Abstract
Background
Neuropathic characteristics are highly involved in the development of chronic pain both physically and psychologically. However, little is known about the relationship between neuropathic characteristics and brain morphological alteration.
Objectives
The aim of this study is to investigate the mechanisms of chronic pain development by examining the above-mentioned relationships by voxel-based morphometry in patients with chronic pain.
Methods
First, we assessed neuropathic characteristics using the painDETECT Questionnaire in 12 chronic pain patients. Second, to assess the gray matter volume changes by voxel-based morphometry, we conducted magnetic resonance imaging of the brain. We applied multiregression analysis of these two assessment methods.
Results
There were significant positive correlations between painDETECT Questionnaire scores and the gray matter volume in the bilateral anterior cingulate cortex and right posterior cingulate cortex.
Conclusions
Our findings suggest that neuropathic characteristics strongly affect the brain regions related to modulation of pain in patients with chronic pain and, therefore, contribute to the severity of chronic pain.
Keywords: Brain, chronic pain, voxel-based morphometry, anterior cingulate cortex, painDETECT Questionnaire, posterior cingulate cortex, neuropathic characteristics
Introduction
Chronic pain is complicated because of its effects on various physical, emotional, and cognitive functions.1 Therefore, the treatment of chronic pain requires multiple approaches, such as pharmacological therapy, interventional therapy, exercise, rehabilitation, and psychological therapy.
In clinical practice, neuropathic pain is regarded as group of disorders characterized by nerve damage including diabetic polyneuropathy, postherpetic neuralgia, and poststroke syndromes2 and defined by the International Association for the Study of Pain as “Pain caused by a lesion or disease of the somatosensory nervous system.” Under this definition, chronic pain has been classified into neuropathic pain or non-neuropathic pain. However, Attal3 provided a novel concept that there are various degrees of neuropathic characteristics in chronic pain, that is, overlapping symptoms such as neuropathic pain and nociceptive pain in various chronic pain conditions. A general population survey4,5 demonstrated that chronic pain patients with neuropathic characteristics showed higher pain intensity, lower quality of life or sleep, and more severe anxiety or depression than those without neuropathic characteristics. Thus, neuropathic characteristics could be present in most chronic pain patients and are highly involved in the development of chronic pain.
Voxel-based morphometry (VBM) is a neuroimaging analytical method of evaluating regional differences in the density or volume of the gray matter throughout the brain. As VBM can assess the anatomical differences throughout the brain, a whole brain analysis can be conducted.6 VBM is widely used in the studies of pain and psychiatric disorders.6 The brain signature hypothesis7 states that chronic pain is closely associated with the structure of the brain. A recent review8 showed that the gray matter volume in a specific region changes under chronic pain condition, as shown by magnetic resonance imaging (MRI). Although the regions showing gray matter morphological alterations differ among various chronic pain patients, there are overlapping areas such as the cingulate cortex, orbitofrontal cortex, insula, and dorsal pons,7 which are considered to interact with each other during the pain experience.
Wu et al.9 reported that the scores of ankylosing spondylitis patients in the painDETECT Questionnaire (PD-Q), a screening questionnaire to identify neuropathic characteristics, positively correlated with the gray matter volume in the anterior cingulate cortex, prefrontal cortex, thalamus, and striatum. Because chronic pain commonly has various degrees of neuropathic characteristics, which play a key role in the development of chronic pain, neuropathic characteristics should not be restricted to ankylosing spondylitis patients, as reported by Wu et al.9 This consideration led us to hypothesize that neuropathic characteristics may be significantly related to the structure of the pain-modulation areas including the anterior cingulate cortex among chronic pain patients with various pathological processes.
To investigate the relationship between neuropathic characteristics and the structures of pain-modulation areas, we conducted a VBM experiment of 12 chronic pain patients. We recruited patients with various types of chronic pain patients. We expected that the volumes of pain-modulation areas, such as the anterior cingulate cortex, correlate with the scores of PD-Q, which is used to identify neuropathic characteristics.
Methods
General design
All patients were recruited among the outpatients in our pain clinic. Each patient completed the PD-Q. Each patient’s MRI data were subsequently acquired on another scheduled day after answering the questionnaire (average, 23 days; SD, 20 days).
Subjects
A total of 12 chronic pain patients who provided their written informed consent participated in this study. The inclusion criteria were right-handedness and pain duration of more than three months. The exclusion criteria were the inability to undergo MRI owing to internal metal plates, psychosis (which interfered with participation in the study), severe physical diseases (heart failure, renal failure, liver failure, and respiratory failure). The details of 12 patients are shown in Table 1. The mean duration of disease was 7.95 years (SD, 7.75 years; range, 5 months to 27 years).
Table 1.
Patient characteristics.
| No. | Main diagnosis | Age | Sex | Side | Painful sites | Radiating pain | Duration |
|---|---|---|---|---|---|---|---|
| 1 | Central neuropathic pain | 60 | M | B | From low back to legs | + | 11 years |
| 2 | CRPS | 75 | F | R | From low back to leg | + | 5 years |
| 3 | CRPS | 27 | F | B | Feet | + | 5 years |
| 4 | CRPS | 43 | F | R | Lower leg | − | 4 years |
| 5 | CRPS | 29 | F | L | Lower leg | + | 5 months |
| 6 | LSS | 73 | F | L B | From buttock to leg, shoulder Hands | + | 16 years |
| 7 | LSS | 72 | F | B L | From low back to buttock Shoulder | + | 3 years |
| 8 | Lumbar disk herniation | 55 | M | B | From low back to legs | + | 5 years |
| 9 | CRPS | 80 | F | L | Lower leg | + | 14 years |
| 10 | FM | 61 | F | B | Everywhere | + | 27 years |
| 11 | FM | 50 | F | B | Everywhere | + | 1 years |
| 12 | Cervical spondylosis | 57 | M | L | Neck | − | 4 years |
| mean ± SD | age: 56.8 ± 17.3 | duration: 7.95 ± 7.75 | |||||
CRPS: Complex regional pain syndrome; LSS: lumbar spinal stenosis; FM: fibromyalgia.
This study was approved by the institutional review board of Gunma University Graduate School of Medicine, Maebashi, Japan (approval No. 1010). This study was conducted in accordance with institutional ethical provisions and the Declaration of Helsinki. The subjects were financially compensated for taking part in this study.
Patients are listed in registration order. Complex regional pain syndrome (CRPS); lumbar spinal stenosis (LSS); fibromyalgia (FM); left (L); right (R), both sides (B), and standard deviation (SD).
Questionnaire
All patients completed PD-Q.10 PD-Q is a screening questionnaire to identify neuropathic characteristics. PD-Q consists of seven neuropathic symptoms such as the pain scale, the pain course, and a section on the existence of radiating pain with a total score between 0 and 38. Each of seven neuropathic symptoms is rated on a 6-point scale: 0 (never) to 5 (very strongly). The pain course is rated from −1 to 1 point and the existence of radiating pain is rated from 0 to 2 points. A score ≥ 19 indicates a high possibility of neuropathic characteristics and ≤ 12 indicates their unlikelihood.10
Neuroimaging assessments
T1-weighted brain MRI was performed using a 3-Tesla scanner (MAGNETOM Trio, A Tim system, Siemens, Germany) at Gunma University Hospital (Gunma, Japan). Magnetization-prepared rapid gradient echo (MPRAGE) images were acquired (176 slices, 1 mm slice thickness with the following parameters: repetition time (TR) = 2300 ms; echo time (TE) = 2.98 milliseconds; flip angle = 9°; field of view = 256 mm2; voxel size = 1 ×1 × 1 mm3, matrix size = 256 × 256). Each image was examined for artifacts.
VBM protocol and analysis
We used VBM8 toolbox revision 435 (http://dbm.neuro.uni-jena.de/vbm/) with Statistical Parametric Mapping (SPM) 8 revision 5236 (The Wellcome Trust Centre for Neuroimaging; http://www.fil.ion.ucl.ac.uk/spm) in MATLAB 2013a (MathWorks, Inc.) to analyze the obtained structural images.
The structural images were corrected for bias-field inhomogeneity and spatially normalized with diffeomorphic anatomical registration through exponentiated Lie algebra (DARTEL) to the Montreal Neurological Institute (MNI) template, and tissues were classified as gray matter, white matter, or cerebrospinal fluid. In the modulation process, nonlinear deformation was used for normalization so that voxel intensities reflected regional gray matter volumes adjusted for individual brain sizes. Images were then smoothed to a Gaussian kernel of 8-mm full width at half maximum. After the preprocessing of structural images, gray matter segments were subjected to second-level analysis. As a primary outcome, multiple regression analysis, with PD-Q score as the effect of interest and gender, age, and pain duration as the effects of no interest, was applied. Considering a possibility that pain duration affect gray matter volume,11 pain duration was included as a covariate. We conducted the regression analysis with pain duration as an effect of interest, but as no brain region was found, pain duration was added as the effect of no interest. The statistical threshold for the MRI data analysis were set at family wise error corrected p < 0.05 at the cluster level with the height threshold as uncorrected p < 0.001 at the peak level. This threshold was applied for the whole-brain analysis. In the analysis, we applied nonstationary correction.12
Results
Scores in painDETECT Questionnaire
The average score in PD-Q was 22.25 (SD = 6.94; range, 10–35). Because we regard chronic pain as having various degrees of neuropathic characteristics, we enrolled all patients regardless of the score.
Correlation analysis between painDETECT score and gray matter volume
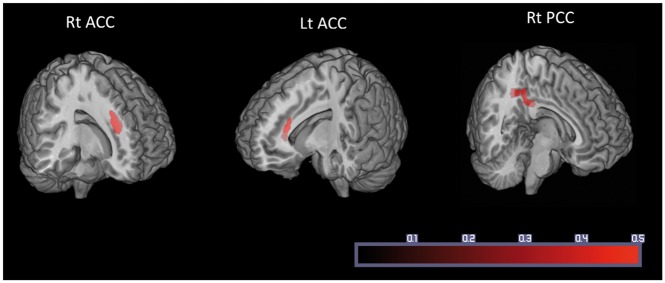
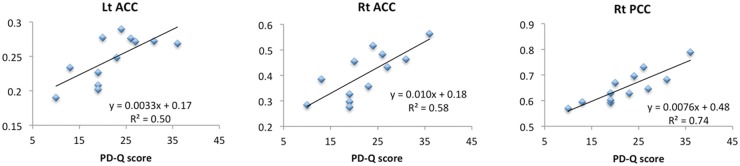
The effect of neuropathic characteristics to gray matter volume was examined by multiple regression analysis. There were significant positive correlations between gray matter volume and painDETECT scores, i.e., increase in gray matter volume with increasing scores) in the right anterior cingulate cortex (ACC), left ACC, and right posterior cingulate cortex (PCC) (Table 2, Figure 1). No significant negative correlation was found between these factors in these regions. For data illustration purpose, we further calculated correlation coefficient between average beta value within each significant cluster and PD-Q scores (Figure 2).
Table 2.
Brain regions showing significant relationship between PD-Q score and gray matter volume.
| Number of voxels | Cluster level FWE corrected p < 0.05 | Brain region | Coordinate (mm) |
T value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| (Positive correlation [painDETECT and gray matter volume]) | ||||||
| 165 | 0.000 | (L) ACC | −14 | 33 | 20 | 9.66 |
| −14 | 30 | 27 | 7.54 | |||
| −7.5 | 30 | 7.5 | 6.55 | |||
| 259 | 0.001 | (R) ACC | 14 | 33 | 36 | 9.40 |
| 280 | 0.000 | (R) PCC | 9 | −54 | 38 | 9.37 |
| 4.5 | −45 | 39 | 6.42 | |||
| 0 | −42 | 24 | 5.82 | |||
FWE: family wise error; ACC: anterior cingulate cortex; PCC: posterior cingulate cortex; PD-Q: painDETECT Questionnaire
Figure 1.
Gray matter volume in three regions was significantly correlated with painDETECT score. ACC: anterior cingulate cortex; PCC: posterior cingulate cortex.
Figure 2.
Correlation coefficient between average beta value within each significant cluster and PD-Q scores. ACC: anterior cingulate cortex; PCC: posterior cingulate cortex; PD-Q: painDETECT Questionnaire.
Discussion
Neuropathic characteristics of participants
In this study, the PD-Q score was 21.5 (median score; interquartile range, 19.0 to 26.3). PD-Q is a screening questionnaire that determines the prevalence of neuropathic characteristics. A high score represents strong neuropathic characteristics.10 Considering that a score ≥ 19 indicates a high possibility of neuropathic characteristics in PD-Q, most of the patients in this study are considered to have strong neuropathic characteristics.
Effect of neuropathic characteristics on chronic pain
Neuropathic characteristics and the severity of chronic pain are closely related. For example, patients with neuropathic characteristics show higher pain intensity and longer pain duration than those without neuropathic characteristics in chronic low back pain,13 fibromyalgia,14 and general chronic pain.15–17 Furthermore, the levels of psychological aspects such as anxiety and depression, as well as pain intensity, were much higher in patients with neuropathic characteristics in chronic pain associated with diabetes mellitus18 and in general chronic pain patients.19 Rasmussen et al.20 showed that general chronic pain and putative neuropathic pain have overlapping neuropathic characteristics. Although the neuropathic characteristics in chronic pain do not always involve neuroanatomical regions and may include psychogenic factors or somatization, these characteristics may be related to the severity of chronic pain. Considering that chronic pain is related to brain morphological changes and neuropathic characteristics are related to the severity of chronic pain, neuropathic characteristics may also be related to brain morphological changes with worsening chronic pain.
Brain regions related to neuropathic characteristics
In this study, we found positive correlations between PD-Q score and gray matter volume in the bilateral ACC and right posterior cingulate cortex by the analysis throughout the brain. Here, we discuss the impact of neuropathic characteristics on the development of chronic pain and the role of these brain regions that show significant positive correlations with neuropathic characteristics.
As we hypothesized, we found significant positive correlations between the volume of ACC and the scores of PD-Q. Findings of functional neuroimaging using recent techniques indicate that ACC is involved in cognition and emotions21; thus, ACC is a key node for the affective pain matrix.22 It was reported that the analgesic effect of hypnosis and placebo treatment is associated with ACC activity.23 Salomons et al.24 showed that the ACC activity was reduced by the manipulation of subjects’ belief that they could control subsequent painful stimulus, although they actually received the same painful stimulus despite their control. These studies suggest that ACC is involved in pain modulation via the cognition of pain. Furthermore, in chronic pain patients as well as healthy subjects, ACC is activated by painful stimulation although the activated area is smaller in chronic pain patients.25 In our study, the gray matter volume and the neuropathic characteristics positively correlated in ACC. We speculate that cognition and emotions are induced by sudden pain attacks, and typical neuropathic characteristics such as burning pain sensation and allodynia might have activated the ACC and consequently increased the gray matter volume in chronic pain patients.
PCC is considered to regulate attention and cognition.26 In a study of healthy volunteers, only PCC showed a higher activity during the anticipation period for noxious pain than during the noxious pain period, although several other brain regions were activated during both periods.27 The patients with a focal region in PCC showed impairment in the performance of multiple tasks that require cognitive ability.28 These studies support the idea that the role of PCC is related to attention and cognition. Meerwijk et al.29 in their review stated that PCC is activated in 31.6% of the studies of psychological pain such as current psychological pain and recalled grief or sadness,29 whereas Apkarian et al.30 found that it is involved in only about 9% of physical pain studies. Thus, attention and cognition, rather than noxious pain itself, activated PCC.
Morphological alterations in these brain regions in chronic pain patients are also shown in other VBM studies of ACC31–35 and PCC.36 These studies also support the idea that these brain regions are related to the processing of chronic pain. The above-mentioned studies indicate that these brain regions interact with each other during pain experience, that is, cognition of and attention to pain.37 Considering that chronic pain involves complex interactions among physical, emotional, cognitive, and social aspects38 and that neuropathic characteristics are related to the severity of chronic pain, it is reasonable to consider that the brain structure is altered in these brain regions. Our findings suggest that chronic pain with neuropathic characteristics activated these brain regions and might have increased gray matter volume.
Alteration of gray matter volume and its mechanisms
We also discuss the gray matter volume alteration and its mechanisms. Although gray matter volume alteration is observed in many chronic pain diseases,34,39–42 its mechanism still remains unclear. Gray matter volume alteration is presumed to occur thorough the change in cell size, neuronal and glial cell genesis, and angiogenesis.43 A rat neuropathic pain model showed longer dendrites with more branches in neurons than the sham-operated rats.44 Stressed mice showed decrease gray matter volume with loss of synaptic spine density of dendrites.45 These studies indicate that gray matter volume alteration relies on activity-dependent dendritic changes.46 In this study, gray matter volume positively correlated with the scores of neuropathic characteristics. Considering the candidate mechanism of gray matter volume alteration above, more severe neuropathic characteristics might have induced higher activities in those brain regions and subsequently increased gray matter volume.
Borsook et al.46 proposed adaptive and maladaptive changes as the brain responses to pain. Adaptive brain responses increase activity-dependent dendritic complexity and maladaptive brain responses decrease dendritic complexity. Several studies indicate that a reduced gray matter volume is reversed to levels seen in healthy states in a relatively short period, for instance, after arthroplasty in painful osteoarthritis patients and improvement of headache in whiplash injury.47–49 These outcomes might have arisen from maladaptive (for reduced volume) and adaptive (for reversed volume) responses. In our study, gray matter volume in brain regions related to pain modulation positively correlated with more severe neuropathic characteristics. It is assumed that our findings might have been derived from adaptive brain responses to activity-dependent dendritic changes.46
Gray matter volume and pain duration
In the current study, patients with a wide range of pain durations (from 5 months to 27 years) were included. Pain duration and pain intensity could be the factors that affect brain morphological changes.50 In this study, we found no positive or negative correlation between gray matter volume and pain duration, whereas we found a strong positive correlation between gray matter volume and neuropathic characteristics in ACC and PCC. Schmidt-Wilcke et al.51 found a correlation between gray matter volume and pain intensity but not between gray matter volume and pain duration, which is similar to our finding. Taking into account, a previous report that daily repeated painful stimulations altered gray matter volume in one week,52 these pain durations would be long enough for the gray matter volume to alter. These reports support our finding of a strong positive correlation between neuropathic characteristics and gray matter volume, but not between pain duration and gray matter volume.
Limitations
Our study has limitations. First, the small number of subjects, 12 patients, is a limitation of this study because statistical power depends on total sample size. Second, the comparison of gray matter volume between the healthy subjects and patients could not be conducted because we can not distinguish PD-Q effects from subjects’ common effects as the PD-Q scores were all zero. Healthy subjects are expected to score zero in PD-Q, as PD-Q was developed to discriminate between neuropathic and nociceptive pain components in chronic low back pain patients and PD-Q consists of questions that address the quality of neuropathic pain symptoms, the pain patterns, and the existence of radiating pain.10
Conclusion
In this study, we found positive correlations between PD-Q scores and gray matter volume in brain regions related to pain experience. Our findings imply that more severe neuropathic characteristics induce greater activity and subsequently induce morphological alterations in those brain regions. Our study, therefore, indicates the importance of neuropathic characteristics in chronic pain.
Acknowledgments
The authors are very grateful to Mr. Koichi Ujita and Mr. Kazuya Asano for devoted support to this project as radiological technologists.
Authors' Note
This study was registered to UMIN clinical Trial Registry (ID; UMIN000010537, title; Comprehensive strategy for chronic pain, URL; http://www.umin.ac.jp/ctr/).
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Grants-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant Number 25462424).
References
- 1.Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth 2008; 101: 17–24. [DOI] [PubMed] [Google Scholar]
- 2.Dworkin RH, Backonja M, Rowbotham MC, et al. Advances in neuropathic pain. Arch Neurol 2003; 60: 1524–1534. [DOI] [PubMed] [Google Scholar]
- 3.Attal N. Can pain be more or less neuropathic? Pain 2004; 112: 223–224. [DOI] [PubMed] [Google Scholar]
- 4.Attal N, Lanteri-Minet M, Laurent B, et al. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain 2011; 152: 2836–2843. [DOI] [PubMed] [Google Scholar]
- 5.Torrance N, Smith BH, Bennett MI, et al. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain 2006; 7: 281–289. [DOI] [PubMed] [Google Scholar]
- 6.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage 2000; 11: 805–821. [DOI] [PubMed] [Google Scholar]
- 7.May A. Chronic pain may change the structure of the brain. Pain 2008; 137: 7–15. [DOI] [PubMed] [Google Scholar]
- 8.Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 2011; 152: S49–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Q, Inman RD, Davis KD. Neuropathic pain in ankylosing spondylitis: a psychophysics and brain imaging study. Arthritis Rheum 2013; 65: 1494–1503. [DOI] [PubMed] [Google Scholar]
- 10.Freynhagen R, Baron R, Gockel U, et al. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006; 22: 1911–1920. [DOI] [PubMed] [Google Scholar]
- 11.May A. Structural brain imaging: a window into chronic pain. Neurosci 2011; 17: 209–220. [DOI] [PubMed] [Google Scholar]
- 12.Hayasaka S, Phan KL, Liberzon I, et al. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage 2004; 22: 676–687. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt CO, Schweikert B, Wenig CM, et al. Modelling the prevalence and cost of back pain with neuropathic components in the general population. Eur J Pain 2009; 13: 1030–1035. [DOI] [PubMed] [Google Scholar]
- 14.Amris K, Jespersen A, Bliddal H. Self-reported somatosensory symptoms of neuropathic pain in fibromyalgia and chronic widespread pain correlate with tender point count and pressure-pain thresholds. Pain 2010; 151: 664–669. [DOI] [PubMed] [Google Scholar]
- 15.Bouhassira D, Lantéri-Minet M, Attal N, et al. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008; 136: 380–387. [DOI] [PubMed] [Google Scholar]
- 16.Harifi G, Amine M, Ait Ouazar M, et al. Prevalence of chronic pain with neuropathic characteristics in the moroccan general population: a national survey. Pain Med 2013; 14: 287–292. [DOI] [PubMed] [Google Scholar]
- 17.Toth C, Lander J, Wiebe S. The prevalence and impact of chronic pain with neuropathic pain symptoms in the general population. Pain Med 2009; 10: 918–929. [DOI] [PubMed] [Google Scholar]
- 18.Bouhassira D, Letanoux M, Hartemann A. Chronic pain with neuropathic characteristics in diabetic patients: a French cross-sectional study. PLoS One 2013; 8: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaygan M, Böger A, Kröner-Herwig B. Clinical features of chronic pain with neuropathic characteristics: a symptom-based assessment using the painDETECT Questionnaire. Eur J Pain 2013; 17: 1529–1538. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen PV, Sindrup SH, Jensen TS, et al. Symptoms and signs in patients with suspected neuropathic pain. Pain 2004; 110: 461–469. [DOI] [PubMed] [Google Scholar]
- 21.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000; 4: 215–222. [DOI] [PubMed] [Google Scholar]
- 22.Singer T, Seymour B, O’Doherty J, et al. Empathy for pain involves the affective but not sensory components of pain. Science 2004; 303: 1157–1162. [DOI] [PubMed] [Google Scholar]
- 23.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci 2006; 7: 268–277. [DOI] [PubMed] [Google Scholar]
- 24.Salomons T V, Johnstone T, Backonja MM, et al. Perceived controllability modulates the neural response to pain. J Neurosci 2004; 24: 7199–7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buffington ALH, Hanlon CA, McKeown MJ. Acute and persistent pain modulation of attention-related anterior cingulate fMRI activations. Pain 2005; 113: 172–184. [DOI] [PubMed] [Google Scholar]
- 26.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain 2014; 137: 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porro CA., Cettolo V, Francescato MP, et al. Functional activity mapping of the mesial hemispheric wall during anticipation of pain. Neuroimage 2003; 19: 1738–1747. [DOI] [PubMed] [Google Scholar]
- 28.Burgess PW, Veitch E, De Lacy Costello A, et al. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia 2000; 38: 848–863. [DOI] [PubMed] [Google Scholar]
- 29.Meerwijk EL, Ford JM, Weiss SJ. Brain regions associated with psychological pain: implications for a neural network and its relationship to physical pain. Brain Imaging Behav 2013; 7: 1–14. [DOI] [PubMed] [Google Scholar]
- 30.Apkarian V, Bushnell MC, Treede RD, et al. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005; 9: 463–484. [DOI] [PubMed] [Google Scholar]
- 31.Barad MJ, Ueno T, Younger J, et al. Complex regional pain syndrome is associated with structural abnormalities in pain-related regions of the human brain. J Pain 2014; 15: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Draganski B, Moser T, Lummel N, et al. Decrease of thalamic gray matter following limb amputation. Neuroimage 2006; 31: 951–957. [DOI] [PubMed] [Google Scholar]
- 33.Kuchinad A, Schweinhardt P, Seminowicz DA, et al. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci 2007; 27: 4004–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obermann M, Rodriguez-Raecke R, Naegel S, et al. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage 2013; 74: 352–358. [DOI] [PubMed] [Google Scholar]
- 35.Valfrè W, Rainero I, Bergui M, et al. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache 2008; 48: 109–117. [DOI] [PubMed] [Google Scholar]
- 36.Absinta M, Rocca MA, Colombo B, et al. Selective decreased grey matter volume of the pain-matrix network in cluster headache. Cephalalgia 2012; 32: 109–115. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Larrea L, Peyron R. Pain matrices and neuropathic pain matrices: a review. Pain 2013; 154(Suppl. 1): S29–S43. [DOI] [PubMed] [Google Scholar]
- 38.Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. Lancet 2011; 377: 2226–2235. [DOI] [PubMed] [Google Scholar]
- 39.Geha PY, Baliki MN, Harden RN, et al. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron 2008; 60: 570–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ivo R, Nicklas A, Dargel J, et al. Brain structural and psychometric alterations in chronic low back pain. Eur Spine J 2013; 22: 1958–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson ME, Craggs JG, Price DD, et al. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. J Pain 2011; 12: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smallwood RF, Laird AR, Ramage AE, et al. Structural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volume. J Pain 2013; 14: 663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 2012; 15: 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Metz AE, Yau H-J, Centeno MV, et al. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci USA 2009; 106: 2423–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kassem MS, Lagopoulos J, Stait-Gardner T, et al. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol Neurobiol 2013; 47: 645–661. [DOI] [PubMed] [Google Scholar]
- 46.Borsook D, Erpelding N, Becerra L. Losses and gains: chronic pain and altered brain morphology. Expert Rev Neurother 2013; 13: 1221–1234. [DOI] [PubMed] [Google Scholar]
- 47.Gwilym SE, Filippini N, Douaud G, et al. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum 2010; 62: 2930–2940. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Raecke R, Niemeier A, Ihle K, et al. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci 2009; 29: 13746–13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Obermann M, Nebel K, Schumann C, et al. Gray matter changes related to chronic posttraumatic headache. Neurology 2009; 73: 978–983. [DOI] [PubMed] [Google Scholar]
- 50.May A. Experience-dependent structural plasticity in the adult human brain. Trends Cogn Sci 2011; 15: 475–482. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt-Wilcke T, Leinisch E, Gänssbauer S, et al. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain 2006; 125: 89–97. [DOI] [PubMed] [Google Scholar]
- 52.Teutsch S, Herken W, Bingel U, et al. Changes in brain gray matter due to repetitive painful stimulation. Neuroimage 2008; 42: 845–849. [DOI] [PubMed] [Google Scholar]