Abstract
Background
Lysophosphatidic acid receptor 1 and Rho/ROCK signaling is implicated in bone cancer pain development. However, it remains unknown whether the two signaling pathways function together in P2X3 receptor-mediated bone cancer pain.
Results
In this study, using a rat model of bone cancer, we examined the expression of P2X3 and lysophosphatidic acid receptor 1 in rat dorsal root ganglion neurons and further dissected whether lysophosphatidic acid receptor 1 and Rho/ROCK-mediated pathways interacted in modulating rat pain behavior. Bone cancer was established by inoculating Walker 256 cells into the left tibia of female Wistar rats. We observed a gradual and yet significant decline in mean paw withdrawal threshold in rats with bone cancer, but not in control rats. Our immunohistochemical staining revealed that the number of P2X3- and lysophosphatidic acid receptor 1-positive dorsal root ganglion neurons was significantly greater in rats with bone cancer than control rats. Lysophosphatidic acid receptor 1 blockade with VPC32183 significantly attenuated decline in mean paw withdrawal threshold. Flinching behavior test further showed that lysophosphatidic acid receptor 1 inhibition with VPC32183 transiently but significantly attenuated α,β-meATP-induced increase in paw lift time per minute. Rho inhibition by intrathecal BoTXC3 caused a rapid reversal in decline in mean paw withdrawal threshold of rats with bone cancer. Flinching behavior test showed that BoTXC3 transiently and significantly attenuated α,β-meATP-induced increase in paw lift time per minute. Similar findings were observed with ROCK inhibition by intrathecal Y27632. Furthermore, VPC32183 and BoTXC3 effectively aborted the appearance of lysophosphatidic acid-induced calcium influx peak.
Conclusions
Lysophosphatidic acid and its receptor LPAR1, acting through the Rho-ROCK pathway, regulate P2X3 receptor in the development of both mechanical and spontaneous pain in bone cancer.
Keywords: Bone cancer pain, P2X3 receptor, lysophosphatidic acid receptor, Rho/ROCK
Background
Bone cancer pain may develop as a result of tumors arising in bone such as osteosarcoma or as a consequence of cancer metastasis, particularly cancers of the breast, prostate, and lungs.1 Apart from direct damage of the sensory fibers by cancer cells, increased local production of inflammatory cytokines and extracellular adenosine triphosphate (ATP) in the tumor microenvironment point to complex pain mechanisms underlying nociceptive and inflammatory pain in bone cancer patients and animal models.2–4
In the peripheral nervous system, extracellular ATP contributes to nociception by activating P2X receptors.5 P2X3 receptor, a member of the purinergic P2X receptors,6 is selectively expressed in small-diameter sensory neurons7 and implicated in bone cancer pain.8,9 In a previous study from this laboratory, we found increased expression of P2X3 receptor in dorsal root ganglion (DRG) neurons in a rat model of bone cancer pain.10 Studies also demonstrate increased expression of P2X3 receptors on epidermal nerve fibers during tumor growth.11 Blockade of P2X3 receptor reduced chronic inflammatory and neuropathic pain behavior in rats, further highlighting a role of P2X3 receptor in pain regulation.12,13
Lysophosphatidic acid (LPA), synthesized by nearly all human cell types under normal conditions, is increased in tumor tissues.14 In bone cancer, LPA increase is associated with increased risk of metastasis and pain development.15,16 LPA also enhances tetrodotoxin receptor-mediated neuronal firing and promotes release of substance P.17 LPA receptor subtype 1 (LPAR1) is implicated in the initiation of peripheral nerve injury-induced neuropathic pain in mice.18 In rats with bone cancer, pain is associated with upregulated LPAR1 expression in DRG cells.19 LPAR1 activation may in turn stimulate mitogen-activated protein kinases (MAPKs), phospholipase C (PLC), and Rho-ROCK, all of which associated with neuropathic pain.20–22 LPA receptor ligand also potentiates ATP-gated P2X receptor channel currents, suggesting that P2X receptor and LPA receptors may functionally interact.
In this study, we hypothesized that LPA and Rho/ROCK may interact in the P2X3 receptor signaling pathway in regulating cancer pain sensitivity and examined the expression of P2X3 and LPAR1 in rat DRG neurons in a rat model of bone pain. Potential interaction between LPAR1 and P2X3 was also investigated.
Methods
Rat bone cancer model
The study protocol was approved by the Administrative Committee of Experimental Animal Care and Use of Shanghai Chest Hospital (Permission No. KS1218), and animal study was performed in accordance with the U.S. National Institutes of Health Guidelines on the Ethical Use of Animals.
Three female Wistar rats (Slaccas Co., Shanghai, China), each weighing 70 – 80 g, received an intraperitoneal inoculation of breast sarcocarcinoma Walker 256 cells (Shanghai Pharmaceutical Industry Research Institute, Shanghai, China). After one week, cells in the ascites were collected and resuspended in normal saline to a final concentration of 2 × 107 cells/mL. Bone cancer was then established by inoculating Walker 256 cells (2 × 105, 10 µL) into the left tibia of female Wistar rats (n = 30), each weighing 150–200 g, as described previously. Control rats (n = 30) were injected with heat-killed cancer cells.
Animal treatments
Twelve days after inoculation, the rats were catheterized by placing a PE-10 polyethylene catheter into the subarachnoid space between vertebrae L5 and L6. Three days later, rats (n = 6 for each group) were randomized to receive via the catheter P2X3 receptor antagonist A-317491 (30 nM; Sigma-Aldrich, St. Louis, MO, USA), LPAR1 receptor blocker VPC32183 (2 mM in 30 µL 3% bovine serum albumin; Avanti Ploar Lipids, Alabaster, AL, USA), Rho-inhibitor botulinum toxin C3 (BoTXC3, 300 pg in 30 µL normal saline; Calbiochem San Diego, CA, USA), ROCK blocker Y27632 (48 µg in 12 µL normal saline; Calbiochem), or normal saline.
Immunohistochemistry
Fourteen and 21 days after inoculation, rats (n = 6 for each group) were anesthetized by intraperitoneal chloral hydrate (300 mg/kg) and perfused with 4% paraformaldehyde. DRG neurons at L4 to L6 were harvested and fixed overnight with 4% paraformaldehyde. Immunohistochemistry was performed as previously depicted, and tissue sections were incubated for 24 h at room temperature with a goat anti-P2X3 receptor antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and a rabbit anti-LPAR1/EDG2 antibody (1:50, Novus Biologicals Inc., Littleton, CO, USA). The slides were then incubated for 1 h at room temperature with a fluorescein isothiocyanate-conjugated donkey anti-goat IgG secondary antibodies (1:200, Jackson ImmunoResearch Lab., West Grove, PA, USA) and Cy3-conjugated donkey anti-goat IgG (1:200; Jackson ImmunoResearch Lab). Images were taken on an Eclipse E600 microscope (Nikon, Tokyo, Japan) equipped with a Nikon DXM1200 digital camera.
Western blotting analysis
Approximately 100 mg of tissues was homogenized in ice-cold M-Per lysis buffer (Pierce Biotechnology). The proteins (50 ng) were denatured and separated by 10% SDS-polyacrylamide gel electrophoresis and subsequently transferred to nitrocellulose membranes. The membranes were incubated with specific antibodies including P2X3 or LPAR1 overnight at 4℃. Membranes were then incubated with a secondary horseradish peroxidase-conjugated antibody and immunoreactive proteins visualized using enhanced chemiluminescence (Santa Cruz Biotechnology). The intensities of light-emitting bands were detected and quantified using Sygene Bio Image system (Synoptics Ltd, UK). To control sampling errors, the ratio of band intensities to β-actin was obtained to quantify the relative protein expression level.
Paw withdrawal threshold and flinching behavior test
Paw withdrawal threshold was assessed before inoculation (day 0) and 1, 4, 7, 10, 13, 17, and 21 days after inoculation. Rats were acclimated to the test environment in individual testing cages for 20 min each day for three consecutive days. On the test day, rats were given at least 20 min to acclimate. Paw withdrawal threshold was measured as the hind paw withdrawal response to von Frey hair monofilaments (Stoelting, IL, USA). The stimuli were applied in a consecutive ascending order (0.6, 1, 2, 4, 6, 8, 10, and 15 g) perpendicularly to the medial surface of the hind paw for 3 s until paw withdrawal occurred. A positive response was defined as a withdrawal of the hind paw upon stimulation accompanied by head turning, biting, and/or licking. Each stimulus was repeated for five times with a 10-min interval, and the lowest force to induce at least three positive responses was defined as the paw withdrawal threshold.
Flinching behavior test (paw lift time per minute, PLTPM) was performed at day 12 postinoculation as described previously.23 α,β-meATP (50 nM in 50 µL; Sigma), α,β-meATP plus P2X3 receptor antagonist A-317491 (100 nM), or an equivalent volume of phosphate-buffered saline was administered subcutaneously into the plantar surface of the left hind paw using a 30-gauge needle. Rats were returned to their cages immediately after injection. Paw lift time and spontaneous withdrawal time were recorded. PLTPM was calculated using the equation:
PLTPM = paw lift time + (1/3) × spontaneous withdrawing time
[Ca2+]i measurement
Primary DRG neurons were dissociated at the L4 and L5 levels 14–16 days after inoculation and cultured as previously described.24 The cells were plated on glass coverslips coated with 0.1 mg/mL polyornithine and 1 mg/mL laminin (Boehringer Mannheim, Mannheim, Germany) and were incubated for 1 h at 37℃ with 5 μM Fura2-AM (Sigma) in HEPES-buffered Krebs solution (pH 7.4). The cells were washed and allowed to stand in a custom-designed perfusion chamber for 20 min at room temperature in the dark. Then, the chamber was mounted on the stage of an inverted microscope equipped with a CCD camera (Olympus, Japan). Cells were treated with α,β-meATP (20 μM) alone, or α,β-meATP (20 μM) followed by treatment with VPC32183 (20 μM), LPA (4 μM, Sigma), or BoTXC3 (5 µg/mL) and then α,β-meATP (20 μM). Fura-2 fluorescence signals were sequentially measured with excitation at 340 and 380 nm and emission at 510 nm to obtain a 340/380 ratio as a surrogate for intracellular calcium concentrations. Data were collected every 5 s. Images were acquired and analyzed using Metafluor imaging software (Universal Imaging Corp., Downingtown, PA, USA).
Statistical analysis
Data were presented as mean ± SD and analyzed using Origin 8.0 (Microcal Software, Northampton, MA, USA). Inter-group differences for PWT and PLTPM were assessed for significance using two-way ANOVA for repeated measures, followed by Scheffe’s multiple comparison test. Inter-group differences in receptor expression levels based on immunofluorescent staining were assessed for significance using Student’s t test. Inter-group differences for peak fold increase of Fura2 340/380 ratio were assessed for significance using one-way ANOVA followed by least significant difference post hoc analysis. Statistical significance was set at p < 0.05.
Results
Bone cancer pain is associated with in increased proportions of LPAR1 and P2X3-positive neurons in DRG
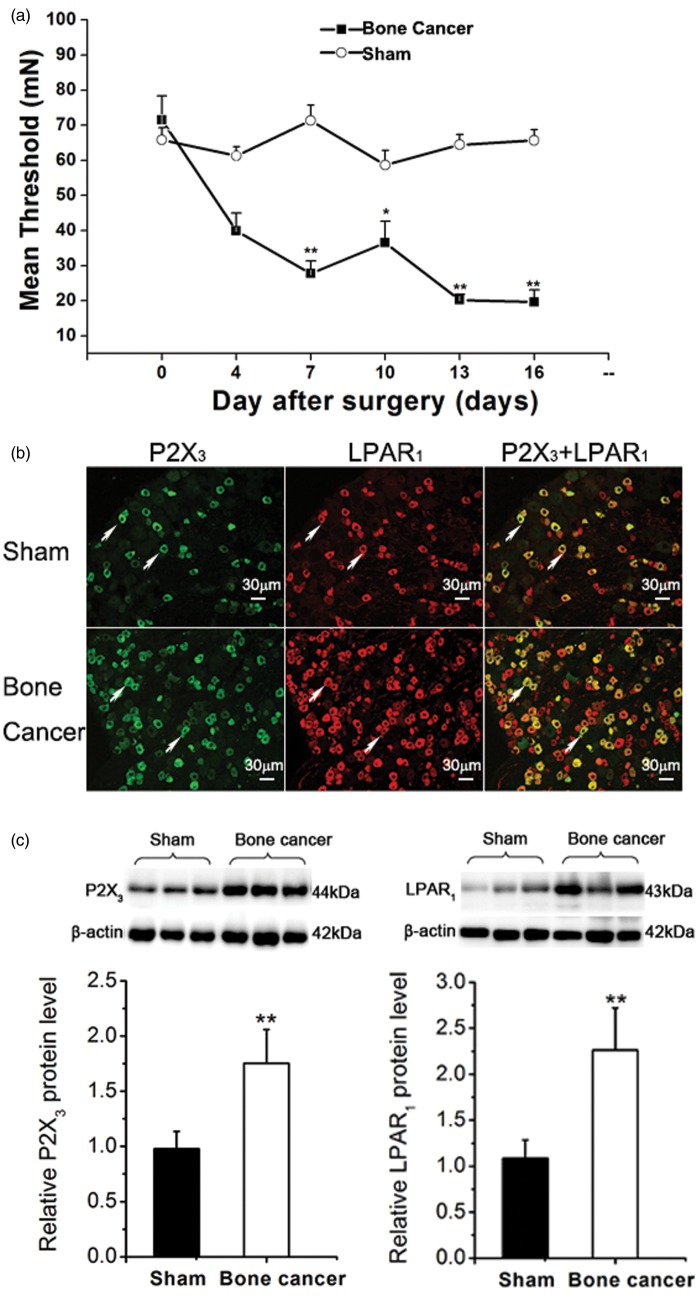
We evaluated paw withdrawal threshold in rats inoculated with live vs. heat-killed Walker 256 cells (each 2 × 105, 10 µL). The mean paw withdrawal threshold (MPWT) at baseline (day 0 postinoculation) was comparable between the two groups (Figure 1(a)). A gradual and yet significant decline in the MPWT was observed in rats inoculated with live tumor cells but not heat-killed tumor cells. At day 16, the MPWT in rats inoculated with 2 × 105 live tumor cells was 29.9% lower than in rats receiving heat-killed tumor cells (p = 2.3244E-8).
Figure 1.
Bone cancer pain is associated with in increased proportions of LPAR1- and P2X3-positive neurons in the dorsal root ganglia of rats. (a) Rats were inoculated with live or heat-killed Walker 256 cells as described in Methods section. The mean paw withdrawal threshold was assessed at the time points indicated. (b) Immunofluorescent microscopy was done using anti-P2X3 receptor and anti-LPAR1 antibodies as described in Methods section. Green: P2X3 receptor; Red: LPAR1. (c) The level of P2X3 and LPAR1 in DRG samples of sham group (n = 10) and bone cancer group (n = 10). P2X3 and LPAR1 were assessed using Western blot analysis. Data (a to c) are presented as mean ± SD (n = 6 rats per group), and inter-group differences are assessed using Student’s t test. *p < 0.05, **p < 0.01 compared with the sham control.
Our previous study and others have previously shown that P2X3 and LPAR1 are upregulated in DRG neurons in rats with bone cancer.10,19 Consistently, immunofluorescent staining in the current study revealed greater number of P2X3-positive cells in rats inoculated with live Walker 256 cells (75%) than in rats receiving heat-killed tumor cells (p = 2.6706E-5) (Figure 1(b) and (c)). Similarly, the number of LPAR1-positive cells was significantly higher in rats inoculated with live tumor cells (126%) (p = 4.92464E-5) (Figure 1(b) and (c)). The diameter of the positively labeled neuronal soma was measured using a calibrated reticule and calculated as an average of the shortest and longest axes. The LPAR1 receptors were predominantly located on small-sized neurons (diameter < 30 µm). Almost all P2X3-positive neurons expressed LPAR1 receptors, whereas only a proportion of the LPAR1-positive neurons also expressed P2X3 receptor.
Blockade of LPAR1 or P2X3 receptor diminishes bone cancer pain and α,β-meATP-induced spontaneous pain
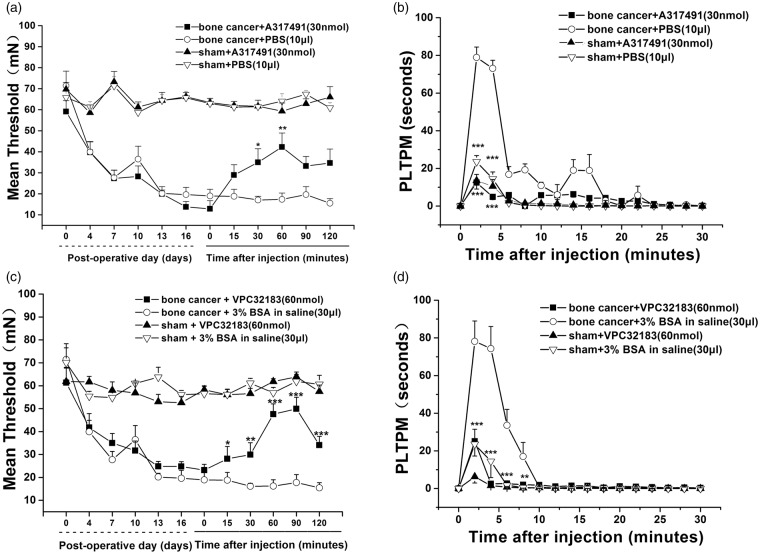
Inhibition of P2X3 receptor by intrathecal A317491 (30 nmol) increased paw withdrawal threshold (Figure 2(a)). At the peak of effects (60 min), the withdrawal threshold was increased by A317491 (n = 6) to 243% (p = 9.67944E-6 vs. rats receiving PBS vehicle; n = 6). We then examined the effect of inhibition of P2X3 receptor on flinching behavior of the rats. Intrathecal A317491 attenuated the transient but yet significant increase in spontaneous pain induced by α,β-meATP in PLTPM (A377491: 12.45 ± 3.45 vs. PBS: 78.94 ± 5.53, 2 min) (p = 2.32139E-8) (Figure 2(b)). LAPR1 blockade with VPC32183 also significantly attenuated the decline in paw withdrawal threshold (p = 8.3823E-8 vs. the BSA vehicle; n = 6) (Figure 2(c)). VPC32183 transiently and significantly attenuated α,β-meATP-induced increase in PLTPM (VPC32183: 25.11 ± 6.45 vs. BSA: 78.16 ± 10.31, 2 min) (p = 9.15659E-7) (Figure 2(d)). These results demonstrated that blockade of LPAR1 or P2X3 receptor could inhibit bone cancer pain and α, β-meATP-induced spontaneous pain.
Figure 2.
P2X3 receptor-mediated pain and spontaneous pain induced by the P2X3 receptor agonist α,β-meATP are reduced by P2X3 receptor inhibitor and LPAR1 antagonist. (a) P2X3 receptor inhibitor A317491 reversed the bone cancer-induced decrease in paw withdrawal threshold. (b) The spontaneous response, PLTPM induced by P2X3 receptor agonist α,β-meATP was significantly longer in cancer rats than in sham control animals, and it was reduced by a P2X3 receptor inhibitor A317491. (c) LPAR1 antagonist VPC32183 reversed the bone cancer-induced decrease in paw withdrawal threshold. (d) P2X3 receptor agonist α,β-meATP induced a spontaneous response that was reduced by LPAR1 antagonist. Data were presented as means ± SD (n = 6 animals per group), and inter-group differences were assessed for significance using two-way ANOVA. *p < 0.05, **p < 0.01 compared with corresponding time points in the PBS group or 3% BSA group.
Rho and ROCK signaling is implicated in bone cancer pain and α, β-meATP-induced spontaneous pain
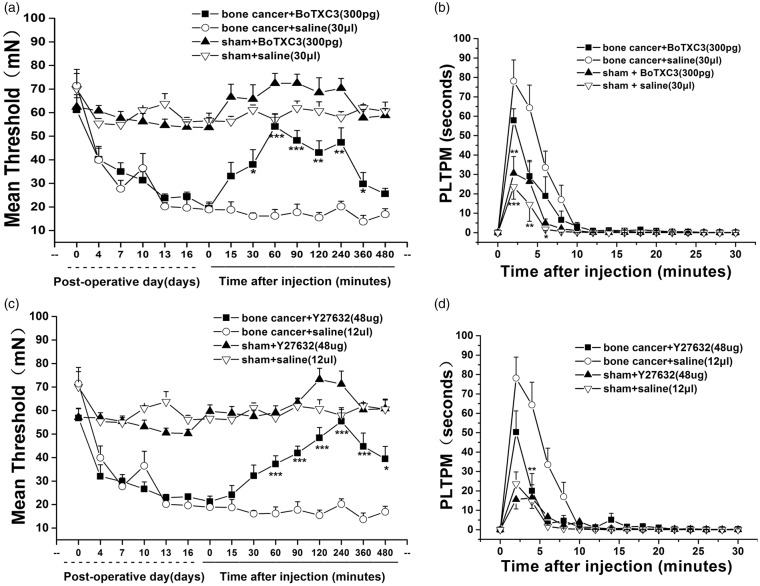
LPAR1 activation is known to stimulate Rho-ROCK, which in turn is associated with neuropathic pain. In our experiments, Rho inhibition by intrathecal BoTXC3 (300 pg) caused a rapid reversal in the decline in mechanical pain threshold in rats inoculated with live tumor cells (Figure 3(a)). The action of BoTXC3 peaked at 60 min after intrathecal administration followed by a gradual decline (BoTXC3: 54.2 ± 5.44 vs. normal saline: 16.13 ± 1.31, 60 min, p = 4.04603E-8 and BoTXC3: 25.542 ± 2.32 vs. normal saline: 16.91 ± 2.36, 480 min, p > 0.05). Rho inhibition with BoTXC3 transiently and significantly attenuated α,β-meATP-induced increase in PLTPM (BoTXC3: 57.88 ± 5.98 vs. normal saline: 78.15 ± 10.86, 2 min) (p = 8.82703E-5) (Figure 3(b)). Consistently, ROCK inhibition by intrathecal Y27632 (48 µg) caused a time-dependent increase in mechanical pain threshold, with a peak at 240 min after intrathecal administration (Y27632: 48.37 ± 4.39 vs. normal saline: 15.49 ± 2.19, 120 min, p = 4.35685E-8). By 24 h, the effects of Y27632 were no longer evident (Figure 3(c)). Flinching behavior test revealed that ROCK inhibition with Y27632 also significantly attenuated α,β-meATP-induced increase in PLTPM (Y27632: 50.29 ± 10.95 vs. normal saline: 78.15 ± 10.87, 2 min) (p = 6.91976E-5) (Figure 3(d)). These results implicated Rho/ROCK signaling in mechanical bone cancer pain and α, β-meATP-induced spontaneous pain.
Figure 3.
P2X3 receptor-mediated bone cancer pain requires Rho-ROCK signaling pathways. The bone cancer-induced decrease in paw withdrawal threshold was reversed by Rho inhibitor BoTXC3 (a) and ROCK inhibitor Y27632 (c). The spontaneous response induced by α,β-meATP was reduced by Rho inhibitor BoTXC3 (b) and ROCK inhibitorY27632 (d). Data were presented at means ± SD (n = 5 animals per group), and inter-group differences were assessed using two-way ANOVA. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with corresponding time points in the saline group or 3% BSA group.
Rho functions downstream of LPAR1 in modulating P2X3 receptor-mediated cellular calcium influx
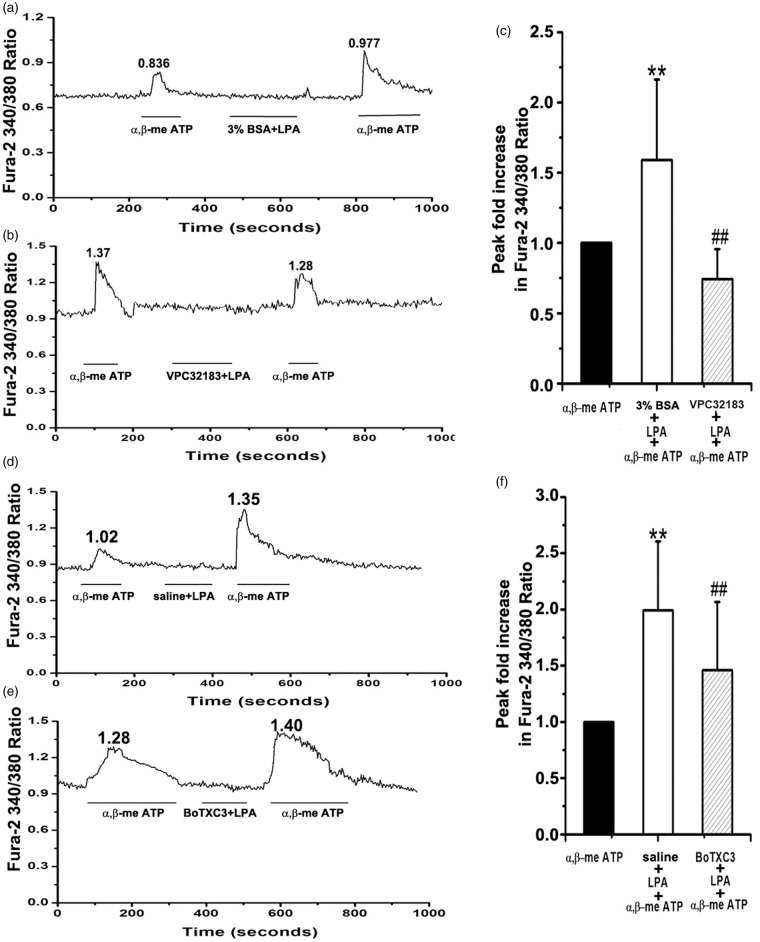
P2X3 receptor agonist α,β-meATP (20 μM) elicited a strong calcium influx in primary DRG neurons of rats with bone cancer with a Fura-2 ratio of 0.836 (peak 1 in Figure 4(a)). Addition of LPA (4 μM) led to marked increase in calcium influx with a Fura-2 ratio of 0.977 (peak 2, Figure 4(a)). Peak 2 was 1.59 ± 0.57 of peak 1 (p = 0.042, n = 47, Figure 4(c)). Addition of LPAR1 antagonist VPC32183 prevented LPA-induced calcium influx (peak 1: 1.37 vs. peak 2: 1.28) (Figure 4(b)). Peak fold increase was reduced from 1.62 ± 0.63 to 0.74 ± 0.21 (p < 0.01, Figure 4(c)). We treated DRG neurons with α,β-meATP (20 μM) and then with LPA (4 μM) and Rho inhibitor BoTXC3 (5 µg/mL). Similar to LPAR1 antagonist VPC32183, BoTXC3 prevented LPA-induced calcium influx (peak 1: 1.28 vs. peak 2: 1.40). Peak fold increase was reduced from 1.99 ± 0.61 to 1.46 ± 0.61 (p < 0.01, Figure 4(f)). These findings indicate that Rho functions downstream of LPAR1 in modulating P2X3 receptor-mediated calcium influx in DRG neurons.
Figure 4.
Rho functions downstream of LPAR1 in modulating P2X3 receptor-mediated cellular calcium influx. Representative tracings of Fura-2 ratio of primary dorsal root ganglion (DRG) neurons treated with α,β-meATP (20 μM) followed by lysophosphatidic acid (LPA, 4 μM) plus vehicle (a, d) or LPA plus LPAR1 antagonist VPC32183 (b), or LPA plus Rho inhibitor BoTXC3 (5 µg/mL) (e) are shown. Pooled data are shown (c, f) measuring the Δ Fura-2 ratio in which peak height is subtracted from basal and then peak fold of increase was Δ Fura-2 ratio of peak2 vs. Δ Fura-2 ratio of peak1; between 23 and 48 cells were analyzed under each condition. Data are presented as mean ± SD, and inter-group differences are assessed using one way ANOVA followed by LSD post hoc analysis. **p < 0.01 α,β-meATP plus LPA vs. α,β-meATP in (c, f), ##p < 0.01 VPC32183 (c) or BoTXC3 (f) vs. α,β-meATP plus LPA.
Discussion
Recent evidence, including evidence from this research group, has implicated P2X3 receptor in modulating bone cancer pain in animal models.10,25 We speculated that P2X3 receptor may represent a promising molecular target, but its upstream and downstream molecules have not been defined. In the current study, by using a rat bone cancer model, we established that LPAR1, which is known to initiate peripheral nerve injury-induced neuropathic pain,6 is also implicated in modulating mechanical allodynia and spontaneous pain in rats with bone cancer. Furthermore, we demonstrated that Rho/ROCK signaling is also involved in modulating bone cancer pain and α,β-meATP-induced spontaneous pain and our calcium influx analysis indicated that Rho/ROCK functions downstream of LPAR1 in modulating P2X3 receptor-mediated cellular calcium influx. Intracellular signaling cascade of P2X3 receptors have not been clearly delineated so far. Rho/ROCK signaling has been recently shown to mediate neuropathic pain in rats by activating p38 MAPK.26 The nociceptive effects of ROCK have been demonstrated in a range of pain models, and ROCK inhibition leads to pain reduction.27 Our findings point to a functional link between P2X3 signaling and the pivotal Rho/ROCK pathway. These findings should help set the stage for further investigations to elucidate the full range of upstream and downstream signaling pathways involved in bone cancer pain, which may in turn unravel novel therapeutic targets for managing and even preventing bone cancer pain.
Inhibition of the P2X3 receptor has been shown to reduce neuropathic pain, chronic inflammatory pain, and formalin-induced pain.13 The present study built on our previous work showing that the P2X3 receptor was functionally up-regulated in DRG neurons of rats with bone cancer.10 Here, we provide additional evidence that bone cancer is associated with an increased proportion of LPAR1 and P2X3 receptor positive neurons. Our results with immunofluorescent assays suggest that LPAR1 and P2X3 receptor are colocalized in DRG cells. However, we do not know whether LPAR1 and P2X3 receptors physically interact with one another in the cells as immunoprecipitation assays were not conducted and whether LPAR1 and P2X3 receptors cross-talk with one another in the development of bone cancer pain.
Cancer pain mechanism is complex and involves nociceptive and inflammatory pain and others. The current study assessed the role of LPAR1 and Rho/ROCK in both mechanical hyperalgesia and α,β-meATP-induced spontaneous pain in the rat bone cancer model. Dissection of changes in calcium influx currents using LPAR1 antagonist VPC32183 and Rho inhibitor BoTXC3 reveals that Rho/ROCK signaling occurs downstream of LAPR1 in P2X3 receptors-mediated spontaneous pain in rats with bone cancer, which is consistent with the finding of a recent study showing that in DRG neurons, LPAR1 activates the Rho-ROCK signaling pathway in the development of neuropathic pain.28 Although there is a limitation that only primary DRG neurons of rats with bone cancer pain were used in calcium influx experiments, the behavior experiments in the control animals do suggest that LPAR1 and Rho/ROCK may interact in the P2X3 receptor signaling pathway in regulating bone cancer pain sensitivity, due to the fact that (1) the bone cancer-induced decrease in paw withdrawal threshold was reversed by A317491, VPC32183, BoTXC3, or Y27632. (2) The PLTPM induced by P2X3 receptor agonist α,β-meATP was significantly longer in cancer rats than in heat-killed cells control animals, and it can be fully reduced by VPC32183, BoTXC3, or Y27632. However, the effect of A317491, VPC32183, BoTXC3, and Y27632 on sham control (heat-killed cell) animals is similar to that of PBS. Hang et al.22 demonstrated that ROCK inhibition significantly attenuated pain behavior in rats with bone cancer, but they did not address the underlying signaling pathways. We extended their findings by showing that ROCK functions downstream of LPAR1 in modulating P2X3 receptor-mediated pain. These findings together suggest that Rho-ROCK signaling modulates pain sensitivity mediated by P2X3 receptors downstream of LPAR1. While LPAR1 is the primary LPA receptor in DRG neurons,29 five other LPARs have been described.30 We cannot completely exclude that α,β-meATP-induced enhancement of spontaneous activity in our experiments reflects activation of other P2X receptors. It remains intriguing to explore whether other LPARs also mediate P2X3 receptor-induced pain in bone cancer and other contexts. Our findings in bone cancer pain help set the stage for delineating LPARs and other signaling pathways in P2X3 receptor-mediated pain.
Conclusions
In conclusion, this study suggests that LPA and its receptor LPAR1, acting through the Rho-ROCK pathway, could regulate P2X3 receptor in the development of both mechanical and spontaneous pain in bone cancer. Antagonizing or inhibiting LPAR signaling or its downstream Rho/ROCK signaling could be developed novel approach to manage bone pain.
Acknowledgments
The authors thank Drs. Jihu Sun and Xingji You (Department of Physiology, Second Military Medical University, Shanghai, China) for their advice and helpful suggestions.
Authors’ contributions
XMY designed and supervised the research project and wrote the paper. WJX performed rat bone cancer model, Western blotting, and data analysis. YXM performed paw withdrawal threshold and flinching behavior test. WQ performed [Ca2+]i measurement, and WW helped to perform rat bone cancer model.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Shanghai Natural Science Foundation (12ZR1428700) and the ‘1050’ Talents Project of Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China.
References
- 1.Sabino MA, Mantyh PW. Pathophysiology of bone cancer pain. J Support Oncol 2005; 3: 15–24. [PubMed] [Google Scholar]
- 2.Guo G, Gao F. CXCR3: latest evidence for the involvement of chemokine signaling in bone cancer pain. Exp Neurol 2015; 265: 176–179. [DOI] [PubMed] [Google Scholar]
- 3.Hu XM, Liu YN, Zhang HL, et al. CXCL12/CXCR4 chemokine signaling in spinal glia induces pain hypersensitivity through MAPKs-mediated neuroinflammation in bone cancer rats. J Neurochem 2015; 132: 452–463. [DOI] [PubMed] [Google Scholar]
- 4.Falk S, Uldall M, Heegaard AM. The role of purinergic receptors in cancer-induced bone pain. JOsteoporos 2012; 2012: 758181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye Y, Ono K, Bernabe DG, et al. Adenosine triphosphate drives head and neck cancer pain through P2X2/3 heterotrimers. Acta Neuropathol Commun 2014; 2: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue M, Rashid MH, Fujita R, et al. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med 2004; 10: 712–718. [DOI] [PubMed] [Google Scholar]
- 7.North RA. P2X3 receptors and peripheral pain mechanisms. J Physiol 2004; 554: 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen RR, Nasser A, Falk S, et al. Chronic administration of the selective P2X3, P2X2/3 receptor antagonist, A-317491, transiently attenuates cancer-induced bone pain in mice. Eur J Pharmacol 2012; 688: 27–34. [DOI] [PubMed] [Google Scholar]
- 9.Kaan TK, Yip PK, Patel S, et al. Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behaviour in rats. Brain 2010; 133: 2549–2564. [DOI] [PubMed] [Google Scholar]
- 10.Wu JX, Xu MY, Miao XR, et al. Functional up-regulation of P2X3 receptors in dorsal root ganglion in a rat model of bone cancer pain. Eur J Pain 2012; 16: 1378–1388. [DOI] [PubMed] [Google Scholar]
- 11.Gilchrist LS, Cain DM, Harding-Rose C, et al. Re-organization of P2X3 receptor localization on epidermal nerve fibers in a murine model of cancer pain. Brain Res 2005; 1044: 197–205. [DOI] [PubMed] [Google Scholar]
- 12.Jarvis MF, Burgard EC, McGaraughty S, et al. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci USA 2002; 99: 17179–17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGaraughty S, Wismer CT, Zhu CZ, et al. Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br J Pharmacol 2003; 140: 1381–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skill N, Wu J, Xu Y, et al. Lysophosphatidic acid aberrancies and hepatocellular carcinoma: studies in the MDR2 gene knockout mouse. Cancer Invest 2013; 31: 145–155. [DOI] [PubMed] [Google Scholar]
- 15.Peyruchaud O, Leblanc R, David M. Pleiotropic activity of lysophosphatidic acid in bone metastasis. Biochim Biophys Acta 2013; 1831: 99–104. [DOI] [PubMed] [Google Scholar]
- 16.Zhao J, Pan HL, Li TT, et al. The sensitization of peripheral C-fibers to lysophosphatidic acid in bone cancer pain. Life Sci 2010; 87: 120–125. [DOI] [PubMed] [Google Scholar]
- 17.Seung Lee W, Hong MP, Hoon, Kim T, et al. Effects of lysophosphatidic acid on sodium currents in rat dorsal root ganglion neurons. Brain Res 2005; 1035: 100–104. [DOI] [PubMed] [Google Scholar]
- 18.Ueda H. Peripheral mechanisms of neuropathic pain – involvement of lysophosphatidic acid receptor-mediated demyelination. Mol Pain 2008; 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan HL, Zhang YQ, Zhao ZQ. Involvement of lysophosphatidic acid in bone cancer pain by potentiation of TRPV1 via PKCepsilon pathway in dorsal root ganglion neurons. Mol Pain 2010; 6: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikelis CM, Palmby TR, Simaan M, et al. PDZ-RhoGEF and LARG are essential for embryonic development and provide a link between thrombin and LPA receptors and Rho activation. J Biol Chem 2013; 288: 12232–12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wuhanqimuge, Itakura A, Matsuki Y, et al. Lysophosphatidylcholine enhances NGF-induced MAPK and Akt signals through the extracellular domain of TrkA in PC12 cells. FEBS Open Biol 2013; 3: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hang LH, Shao DH, Chen Z, et al. Spinal RhoA/Rho kinase signalling pathway may participate in the development of bone cancer pain. Basic Clin Pharmacol Toxicol 2013; 113: 87–91. [DOI] [PubMed] [Google Scholar]
- 23.Xiang Z, Xiong Y, Yan N, et al. Functional up-regulation of P2X 3 receptors in the chronically compressed dorsal root ganglion. Pain 2008; 140: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melli G, Hoke A. Dorsal root ganglia sensory neuronal cultures: a tool for drug discovery for peripheral neuropathies. Expert Opin Drug Discov 2009; 4: 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M, Yang H, Fang D, et al. Upregulation of P2X3 receptors by neuronal calcium sensor protein VILIP-1 in dorsal root ganglions contributes to the bone cancer pain in rats. Pain 2013; 154: 1551–1568. [DOI] [PubMed] [Google Scholar]
- 26.Tatsumi E, Yamanaka H, Kobayashi K, et al. RhoA/ROCK pathway mediates p38 MAPK activation and morphological changes downstream of P2Y12/13 receptors in spinal microglia in neuropathic pain. Glia 2015; 63: 216–228. [DOI] [PubMed] [Google Scholar]
- 27.Paiva-Lima P, Bakhle YS, Francischi JN. Dual effects of Rho-kinase inhibitors on a rat model of inflammatory pain. Pain Res Manag 2014; 19: e172–e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahn DK, Lee SY, Han SR, et al. Intratrigeminal ganglionic injection of LPA causes neuropathic pain-like behavior and demyelination in rats. Pain 2009; 146: 114–120. [DOI] [PubMed] [Google Scholar]
- 29.Xie W, Matsumoto M, Chun J, et al. Involvement of LPA1 receptor signaling in the reorganization of spinal input through Abeta-fibers in mice with partial sciatic nerve injury. Mol Pain 2008; 4: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oude Elferink RP, Bolier R, Beuers UH. Lysophosphatidic acid and signaling in sensory neurons. Biochimi Biophys Acta 2015; 1851: 61–65. [DOI] [PubMed] [Google Scholar]