Abstract
Background
Pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptors are present in the spinal dorsal horn and dorsal root ganglia, suggesting an important role of PACAP–PACAP receptors signaling system in the modulation of spinal nociceptive transmission. We have previously reported that a single intrathecal injection of PACAP or a PACAP specific (PAC1) receptor selective agonist, maxadilan, in mice induced dose-dependent aversive behaviors, which lasted more than 30 min, and suggested that the maintenance of the nociceptive behaviors was associated with the spinal astrocytic activation.
Results
We found that a single intrathecal administration of PACAP or maxadilan also produced long-lasting hind paw mechanical allodynia, which persisted at least 84 days without affecting thermal nociceptive threshold. In contrast, intrathecal application of vasoactive intestinal polypeptide did not change mechanical threshold, and substance P, calcitonin gene-related peptide, or N-methyl-D-aspartate induced only transient mechanical allodynia, which disappeared within 21 days. Western blot and immunohistochemical analyses with an astrocytic marker, glial fibrillary acidic protein, revealed that the spinal PAC1 receptor stimulation caused sustained astrocytic activation, which also lasted more than 84 days. Intrathecal co-administration of L-α-aminoadipate, an astroglial toxin, with PACAP or maxadilan almost completely prevented the induction of the mechanical allodynia. Furthermore, intrathecal treatment of L-α-aminoadipate at 84 days after the PAC1 stimulation transiently reversed the mechanical allodynia accompanied by the reduction of glial fibrillary acidic protein expression level.
Conclusion
Our data suggest that spinal astrocytic activation triggered by the PAC1 receptor stimulation contributes to both induction and maintenance of the long-term mechanical allodynia.
Keywords: ERK, extracellular signal-regulated kinase, GFAP, glial fibrillary acidic protein, JNK, c-Jun-N-terminal kinase, MAP kinase, PACAP, PAC1 receptor
Background
Pituitary adenylate cyclase-activating polypeptide (PACAP) was originally isolated from ovine hypothalamic extracts based on its ability to stimulate adenylate cyclase in rat anterior pituitary cell cultures.1 PACAP exists as two variants, 38 amino acid form (PACAP38) and C-terminal truncated form PACAP27,1,2 both of which share 68% homology with vasoactive intestinal polypeptide (VIP), suggesting that PACAP belongs to the VIP/secretin/glucagon superfamily.3
Three distinct G-protein-coupled receptors mediate the actions of PACAP and VIP. The PACAP type I (PAC1) receptor, which is coupled mainly to adenylate cyclase/protein kinase A, binds the two forms of PACAP with high affinity and selectivity. Meanwhile, VPAC1 and VPAC2 receptors, which are also primarily coupled to adenylate cyclase, can bind both PACAP and VIP with similar affinities.3,4
In normal state, PAC1 receptor is particularly abundant in central nervous system including spinal dorsal horn,3,5,6 where PACAP-immunoreactive (IR) fibers are also considerably localized.7–10 These fibers are thought to be predominantly primary afferents in origin, since capsaicin treatment releases PACAP from rat spinal cord11,12 and induces a significant decrease in the number of PACAP-IR nerve fibers within the spinal cord.9 Furthermore, PACAP mRNA/immunoreactivity is localized primarily in calcitonin gene-related peptide (CGRP) or substance P (SP)-IR containing neurons in rat dorsal root ganglion9,13 and is markedly upregulated in peripheral nerve injury or inflammation.14–18 These observations coupled with other lines of evidence propose that PACAP-PAC1 receptor system could play an important role in the modulation of spinal nociceptive transmission.
We have previously demonstrated in mice that a single intrathecal injection of PACAP38 or a PAC1 receptor specific agonist, maxadilan (Max),19 induced spontaneous aversive behaviors, such as licking, biting, and scratching directed toward the caudal part of the body for more than 30 min.20,21 Pre- and post-treatment of a PAC1 receptor antagonist, max.d.4,22,23 almost completely inhibited the aversive behaviors. Immunohistochemical and immunoblotting studies revealed that spinal application of Max induced phosphorylation of both extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK), as well as upregulation of an astrocyte marker, glial fibrillary acidic protein (GFAP) within 30 min. Intriguingly, co-administration of a protein kinase A inhibitor (Rp-8-Br-cAMPS), a MAP kinase/ERK kinase (MEK) inhibitor (PD98059), or a JNK inhibitor (SP600125) with Max markedly inhibited the inductions of the aversive behaviors. Furthermore, L-α-aminoadipate (L-α-AA), an astroglial toxin, attenuated both induction of the aversive behaviors and phosphorylation of ERK, suggesting astroglial activation maintained the ERK phosphorylation, a marker for central sensitization of spinal dorsal horn.24,25
Further to address the nature of the PAC1 receptor-mediated nociceptive responses, we examined whether the single intrathecal administration of PACAP or Max would induce evoked-nociceptive behaviors, after subsidence of the spontaneous aversive behaviors within 24 h,20 by measuring hind paw mechanical and thermal thresholds in mice.
Methods
Animals
Male ddY mice (five weeks old) were purchased from Kyudo Co. Ltd. (Kumamoto, Japan) and housed in standard polycarbonate cages (four mice/cage) under controlled temperature (24 ± 1℃) and humidity (55 ± 10%) with a 12-h light–dark cycle (lights on at 07:00 h) with food and water freely available. Mice were habituated to the animal facility for at least one week before experimentation. The animal experiments were approved by the Animal Care Committee of Kagoshima University (approval no. MD 15011) and were conducted in accordance with the ethical guidelines for the study of experimental pain in conscious animals of the International Association for the Study of Pain.
Intrathecal injection and behavioral observation
Intrathecal injection was given in a volume of 5 μl by percutaneous puncture through an intervertebral space at the level of the fifth or sixth lumbar vertebra, according to a previously reported procedure.20,26
An investigator, who was unaware of the drug treatment, performed all of the behavioral experiments. The assessment of mechanical and thermal thresholds was carried out according to previously described methods.27 Briefly, mechanical sensitivity was evaluated with calibrated von Frey hairs (Stoelting, Wood Dale, IL, USA) by measuring the tactile stimulus producing a 50% likelihood of hind paw withdrawal response (50% gram threshold), which was determined using the up-down paradigm.28 Thermal sensitivity was evaluated by measuring withdrawal latency with a Paw Thermal Stimulator (UCSD, San Diego, CA, USA). Data from right and left hind paws were combined and averaged in both tests.
Drugs
PACAP38, VIP, SP, and CGRP were purchased from Peptide Institute Inc. (Osaka, Japan). N-Methyl-D-aspartic acid (NMDA) and L-α-AA were obtained from Sigma (St. Louis, MO, USA). PD98059 and SP600125 were from Millipore (Billerica, MA, USA). Max and max.d.4 were kindly donated by Dr M Tajima (Shiseido, Japan).19,23 These drugs were made up as concentrated stock solution in MilliQ water or dimethyl sulfoxide, aliquoted, and stored at –70℃. An aliquot was diluted to the desired concentration in artificial cerebrospinal fluid (ACSF: NaCl 138 mM, KCl 3 mM, CaCl2 1.25 mM, MgCl2 1 mM, D-glucose 1 mM) immediately prior to use. The doses for PACAP (100 pmol) and Max (50 pmol) we chose in this study were determined according to our previous reports.20,21 Intrathecal injection of PACAP or Max at the dosage induced marked spontaneous aversive behaviors (see Background; Figure S1). As repeatedly demonstrated by previous studies including ours,21,29–36 intrathecal injection of SP or NMDA induced only short-lasting spontaneous aversive behaviors, which disappeared within 5 min (Figure S1). Then, we employed 100 pmol for SP and even higher dosage (1 nmol) for NMDA. VIP and CGRP have been reported that they produce few spontaneous aversive behaviors when intrathecally injected by themselves.36–40 Thus, we intrathecally injected these peptides at the same dosage (100 pmol) as PACAP. In agreement with the previous studies, 100 pmol of VIP or CGRP induced only small or no aversive behaviors (Figure S1). The maximum concentration of the vehicle used to dilute drugs (1–2% MilliQ water or dimethyl sulfoxide) did not have any effects on the mechanical or thermal threshold and did not interfere with the effects of drugs (see Results section).
Immunohistochemistry
Immunohistochemistry was performed as previously described.41 The animals were deeply anesthetized with sodium pentobarbital (60 mg/kg, intraperitoneal [i.p.]) and perfused intracardially with heparinized saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). After laminectomy, the spinal cord (L4–L6) was identified, excised, and postfixed over night at 4℃ in the same fixative and then replaced sequentially with 10%, 15%, and 20% sucrose in 0.1 M phosphate buffered saline at 4℃ for cryoprotection. Transverse spinal sections (10 µm) were cut on a cryostat and collected on silane-coated glass sides (Matsunami glass, Japan).
Sections were blocked in phosphate buffered saline containing 1% normal donkey serum (ImmunoBioScience, Mukilteo, WA, USA), 1% bovine serum albumin (Sigma), and 1% saponin (Sigma) for 1 h at room temperature, and incubated for three days at 4℃ with the primary antibodies against PAC1 receptor (rabbit polyclonal, 1:1,000, nos. 93093, which was provided by Dr A Arimura),42–45 neuronal nuclei (NeuN, mouse monoclonal, 1:200, Millipore), GFAP (mouse monoclonal, 1:500, Millipore), and ionized calcium binding adaptor molecule 1 (Iba1, goat polyclonal, 1:500, Abcam, Cambridge, UK). The sections were then incubated for 1 h at room temperature with Alexa Fluor 488-labeled donkey anti-rabbit IgG antibody (1: 1,000, Invitrogen, Carlsbad, CA), Alexa Fluor 594-labeled donkey anti-mouse IgG antibody (1:1,000, Invitrogen), and Alexa Fluor 594-labeled donkey anti-goat IgG antibody (1:1,000, Invitrogen). Stained sections were examined with a fluorescence microscope (Pulse-SIM BZ-X700, Keyence Co., Osaka, Japan) or a Zeiss LSM 700 confocal microscope (Carl Zeiss Microscopy, Jena, Germany).
Western blot analysis
Western blot analysis was conducted as previously described.20,41 Mice were deeply anesthetized with sodium pentobarbital (60 mg/kg, i.p.), and the lumbar spinal cords (L3–L5) were quickly removed. Each spinal cord sample was homogenized in a lysis buffer (150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and 50 mM Tris-HCl, pH 8.0) with a mixture of protease and phosphatase inhibitors (Roche Diagnostics, Mannheim, Germany). Protein concentrations were determined with a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). Proteins (6.3 µg) were separated by SDS-polyacrylamide gel electrophoresis (12.5% gel) and then transferred to a polyvinylidene difluoride membrane (Millipore) and incubated with a mouse anti-GFAP monoclonal antibody (1:10,000, Millipore). Immunoreactivity was detected by using an ECL prime kit (GE Healthcare, Buckinghamshire, UK). An anti-β-actin antibody (mouse monoclonal, 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX) was used to normalize protein loading. Relative intensities of the bands were quantified by using an image analysis system with Image J software, version 1.46 (National Institutes of Health, Bethesda, MD). At least two independent immunoblot experiments of three individual spinal cord samples were analyzed.
Statistical analysis
Experimental data are expressed as mean ± SEM. For behavioral analyses, we employed the Mann–Whitney U-test for single comparisons or the Friedman test followed by the Steel test for multiple comparisons. For Western blot analyses, single comparisons were made using the Student’s two-tailed unpaired t-test, and for multiple comparisons, one-way analysis of variance followed by the Dunnett test was used. P < 0.05 was considered statistically significant.
Results
A single intrathecal injection of PACAP or the PAC1 receptor selective agonist, maxadilan, induces long-lasting mechanical allodynia
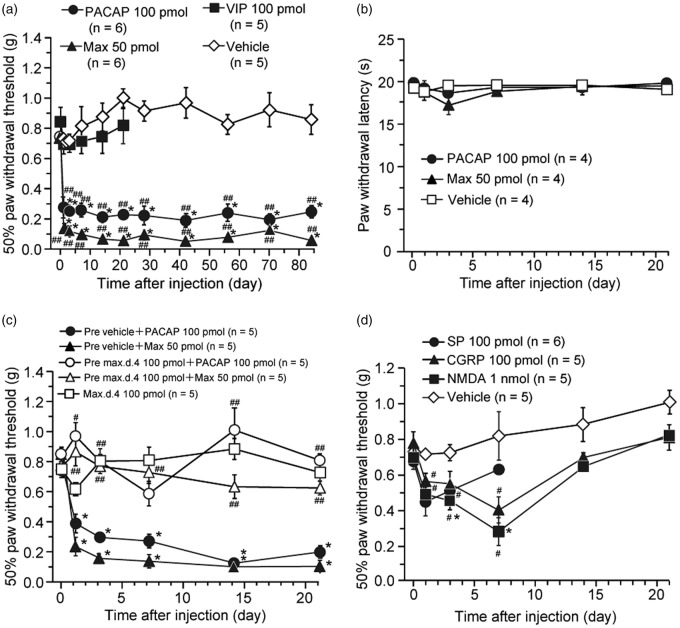
We first examined whether the intrathecal treatment with PACAP or Max could cause a hyperalgesic or allodynic response in normal mice. A single intrathecal administration of PACAP (100 pmol) or Max (50 pmol) markedly decreased mechanical threshold (induction of mechanical allodynia) from day 1, and this decrease persisted at least for 84 days after the administration (Figure 1(a)). In contrast, spinal delivery of the same dosage of each compound, intriguingly, did not change thermal threshold (Figure 1(b)).
Figure 1.
A single intrathecal injection of PACAP and a PAC1 receptor agonist, maxadilan (Max), produces long-lasting mechanical allodynia in mice. (a, b) Effects of PACAP, Max, or VIP on paw withdrawal responses to the mechanical (a) and thermal (b) stimuli in mice. Intrathecal VIP or vehicle had no significant effect on the mechanical threshold. (c) Spinal pretreatment of a PAC1 receptor antagonist, max.d.4, prevented the induction of PACAP- or Max-induced long-lasting mechanical allodynia. Vehicle or max.d.4 was intrathecally pretreated 5 min before PACAP or Max injection. Intrathecal max.d.4 had no significant effect on the mechanical threshold. (d) Effect of SP, CGRP, or NMDA on paw withdrawal responses to the mechanical stimuli. Paw withdrawal threshold to mechanical stimulation (a, c, d) or paw withdrawal latency to thermal stimuli (b) are plotted against the time after each intrathecal treatment. For clarity, the same vehicle control in Figure 1(a) is depicted again in Figure 1(d). After confirming the acute behavioral effects of these treatments (see Figure S1), we performed Figure 1 experiments. *P < 0.05, when compared with pre-drug (at 0 h) data. #P < 0.05 and ##P < 0.01, when compared with vehicle control in Figure 1(a) and (d) or corresponding vehicle pre-treated control in Figure 1(c). Data are mean ± SEM.
To confirm that the PACAP- or Max-induced mechanical allodynia were PAC1 receptor-mediated, we examined the effects of the selective PAC1 receptor antagonist, max.d.4.4,22,23 In accordance with our previous study,20 intrathecal pre-treatment of max.d.4 (100 pmol) markedly prevented the expression of PACAP- or Max-induced aversive behaviors (Figure S1). We further found in this study that intrathecal pre-treatment of max.d.4 (100 pmol) also almost completely blocked the induction of PACAP- or Max-induced mechanical allodynia (Figure 1(c)). However, a single intrathecal administration of VIP (100 pmol) did not affect the mechanical threshold (Figure 1(a)). Neither aversive behaviors (Figure S1) nor mechanical allodynia (Figure 1(c)) were induced after intrathecal application of max.d.4 (100 pmol) alone.
To compare the effects of the PACAP and Max with those of sensory neuropeptides, SP and CGRP, and with an excitatory amino acid agonist, NMDA, temporal changes in hind paw withdrawal to mechanical stimuli were examined over three weeks (Figure 1(d)). In general agreement with previous reports,29,30,37,46,47 spinal injection of SP (100 pmol), CGRP (100 pmol), or NMDA (1 nmol) resulted in only a transient mechanical allodynia, lasting 3 to 14 days.
Intrathecal PACAP or maxadilan induces long-lasting astroglial activation
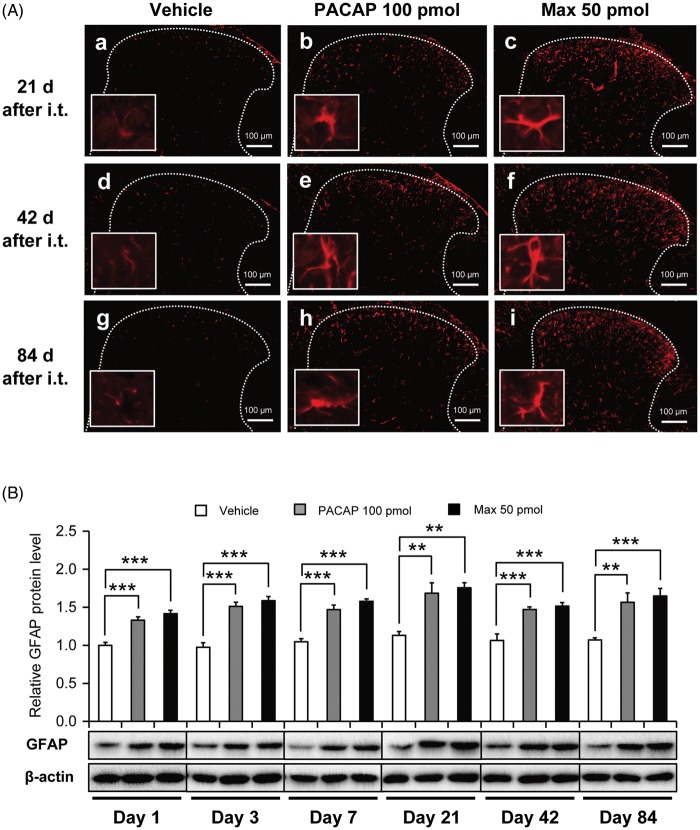
We have previously demonstrated that intrathecal administration of Max elicited rapid upregulation of spinal GFAP protein expression level as early as 30 min after the injection.20 Further to investigate how long does the GFAP upregulation persist after the spinal PAC1 receptor activation, we investigated temporal changes in GFAP expression level by immunohistochemical and immunoblot methods (Figure 2).
Figure 2.
GFAP immunostaining in the lumbar dorsal horn of the spinal cord after a single intrathecal administration of PACAP (100 pmol) or Max (50 pmol). (A) Representative photomicrographs from the spinal cord sections of the mice administered intrathecally with vehicle (a, d, g), PACAP (b, e, h), or Max (c, f, i) at the times indicated. Insets are high power images of a randomly selected GFAP-positive cell from each section. White dotted lines indicate the border of the dorsal horn gray matter. Scale bar = 100 µm. (B) Western blot analysis of GFAP immunoreactivity. β-actin was used as a control for sample loading. In the upper panel, the value obtained for vehicle control serves as the control, and the results are presented as the mean ± SEM (vehicle, n = 4–6; PACAP, n = 5–6; Max, n = 6). The lower panel shows representative blots. The densities of specific GFAP and actin bands were measured, and GFAP levels were normalized against the corresponding actin levels. **P < 0.01 and ***P < 0.001, when compared with vehicle control.
In the vehicle-treated spinal dorsal horn, the GFAP immunostaining was relatively low at 21, 42, and 84 days after the intrathecal administration, and the stained astrocytes appeared to show no overt morphological sign of astrocytic activation (Figure 2(Aa), (Ad), and (Ag)). In contrast, following intrathecal PACAP (100 pmol) or Max (50 pmol), spinal astrocytes became intensely GFAP immuno-positive and appeared to have an altered shape, suggesting an activated state of the astrocytes (Figure 2(Ab) and (Ac), (Ae) and (Af), (Ah) and (Ai)). By contrast, these intrathecal manipulation did not change morphology and intensity of immunoreactivity of a microglial marker (Iba1)-positive cells (Figure S2).
Upregulation of GFAP expression level was also confirmed by Western blot analyses (Figure 2(B)). Significant increase of GFAP expression level after PACAP or Max treatment was sustained at least for 84 days.
An astroglial toxin, L-α-aminoadipate, blocks both induction and maintenance of the spinal PAC1 receptor-induced mechanical allodynia
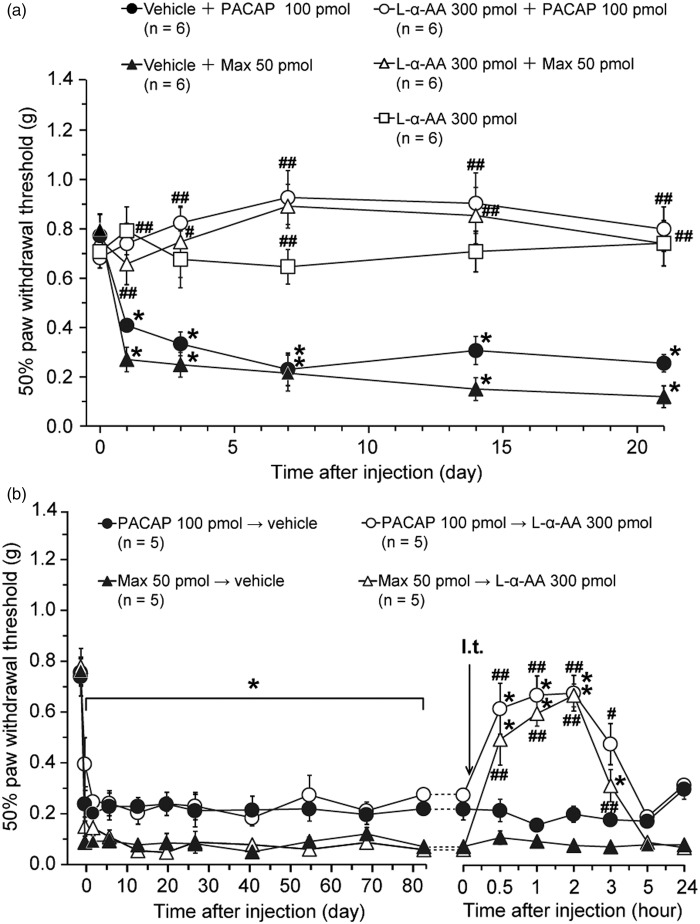
Further to explore whether spinal astrocyte activation contributes to the PAC1 receptor-induced mechanical allodynia, effects of co- and post-treatment of L-α-AA (300 pmol) on the mechanical allodynia were examined (Figure 3). L-α-AA is reported to be a cytotoxin relatively specific for astrocytes.48–52
Figure 3.
Effects of co- or post-treatment of an astroglial toxin, L-α-AA, on the PAC1 receptor-induced mechanical allodynia. (a) Intrathecal co-administration of L-α-AA with PACAP or Max blocked the induction of the long-lasting mechanical allodynia. Paw withdrawal threshold to mechanical stimulation is plotted against the time after intrathecal co-administration of L-α-AA or vehicle with PACAP or Max. After confirming the acute behavioral effects of these treatments (see Figure S3), we performed Figure 3(a) experiments. (b) Reversal of the established PAC1 receptor-induced mechanical allodynia by intrathecal L-α-AA. L-α-AA was injected 84 days after intrathecal PACAP or Max. *P < 0.05, when compared with pre-drug (at 0 h) data. #P < 0.05 and ##P < 0.01, when compared with corresponding vehicle co-administered control (a) or vehicle-treated control (b). Data are mean ± SEM.
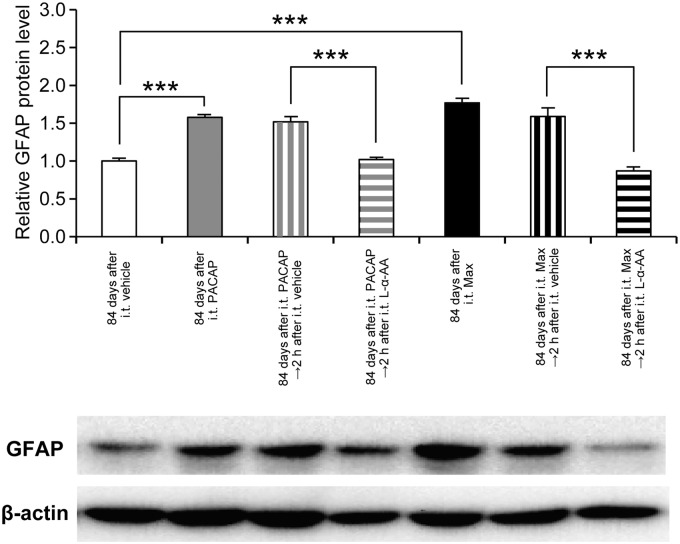
We have confirmed our previous study that intrathecal co-administration of L-α-AA with PACAP (100 pmol) or Max (50 pmol) markedly attenuated the expression of the nociceptive behaviors (Figure S3).20 Furthermore, the simultaneous intrathecal injection of L-α-AA almost completely prevented the induction of the mechanical allodynia, while intrathecal L-α-AA alone neither induced aversive behaviors (Figure S3) nor affected the mechanical threshold (Figure 3(a)). Intriguingly, intrathecal administration of L-α-AA at 84 days after the spinal PAC1 receptor activation transiently alleviated the mechanical allodynia (Figure 3(b)), suggesting that the spinal astroglial activation evoked by a single intrathecal administration of PACAP or Max persisted for as long as 84 days. Western blot analyses further confirmed that intrathecal L-α-AA decreased the elevated expression of GFAP to control level at 2 h post-treatment (Figure 4).
Figure 4.
Effects of intrathecal L-α-AA on the GFAP expression levels at 84 days after the spinal PAC1 receptor activation. Sustained up-regulation of the spinal GFAP expression at 84 days after intrathecal PACAP (100 pmol) or Max (50 pmol) was reversed by L-α-AA (300 pmol) at 2 h post-treatment. β-actin was used as a control for sample loading. In the upper panel, the value obtained for vehicle control serves as the control, and the results are presented as the mean ± SEM (n = 6 for each group). The lower panel shows a representative blot. The densities of specific GFAP and actin bands were measured, and GFAP levels were normalized against the corresponding β-actin levels. ***P < 0.001, compared with respective control.
Expression of PAC1 receptor in mouse spinal cord
The localization of PACAP binding sites and PAC1 receptor mRNA has been extensively investigated in rat and mouse brain.3 However, there is only limited number of in situ hybridization and immunohistochemical studies which demonstrate the localization of PAC1 receptor in the rat spinal cord.6,53 Furthermore, to our knowledge, there is still no concrete immunohistochemical report for the expression of PAC1 receptor in the mouse spinal cord.
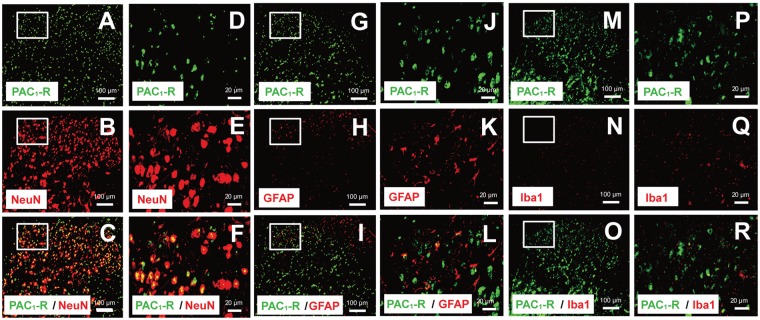
To identify the cell type on which PAC1 receptor is expressed, we performed double-immunohistochemical studies using a previously characterized anti-PAC1 receptor antibody42,43 and cell type-specific markers (Figure 5). In accordance with a previous in situ hybridization study showing that numerous dorsal horn nerve cell bodies labeled for PAC1 receptor mRNA,6 PAC1 receptor-like immunoreactivity were shown to be widely distributed in the dorsal horn. Double staining experiments revealed that PAC1 receptor-like immunoreactivity was principally colocalized with a neural marker, neuronal specific nuclear protein (Neu N) (Figure 5(a)–(f)), but rarely with Iba1 (Figure 5(m)–(r)). However, PAC1 receptor-like immunoreactivity was observed occasionally in GFAP-positive cells, suggesting the presence of PAC1 receptor on a part of spinal dorsal horn astrocytes (Figure 5(g)–(l)).
Figure 5.
Double immunofluorescence staining performed with a specific PAC1 receptor antibody using lumbar spinal dorsal horn of naïve mice. Double-staining for PAC1 receptor (green) and NeuN (a–f), GFAP (g–l), or Iba1 (m–r) (red) showed PAC1 receptor immunoreactivity mainly colocalized with a neuronal marker, NeuN (c, f), but occasionally with an astrocytic marker, GFAP (i, l). PAC1 receptor immunoreactivity rarely colocalized with a microglial marker, Iba1 (o, r), in the spinal dorsal horn. (d–f), (j–l), and (p–r) are the magnified images of the rectangles indicated in each left panel. Scale bars = 100 µm (a–c, g–h, and m–o) and 20 µm (d–f, j–l, and p–r).
Spinal ERK and JNK activation contribute to the induction of PAC1 receptor-induced mechanical allodynia
As mentioned in the Background section, we have previously demonstrated that spinal administration of Max induced upregulation of phosphorylated forms of both ERK (within 5 min) and JNK (within 30 min), an indicator of respective MAP kinase activation. In addition, co-spinal application of an MEK inhibitor (PD98059)54 or a JNK inhibitor (SP600125)55 with Max markedly prevented the induction of the spontaneous aversive behaviors (Figure S4).20 Thus, in this study, we have examined whether co-spinal application of PD98059 or SP600125 with PACAP or Max also affected the induction of the mechanical allodynia (Figure 6).
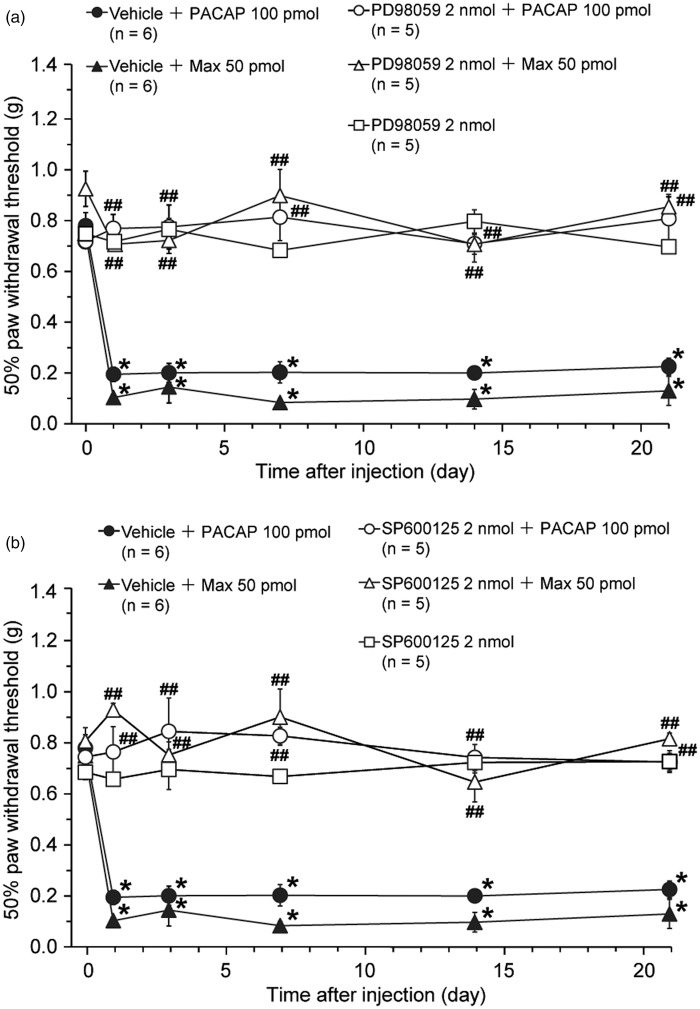
Figure 6.
Intrathecal co-administration of a MEK inhibitor, PD98059, or a JNK inhibitor, SP600125, with PACAP or Max blocks the induction of the PCA1 receptor-induced long-lasting mechanical allodynia. Effects of PD98059 (a) or SP600125 (b) on the PACAP- or Max-induced mechanical allodynia. Paw withdrawal threshold to mechanical stimulation is plotted against the time after intrathecal co-administration of each inhibitor or vehicle with PACAP or Max. Since we have made up stock solutions of the same concentration and used the same final dose for both inhibitors, we employed the same vehicle control in both Figure 6(a) and (b). After confirming the acute behavioral effects of these treatments (see Figure S4), we performed Figure 6 experiments. *P < 0.05, compared with pre-drug (at 0 h) data. ##P < 0.01, compared with respective vehicle co-administered control group. Data are mean ± SEM.
Both PD98059 (2 nmol) and SP600125 (2 nmol) almost completely blocked the induction of the PACAP- or Max-induced mechanical allodynia. Neither PD98059 nor SP600125 administered intrathecally induced aversive behaviors (Figure S4) and mechanical allodynia (Figure 6)
Discussion
In this study, we have further characterized the nociceptive responses induced by the spinal PAC1 receptor activation and made the following findings. First, a single intrathecal administration of PACAP or Max induced long-lasting hind paw mechanical allodynia, which persisted more than 84 days through PAC1 receptor activation. Second, a single intrathecal administration of each agonist also induced a marked upregulation of GFAP expression, which again lasted for at least 84 days. Third, intrathecal co-administration of L-α-AA, an astroglial inhibitor, with PACAP or Max almost completely prevented the induction of mechanical allodynia. Furthermore, intrathecal injection of L-α-AA at 84 days after the PAC1 stimulation transiently reversed the mechanical allodynia along with the reduction of the elevated expression of GFAP to control level. Finally, spinal co-application of a MEK inhibitor, PD98059, or a JNK inhibitor, SP600125, with each stimulant almost completely prevented the induction of the mechanical allodynia. These findings together with our previous data indicate that spinal PACAP-PAC1 receptor system activates ERK and JNK signaling pathway to induce mechanical allodynia and that spinal astrocytic activation contributes to both initiation and maintenance of the PAC1 receptor-evoked long-term mechanical allodynia.
Spinal PAC1 receptor activation and hypersensitivity to mechanical and thermal stimulation
In the present study, we have demonstrated that a single intrathecal administration of PACAP (100 pmol) or Max (50 pmol) induced long-lasting hind paw mechanical allodynia without affecting the thermal nociceptive threshold from 1 day post-administration. The reason why we did not perform mechanical and thermal tests on the day of PACAP or Max injection was because a single intrathecal injection of these agonists evoked aversive behaviors such as licking and biting,10 which gradually appeared within 3–5 min, reached a plateau between 15 and 30 min, and sustained for more than 60 min.20,21 In addition, previous studies by tail-flick or paw-flick test showed that a single intrathecal PACAP (0.05–0.5 µg; roughly comparable to 11–110 pmol) evoked a short-term thermal hyperalgesia, which lasted about 90 min after the injection in mice.10,56
Although it is still uncertain whether there is a significant difference in the contribution of the spinal PAC1 receptor signaling between mechanical and thermal nociception, our observations may be in agreement with the reports that mice lacking PACAP gene did not exhibit mechanical allodynia and thermal hyperalgesia after peripheral inflammation or nerve injury15 and that PACAP6-38, a PACAP receptor antagonist, reduced mechanical allodynia in a neuropathic pain model and thermal hyperalgesia in an inflammatory pain model.57 These considerations also suggest that PACAP-PAC1 receptor signaling might be critically involved in the initiation and maintenance of pathological pain.
The contribution of astrocytes to the PAC1 receptor-induced long-lasting mechanical allodynia
Recent progress points to an important role of astrocytes in the spinal cord for the maintenance of inflammatory and neuropathic pain.58–62 In accordance with this notion, we have shown that intrathecal injection of PACAP or Max induced persistent upregulation of GFAP expression level in parallel with the long-lasting mechanical allodynia, and simultaneous application of L-α-AA with these PAC1 agonists markedly repressed the induction of the mechanical allodynia. Furthermore, intrathecal application of L-α-AA at 84 days after the PAC1 receptor stimulation transiently reversed the mechanical allodynia concomitant with the reduction of the elevated expression of GFAP to control level. These observations suggest that spinal PAC1 receptor-mediated astroglial activation contributes to both induction and maintenance of the prolonged mechanical allodynia. Although the underlying mechanisms of such a long-term activation of spinal astrocytes remain to be determined, we hypothesize that interactions between spinal dorsal horn neurons and astrocytes by signaling molecules might be important to maintain the astroglial activation.
Since spinal PACAP-PAC1 receptor signaling induced GFAP upregulation after neuronal ERK activation (see below) as shown in a previous our study,20 it may be reasonable to speculate that the activation of spinal astrocytes would be primarily induced by indirect effects of neuronal PAC1 receptor activation rather than direct effects through the astroglial PAC1 receptor. However, we cannot completely exclude a possible contribution of the direct activation of PAC1 receptor on spinal astrocytes to the astroglial activation because we found the occasional detection of PAC1 receptor-like immunoreactivity on spinal astrocytes in this study. Further study would be required to understand the functional significance of the spinal astroglial PAC1 receptor.
In any case, we currently speculate that spinal PAC1 activation initiates rapid crosstalk between dorsal horn neurons and astrocytes, contributing to the onset of spontaneous aversive behaviors,20 which then develops into mechanical allodynia. Interfering this crosstalk by L-α-AA may effectively alleviate the initiation of the aversive behaviors, eventually result in the blockade of the mechanical allodynia induction.
Involvement of ERK and JNK activation in the induction of the PAC1 receptor-induced mechanical allodynia
MAP kinases represent a family of serine-threonine kinases that are activated by a broad array of extracellular stimuli.25,63,64 There are three major MAP kinase members, ERK, JNK, and p38, and accumulating evidence suggest that all three MAP kinase pathways contribute to neuronal plasticity associated with chronic pain. Our previous study showed that spinal co-administration of PD98059 (a MEK inhibitor) or SP600125 (a JNK inhibitor) with Max largely suppressed the Max-induced nociceptive behaviors as shown in Figure S4, and these and other lines of evidence suggested that activation of ERK and JNK in the spinal dorsal horn neurons and astrocytes, respectively, contributes to the induction of the spinal PAC1 receptor-induced nociceptive behaviors.20 As expected, the same treatment of MAP kinase inhibitors with PACAP or Max almost completely blocked the development of the mechanical allodynia. Thus, spinal ERK and JNK signaling pathways also play important roles in the induction of the PAC1 receptor-induced long-term mechanical allodynia.
At present, we do not know whether ERK and JNK are activated in other spinal cell types at different times following the PAC1 activation. It was demonstrated that spinal nerve injury induced an immediate (< 10 min) but transient (< 6 h) activation of ERK, which was restricted to spinal dorsal horn neurons. This was followed by a widespread activation of ERK in spinal microglia, which peaked between one and three days after the nerve injury. On day 10, activation of ERK was observed in both microglia and astrocytes, but by day 21, predominantly in astrocytes in the dorsal horn.65 Further extensive immunohistochemical analyses are necessary to determine the contribution of temporal activation of ERK and JNK to the maintenance of the PAC1 receptor-induced mechanical allodynia.
Taken together, our present data show that spinal PAC1 receptor-mediated astroglial activation contributes to both induction and maintenance of the long-lasting mechanical allodynia. Our data also suggest that the PAC1 receptor-mediated spinal ERK and JNK signal transduction system play important roles in the induction of the long-term mechanical allodynia. Thus, targeting PAC1 receptor signaling pathway may provide a new avenue for chronic pain therapy.
Conclusions
The interaction between spinal dorsal horn neurons and astrocytes evoked by PACAP-PAC1 receptor-mediated signal transduction is critically involved in the induction and maintenance of the long-lasting mechanical allodynia. The signaling pathway linking between PAC1 receptor stimulation and astroglial activation may offer a new opportunity to treat intractable chronic pain.
Acknowledgements
The authors thank Dr Ichiro Takasaki for critically reading the manuscript; Drs Kazuhiko Inoue and Yuki Kambe for helpful discussions; Mr Toshihide Asada and Tetsuya Kawamura for excellent technical assistance; and all the staff members of the Joint Research Laboratory and the Division of Laboratory Animal Sciences, Kagoshima University.
Authors’ contributions
MY carried out all experiments, performed statistical analysis, and drafted the manuscript. TK conceived, participated in the design of the study, performed behavioral studies, and wrote the manuscript. AM participated in the design of the study and reviewed the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by JSPS KAKENHI Grant Numbers 22600001, 25460723, and 26462384.
References
- 1.Miyata A, Arimura A, Dahl RR, et al. Isolation of a novel 38 residue-hypothalamic polypeptides which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 1989; 164: 567–574. [DOI] [PubMed] [Google Scholar]
- 2.Miyata A, Jiang L, Dahl RD, et al. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem Biophys Res Commun 1990; 170: 643–648. [DOI] [PubMed] [Google Scholar]
- 3.Vaudry D, Falluel-Morel A, Bourgault S, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 2009; 61: 283–357. [DOI] [PubMed] [Google Scholar]
- 4.Dickson L, Finlayson K. VAPAC and PAC receptors: from ligands to function. Pharmacol Ther 2009; 121: 294–316. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson T, Mitchell R, Robberecht P, et al. The role of VIP/PACAP receptor subtypes in spinal somatosensory processing in rats with an experimental peripheral mononeuropathy. Neuropharmacology 1999; 38: 167–180. [DOI] [PubMed] [Google Scholar]
- 6.Jongsma H, Danielsen N, Sundler F, et al. Alteration of PACAP distribution and PACAP receptor binding in the rat sensory nervous system following sciatic nerve transection. Brain Res 2000; 853: 186–196. [DOI] [PubMed] [Google Scholar]
- 7.Dun EC, Huang RL, Dun SL, et al. Pituitary adenylate cyclase activating polypeptide-immunoreactivity in human spinal cord and dorsal root ganglia. Brain Res 1996; 721: 233–237. [DOI] [PubMed] [Google Scholar]
- 8.Dun NJ, Miyazaki T, Tang H, et al. Pituitary adenylate cyclase activating polypeptide immunoreactivity in the rat spinal cord and medulla: implication of sensory and autonomic functions. Neuroscience 1996; 73: 677–686. [DOI] [PubMed] [Google Scholar]
- 9.Moller K, Zhang YZ, Håkanson R, et al. Pituitary adenylate cyclase activating peptide is a sensory neuropeptide: immunocytochemical and immunochemical evidence. Neuroscience 1993; 57: 725–732. [DOI] [PubMed] [Google Scholar]
- 10.Narita M, Dun SL, Dun NJ, et al. Hyperalgesia induced by pituitary adenylate cyclase-activating polypeptide in the mouse spinal cord. Eur J Pharmacol 1996; 311: 121–126. [DOI] [PubMed] [Google Scholar]
- 11.Sakashita Y, Kurihara T, Uchida D, et al. Involvement of PACAP receptor in primary afferent fibre-evoked responses of ventral roots in the neonatal rat spinal cord. Br J Pharmacol 2001; 132: 1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Malmberg AB, Yaksh TL, et al. Capsaicin-evoked release of pituitary adenylate cyclase activating peptide (PACAP) and calcitonin gene-related peptide (CGRP) from rat spinal cord in vivo. Regul Pept 1997; 69: 83–87. [DOI] [PubMed] [Google Scholar]
- 13.Mulder H, Uddman R, Moller K, et al. Pituitary adenylate cyclase activating polypeptide expression in sensory neurons. Neuroscience 1994; 63: 307–312. [DOI] [PubMed] [Google Scholar]
- 14.Jongsma Wallin H, Pettersson LM, Verge VM, et al. Effect of anti-nerve growth factor treatment on pituitary adenylate cyclase activating polypeptide expression in adult sensory neurons exposed to adjuvant induced inflammation. Neuroscience 2003; 20: 325–331. [DOI] [PubMed] [Google Scholar]
- 15.Mabuchi T, Shintani N, Matsumura S, et al. Pituitary adenylate cyclase-activating polypeptide is required for the development of spinal sensitization and induction of neuropathic pain. J Neurosci 2004; 24: 7283–7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Shi TJ, Ji RR, et al. Expression of pituitary adenylate cyclase activating polypeptide in dorsal root ganglia following axotomy: time course and coexistence. Brain Res 1995; 705: 149–158. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Danielsen N, Sundler F, et al. Pituitary adenylate cyclase-activating peptide is upregulated in sensory neurons by inflammation. Neuroreport 1998; 9: 2833–2836. [DOI] [PubMed] [Google Scholar]
- 18.Zhang YZ, Hannibal J, Zhao Q, et al. Pituitary adenylate cyclase-activating peptide expression in the rat dorsal root ganglia: up-regulation after peripheral nerve injury. Neuroscience 1996; 74: 1099–1110. [DOI] [PubMed] [Google Scholar]
- 19.Moro O, Lerner EA. Maxadilan, the vasodilator from sand flies, is a specific pituitary adenylate cyclase activating peptide type I receptor agonist. J Biol Chem 1997; 272: 966–970. [DOI] [PubMed] [Google Scholar]
- 20.Ohnou T, Yokai M, Kurihara T, et al. Pituitary adenylate cyclase-activating polypeptide type 1 receptor signaling evokes long-lasting nociceptive behaviors through the activation of spinal astrocytes in mice. J Pharmacol Sci. Epub ahead of print 3 February 2016. DOI: 10.1016/j.jphs.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu T, Katahira M, Sugawara H, et al. Diverse effects of intrathecal pituitary adenylate cyclase-activating polypeptide on nociceptive transmission in mice spinal cord. Regul Pept 2004; 123: 117–122. [DOI] [PubMed] [Google Scholar]
- 22.Moro O, Wakita K, Ohuma M, et al. Functional characterization of structural alterations in the sequence of the vasodilatory peptide maxadilan yields a pituitary adenylate cyclase-activating peptide type 1 receptor-specific antagonist. J Biol Chem 1999; 274: 23103–23110. [DOI] [PubMed] [Google Scholar]
- 23.Uchida D, Tatsuno I, Tanaka T, et al. Maxadilan is a specific agonist and its deleted peptide (M65) is a specific antagonist for PACAP Type 1 receptor. Ann NY Acad Sci 1998; 865: 253–258. [DOI] [PubMed] [Google Scholar]
- 24.Ji RR, Baba H, Brenner GJ, et al. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci 1999; 2: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 25.Ji RR, Gereau RW IV, Malcangio M, et al. MAP kinase and pain. Brain Res Rev 2009; 60: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hylden JLK, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol 1980; 67: 313–316. [DOI] [PubMed] [Google Scholar]
- 27.Saegusa H, Kurihara T, Zong S, et al. Altered pain responses in mice lacking α1E subunit of the voltage-dependent Ca2+ channel. Proc Natl Acad Sci USA 2000; 97: 6132–6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 29.Aanonsen LM, Wilcox GL. Nociceptive action of excitatory amino acids in the mouse: effects of spinally administered opioids, phencyclidine and sigma agonists. J Pharmacol Exp Ther 1987; 243: 9–19. [PubMed] [Google Scholar]
- 30.Li S, Cao J, Yang X, et al. NR2B phosphorylation at tyrosine 1472 in spinal dorsal horn contributed to N-methyl-D-aspartate-induced pain hypersensitivity in mice. J Neurosci Res 2011; 89: 1869–1876. [DOI] [PubMed] [Google Scholar]
- 31.Aanonsen LM, Wilcox GL. Phencyclidine selectively blocks a spinal action of N-methyl-D-aspartate in mice. Neurosci Lett 1986; 67: 191–197. [DOI] [PubMed] [Google Scholar]
- 32.Dobry JKP, Piercey MF, Schroeder LA. Pharmacological characterization of scratching behaviour induced by intrathecal injection of substance P and somatostatin. Neuropharmacol 1981; 20: 267–272. [DOI] [PubMed] [Google Scholar]
- 33.Frenk H, Bossut D, Urca G, et al. Is substance P a primary afferent neurotransmitter for nociceptive input? I. Analysis of pain-related behaviors resulting from intrathecal administration of substance P and 6 excitatory compounds. Brain Res 1988; 455: 223–231. [DOI] [PubMed] [Google Scholar]
- 34.Hylden JLK, Wilcox GL. Intrathecal substance P elicits a caudally directed biting and scratching behavior in mice. Brain Res 1981; 217: 212–215. [DOI] [PubMed] [Google Scholar]
- 35.Piercey MF, Dobry PJK, Schroeder LA, et al. Behavioral evidence that substance P may be a spinal cord sensory neurotransmitter. Brain Res 1981; 210: 407–412. [DOI] [PubMed] [Google Scholar]
- 36.Seybold VS, Hylden JLK, Wilcox GL. Intrathecal substance P and somatostatin in rats: behaviors indicative of sensation. Peptides 1982; 3: 49–54. [DOI] [PubMed] [Google Scholar]
- 37.Oku R, Satoh M, Fujii N, et al. Calcitonin gene-related peptide promotes mechanical nociception by potentiating release of substance P from the spinal dorsal horn in rats. Brain Res 1987; 403: 350–354. [DOI] [PubMed] [Google Scholar]
- 38.Beyer C, Caba M, Banas C, et al. Vasoactive intestinal polypeptide (VIP) potentiates the behavioral effect of substance P intrathecal administration. Pharmacol Biochem Behav 1991; 39: 695–698. [DOI] [PubMed] [Google Scholar]
- 39.Mao J, Coghill RC, Kellstein DE, et al. Calcitonin gene-related peptide enhances substance P-induced behaviors via metabolic inhibition: in vivo evidence for a new mechanism of neuromodulation. Brain Res 1992; 574: 157–163. [DOI] [PubMed] [Google Scholar]
- 40.Welch SP, Singha AK, Dewey WL. The antinociception produced by intrathecal morphine, calcium, A23187, U50,488H, [D-Ala2, N-Me-Phe4, Gly-ol]enkephalin and [D-Pen2, D-Pen5]enkephalin after intrathecal administration of calcitonin gene-related peptide in mice. J Pharmacol Exp Ther 1989; 251: 1–8. [PubMed] [Google Scholar]
- 41.Karki P, Kurihara T, Nakamachi T, et al. Attenuation of inflammatory and neuropathic pain behaviors in mice through activation of free fatty acid receptor GPR40. Mol Pain 2015; 11: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M, Shioda S, Somogyvári-Vigh A, et al. Specific antibody recognition of rat pituitary adenylate cyclase activating polypeptide receptors. Endocrine 1997; 7: 183–190. [DOI] [PubMed] [Google Scholar]
- 43.Shioda S, Yada T, Nakajo S, et al. Pituitary adenylate cyclase activating polypeptide (PACAP): a novel regulator of vasopression-containing neurons. Brain Res 1997; 765: 81–90. [DOI] [PubMed] [Google Scholar]
- 44.Miura A, Kambe Y, Inoue K, et al. Pituitary adenylate cyclase-activating polypeptide type 1 receptor (PAC1) gene is suppressed by transglutaminase 2 activation. J Biol Chem 2013; 288: 32720–32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miura A, Odahara N, Tominaga A, et al. Regulatory mechanism of PAC1 gene expression via Sp1 by nerve growth factor in PC12 cells. FEBS Lett 2012; 586: 1731–1735. [DOI] [PubMed] [Google Scholar]
- 46.Gil DW, Cheevers CV, Donello JE. Transient allodynia pain models in mice for early assessment of analgesic activity. Br J Pharmacol 2008; 153: 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun R-Q, Tu Y-J, Lawand NB, et al. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol 2004; 92: 2859–2866. [DOI] [PubMed] [Google Scholar]
- 48.Huck S, Grass F, Hortnagl H. The glutamate analogue α-aminoadipic acid is taken up by astrocytes before exerting its gliotoxic effect in vivo. J Neurosci 1984; 4: 2650–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji RR, Kawasaki Y, Zhuang ZY, et al. Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol 2006; 2: 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khurgel M, Koo AC, Ivy GO. Selective ablation of astrocytes by intracerebral injections of alpha-aminoadipate. Glia 1996; 16: 351–358. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez MJ, Martinez-Sanchez M, Bernal F, et al. Heterogeneity between hippocampal and septal astroglia as a contributing factor to differential in vivo AMPA excitotoxicity. J Neurosci Res 2004; 77: 344–353. [DOI] [PubMed] [Google Scholar]
- 52.Zhuang ZY, Wen YR, Zhang DR, et al. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci 2006; 26: 3551–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu N-J, Schnell SA, Schulz S, et al. Regulation of spinal dynorphin 1-17 release by endogenous pituitary adenylyl cyclase-activating polypeptide in the male rat: relevance of excitation via disinhibition. J Pharmacol Exp Ther 2011; 336: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alessi DR, Cuenda A, Cohen P, et al. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. J Biol Chem 1995; 270: 27489–27494. [DOI] [PubMed] [Google Scholar]
- 55.Bennett BL, Sasaki DT, Murray BW, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 2001; 98: 13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohsawa M, Brailoiu GC, Shiraki M, et al. Modulation of nociceptive transmission by pituitary adenylate cyclase activating polypeptide in the spinal cord of the mouse. Pain 2002; 100: 27–34. [DOI] [PubMed] [Google Scholar]
- 57.Davis-Taber R, Baker S, Lehto SG, et al. Central pituitary adenylate cyclase 1 receptors modulate nociceptive behaviors in both inflammatory and neuropathic pain states. J Pain 2008; 9: 449–456. [DOI] [PubMed] [Google Scholar]
- 58.Grace PM, Hutchinson MR, Maier SF, et al. Pathological pain and the neuroimmune interface. Nat Rev Immun 2014; 14: 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discovery 2014; 13: 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mika J, Zychowska M, Popiolek-Barczyk K, et al. Importance of glial activation in neuropathic pain. Eur J Pharmacol 2013; 716: 106–119. [DOI] [PubMed] [Google Scholar]
- 61.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 2009; 10: 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med 2010; 16: 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edelmayer RM, Brederson J-D, Jarvis MF, et al. Biochemical and pharmacological assessment of MAP-kinase signaling along pain pathways in experimental rodent models: a potential tool for the discovery of novel antinociceptive therapeutics. Biochem Pharmacol 2014; 87: 390–398. [DOI] [PubMed] [Google Scholar]
- 64.Hansen RR, Malcangio M. Astrocytes-Multitaskers in chronic pain. Eur J Pharmacol 2013; 716: 120–128. [DOI] [PubMed] [Google Scholar]
- 65.Zhuang ZY, Gerner P, Woolf CJ, et al. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 2005; 114: 149–159. [DOI] [PubMed] [Google Scholar]