absence of functional CB1R would unveil hypoalgesia to innocuous cold and mechanical stimuli and protect against the development of persistent neuropathic pain.
Methods
Animals
All experiments were approved by and conducted in accordance with guidelines set forth by the New York University Animal Care and Usage Committee. Cannabinoid 1 receptor knockout (CB1R−/−) mice were generated from CB1 heterozygous (+/−) breeding pairs obtained from Dr Andreas Zimmer, University of Bonn, Germany, through the European Mutant Mouse Archive. Genotypes were confirmed with PCR as previously described.26 Adult male mice (8–12 weeks old) were used for experiments. Food and water were available ad libitum.
Behavioral measurements: Mechanical and thermal thresholds
All behavior tests were performed during the day portion of the circadian cycle (06:00 h to 18:00 h), and the experimenter was blinded to the genotype during the tests.
To measure mechanical thresholds, the electronic Von Frey method was used. Mice were first weighed on a scale before being placed in separate plexiglass boxes on top of a raised wire mesh. They were left to acclimate for approximately 30 minutes prior to testing. Stimulation of the lateral area of the hind paws was done using the 90 g arm and the size 8 filament. The maximum threshold readout that accompanied a paw flick was recorded. Both paws of each animal were stimulated three times, with a 2-minute rest in-between stimulations.
To measure for cold hypersensitivity, the acetone test of evaporative cooling was used. After the von Frey test, mice were allowed to rest for approximately 20 minutes before commencing with acetone applications. A small drop of acetone was placed on the plantar surface of the hind paws with a pipette, making sure that only the acetone drop touched the paw, and not the pipette tip. The duration of paw withdrawal was recorded for 30 seconds afterwards. As a control, we placed a drop of water at 37℃ on the paws. This induced a quick paw flick (<1 s), but no further paw withdrawal (data not shown).
Spared-nerve injury
Using aseptic surgical technique, under 2% isoflurane anesthesia, the skin on the lateral surface of the right thigh was incised, and the biceps femoris muscle separated to expose the sciatic nerve and its three terminal branches: the sural, common peroneal, and tibial nerves. The common peroneal and tibial branches were ligated together with a 9-0 silk suture, transected distal to the ligature, and 2 mm of each distal nerve stump was removed.19 The overlying muscle was closed with an absorbable suture 6.0 PDS Plus Antibacterial (Polydioxanone) by Ethicon, and the skin closed with non-absorbable monofilament, nylon size 5 sutures. After the surgeries, mice were housed separately and monitored for signs of autotomy or distress.
Dissociated dorsal root ganglia primary cultures
Primary cultures of dorsal root ganglion (DRG) from lumbar areas of the spinal column were extracted from CB1R +/+and CB1R−/− mice at 3–4 months of age. The tissues were enzymatically digested with trypsin and collagenase at 37℃, subjected to a gradient to remove myelin and plated on poly-D-lysine coated coverslips (12.5 µg/ml or 25 µg/ml).27 Cells were grown for two days in Neurobasal A media supplemented with 2% B27, 1% Pen/Strep, 0.5 mM Glutamax, and NGF (100 ng/ml)28and GDNF (10 ng/ml),29 as previously described.
Calcium imaging
To measure changes in intracellular calcium levels in DRG cultures derived from CB1+/+ and CB1−/− mice, the ratiometric calcium indicator dye FURA-2 AM and the PTI (Photon Technology International, Inc. Lawrenceville, NJ, USA) imaging system were used. All measurements were performed at 48 hours in vitro within a temperature range of 30℃ to 32℃. FURA-2 was excited at 340 nm and 380 nm by using a high-speed multi-wavelength illuminator alternated every 0.5 seconds. The emitted fluorescence of single cells was filtered with a fluorescence barrier filter at 515 nm, collected with a Cool-Snap HQ2 camera, and analyzed with ImageMaster 5 software. Cells were continually perfused with a HEPES buffer (pH 7.4) containing (in mM): 140 NaCl, 5 KCl, 5 NaHCO3, 10 HEPES, 1 MgCl2, 1.5 CaCl2, and 10 glucose.30 Cells were “pulsed” using a pressurized superperfusion system (AutoMate Scientific, Inc) with the following compounds (dissolved in HEPES buffer): KCl (50 mM) for 5 seconds to confirm the presence of viable neurons and menthol (250 µM)28 for 7 seconds to stimulate transient receptor potential channel of melastatin 8 (TRPM8) channels. Cells were allowed to recuperate for 10 minutes between stimulations.
Data analysis
For behavioral tests, a total of three stimulations per paw were administered to each animal on the testing day. Averages per animal were calculated from these data and pooled according to genotype. Data are presented as the mean ± SEM. Data were pooled, and t tests performed using Graph Pad Prism 6 software. A p value less than 0.05 was considered statistically significant. Percentage decreases in mechanical thresholds post-SNI were calculated from baseline. The two-way ANOVA test was used for time course of mechanical and cold-hypersensitivity following SNI, with genotype and time as main factors; Tukey’s post hoc test with multiple comparisons was used.
For the imaging experiments, calcium traces from the 340 nm and 380 nm signals, and the derived ratio were exported from the Image Master 5 software into Microsoft Excel. Traces were analyzed in pClamp 10.5.2.6 and Graph Pad Prism 6. Neurons were identified on the basis of their increased cytoplasmic response to 50 mM KCl. Neurons were considered menthol sensitive (MS) if cytoplasmic calcium levels increased at least 5% in the period after drug administration relative to the pre-stimulation baseline. Neurons that were not responsive were designated as menthol insensitive (MI). Differences in menthol sensitivity between genotypes were assessed using the two-tailed, Fisher’s exact test.
Footprint analysis
For footprint analysis, mice performed several pre-training trials during which they were habituated to a runway. A total of N = 4 mice per genotype were used. The start of the runway was brightly lit and the end contained a darkened enclosed chamber. Immediately after the pre-training sessions, footprints were recorded on a white paper on the runway floor after applying non-toxic tempera paint to the paws. Front and rear paws were distinguished by their differing paint color. Between 1 and 3 sets of footprints were collected for each animal in order to obtain footsteps that provided complete print of the foot as well as contiguous steps. From these trials, the highest quality footprints were chosen and used for analysis for each. The footprint patterns were analyzed using the program Image J (NIH). Toe spread was defined as the distance between the most extreme digits. Paw length was measured beginning from the tip of the longest digit to the end of the heel. Sciatic functional indices (SFI) were calculated from the footprint patterns as previously described, by using the following formula: , where ETS is the experimental toe spread, NTS is the normal toe spread, EPL is experimental toe length, and NPL normal paw length.31 An SFI near “0” is a normally functioning nerve, whereas a value of −100 indicates complete impairment.
The distance from the R → L (horizontal distance of the step) foot was measured in addition to the R → R (forward distance of the step). An N of three measurements for each horizontal and forward was obtained when possible. The alternation coefficient was then derived by calculating the absolute value of 0.5 minus the ratio of the average horizontal to average forward distance for each mouse, as previously described.32 The purpose of the alternation coefficient is to show the level of variability or uniformity between each step. A gait which is completely in tandem will have a horizontal to forward ratio of 0.5 resulting in an alternation coefficient of 0. The coefficient was calculated pre-operatively in the week prior to injury, as well as on the following post-operative days (POD): 1, 3, 7, 14, 21, and 28.The coefficients were pooled by genotype and averaged for each day of the study. The average coefficients by genotype and day were then plotted on an x–y line graph in Graph Pad Prism 6. Standard errors of the mean were calculated for each genotype by day.
Results
CB1R−/− mice are hypersensitive to evaporative cooling, but exhibit no differences in mechanical thresholds
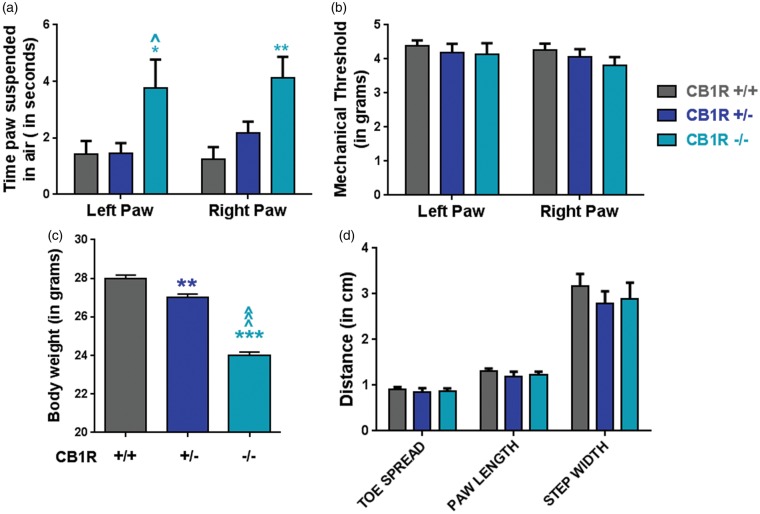
Before performing the peripheral nerve injury, baseline cold responses, mechanical thresholds, body weights, and footprints patterns were measured in adult male CB1R+/+, CB1+/−, and CB1R−/− mice to determine the extent to which CB1R expression affected these parameters. The innocuous cooling agent acetone was used to measure cold sensitivity. The homozygous (CB1R−/−) knockouts, but not the heterozygous knockouts (CB1R+/−), displayed significant cold allodynia in both paws relative to the CB1R+/+ mice (Figure 1(a)). The average duration of paw withdrawals for the CB1R−/− versus the CB1R+/+ mice was 3.75 ± 1.01 versus 1.42 ± 0.47 s in the left paw and 4.14 ± 0.84 s versus 1.26 ± 0.41 s in the right paw. While there was a significant difference in cold responses, mechanical thresholds, measured with the electronic von Frey, were the same among the genotypes (Figure 1(b)). Thresholds in the left and right paws of the mice were 4.39 g ± 0.16 and 4.26 g ± 0.19 in CB1R+/+ mice; 4.20 g ± 0.25 and 4.05 g ± 0.24 in CB1R+/− mice; and 4.14 g ± 0.33 and 3.81 g ± 0.25 in the CB1R−/− mice (Figure 1(b)). The heterozygous and homozygous knockouts weighed significantly less than the CB1R+/+ mice (Figure 1(c)), but this finding did not affect the mechanical thresholds (Figure 1(b)). As a measure of potential gait abnormalities, footprint patterns were measured. No significant differences were observed in toe spreads, paw lengths, or step widths (Figure 1(d)).
Figure 1.
Baseline effects of CB1R deletion in adult male mice. (a) Duration of paw withdrawal after application of acetone to the ipsilateral (right) and contralateral (left) paws in adult male mice. CB1R+/+, N = 9; CB1R+/−, N = 7; CB1R−/−, N = 6. One-way ANOVAs comparing genotypes per paw. (*p < 0.05, **p < 0.01 CB1R−/− vs. CB1R+/+; ^p < 0.05 CB1R−/− vs. CB1R+/−). (b) Mechanical thresholds assessed with the electronic von Frey apparatus are not affected by significantly lower body weights (c) in the knockouts, CB1R+/+, N = 11; CB1R+/−, N = 9; CB1R−/−, N = 8. One-way ANOVA, with Tukey’s comparison (**p < 0.01 vs CB1R+/+; ^p < 0.001 vs. CB1R+/−; ***p < 0.001. Data are expressed as mean ± S.E.M. (d) Measurements from footprint patterns, per genotype.
An increased percentage of CB1R−/− DRG neurons are sensitive to menthol relative to CB1R+/+ neurons
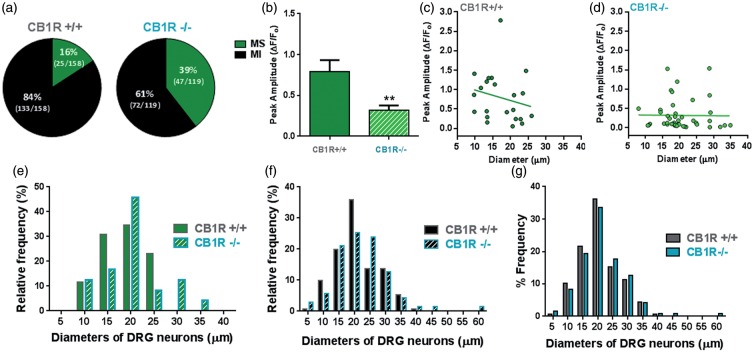
Before SNI, the only significant behavioral difference found to be affected by a global absence of CB1R was cold sensitivity. This was suggestive of a change in the function of a temperature sensitive channel within primary sensory neurons of the DRG. A drop of acetone on the hindpaws is an innocuous cold stimulus, and the TRPM8 is activated upon innocuous and noxious cooling33; hence, we tested whether TRPM8 sensitivity in DRG neurons is correlated with the cold allodynia in the CB1R−/− mice. Primary dissociated cultures generated from the lumbar DRG of CB1R+/+ and CB1R−/− mice were challenged with the TRPM8 agonist menthol.28,33 A significantly higher percentage of neurons from the CB1R−/− cultures were sensitive to menthol compared to CB1R+/+ (39% (47/119) versus 16% (25/158) as measured by Ca2+ imaging (p < 0.0001, Figure 2(a)). Interestingly, the mean amplitude of the menthol responses in the CB1R−/− neurons was significantly lower compared to CB1R+/+ neurons (Figure 2(b)), but more neurons with a diameter of 20 µm and above responded to menthol in CB1R−/− cultures (Figures 2(c) to (e)). Neurons that were MI had a similar diameter frequency distribution between the genotypes (Figure 2(f)). Overall, lumbar DRG cultures from both genotypes contained neurons with similar diameter distributions, with a mean of 20.4 ± 0.5 µm in CB1R+/+ cultures and 21.4 ± 0.7 µm in CB1R−/− cultures (Figure 2(g)). Similar frequencies across a range of diameters, but different responses to menthol stimulation indicate that CB1R genotype affected TRPM8 sensitivity rather than a preferential survival of a subset of neurons in culture. An increase in the percentage of menthol-sensitive DRG neurons (Figure 2) is related to the observed cold allodynia phenotype displayed by CB1R−/− mice (Figure 1(a)).
Figure 2.
Increased percentage of menthol sensitive neurons in CB1R−/− DRG cultures. Primary cultures were generated from lumbar DRG isolated from CB1R+/+ (N = 4 mice, 11 coverslips) and CB1R−/− (N = 5 mice, 10 coverslips), loaded with FURA-2 and stimulated with the TRPM8 agonist menthol. (a) Pie-graphs depicting the percentage of menthol sensitive (MS) and menthol-insensitive (MI) neurons per genotype with the number of neurons imaged placed in parentheses; CB1R−/− had a significantly higher percentage of menthol sensitive neurons, p < 0.0001, Fisher’s exact test. (b) Average amplitude of peak menthol responses in CB1R+/+ and CB1R−/− cultures. Data are presented as mean amplitude of the ΔF/Fo ± SEM. ** p < 0.05, Student’s t-test. Scatter plot of neuronal diameters versus peak menthol response; each point represents a single neuron from (c) CB1R+/+ and (d) CB1R−/− cultures. Relative frequency of (e) ‘MS’ and (f) ‘MI’ DRG neurons in CB1R+/+ and CB1R−/− according to neuron diameter (in µm). (e) Frequency distribution (in percentage) of all DRG neurons in culture per diameter and per genotype; bin centers set at 5 µm.
CB1R expression protects against the development of cold allodynia, but contributes to persistent mechanical hypersensitivity following SNI in mice
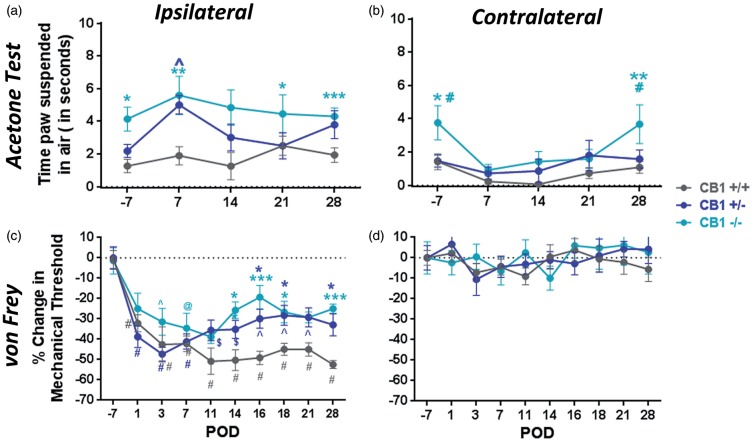
After collecting pre-surgery data, mice were subjected to SNI as described in methods. Following SNI, the high number of cold responses was maintained in the ipsilateral paws of the CB1R−/− mice for the four-week observation period (Figure 3(a)), but not in the contralateral paws (Figure 3(b)). At POD 7, 14, and 21, the contralateral paws lost the cold allodynia phenotype, but regained it by POD 28 (Figure 3(b)). Interestingly, though CB1R+/− protected against cold hypersensitivity before surgery (Figure 1(a)), this genotype was sufficient to produce transient, significant cold hypersensitivity at POD 7 comparable to CB1R−/− mice. The cold allodynia phenotype never developed in the CB1R+/+ mice (Figure 3(a)). However, in all the mice, the contralateral paws displayed a decrease in their cold sensitivity between POD 7 and 21 (Figure 3(b)), which resulted in significant differences between the ipsilateral and contralateral paws in CB1R+/− (POD7, p < 0.0001) and CB1R−/− mice (POD7, p < 0.05).
Figure 3.
CB1R expression affects the development of cold and mechanical hypersensitivity following SNI in mice. (a) Cold responses in ipsilateral paws of CB1R−/− mice are significantly higher than CB1R+/+ responses (*p < 0.05; **p < 0.01; ***p < 0.001) and CB1R−/− mice develop this phenotype at day 7 (^p < 0.05 vs. CB1R+/+). Two-way ANOVA with Tukey’s post hoc test with multiple comparisons. (b) Cold responses in the contralateral paws, *p < 0.05, **p < 0.01 CB1R−/− vs. CB1R +/+; #p < 0.05 CB1R−/− vs. CB1R+/−. Significances between ipsilateral and contralateral paws are indicated in results. (c) Percentage change in mechanical thresholds calculated from baseline before surgery (POD -3). All genotypes develop mechanical hypersensitivity, but beginning at POD14 up to POD28, thresholds are significantly higher in the knockouts (CB1R−/− and CB1R+/−) compared to CB1R+/+. ^p < 0.05; @ p < 0.01; $ p < 0.001; #p < 0.0001 vs. baseline; *p < 0.05; ***p < 0.001 versus CB1R+/+ at particular time point. (d) Percentage change of contralateral paw mechanical thresholds.
With respect to mechanical thresholds, all genotypes developed a similar magnitude of hypersensitivity in the ipsilateral paws, indicated by a significant decrease in thresholds immediately after the injury (Figure 3(c)). However, beginning at POD 14, the threshold of the CB1R−/− and CB1R+/− mice began to significantly increase relative to CB1R+/+ mice, reaching approximately 50% recovery (Figure 3(c)). The contralateral paws of mice from each genotype never developed mechanical hypersensitivity (Figure 3(d)). Although CB1R+/+ mice weighed, on average, more than the knockouts at every time point, animals in all groups maintained their weight following SNI, indicating that the surgery did not limit their access to and interest in food (data not shown). Moreover, for a given animal per genotype, changes in mechanical thresholds over time are not due to differences in body weight.
CB1R expression is not involved in SNI-induced motor impairment
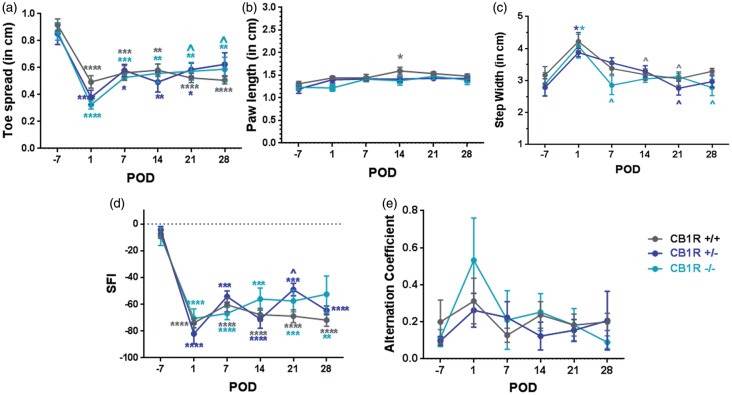
Following SNI, toe spread significantly decreased for each genotype relative to their baselines, throughout the four week observation period (Figure 4(a)). There was a tendency for the injured paw length to increase over time, but was only significantly higher in CB1R+/+ at POD14 relative to pre-surgery length (Figure 4(b), p < 0.05). Step width also significantly increased immediately after SNI, but resolved to pre-surgical distances by POD28 (Figure 4(c)). A two-way ANOVA was conducted that examined the effect of genotype and time on toe-spread, paw length, or step width. There was no statistically significant interaction between genotype and time, on these parameters. As an overall measure of motor nerve health, SFIs significantly decreased to a similar extent after SNI in all genotypes. This decrease was maintained over the entire observation period (Figure 4(d)). Moreover, uniformity of strides, measured by alternation coefficients was similar between genotypes (Figure 4(e)).
Figure 4.
Motor function after SNI is not differentially affected by CB1R expression. The (a) total toe spread, (b) length of ipsilateral paws, and (c) step width following SNI. (d) Sciatic Functional Indices (SFI) calculated from A & B, using the left uninjured paw as the control, described in Methods. (e) Alternation coefficients calculating uniformity of gait were not significantly affected by genotype or SNI. Two-way ANOVA using Tukey’s post hoc multiple comparisons test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 for POD vs. pre-SNI values; ^p < 0.05 vs. POD 1. N = 4 mice per genotype.
Discussion
In this study, we found that cold allodynia and significant recovery from SNI-induced mechanical hypersensitivity are two novel phenotypes which characterize the global CB1R−/− mice. In the acetone test of evaporative cooling, the CB1R−/− mice lift their paws significantly longer in response to this innocuous stimulus. The cold allodynia is related to an increase in functional TRPM8 channels in DRG neurons, as assessed by calcium imaging of menthol-stimulated cultures. After SNI, the ipsilateral paw maintained cold hypersensitivity for at least four weeks, but the contralateral paws lost this phenotype at weeks 1, 2, and 3. Thus, central CB1R expression may also be contributing to the sensory phenotypes following SNI. Moreover, it was also found that CB1R expression and constitutive activity do not affect baseline mechanical thresholds or the level of injury-induced mechanical hypersensitivity during the first two weeks following SNI. However, the presence of CB1R appears to contribute to persistent mechanical allodynia in the SNI model, since significant recovery was evident only in mice lacking CB1R. Collectively, these results indicate that CB1R may protect against the development of some types of pain such as cold allodynia, but it may contribute to the development of other types of pain such as nerve injury-induced mechanical hypersensitivity. While global deletion of CB1R may promote significant recovery from SNI-induced mechanical hypersensitivity in mice, it does not promote significant motor recovery.
With respect to the thermal phenotype of CB1R−/− mice, because the acetone test measures responses to innocuous cooling, the TRPM8 channel is a likely candidate involved in the behavioral responses that were observed.34,35 TRPM8 within the DRG contributes to the perception of cold sensation under normal conditions,36 and increases in this channel's activity has been associated with the development of cold allodynia.37 In order to further probe this behavioral finding, menthol stimulation of DRG primary cultures was used to determine whether TRPM8 channel sensitivity differs between the genotypes. It was found that CB1R−/− DRG cultures have a significantly greater percentage of neurons sensitive to menthol, including larger diameter neurons; however, the peak menthol-induced calcium responses are lower in the knockouts. Menthol responses in the larger diameter neurons from CB1R−/− are suggestive of a phenotypic switch, since in vivo TRPM8 expression is found in approximately 10% to 15% of the total adult DRGs and mostly in small diameter, non-peptidergic, and un-myelinated neurons of C and Aδ fibers.28,36,38 Our data are the first to demonstrate that levels of CB1R in sensory neurons significantly affect menthol responses and thus TRPM8 channel activity. An increase in the proportion of sensory neurons with functional TRPM8 channels correlates with the presence of cold allodynia in CB1R−/− animals. In prior studies using TRPM8-transfected HEK cells, a relationship between CB1R activity and TRPM8 channel function has been suggested.39,40 While cannabinoids can directly inhibit TRPM8 channel activity, this inhibition is augmented with increased CB1R expression.39–41 These findings are consistent with our observations in that a complete down-regulation of CB1R expression (CB1R−/−) results in an increase in TRPM8 activity.
Presently, we cannot rule out compensatory changes of other ion channels in CB1R−/− that may contribute to the cold responses in the mice and increased menthol sensitivity in the DRG sensory neurons.15,16 However, CB1R expression levels indeed appear to be important for cold hypersensitivity and mechanical thresholds, since we also show that partial CB1R knockdown (CB1R+/−) is sufficient to induce transient cold allodynia after SNI and promote recovery from mechanical allodynia.
While pre-surgical mechanical thresholds are similar among genotypes, significant recovery from SNI-induced mechanical hypersensitivity occurs only in the CB1R−/− and CB1R+/− mice. To our knowledge, this is the first study to demonstrate these phenotypes. The data suggest that constitutive activity of CB1Rs or their stimulation by endocannabinoids following SNI contributes to persistent mechanical hypersensitivity. In contrast, studies using global CB1R−/− mice and other models of peripheral nerve injuries fail to demonstrate any differences in mechanical threshold changes relative to wildtype.42–44 These studies include partial sciatic nerve ligation (PSL) performed on the same mouse line used in our study43; and CCI of the sciatic nerve performed on global CB1R−/− mouse generated on a C57Bl/6 background9 and on a CD-1 background.44 PSL and CCI involve ligating different proportions of sciatic nerve fibers at the mid-thigh level.45 The SNI model used here involves the ligation, transection, and partial removal of two of the three terminal branches of the sciatic nerve distal to the ligation.19 In PSL and CCI, there is intermingling of the injured and intact axons both proximal and distal to the injury, but in SNI, this interaction only occurs proximal to the injury. During the behavioral testing, the SNI-evoked responses are from the uninjured sciatic nerve fibers and possibly from the sprouting sympathetic and saphenous nerves, whereas responses in the PSL and CCI models include both the intact and regenerating sciatic nerve axons.22 We show in the SNI model that CB1R−/− and CB1R+/− promotes significant recovery of mechanical thresholds. Collectively, irrespective of mouse strain, in peripheral-nerve injuries that do not involve transection of sciatic nerve fibers (i.e., PSL and CCI), global CB1R expression perturbation does not significantly contribute to sensory changes. In contrast, hypersensitivities resulting from sciatic nerve fiber transection injuries (i.e., SNI) appear to be significantly affected by CB1R expression. It is, therefore, possible that there are different mechanisms by which CB1R is involved in the development of nerve injury-evoked functional changes. In fact, it has been suggested that analgesic sensitivity is dependent on the nerve injury model,22 and this may partly explain why CBR agonists have been reported as ineffective in SNI-induced neuropathic pain in mice.8
Because the data presented in the current study are derived from global CB1R−/− mice, the distinct contributions of peripheral and central nervous system regions are not clear.46 Several mouse lines have been generated in which CB1R is conditionally deleted from a particular type of neuron; these include deletions in principal forebrain neurons,47 GABAergic neurons in the brain,48 GABAergic neurons in the dorsal horn,9 and in peripheral nociceptors.49 Of those, SNI has only been performed on the mice with conditional CB1R deletion in peripheral nociceptors. This mouse line targeted DRG that also co-expressed IB4, TRPV1, Substance P, and Nav1.8 channels; however, CB1R expression was not affected in large diameter A neurons expressing NF200.49 In these mice, pre-surgical mechanical thresholds were lower while acetone responses were the same relative to control wild-type mice. After SNI, no differences in mechanical thresholds were evident in the three week observation period and only the conditional knockouts had significant cold allodynia two weeks after injury.49 However, within the DRG, CB1R expression is not limited to small diameter, nociceptors50; CB1R is expressed across of range of DRG neurons in vivo, including those expressing calcitonin gene related peptide (CGRP).51 Though mice with a conditional deletion of CB1R on CGRP expressing sensory neurons have not been generated, selective ablation of the CGRP neuron population caused increased responses in the acetone test, but had no effect on mechanical thresholds pre- and post-SNI.52 Taken together, CB1R expressing cells play a complex role in somatic sensation, since global or conditional deletion produces varying phenotypes, when assessed before and after SNI. Hence, the distinct pain phenotypes of CB1R knockout mice observed in this study may involve both peripheral and central mechanisms.
Though we found significant genotypic differences in sensory tests, gross motor function was not differentially affected following SNI. The motor findings are not completely unexpected. In nerve transection injuries proper synapse formation is hindered and motor function continues to be significantly impaired at two months.53 In contrast, if the sciatic nerve is crushed, sensory and motor functions are completely restored within one month.53 The SNI model in the mouse further ensures prolonged dennervation of sciatic fibers because the tibial and peroneal branches are also ligated before being transected, and pieces of the nerves are also removed. Importantly, SNI does not preclude the capacity for motor recovery since effective pharmacological interventions have been found.54 However, by computing sciatic functional indices and assessing alternation coefficients, we showed that expression of CB1R does not significantly improve motor function, and is therefore not involved in SNI-induced motor impairment.
Given that cannabinoids acting at CB1R are attractive therapeutic compounds for the relief of pain, yet neuropathic pain conditions can manifest as a complex mixture of mechanical and thermal allodynia and hyperalgesia, further studies are warranted to understand how acute and chronic treatments with CB1R agonists affect the distinct symptoms experienced by pain patients. Our data add to the findings of several neuropathic pain animal models, but emphasize that differences in the regulation of endocannabinoid system exist across models and likely across humans. Within the spectrum of neuropathic pain disorders in patients, there appears to be mechanistic heterogeneity which may invariably contribute to the efficacy of cannabinoids.55–57
At this point, it is difficult to extrapolate for which conditions and symptoms CB1R activation will be most efficacious. With respect to thermal sensitivity, our present findings in the knockout mice, other pre-clinical work and experimental pain models in humans suggest that CB1R activation may oppositely modulate the thermo-TRP channels TRPM8 and TRPV1.11,25,39–41,58,59 Additional clinical studies are needed to measure the effect of CB1R activation specifically on thermal and mechanical thresholds.60 Our data also suggest that CB1R antagonism may prevent the development or ameliorate established nerve-injury induced mechanical hypersensitivity. While animal studies show this intervention to be promising,15–17 further drug development of CB1R neutral antagonists or negative allosteric modulators may be key, since severe psychiatric side effects can precipitate in vulnerable patients taking CB1R inverse agonists.61,62
When discussing the place of individual cannabinoids, whole plant, or extracts of marijuana in pain management, it is of great importance to emphasize that medical marijuana throughout the USA and in other countries is not universally regulated with respect to cannabinoid content and ratio, or the form and route of administration. Marijuana contains many cannabinoids and other chemicals that interact to produce their effects in the body.63 Marijuana is as heterogeneous and complex as the pain patient, urging both caution and continued efforts to elucidate pain mechanisms that will ultimately inform optimal, personalized cannabinoid therapy.
Conclusion
Compounds acting at CB1R may have therapeutic potential for the relief of pain, including neuropathic origin. It is important to decipher how CB1R expression correlates with behavioral endpoints. To our knowledge, the data presented here are the first to demonstrate that global CB1R−/− mice are hypersensitive to evaporative cooling and recover significantly from SNI-induced mechanical hypersensitivity. Our data suggest that CB1R expression contributes to distinct aspects of persistent neuropathic pain in the SNI model in mice.
Acknowledgments
The authors thank Department of Anesthesiology at NYU Langone Medical Center for research support; Dr Andreas Zimmer, University of Bonn, Germany and the European Mouse Mutant Archive for providing CB1 heterozygous breeding pairs for experiments; Dr Jin Zhang for technical and administrative support; Dr Brian Schimdt and Lab at the Bluestone Center for Clinical Research at NYUCD for helping us set-up the electronic von Frey; Joyce Chen and Shanelle Mason for assisting with behavioral experiments, and Daniel Choi for writing a macro to transform Image Master 5 data for pClamp analysis.
Authors’ contributions
AS performed the surgeries, the behavioral tests, calcium imaging, data analysis, and wrote the manuscript; BP assisted in primary culture preparation, calcium imaging, and footprint analysis; LR assisted in initial surgeries, behavioral testing, and footprint analysis; MN taught the surgeries and assisted in behavioral testing; TJJB and ERP were involved in conceptualizing the study and providing support. All authors read and contributed to the final version of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support was provided by the NYULMC Department of Anesthesiology Research Funds.
References
- 1.Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 2002; 54: 161–202. [DOI] [PubMed] [Google Scholar]
- 2.Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience 1999; 90: 923–931. [DOI] [PubMed] [Google Scholar]
- 3.Farquhar-Smith WP, Egertova M, Bradbury EJ, et al. Cannabinoid CB(1) receptor expression in rat spinal cord. Mol Cell Neurosci 2000; 15: 510–521. [DOI] [PubMed] [Google Scholar]
- 4.Miller LK, Devi LA. The highs and lows of cannabinoid receptor expression in disease: mechanisms and their therapeutic implications. Pharmacol Rev 2011; 63: 461–470. doi:10.1124/pr.110.003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rani Sagar D, Burston JJ, Woodhams SG, et al. Dynamic changes to the endocannabinoid system in models of chronic pain. Philos Trans R Soc B 2012; 367: 3300–3311. doi:10.1098/rstb.2011.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics 2009; 6: 713–737. doi:10.1016/j.nurt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 2015; 313: 2456–2473. doi:10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 8.Hald A, Ding M, Egerod K, et al. Differential effects of repeated low dose treatment with the cannabinoid agonist WIN 55,212-2 in experimental models of bone cancer pain and neuropathic pain. Pharmacol Biochem Behav 2008; 91: 38–46. doi:10.1016/j.pbb.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Pernia-Andrade AJ, Kato A, Witschi R, et al. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science 2009; 325: 760–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang G, Chen W, Lao L, et al. Cannabinoid CB1 receptor facilitation of substance P release in the rat spinal cord, measured as neurokinin 1 receptor internalization. Eur J Neurosci 2010; 31: 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace M, Schulteis G, Atkinson JH, et al. Dose-dependent effects of smoked cannabis on capsaicin-induced pain and hyperalgesia in healthy volunteers. Anesthesiology 2007; 107: 785–796. doi:10.1097/01.anes.0000286986.92475.b7. [DOI] [PubMed] [Google Scholar]
- 12.Azim S, Nicholson J, Rebecchi MJ, et al. Endocannabinoids and acute pain after total knee arthroplasty. Pain 2015; 156: 341–347. doi:10.1097/01.j.pain.0000460315.80981.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraft B, Frickey NA, Kaufmann RM, et al. Lack of analgesia by oral standardized cannabis extract on acute inflammatory pain and hyperalgesia in volunteers. Anesthesiology 2008; 109: 101–110. doi:10.1097/ALN.0b013e31817881e1. [DOI] [PubMed] [Google Scholar]
- 14.Lim G, Sung B, Ji RR, et al. Upregulation of spinal cannabinoid-1-receptors following nerve injury enhances the effects of Win 55,212-2 on neuropathic pain behaviors in rats. Pain 2003; 105: 275–283. [DOI] [PubMed] [Google Scholar]
- 15.Costa B, Trovato AE, Colleoni M, et al. Effect of the cannabinoid CB1 receptor antagonist, SR141716, on nociceptive response and nerve demyelination in rodents with chronic constriction injury of the sciatic nerve. Pain 2005; 116: 52–61. [DOI] [PubMed] [Google Scholar]
- 16.Comelli F, Bettoni I, Colombo A, et al. Rimonabant, a cannabinoid CB1 receptor antagonist, attenuates mechanical allodynia and counteracts oxidative stress and nerve growth factor deficit in diabetic mice. Eur J Pharmacol 2010; 637: 62–69. doi:10.1016/j.ejphar.2010.03.061. [DOI] [PubMed] [Google Scholar]
- 17.Toniolo EF, Maique ET, Ferreira WA, Jr, et al. Hemopressin, an inverse agonist of cannabinoid receptors, inhibits neuropathic pain in rats. Peptides 2014; 56: 125–131. doi:10.1016/j.peptides.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueda M, Iwasaki H, Wang S, et al. Cannabinoid receptor type 1 antagonist, AM251, attenuates mechanical allodynia and thermal hyperalgesia after burn injury. Anesthesiology 2014; 121: 1311–1319. doi:10.1097/ALN.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourquin AF, Suveges M, Pertin M, et al. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain 2006; 122: 14.e1–14. [DOI] [PubMed] [Google Scholar]
- 20.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 2000; 87: 149–158. doi:S0304-3959(00)00276-1 [pii]. [DOI] [PubMed] [Google Scholar]
- 21.Shields SD, Eckert WA, 3rd, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain 2003; 4: 465–470. doi: S1526590003007818 [pii]. [DOI] [PubMed] [Google Scholar]
- 22.Decosterd I, Allchorne A, Woolf CJ. Differential analgesic sensitivity of two distinct neuropathic pain models. Anesth Analg 2004; 99: 457–463. doi: 10.1213/01.ANE.0000131967.69309.4F. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed MM, Rajpal S, Sweeney C, et al. Cannabinoid subtype-2 receptors modulate the antihyperalgesic effect of WIN 55,212-2 in rats with neuropathic spinal cord injury pain. Spine J 2010; 10: 1049–1054. doi: 10.1016/j.spinee.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Lever IJ, Pheby TM, Rice AS. Continuous infusion of the cannabinoid WIN 55,212-2 to the site of a peripheral nerve injury reduces mechanical and cold hypersensitivity. Br J Pharmacol 2007; 151: 292–302. doi: 10.1038/sj.bjp.0707210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmer A, Zimmer AM, Hohmann AG, et al. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA 1999; 96: 5780–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sideris A, Bekker T, Chan WS, et al. A role for the cannabinoid 1 receptor in neuronal differentiation of adult spinal cord derived progenitors in vitro is revealed through pharmacological inhibition and genetic deletion. Front Neuropharmacol 2012; 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo C, Norcini M, Baquero-Buitrago J, et al. The N-methyl-D-aspartate-evoked cytoplasmic calcium increase in adult rat dorsal root ganglion neuronal somata was potentiated by substance P pretreatment in a protein kinase C-dependent manner. Neuroscience 2011; 177: 308–320. doi: 10.1016/j.neuroscience.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 28.Dhaka A, Earley TJ, Watson J, et al. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci 2008; 28: 566–575. doi: 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baudet C, Mikaels A, Westphal H, et al. Positive and negative interactions of GDNF, NTN and ART in developing sensory neuron subpopulations, and their collaboration with neurotrophins. Development 2000; 127: 4335–4344. [DOI] [PubMed] [Google Scholar]
- 30.Sutachan JJ, Montoya GJ, Xu F, et al. Pluronic F-127 affects the regulation of cytoplasmic Ca2+ in neuronal cells. Brain Res 2006; 1068: 131–137. doi: 10.1016/j.brainres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Yao M, Inserra MM, Duh MJ, et al. A longitudinal, functional study of peripheral nerve recovery in the mouse. Laryngoscope 1998; 108: 1141–1145. [DOI] [PubMed] [Google Scholar]
- 32.Patel S, Hillard CJ. Cannabinoid CB(1) receptor agonists produce cerebellar dysfunction in mice. J Pharmacol Exp Ther 2001; 297: 629–637. [PubMed] [Google Scholar]
- 33.Bautista DM, Siemens J, Glazer JM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007; 448: 204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 34.Colburn RW, Lubin ML, Stone DJ, Jr, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron 2007; 54: 379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Dhaka A, Murray AN, Mathur J, et al. TRPM8 is required for cold sensation in mice. Neuron 2007; 54: 371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 36.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002; 416: 52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 37.Su L, Wang C, Yu YH, et al. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci 2011; 12: 120, doi: 10.1186/1471-2202-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell 2002; 108: 705–715. [DOI] [PubMed] [Google Scholar]
- 39.De Petrocellis L, Vellani V, Schiano-Moriello A, et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther 2008; 325: 1007–1015. doi: 10.1124/jpet.107.134809. [DOI] [PubMed] [Google Scholar]
- 40.De Petrocellis L, Starowicz K, Moriello AS, et al. Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp Cell Res 2007; 313: 1911–1920. doi: 10.1016/j.yexcr.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 41.De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol 2011; 163: 1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng L, Cornett BL, Mackie K, et al. CB1 knockout mice unveil sustained CB2-mediated anti-allodynic effects of the mixed CB1/CB2 agonist CP55,940 in a mouse model of paclitaxel-induced neuropathic pain. Mol Pharmacol 2015; 88: 64–74. doi: 10.1124/mol.115.098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Racz I, Nent E, Erxlebe E, et al. CB1 receptors modulate affective behaviour induced by neuropathic pain. Brain Res Bull 2015; 114: 42–48. doi: 10.1016/j.brainresbull.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Castane A, Celerier E, Martin M, et al. Development and expression of neuropathic pain in CB1 knockout mice. Neuropharmacology 2006; 50: 111–122. [DOI] [PubMed] [Google Scholar]
- 45.Malmberg AB, Basbaum AI. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: behavioral and neuroanatomical correlates. Pain 1998; 76: 215–222. [DOI] [PubMed] [Google Scholar]
- 46.Sideris A NM, Russo L, Chen J, et al. In E Recio-Pinto (ed) Knock out of the cannabinoid 1 receptor promotes recovery from mechanical but not from cold allodynia following sural spared nerve injury in mice. Toronto, Canada: Neuropathic Pain Special Interest Group Congress, 2013.
- 47.Marsicano G, Goodenough S, Monory K, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 2003; 302: 84–88. [DOI] [PubMed] [Google Scholar]
- 48.Monory K, Massa F, Egertova M, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 2006; 51: 455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agarwal N, Pacher P, Tegeder I, et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci 2007; 10: 870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahluwalia J, Urban L, Capogna M, et al. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience 2000; 100: 685–688. [DOI] [PubMed] [Google Scholar]
- 51.Bridges D, Rice AS, Egertova M, et al. Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neuroscience 2003; 119: 803–812. [DOI] [PubMed] [Google Scholar]
- 52.McCoy ES, Taylor-Blake B, Street SE, et al. Peptidergic CGRPalpha primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron 2013; 78: 138–151. doi: 10.1016/j.neuron.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma CH, Omura T, Cobos EJ, et al. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J Clin Invest 2011; 121: 4332–4347. doi: 10.1172/JCI58675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hou H, Zhang L, Ye Z, et al. Chitooligosaccharide inhibits scar formation and enhances functional recovery in a mouse model of sciatic nerve injury. Mol Neurobiol 2016; 53: 2249–2257. doi: 10.1007/s12035-015-9196-0. [DOI] [PubMed] [Google Scholar]
- 55.Woolf CJ, Max MB. Mechanism-based pain diagnosis: issues for analgesic drug development. Anesthesiology 2001; 95: 241–249. [DOI] [PubMed] [Google Scholar]
- 56.Serpell M, Ratcliffe S, Hovorka J, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 2014; 18: 999–1012. doi: 10.1002/j.1532-2149.2013.00445.x. [DOI] [PubMed] [Google Scholar]
- 57.Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage 2014; 47: 166–173. doi: 10.1016/j.jpainsymman.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 58.Fioravanti B, De Felice M, Stucky CL, et al. Constitutive activity at the cannabinoid CB1 receptor is required for behavioral response to noxious chemical stimulation of TRPV1: antinociceptive actions of CB1 inverse agonists. J Neurosci 2008; 28: 11593–11602. doi: 10.1523/JNEUROSCI.3322-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cooper ZD, Comer SD, Haney M. Comparison of the analgesic effects of dronabinol and smoked marijuana in daily marijuana smokers. Neuropsychopharmacology 2013; 38: 1984–1992. doi: 10.1038/npp.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clark WC, Janal MN, Zeidenberg P, et al. Effects of moderate and high doses of marihuana on thermal pain: a sensory decision theory analysis. J Clin Pharmacol 1981; 21: 299S–310S. [DOI] [PubMed] [Google Scholar]
- 61.Mitchell PB, Morris MJ. Depression and anxiety with rimonabant. Lancet 2007; 370: 1671–1672. doi: 10.1016/S0140-6736(07)61705-X. [DOI] [PubMed] [Google Scholar]
- 62.Christensen R, Kristensen PK, Bartels EM, et al. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 2007; 370: 1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 63.Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011; 163: 1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]