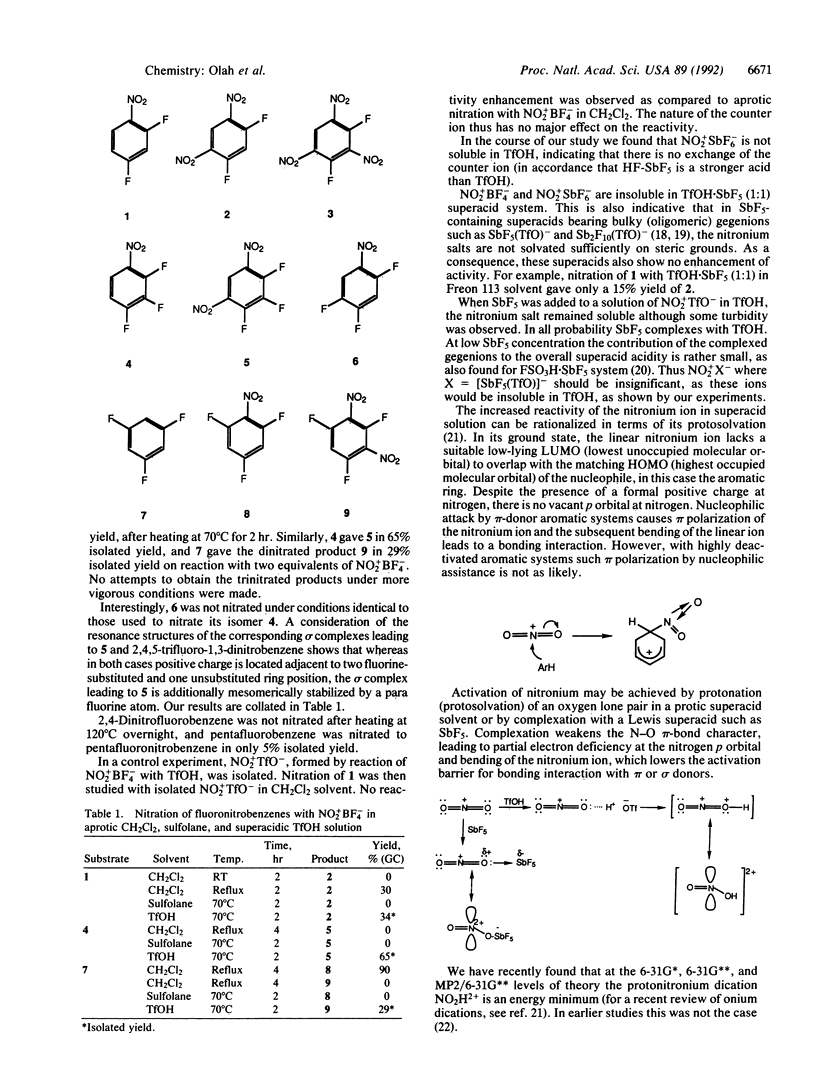
Abstract
The reactivity of nitronium tetrafluoroborate in the nitration of deactivated di- and trifluoronitrobenzenes is enhanced in superacidic trifluoromethanesulfonic (triflic) acid compared with aprotic methylene chloride and sulfolane solutions. The enhanced reactivity is discussed in terms of better solubility and higher dissociation of the nitronium salts, as well as protosolvation of NO2+ by superacids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Olah G. A., Lin H. C., Olah J. A., Narang S. C. Electrophilic and free radical nitration of benzene and toluene with various nitrating agents. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1045–1049. doi: 10.1073/pnas.75.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]



