Abstract
The extracellular signal-regulated kinase is an important protein kinase for cortical plasticity. Long-term potentiation in the anterior cingulate cortex is believed to play important roles in chronic pain, fear, and anxiety. Previous studies of extracellular signal-regulated kinase are mainly focused on postsynaptic form of long-term potentiation (post-long-term potentiation). Little is known about the relationship between extracellular signal-regulated kinase and presynaptic long-term potentiation (pre-long-term potentiation) in cortical synapses. In this study, we examined whether pre-long-term potentiation in the anterior cingulate cortex requires the activation of presynaptic extracellular signal-regulated kinase. We found that p42/p44 mitogen-activated protein kinase inhibitors, PD98059 and U0126, suppressed the induction of pre-long-term potentiation. By contrast, these inhibitors did not affect the maintenance of pre-long-term potentiation. Using pharmacological inhibitors, we found that pre-long-term potentiation recorded for 1 h did not require transcriptional or translational processes. Our results strongly indicate that the activation of presynaptic extracellular signal-regulated kinase is required for the induction of pre-long-term potentiation, and this involvement may explain the contribution of extracellular signal-regulated kinase to mood disorders.
Keywords: LTP, anterior cingulate cortex, ERK, mouse, presynaptic release
Background
Extracellular signal-regulated kinase (ERK) is one of mitogen-activated protein kinase (MAPK) family of serine/threonine protein kinases that transduce extracellular signals from cell surface receptors to the cell nucleus1,2 and is widely expressed in the central nervous system. ERK pathway associates with a variety of cellular activities including cell proliferation, differentiation, survival, and functional plasticity of neurons.3 ERK pathway is also known as one of several common targets of mood stabilizers. Actually, previous studies reported that mood stabilizers, lithium, and valproate increased the activated forms of ERK1/2 and phosphorylation of ERK pathway targets in rat prefrontal cortex and hippocampus and in cultured neuronal cells.3,4 In anterior cingulate cortex (ACC), ERK pathway has been reported to contribute to behavioral excitement and hedonic activity.5
Among several cortical regions, ACC is an important area for pain perception, chronic pain, and emotional disorders.6–12 Two major forms of LTP have been reported in ACC synapses: NMDA receptor-dependent postsynaptic LTP (post-LTP) and NMDA receptor-independent presynaptic LTP (pre-LTP).13,14 Especially, pre-LTP is thought to be related to behavioral anxiety, and post-LTP is related to chronic pain. In previous studies, it has been reported that activation of MAPK including ERK is critical for the induction of post-LTP in the ACC.15 There is no report of the involvement of ERK in ACC pre-LTP. However, several previous reports suggest the possible roles of ERKs in presynaptic regulation or plasticity. For example, in Aplysia, Serotonin (5-HT) gates long-term presynaptic plasticity through presynaptic regulation of MAPK/ERK.16–18 In hippocampus, presynaptic H-ras/ERK/synapsin I signaling pathway adjusts the presynaptic plasticity.19
In the present study, we performed whole-cell patch-clamp recording from cingulate pyramidal neurons of adult mice and investigated the role of ERK for pre-LTP in the ACC. We showed that the induction of pre-LTP in ACC was blocked by the MAPK and ERK kinase (MEK) inhibitors. By contrast, we found that the MEK inhibitors did not affect the maintenance of ACC pre-LTP. These results suggest that the activation of MAPK including ERK is critical for the induction of pre-LTP in the ACC and indicate that ERK inhibitors may affect mood-related behaviors by inhibiting pre-LTP in the ACC.
Methods
Animals and slice preparation
C57BL/6 mice (6–14 weeks old) were anesthetized with isoflurane, and coronal brain slices (300 µm) containing the ACC were prepared using our previous methods.13,20–22 Slices were transferred to a submerged recovery chamber with oxygenated (95% O2 and 5% CO2) artificial cerebrospinal fluid (ACSF) containing (in mM) 124 NaCl, 2.5 KCl, 2 CaCl2, 1 MgSO4, 25 NaHCO3, 1 NaH2PO4, and 10 glucose at room temperature for at least 1 h. The Animal Care and Use Committee of University of Toronto approved the mouse protocols.
Whole-cell patch-clamp recording
Experiments were performed in a recording chamber on the stage of an Axioskop 2FS microscope with infrared DIC optics for visualization of whole-cell patch-clamp recording. Neurons of the ACC in the layers II, III, and V receive afferent input from the thalamus.23 In the present study, EPSCs were recorded from the layer II/III neurons with an Axopatch 200B amplifier (Molecular Devices, CA), and the stimulations were delivered by a bipolar tungsten stimulating electrode placed in the layer V of the ACC slices.20–23 The recording pipettes (3–5 MΩ) were filled with solution containing (mM) 145 K-gluconate, 5 NaCl, 1 MgCl2, 0.2 EGTA, 10 HEPES, 2 Mg-ATP, and 0.1 Na3-GTP (adjusted to pH7.2 with KOH). In most of the experiments, picrotoxin (100 μM) was used to block the γ-aminobutyric acid (A) (GABAA) receptor-mediated inhibitory currents. In some experiments, LTP was induced in the absence of picrotoxin. Access resistance was 15–30 MΩ and was monitored throughout the experiment.
Pharmacological inhibition
PD98059 and U0126 were obtained from Hello Bio (Bristol, UK). Actinomycin-D and aminomycin were obtained from Sigma-Aldrich (St. Louis, MO). These drugs were dissolved in dimethyl sulfoxide (DMSO) and diluted more than 1000-fold to give a final concentration in ACSF. The diluted DMSO in ACSF had no effect on synaptic transmission and plasticity.
Data analysis
Data were collected and analyzed using pClamp 9.2 software (Axon Instruments). Data were discarded if access resistance changed more than 15% during an experiment. The change in frequency and amplitude of postsynaptic currents following the application of drugs was analyzed using the Mini Analysis Program 6.0 (Synaptosoft, Decatur, GA). Statistical comparisons were performed using the Student’s t-test, one-way analysis of variance (ANOVA), or two-way ANOVA. The Kolmogorov–Smirnov test was used to compare the cumulative distributions of the postsynaptic current parameters in the absence and presence of the test drugs. The level of significance was set at p < 0.05.
Results
MAP kinase activity is required for cingulate pre-LTP
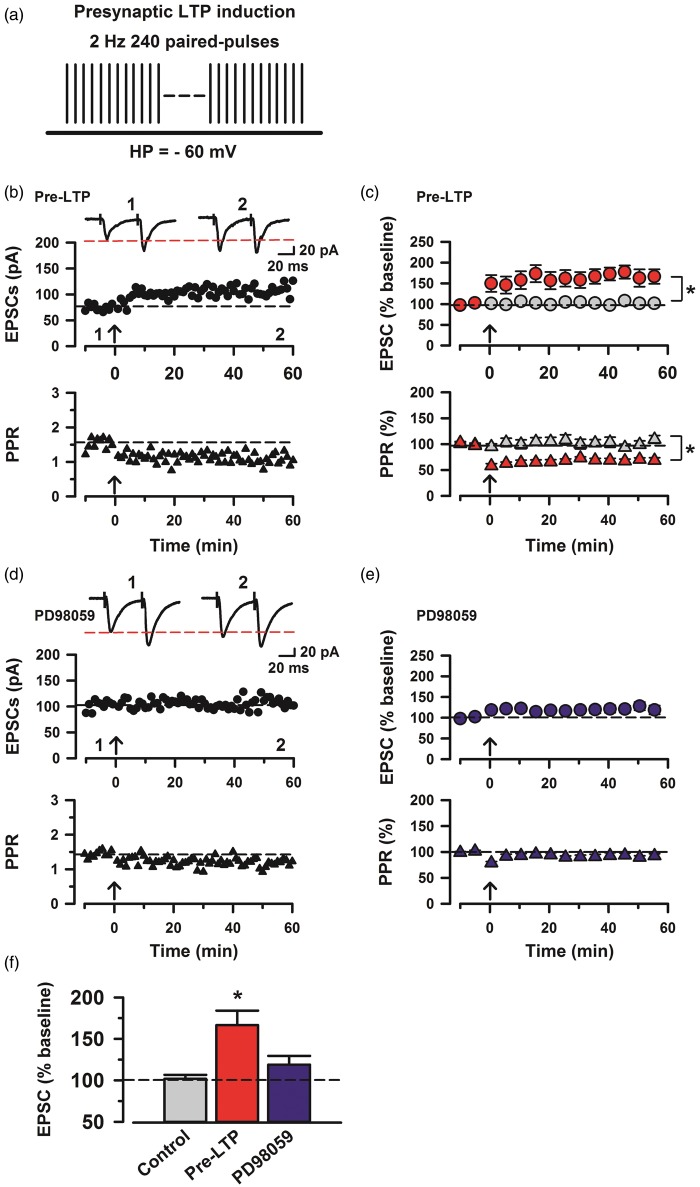
A previous study demonstrates that LTP at synapses onto ACC pyramidal cells is postsynaptically induced and depends on the activity of ERK.15 To investigate the effects of MAP kinase inhibition on pre-LTP in ACC synapses, we employed a stimulating protocol for inducing pre-LTP in the ACC.13 We recorded evoked EPSCs (eEPSCs) in pyramidal neurons of layer II/III at a holding potential of −60 mV by applying local stimulation in layer V/VI in the presence of a GABAA receptor antagonist picrotoxin (100 µM) (Figure 1(b)). After achieving a stable baseline recording in response to paired-pulse stimulation (interpulse interval of 50 ms) for at least 10 min, we then applied low-frequency paired-pulse stimulation (2 Hz for 2 min) at a holding potential of −60 mV (Figure 1(a)). Similar to previous reports,13,14 we found that this stimulation robustly increased the amplitude of eEPSCs in ACC neurons and that the LTP lasted for at least 1 h (mean 164.7% ± 12.5% of baseline, n = 7; Figure 1(c) and (f)). In contrast, control neurons, which did not receive the pre-LTP induction protocol, showed no change in the amplitude of eEPSCs (mean 101.7% ± 4.8%, n = 6; Figure 1(c) and (f)). The low-frequency stimulation and control groups were significantly different (two-way ANOVA, F1,22 = 19.1, p < 0.05; Figure 1(c)). In addition, the potentiation induced by the stimulation was associated with a reduction in the paired-pulse ratio (PPR) (Figure 1(b)). In contrast, control neurons, which did not receive the LTP induction protocol, showed no change in the PPR (109.3% ± 7.4%; Figure 1(c)). The low-frequency stimulation statistically altered the PPR (68.6% ± 5.1% of baseline, two-way ANOVA, F1,22 = 36.33, p < 0.05; Figure 1(c)).
Figure 1.
Induction of cingulate pre-LTP requires MAP kinase activity. (a) A scheme illustrating the pre-LTP induction protocol consisting of low-frequency paired-pulse stimulation (2 Hz for 2 min) at a holding potential of −60 mV. (b) Top: sample traces of eEPSCs with paired-pulse stimulation at 50 ms interstimulus interval during baseline (1) and 60 min after the pre-LTP (2) at a holding membrane potential of −60 mV. Middle: a time course plot of a representative single example. Bottom: time course plot of PPR for this neuron. The arrow donates the time of pre-LTP induction. (c) Pooled data to illustrate the time course of pre-LTP and changes in PPR. Top: pre-LTP (red, n = 7 neurons/5 mice) and control (grey, n = 6/4). Bottom: PPR for these neurons. *p < 0.05 for PPR change estimated for the 10 min intervals baseline and between 50 min and 60 min after the induction stimulation. (d) Top: a time course plot of a representative single example with application of ERK inhibitor, PD98059 (100 µM). Bottom: time course plot of PPR for this neuron. (e) PD98059 (blue, n = 10/6) in the bath solution blocks pre-LTP induction. (f) Summary of the effects of an ERK inhibitor, PD98059 on pre-LTP. There was statistical difference when comparing control with pre-LTP, PD98059 groups (one-way ANOVA, F2,43 = 12.03, *p < 0.05). The mean amplitudes of eEPSCs were determined at 50–60 min after the pre-LTP induction stimulation. Error bars represent SEM.
Next, we tested whether pre-LTP induced by low-frequency stimulation (2 Hz for 2 min) is prevented by bath application of a MAPK inhibitor, PD98059 (100 µM).15,24 First, we applied PD98059 into bath solution before low-frequency stimulation. We found that PD98059 blocked pre-LTP induced by low-frequency stimulation in ACC neurons (mean 118.9% ± 10.6% of baseline, n = 10, one-way ANOVA, F2,43 = 12.03, p < 0.05, PD98059 group versus pre-LTP group; Figure 1(f)). In addition, the reduction in PPR was also blocked (Figure 1(d)). The PD98059 application groups statistically altered the PPR compared with the pre-LTP group (92.3% ± 4.1% of baseline, two-way ANOVA, F1,30 = 27.87, p < 0.05, PD98059 group versus pre-LTP group; Figure 1(e)).
Effect of MAP kinase on the maintenance of pre-LTP
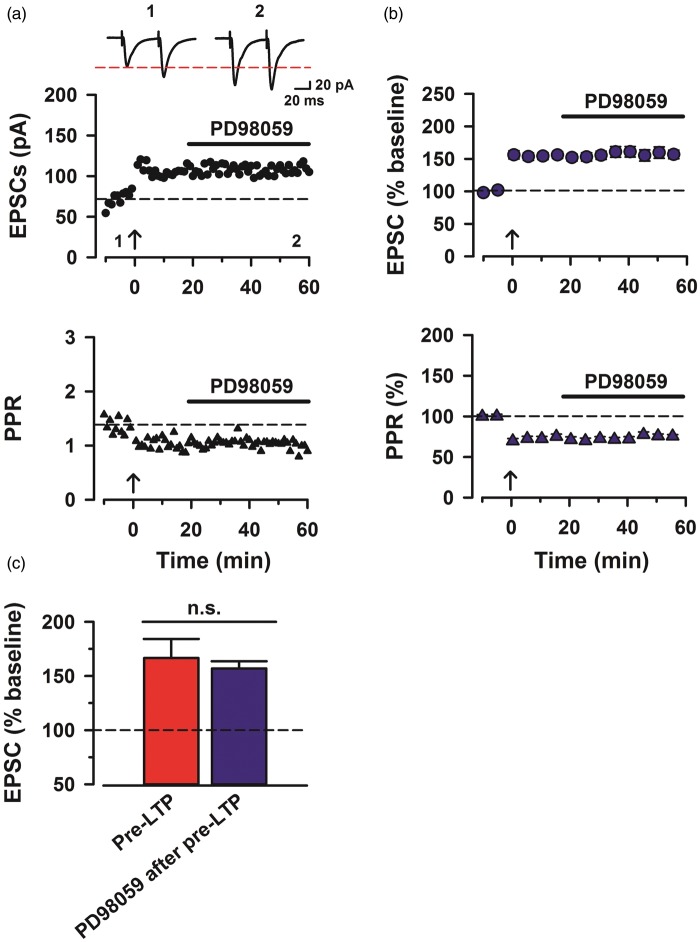
To test whether MAPK activity is required for the maintenance of pre-LTP, we applied PD98059 (100 µM) into bath solution after the induction (Figure 2(a)). Interestingly, we found that PD98059 did not affect the maintenance of pre-LTP in ACC neurons (156.9% ± 6.7% of baseline, n = 8, one-way ANOVA, F1,28 = 0.24, p > 0.05, NS; Figure 2(b) and (c)). In addition, the reduction in PPR did not change by PD98059 (75.6% ± 1.9% of baseline; Figure 2(b)). There is no significant difference between PD98059-treated group and pre-LTP control (75.8% ± 2.2% of baseline, two-way ANOVA, F1,26 = 3.60, p > 0.05, NS; Figure 2(b)). These results indicate that ERK activity is not required for the maintenance of pre-LTP in ACC.
Figure 2.
MAP kinase activity is not required for the maintenance of pre-LTP. (a) Bath application of PD98059 (100 µM) did not affect the maintenance of pre-LTP. Top: sample traces of eEPSCs with paired-pulse stimulation at 50 ms interstimulus interval during baseline (1) and 60 min after the pre-LTP (2) at a holding membrane potential of −60 mV. Middle: a time course plot of a representative single example. Bottom: time course plot of PPR for this neuron. The arrow donates the time of pre-LTP induction. (b) Pooled data to illustrate the time course of pre-LTP and changes in PPR with application of PD98059 after pre-LTP induction stimulation. Top: pre-LTP of PD98059 (n = 8/4) after the stimulation. Bottom: PPR for these neurons. (c) Summary of the effects of pre-LTP and PD98059 after pre-LTP induction stimulation. The amplitudes of eEPSCs were not significantly different between pre-LTP and PD98059 after pre-LTP (one-way ANOVA, F1,28 = 0.238, p > 0.05, NS). The mean amplitudes of eEPSCs were determined at 50–60 min after the pre-LTP induction stimulation. Error bars represent SEM.
Effects of another MEK inhibitor
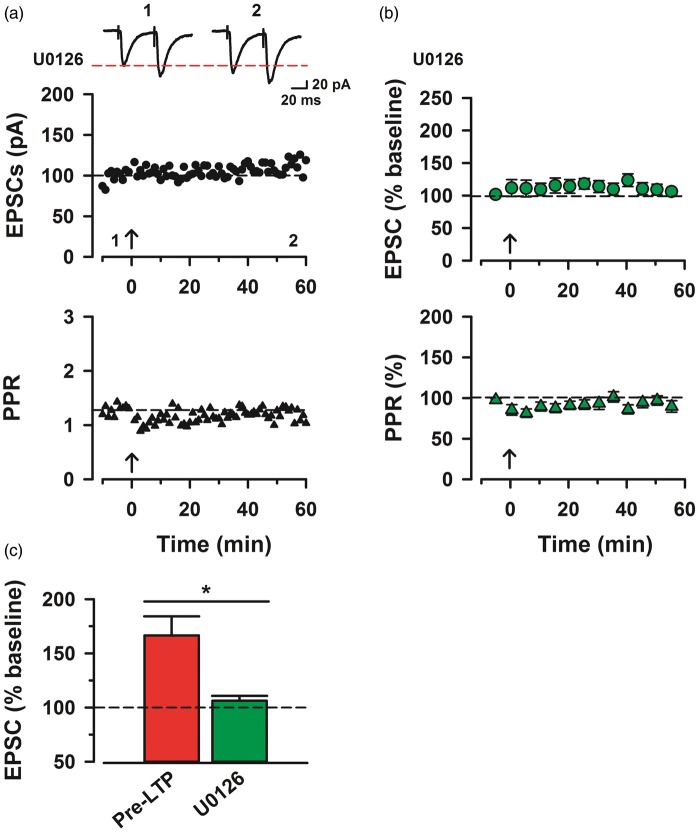
We also used another MEK inhibitor U0126 (100 μM) to investigate whether pre-LTP can be prevented.25 Bath application of U0126 blocked the induction of pre-LTP and the reduction in PPR generated by low-frequency stimulation in ACC neurons (106.2% ± 4.5% of baseline, n = 8, one-way ANOVA, F1,32 = 11.93, p < 0.05; Figure 3(b) and (c)). In addition, the reduction in PPR was also blocked by U0126 (Figure 3(a)). There is significant difference between U0126 application group and pre-LTP control group (89.6% ± 7.1% of baseline, two-way ANOVA, F1,30 = 17.0, p < 0.05; Figure 3(b)).
Figure 3.
U0126, another MAP kinase inhibitor also blocked cingulate pre-LTP. (a) Top: a time course plot of a representative single example with application of MEK inhibitor, U0126 (100 µM). Bottom: a time course plot of PPR for this neuron. (b) Pooled data to illustrate the time course of pre-LTP and changes in PPR with application of U0126. U0126 (n = 10/5) in the bath solution blocks pre-LTP induction. (c) Summary of the effects of an MEK inhibitor, U0126 on pre-LTP. There was statistical difference when comparing pre-LTP with U0126 group (one-way ANOVA, F1,32 = 11.93, *p < 0.05). The mean amplitudes of eEPSCs were determined at 50–60 min after the pre-LTP induction stimulation. Error bars represent SEM.
Effects of transcriptional and translational inhibitors
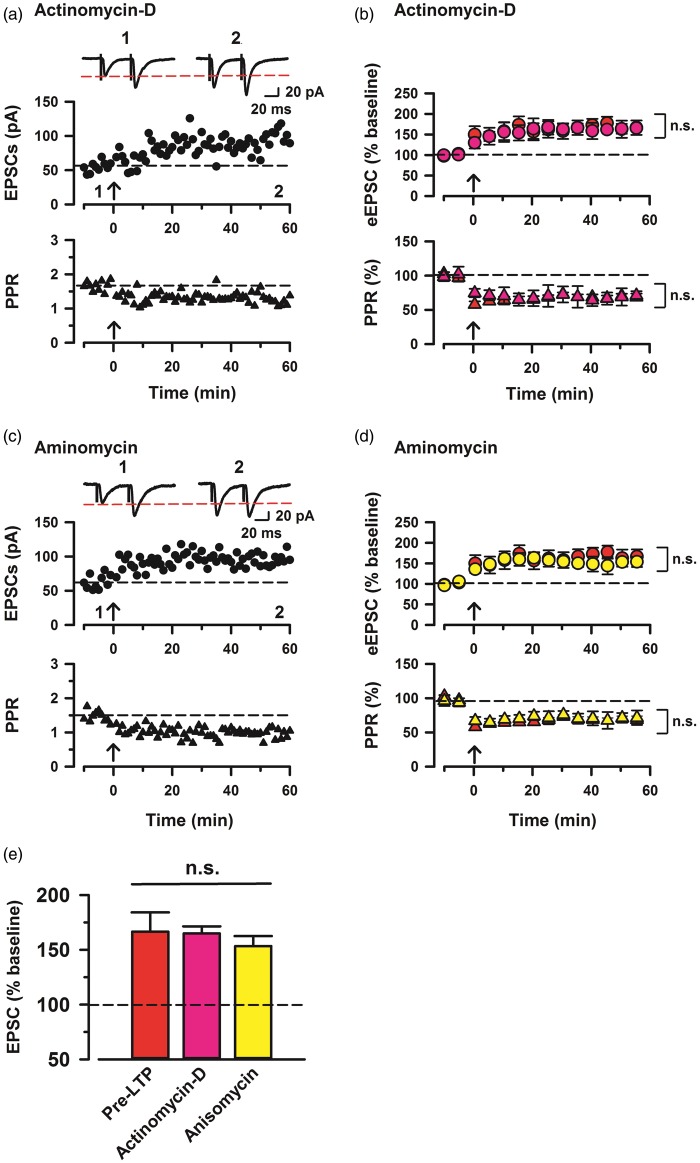
Post-LTP in ACC is reported to be sensitive to the inhibition of protein synthesis.26 Therefore, to investigate whether protein synthesis also is involved in pre-LTP, we tested the effects of transcription and translation inhibitors. First, we investigated if actinomycin-D, a transcription inhibitor, affects synaptic potentiation in ACC neurons induced by the paired training. Actinomycin-D was used at a concentration of 40 μM, a dose that reduces uridine incorpolation into RNA by 77%.27 In ACC slices pretreated with 40 μM actinomycin-D for 30 min, pre-LTP induced by low-frequency stimulation was not affected as compared with pre-LTP group (164.9% ± 6.4% of baseline, n = 11; Figures 4(a), (b), and (e)). In addition, there was no significant difference of the PPR between actinomycin-D and pre-LTP (68.4% ± 4.0% of baseline, two-way ANOVA, F1,32 = 0.06, NS; Figure 4(b)). We also investigated the effects of a translation inhibitor, anisomycin, on presynaptic potentiation in the ACC. Anisomycin was used at a concentration of 20 μM, which inhibited the maintenance phase of hippocampal LTP.28,29 In ACC slices pretreated with 20 μM anisomycin, pre-LTP was not affected as compared with control (153.4 ± 10.3% of base line, n = 9; Figure 4(c) to (e)). Also, there is no significant difference of the PPR between aminomycin and pre-LTP, similar to the results obtained in the presence of actinomycin-D (68.5% ± 4.1% of baseline, two-way ANOVA, F1,28 = 0.03, NS; Figure 4(d)). These results suggest that protein synthesis is not required for presynaptic potentiation in the ACC neurons.
Figure 4.
Enhanced presynaptic potentiation in the ACC neurons mediated by transcription and translation activities. (a) Top: a time course plot of a representative single example with application of a transcription inhibitor, actinomycin-D (40 µM). Bottom: a time course plot of PPR for this neuron. (b) Pooled data to illustrate the time course of pre-LTP and changes in PPR with application of actinomycin. Top: actinomycin-D (pink, n = 11 neurons/7 mice) and pre-LTP (red, n = 7/5). Bottom: PPR for these neurons. (c) Top: a time course plot of a representative single example with application of a translation inhibitor, anisomycin (20 µM). Bottom: a time course plot of PPR for this neuron. (d) Pooled data to illustrate the time course of pre-LTP and changes in PPR with application of anisomycin. Top: anisomycin (yellow, n = 9/6) and pre-LTP. Bottom: PPR for these neurons. (e) Summary of the effects of pre-LTP, actinomycin-D, and anisomycin after pre-LTP induction stimulation. The amplitudes of eEPSCs were not significantly different between pre-LTP, actinomycin-D, and anisomycin (one-way ANOVA, F2,51 = 0.548, p > 0.05, NS). The mean amplitudes of eEPSCs were determined at 50–60 min after the pre-LTP induction stimulation. Error bars represent SEM.
Effects of MAP kinases on baseline paired-pulse facilitation (PPF) and spontaneous EPSCs
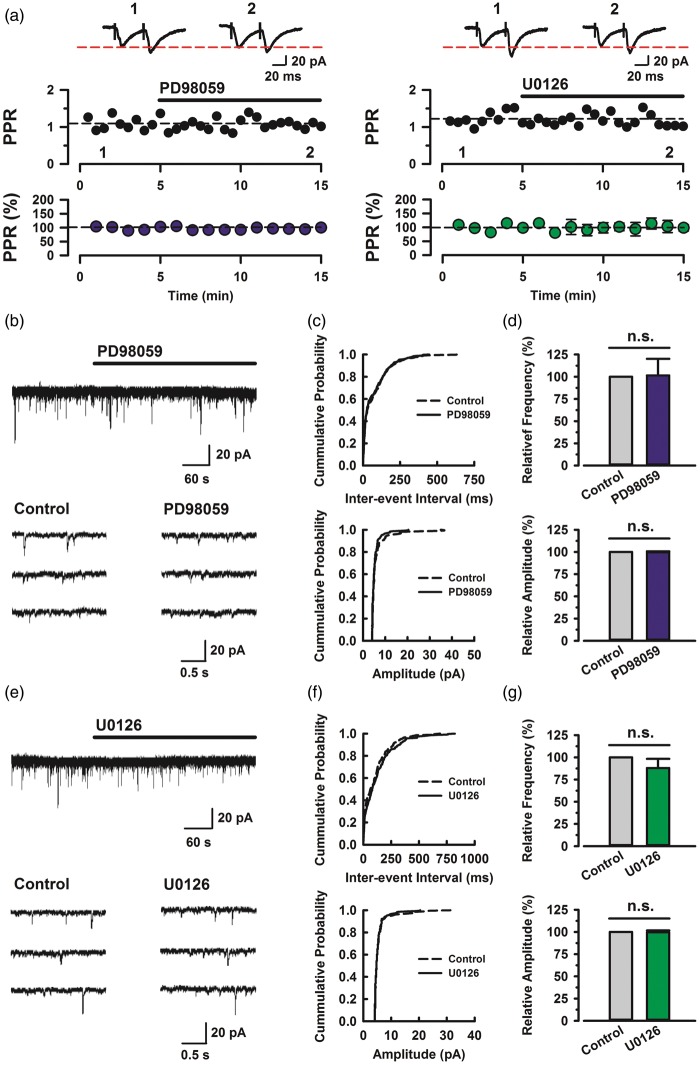
To examine the effect of PD98059 and U0126 on PPF, a simple form of synaptic plasticity, we applied PD98059 (100 µM) or U0126 (100 µM) into bath solution. Both PD98059 and U0126 did not affect PPF (PD98059: n = 5, U0126: n = 5; Figure 5(a)). These results suggest that the MEK inhibitors had no effect on basal synaptic transmissions in ACC synapses. To assess the impact of MAPK on spontaneous excitatory synaptic transmission, we applied PD98059 (100 μM) or U0126 (100 μM) into bath solution. Application of PD98059 resulted in no effect on the frequency and amplitude of spontaneous EPSCs (sEPSCs) (Figure 5(b)). Figure 5(c) demonstrates the effect of PD98059 on the cumulative distribution of the inter-event interval and amplitude of sEPSCs. PD98059 did not affect the proportion of inter-event interval (p > 0.05) and amplitude (p > 0.05) when compared with the control (same neuron as in Figure 5(b)). When measured for 5 min in the presence of PD98059, frequency and amplitude were no significant difference with control (101.3 ± 18.7% and 99.5 ± 1.2% (n = 6), respectively; Figure 5(d)). Similarly, U0126 also did not affect sEPSCs and the proportion of inter-event interval (p > 0.05) and amplitude (p > 0.05) when compared with the control (same neuron as in Figure 4(e)) (Figure 5(e) and (f)). When measured for 5 min in the presence of PD98059, frequency and amplitude were no significant difference with control (87.9 ± 10.4% and 99.6 ± 2.0% (n = 6), respectively; Figure 5(g)). These results suggest that the ERK inhibitors have no effect on the sEPSCs in ACC.
Figure 5.
Effects of MAP kinases on baseline PPF and spontaneous EPSCs. (a) Map kinase did not affect the baseline PPF. Top: sample traces of eEPSCs with paired-pulse stimulation at 50 ms interstimulus interval during baseline (1) and 10 min after the application of PD98059 (left, 100 µM) and U0126 (left, 100 µM) (2) at a holding membrane potential of −60 mV. Middle: a time course plot of PPR for a single example neuron. Bottom: pooled data to illustrate the time course of changes in PPR with application of PD98059 (left, n = 5/3) and U0126 (right, n = 5/3). (b) Top: continuous chart recording of spontaneous EPSCs before and during the action of PD98059 (100 µM). Three consecutive traces of EPSCs are shown in an expanded scale in time, before (bottom left) and under the action of PD98059 (bottom right). (c) Cummulative distribution of the inter-event interval (left) and amplitude (right) of EPSCs in control (dotted line) and during (continuous line) the action of PD98059 (same neuron as in Figure 4(b)). (d) Summary of EPSC frequency (left) and amplitude (right) under the action of PD98059 (n = 6/4) relative to control. Both the frequency and amplitude of EPSC were not significantly different between control and PD98059 (*p > 0.05, NS). (e) Top: continuous chart recording of spontaneous EPSCs before and during the action of U0126 (100 µM). Three consecutive traces of EPSCs are shown in an expanded scale in time, before (bottom left) and under the action of U0126 (bottom right). (f) Cummulative distribution of the inter-event interval (left) and amplitude (right) of EPSCs in control (dotted line) and during (continuous line) the action of U0126 (same neuron as in Figure 4(e)). (g) Summary of EPSC frequency (left) and amplitude (right) under the action of U0126 (n = 6/3) relative to control. Both The frequency and amplitude of EPSC were not significantly different between control and U0126 (*p > 0.05, NS). Error bars represent SEM.
Presynaptic glutamate release is altered in ACC during training
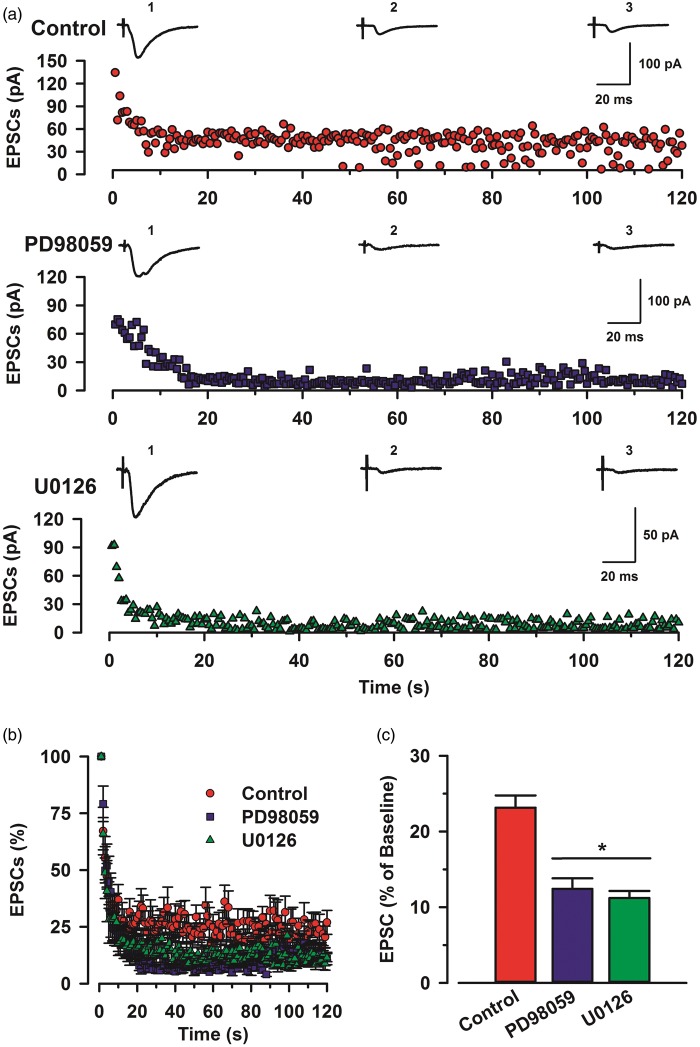
To investigate whether the readily releasable pool of glutamate vesicles would be affected by ERK inhibitors, we applied the low-frequency stimulation which induces pre-LTP (Figure 6(a)). This stimulation protocol, which causes a continuous decline in neurotransmitter release, resulted in a rapid and pronounced activity-dependent depression. Interestingly, glutamate releases of PD98059 group (n = 6) and U0126 (n = 6) group significantly decreased less than control group (n = 6) (one-way ANOVA, F2,177 = 12.53, p < 0.05; Figure 6(b) and (c)). This result suggests that ERK affects glutamate release mechanism in the ACC, in addition to its contribution to the induction of pre-LTP in ACC.
Figure 6.
Effects of MAP kinase inhibitor on eEPSCs during training. (a) Sample traces of eEPSCs during stimulation among control, PD98059, and U0126 at a holding membrane potential of −60 mV. Average amplitude of 0–10 s (1), 50–60 s (2), and 110–120 s (3). Top: a time course plot of a representative single example of control. Middle: a time course plot of a representative single example of PD98059 (100 µM). Bottom: a time course plot of a representative single example of U0126 (100 µM). (b) Pooled data to illustrate the time course of amplitude during pre-LTP induction stimulation. (c) Summary of the effects of ERK inhibitors, PD98059 and U0126 at last 10 s of repetitive stimulation. There was statistical difference when comparing control with PD98059 groups, U0126 (one-way ANOVA, F2,177 = 12.53, *p < 0.05). Error bars represent SEM.
Discussion
In the present study, we demonstrate that ERK activity is required for the induction of pre-LTP in the ACC. Interestingly, inhibiting ERK activity affects synaptic responses to repetitive stimulation, suggesting possible presynaptic involvement of ERK. By contrast, the maintenance of pre-LTP does not require the ERK activity. Inhibiting ERK did not affect the spontaneous glutamate releases and baseline PPF induced by paired-pulse stimulation. Together with previous report of ERK requirement for post-LTP, our results suggest that ERK play important roles in both pre- and post-LTP in ACC. Unlike post-LTP, we found that protein synthesis is not required for pre-LTP, at least for the period time we recorded (1 h). We cannot rule out the possibility that late-phase pre-LTP (more than 3 h) may be sensitive to inhibition of ERK and protein synthesis.
The molecular mechanism of pre-LTP in the ACC
The current study provides strong evidence that pre-LTP in ACC of adult mice requires ERK activity. Our recent study has reported low-frequency stimulation (2 Hz for 2 min) induced LTP presynaptically.13 The induction of ACC pre-LTP is independent of NMDARs. Furthermore, postsynaptic G protein-coupled receptors, postsynaptic Ca2+ influx, metabotropic glutamate receptors, GABABRs, and protein kinase M zeta (PKMζ) that are important for the induction of post-LTP in ACC are not required for pre-LTP.13 For pre-LTP, activation of GluK-1 containing Kainate receptors (KARs) and calcium-stimulated AC1 are necessary.13 One key protein kinase involved in pre-LTP is cAMP-dependent protein kinase (PKA). It has been reported that PKA is required for long-lasting increases in transmitter release at many cortical synapses30–33 as well as in ACC.13 In addition, it has been reported that presynaptic MAPK is activated by cAMP independently of PKA.34,35 In rat prefrontal cortex neurons, cAMP activates ERK signaling pathways to induce presynaptic potentiation.36 Our current results provide new roles for MAPK in ACC pre-LTP. One possible downstream protein is synapsin I. Activation of ERK was reported to facilitate glutamate release from synaptosomes in rat brain by phosphorylating the synaptic vesicle membrane protein synapsin I, thereby regulating its interaction with the actin cytoskeleton, leading to the recruitment of releasable synaptic vesicles from a distal pool.37 In the present study, PD98059 (ERK inhibitor) and U0126 (MEK inhibitor) inhibited pre-LTP in ACC, indicating that activation of MAPK/ERK may contribute to enhanced release of glutamate in ACC pre-LTP.
ERK is not required for the maintenance of pre-LTP
We previously reported that hyperpolarization-activated cyclic nucleotide-gated (HCN) channels were important for the maintenance of pre-LTP in ACC.13 On the other hand, previous Aplysia studies have reported that maintenance of memory-related long-term facilitation of presynapses requires upregulation and prion-like activation of CPEB, a synaptic translational regulator through MAPK/ERK signaling.16 Therefore, in the present study, we tested whether MAP/ERK signaling cascade is also related to the maintenance in addition to HCN channels and found that the maintenance of cingulate pre-LTP was not affected by both PD98059 and U0126. This suggests that the MAPK/ERK signaling cascade is not persistently activated during pre-LTP in the ACC.
Glutamate releasable vesicles are increased by ERK
Presynaptic vesicle mobilization is a complex phenomenon that is regulated by a number of protein kinases. One of most important kinase is MAPK, highly expressed at the presynaptic terminal.38,39 Previous studies have demonstrated that MAPK can increase releasable vesicles and induce glutamate exocytosis by phosphorylation of synapsin I which is a major substrate for MAPK and a presynaptic protein regulating the vesicle cycle and neurotransmitter release.38,40 Although under the inactive condition, synapsin I anchors synaptic vesicles to cytoskeletal elements, once phosphorylated by MAPK, it dissociates from synaptic vesicles and increases more releasable vesicles at presynaptic active zone for neurotransmitter release.38,41 In this study, we showed that ERK/MEK inhibitors did not affect baseline PPF and spontaneous EPSC in the ACC neurons under resting condition. This means that these kinase can be activated by a condition such a low-frequency stimulation and then enhance glutamate releases on presynaptic neurons.
Physiological and pathological significance
ACC is known to play an important role as the higher brain function in chronic pain, fear, and anxiety.6–12 Previous behavioral studies show that in ACC ERK signaling pathway contributes to behavioral excitement and hedonic activity.5 Since pre-LTP has been shown to contribute to behavioral anxiety, especially chronic pain-related anxiety,13 the present study provides a possible explanation that ERK may contribute to mood control by triggering pre-LTP in ACC pyramidal cells. Furthermore, for pain perception and pain unpleasantness, there are strong evidence that ERK activity in the ACC are critical in addition to spinal cord dorsal horn ERK activity.42 It has been reported that ERK signaling pathway is activated in the ACC after peripheral tissue or nerve injury.43 Furthermore, mechanical allodynia significantly activated ERK activity at synaptic sites at two weeks after the injury, suggesting that ACC activation of ERK may contribute to both induction and expression of chronic pain. Activation of ACC ERK pathway has also been reported in animal model of visceral pain.44 Finally, inhibiting ERK activation in ACC blocked the expression of formalin-induced conditioned place avoidance in freely moving animals.45 Future studies are clearly needed to further determine molecular mechanism for the roles of ERK activity in pain, mood, as well as memory.
Author Contributions
MY performed electrophysiological experiments and drafted the manuscript. MY and MZ designed the project and finished the final vision of the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the EJLB-CIHR Michael Smith Chair in Neurosciences and Mental Health, Canada Research Chair, Canadian Institute for Health Research operating Grants (MOP-258523), NSERC Discovery Grant (RGPIN 402555), and the Azrieli Neurodevelopmental Research Program and Brain Canada.
References
- 1.Impey S, et al. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron 1998; 21: 869–883. [DOI] [PubMed] [Google Scholar]
- 2.Widmann C, et al. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev 1999; 79: 143–180. [DOI] [PubMed] [Google Scholar]
- 3.Einat H, et al. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci 2003; 23: 7311–7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao Y, et al. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci 2004; 24: 6590–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creson TK, et al. The anterior cingulate ERK pathway contributes to regulation of behavioral excitement and hedonic activity. Bipolar Disord 2009; 11: 339–350. [DOI] [PubMed] [Google Scholar]
- 6.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013; 14: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damsa C, Kosel M, Moussally J. Current status of brain imaging in anxiety disorders. Curr Opin Psychiatry 2009; 22: 96–110. [DOI] [PubMed] [Google Scholar]
- 8.Frankland PW, et al. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 2004; 304: 881–883. [DOI] [PubMed] [Google Scholar]
- 9.Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci 2013; 14: 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 2005; 6: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhuo M. Cortical excitation and chronic pain. Trends Neurosci 2008; 31: 199–207. [DOI] [PubMed] [Google Scholar]
- 12.Zhuo M. Long-term potentiation in the anterior cingulate cortex and chronic pain. Philos Trans R Soc Lond B Biol Sci 2014; 369: 20130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koga K, et al. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron 2015; 85: 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koga K, et al. Impaired presynaptic long-term potentiation in the anterior cingulate cortex of Fmr1 knock-out mice. J Neurosci 2015; 35: 2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toyoda H, et al. Requirement of extracellular signal-regulated kinase/mitogen-activated protein kinase for long-term potentiation in adult mouse anterior cingulate cortex. Mol Pain 2007; 3: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiumara F, et al. MicroRNA-22 gates long-term heterosynaptic plasticity in aplysia through presynaptic regulation of CPEB and downstream targets. Cell Rep 2015; 11: 1866–1875. [DOI] [PubMed] [Google Scholar]
- 17.Martin KC, et al. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron 1997; 18: 899–912. [DOI] [PubMed] [Google Scholar]
- 18.Sharma SK, et al. Differential role of mitogen-activated protein kinase in three distinct phases of memory for sensitization in Aplysia. J Neurosci 2003; 23: 3899–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kushner SA, et al. Modulation of presynaptic plasticity and learning by the H-ras/extracellular signal-regulated kinase/synapsin I signaling pathway. J Neurosci 2005; 25: 9721–9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toyoda H, et al. Long-term depression requires postsynaptic AMPA GluR2 receptor in adult mouse cingulate cortex. J Cell Physiol 2007; 211: 336–343. [DOI] [PubMed] [Google Scholar]
- 21.Toyoda H, et al. Time-dependent postsynaptic AMPA GluR1 receptor recruitment in the cingulate synaptic potentiation. Dev Neurobiol 2007; 67: 498–509. [DOI] [PubMed] [Google Scholar]
- 22.Zhao MG, et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 2005; 47: 859–872. [DOI] [PubMed] [Google Scholar]
- 23.Wang CC, Shyu BC. Differential projections from the mediodorsal and centrolateral thalamic nuclei to the frontal cortex in rats. Brain Res 2004; 995: 226–235. [DOI] [PubMed] [Google Scholar]
- 24.Davis S, et al. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci 2000; 20: 4563–4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies SP, et al. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 2000; 351: 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toyoda H, et al. Calcium/calmodulin-dependent kinase IV contributes to translation-dependent early synaptic potentiation in the anterior cingulate cortex of adult mice. Mol Brain 2010; 3: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science 1994; 265: 1104–1107. [DOI] [PubMed] [Google Scholar]
- 28.Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell 2002; 108: 689–703. [DOI] [PubMed] [Google Scholar]
- 29.Huang YY, Li XC, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell 1994; 79: 69–79. [DOI] [PubMed] [Google Scholar]
- 30.Chavez-Noriega LE, Stevens CF. Increased transmitter release at excitatory synapses produced by direct activation of adenylate cyclase in rat hippocampal slices. J Neurosci 1994; 14: 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colwell CS, Levine MS. Excitatory synaptic transmission in neostriatal neurons: regulation by cyclic AMP-dependent mechanisms. J Neurosci 1995; 15: 1704–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron 1996; 16: 797–803. [DOI] [PubMed] [Google Scholar]
- 33.Weisskopf MG, et al. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science 1994; 265: 1878–1882. [DOI] [PubMed] [Google Scholar]
- 34.Iacovelli L, et al. Thyrotropin activates mitogen-activated protein kinase pathway in FRTL-5 by a cAMP-dependent protein kinase A-independent mechanism. Mol Pharmacol 2001; 60: 924–933. [DOI] [PubMed] [Google Scholar]
- 35.Troadec JD, et al. Activation of the mitogen-activated protein kinase (ERK(1/2)) signaling pathway by cyclic AMP potentiates the neuroprotective effect of the neurotransmitter noradrenaline on dopaminergic neurons. Mol Pharmacol 2002; 62: 1043–1052. [DOI] [PubMed] [Google Scholar]
- 36.Huang CC, Hsu KS. Presynaptic mechanism underlying cAMP-induced synaptic potentiation in medial prefrontal cortex pyramidal neurons. Mol Pharmacol 2006; 69: 846–856. [DOI] [PubMed] [Google Scholar]
- 37.Jovanovic JN, et al. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci 2000; 3: 323–329. [DOI] [PubMed] [Google Scholar]
- 38.Chi P, Greengard P, Ryan TA. Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron 2003; 38: 69–78. [DOI] [PubMed] [Google Scholar]
- 39.Grewal SS, York RD, Stork PJ. Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol 1999; 9: 544–553. [DOI] [PubMed] [Google Scholar]
- 40.Jovanovic JN, et al. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci USA 1996; 93: 3679–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chi P, Greengard P, Ryan TA. Synapsin dispersion and reclustering during synaptic activity. Nat Neurosci 2001; 4: 1187–1193. [DOI] [PubMed] [Google Scholar]
- 42.Wei F, et al. Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice. J Neurosci 2006; 26: 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei F, Zhuo M. Activation of Erk in the anterior cingulate cortex during the induction and expression of chronic pain. Mol Pain 2008; 4: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang XJ, et al. Analgesic effect of paeoniflorin in rats with neonatal maternal separation-induced visceral hyperalgesia is mediated through adenosine A(1) receptor by inhibiting the extracellular signal-regulated protein kinase (ERK) pathway. Pharmacol Biochem Behav 2009; 94: 88–97. [DOI] [PubMed] [Google Scholar]
- 45.Cao H, et al. Activation of extracellular signal-regulated kinase in the anterior cingulate cortex contributes to the induction and expression of affective pain. J Neurosci 2009; 29: 3307–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]