Introduction
Cardiovascular disease is the leading cause of death in the United States and a growing health problem world-wide1. Following an adverse event such as myocardial infarction, there is a loss of nearly 1 billion cardiomyocytes that results in significant compromise to cardiac function. In humans, unlike some lower organisms such as the newt and zebrafish, these lost cardiomyocytes are not fully replenished through division of existing adult myocytes2, 3. Adult human cardiomyocytes are among the handful of cell types in the human body with extremely limited mitotic potential. Hence, the postnatal heart largely increases its size through hypertrophy and not hyperplasia4. Interestingly, studies in mouse models have demonstrated that the mammalian heart exhibits a robust regenerative response during embryonic development but this ability is quickly lost after birth and declines further with adult maturation5, 6. Some investigators have reported the possibility of adult human myocyte replenishment through the division of rare populations of adult cardiac stem cells7 or specialized populations of mitotically active myocytes8. While the precise contribution to new adult cardiomyocytes by these specialized populations of cells remains to be determined, their effect on cardiomyocyte replacement after injury is expected to be low and insufficient to compensate for the one billion cardiomyocytes lost during myocardial infarction. Consequently, the replenishment of lost cardiomyocytes in adult heart, remains a major unmet need in cardiovascular regenerative medicine.
Modern post-infarction treatment options target the neurohormonal physiology of residual cardiomyocytes without attempting to replenish the lost myocytes. For example, standard treatment regimens for post-MI heart failure due to cardiomyocyte loss include beta blocker, ACE inhibitors, and diuretics to 1) reduce oxygen demand, sympathetic overstimulation, and arrhythmias by managing heart rate and blood pressure, 2) reduce activation of the renin-angiotensin-aldosterone pathway, and 3) improve symptoms by managing the body fluid volume overall. In the most extreme cases of post-infarction heart failure where medical therapy is inadequate, replacement of the whole organ function by left ventricular assist device (LVAD) or heart transplantation may be necessary9. However, heart transplantation is associated with a host of complex issues, the most pressing of these being the limited availability of replacement organs and significant financial costs associated with such procedures10. As such, novel approaches for restoring cardiac function following myocardial infarction are in dire need.
Current Applications of Human Pluripotent Stem Cells for Studying Cardiomyocyte Proliferation and Cell Therapy
Recently, advances in stem cell biology and regenerative medicine have allowed, for the first time, mass production of human cardiomyocytes from pluripotent stem cells. Early cardiomyocyte differentiation protocols were limited in terms of their ability to create large numbers of cardiomyocytes as they employed cumbersome embryoid body-based methods and expensive growth factor treatment regimens11, 12. Current differentiation protocols can generate millions of cardiomyocytes from both human embryonic stem cells and patient specific human induced pluripotent stem cells using chemically-defined and small molecule-based approaches13, 14. Future refinements in methods for pluripotent stem cell culture and differentiation should easily lead to the production of billions of cardiomyocytes within two to three weeks from the onset of differentiation15. Although these stem cell-derived cardiomyocytes exhibit fetal-like phenotypes in terms of their structural and electrophysiological properties, they are currently the only reliable source of human cardiomyocytes that can be used to study human cardiovascular disease mechanisms16. For example, human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) have been shown to recapitulate the molecular mechanisms driving cardiovascular diseases such as long QT syndrome, viral myocarditis, and various other types of cardiomyopathies17–19. Additionally, because cardiotoxicity is the leading cause of drug withdrawal from the market, there is much interest within the pharmaceutical industry for using hiPSC-CMs for high throughput cardiotoxicity screening20. Finally, early stage clinical trials focusing on directly using stem cell-derived cardiomyocytes for regenerative therapy following myocardial infarction are underway. A recent study demonstrated in a non-human primate model that stem cell-derived cardiomyocytes are able to integrate into the host heart for a number of months21. Additionally, bioengineering-based approaches, utilizing stem cell-derived cardiomyocytes in combination with various biocompatible scaffolding technologies such as patches, are being tested as a way to introduce cardiomyocytes into damaged areas of the myocardium following infarction22, 23. Such engineered heart muscle could also be used for in vitro drug screening to test the efficacy or toxicity of compounds, with the assumption that 3D tissue slices made from PSC-CMs may be more likely to predict the behavior of three-dimensional tissue structure of the native heart. Given these possibilities, there is much hope that stem cell-derived cardiomyocytes can be used for 1) cardiovascular disease modeling, 2) drug toxicity screening, and 3) cell therapy (Figure 1).
Figure 1. Applications of stem cell-derived cardiomyocytes.
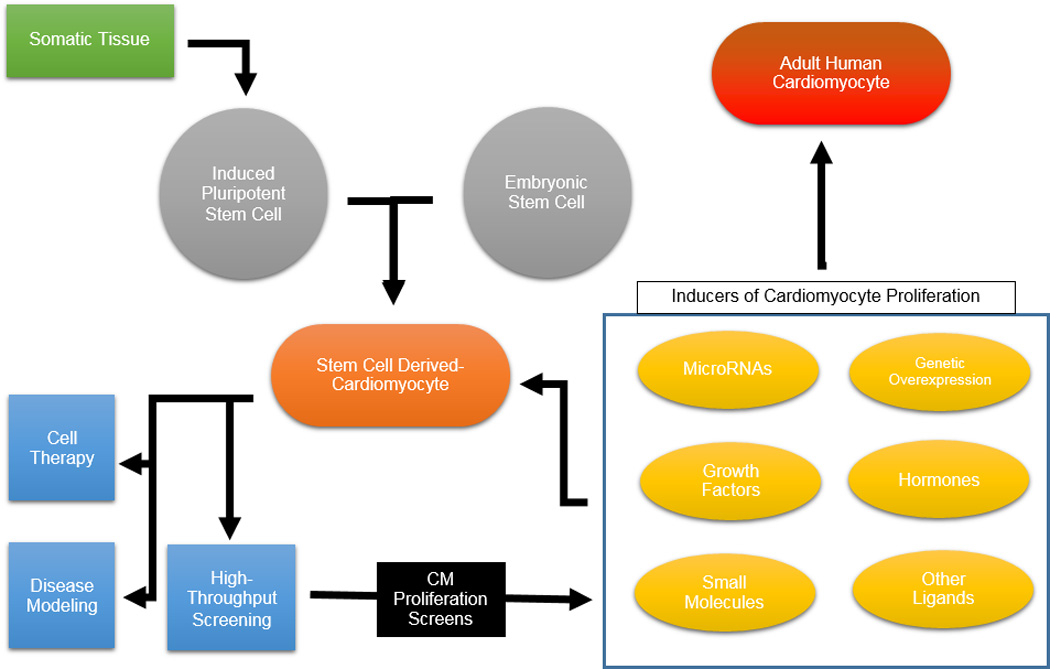
Human pluripotent stem cell-derived cardiomyocytes can be produced from induced pluripotent stem cells derived from somatic tissue or from embryonic stem cells. Following differentiation, PSC-CMs can be used for cardiovascular disease modeling, potentially for cell therapy, or for high-throughput screening purposes. As an extension of high-throughput screening applications, PSC-CMs can be utilized to identify novel regulators of cardiomyocyte proliferation. These pro-proliferative compounds could then be tested on adult human cardiomyocytes.
Screening for Novel Regulators of Cardiomyocyte Cell Cycle Activity
Beyond the aforementioned applications, PSC-CM could also be used to screen for novel regulators of cardiomyocyte proliferation. A number of studies have previously shown that cardiomyocytes can exhibit cell cycle activity in response to treatments with an array of compounds in vitro and in vivo (Table 1).
Table 1.
Studies of cardiomyocyte cell cycle reentry in in vitro platforms and animal models
| Name | In Vivo/ In Vitro | Summary | Citation |
|---|---|---|---|
| ANP/BNP | In vitro (neonatal rat ventricular CMs) In vivo (zebra fish) |
Low levels of the natriuretic peptides enhances CM proliferation via activation of Npr3 and cAMP pathways. |
Becker et al., 2013 |
| BMP10 (Bone morphogenetic protein-10) |
In vitro (rat ventricular CMs) In vivo (rat) |
BMP10 induces dose-dependent CM proliferation and cell cycle reentry of differentiated CMs in vitro, and stimulates CM cell-cycle reentry and mitosis post MI in vivo, by enhancing Tbx20 expression. |
Sun et al., 2014 |
| Cyclin A2 | In vivo (mouse) | Cyclin A2 induces postnatal mitosis, stimulates CM proliferation, and provides benefit after myocardial infarction. |
Chaudhry et al., 2004; Woo et al., 2006 |
| Cyclin B1 | In vitro | Overexpression of Cyclin B1 induces cell cycle reentry, DNA synthesis, and multinucleation. |
Bicknell et al., 2004 |
| Cyclin D1 | In vitro (neonatal rat CMs) In vivo |
Nuclear import of cyclin D1 and its partner CDK4 promotes cell-cycle reentry by activating the Rb regulatory pathway, which is required for cell cycle progression in both fetal CMs and adult heart. |
Tamamori-Adachi et al., 2003 |
| Cyclin D2 | In vivo (mouse) | Cyclin D2 promotes CM DNA synthesis in uninjured hearts and promotes regression growth post MI. |
Pasumarthi et al., 2004 |
| C3orf58 (Hypoxia and Akt induced stem cell factor, HASF) |
In vitro (neonatal rat ventricular CMs) In vivo (mouse) |
HASF increases DNA synthesis and promotes mitosis and cell division in vitro and enhances DNA synthesis and karyokinesis of neonatal and adult CMs in vivo through the PI3K/Akt-dependent CDK7 pathway. |
Beigi et al., 2015 |
| FOXO 1 | In vitro (mouse embryonic CMs) |
Absence of FOXO 1 downregulates cyclin kinase inhibitor expression and increases CM proliferation. |
Evans-Anderson et al., 2008 |
| JMJ (Jumonji) |
In vitro (primary mouse CMs) |
JMC knock-out increases mitosis via derepression of cell cycle progression by the interaction between JMC and Rb, with up-regulation of cyclin D1, cyclin D2, and Cdc2. |
Jung et al., 2005 |
| IL13 (Interleukin 13) |
In vitro (neonatal rat CMs) |
IL13 stimulates cell cycle reentry of CMs and preferentially induces either proliferation or survival of CMs compared with other cell types. |
O’Meara 2015 |
| FGF-1 (Fibroblast growth factor- 1) |
In vitro (rat neonatal ventricular CMs) In vivo (mouse) |
FGF-1 mediates CM cell cycle reentry through TWEAK/Fn14 and FGF1/FGFR-1 signaling cascades crosstalk. |
Novoyatleva et al., 2014 |
| FGF-10 | In vivo (mouse) | FGF10 regulates regional CM proliferation in the fetal heart through a FOXO3/p27 (kip1) pathway, and its overexpression in adult mice promotes CM but not cardiac fibroblast cell-cycle re-entry. |
Rochais et al., 2014 |
| G-CSF (Granulocyte colony stimulating factor) |
In vitro (primate ESC-CM, human iPSC-CM) In vivo (mouse) |
G-CSF promotes proliferation of developing CMs by activating the JAK/STAT signaling pathway. |
Shimoji et al., 2010 |
| IGF1/2 (Insulin-like growth factor 1/2) |
In vitro (hESC-CM) | IGF1 or IGF1 stimulates proliferation of terminally differentiated hESC-CMs in a dose-dependent manner by triggering PI3K/Akt signaling. |
McDevitt et al., 2005 |
| IGF1/2 | In vitro (murine ESCs) |
IGF treatment during early differentiation of ESCs increases mesodermal cell proliferation and, consequently, CPC formation through activation of downstream targets such as Akt and mTOR. |
Engels et al., 2014 |
| NRG1 (Neuregulin1) |
In vitro (rat ventricular CMs) In vivo (mouse) |
NRG1 stimulates cell cycle reentry, karyokinesis and cytokinesis of differentiated CMs by activating tyrosine kinase signaling downstream of the ErbB4 receptor, and leads to improved cardiac function post MI. |
Bersell et al., 2009 |
| SDF-1α (Stromal cell-derived factor-1α) |
In vitro (rat CMs) | SDF-1α enhances the proliferation ability of CMs with physical injury through regulating PI3K/Akt and ERK signaling pathway. Proliferation rate increases most markedly at 80 µg/L. |
Hou et al., 2015 |
| BIO (6-bromoindirubin-30- oxime) |
In vitro (neonatal and adult rat CMs; mESC-CMs, hiPSC- CMs) |
BIO, a GSK3B inhibitor, promotes cell cycle reentry and progression and increases the number of mitoses of CMs through activation of the canonical Wnt pathway. |
Uosaki et al., 2013 |
| KN93 | In vitro (mESC-CMs, hiPSC-CMs) |
KN93 increases CM proliferation by inhibiting CAMKII. |
Uosaki et al., 2013 |
| SB203580 | In vitro (adult rat ventricular CMs) |
SB203580 inhibits p38 MAPK, by which promotes growth factor induced DNA synthesis, karyokinesis, cytokinesis of CMs through dedifferentiation of sarcomeric structures. |
Engel et al., 2005 |
| SU 1498 | In vitro (mESC-CMs, hiPSC-CMs) |
SU1498 increases CM proliferation through activation of Raf-MEK-ERK signal cascade. |
Uosaki et al., 2013 |
| miR-15 family | In vivo (mouse) | Knockdown of the miR-15 family increases number of mitotic cardiomyocytes and derepression of Chek1. |
Porrello et al., 2011 |
| miR-17–29 | In vitro (neonatal rat cardiomyocytes) In vivo (mouse) |
Overexpression of miR-17–92 is sufficient to induce CM proliferation in embryonic, postnatal, and adult hearts by repressing tumor suppressor Phosphatase and Tensin Homolog (PTEN). |
Chen et al., 2013 |
| miR-34a | In vivo (mouse) | Anti-miR-34a treatment improves post- MI remodeling in adult heart by interfering with miR-34a’s regulation of cell activity and death via its target genes including Bcl2, Cyclin D1, and Sirt1. |
Yang et al., 2015 |
| miR-99/100 and Let-7a/c and their downstream protein targets SMARCA5 and FNTβ |
In vivo (mouse) | Anti-miR delivery or overexpression of FNTβ and SMARCA5 results in adult CM dedifferentiation and the activation of a proliferative response. |
Aguirre et al., 2014 |
| miR-133a | In vivo (mouse) | Knockout of miR-133a promotes CM proliferation by elevating expression of SRF and cyclin D2. |
Liu et al., 2008 |
| miR-199a-3p and miR- 590-3p |
In vitro (mouse and rat CMs) In vivo (mouse) |
miR induces cell cycle reentry and cytokinesis of neonatal and adult cardiomyocytes in vitro and promotes CM proliferation in both neonatal and adult hearts following MI in vivo. |
Eulalio et al., 2012 |
| miR302–367 | In vivo (mouse) | Postnatal reexpression of miR302–367 reactivated the cell cycle in CMs and induces proliferation by targeting several components of the Hippo signal transduction pathway, resulting in reduced scar formation after experimental MI. |
Tian et al., 2015 |
| miR-499 | In vitro (mouse P19CL6 cells and neonatal rat ventricular CMs) |
miR-499 promotes the proliferation of neonatal CMs via its effect on Sox6 and cyclin D1. |
Li et al., 2013 |
| T3 (Triiodothyronine) |
In vivo (mouse) | A thyroid hormone surge activates the IGF-1/IGF-1-R/Akt pathway on postnatal day 15 and initiates a brief but intense proliferative burst of predominantly binuclear CMs. |
Naqvi et al., 2014 |
Treatments with extracellular ligands, secreted hormones, growth factors have been shown to activate critical signaling pathways that up-regulate cell cycle activity in cardiomyocytes. Insulin-like growth factor (IGF) enhances proliferation of terminally-differentiated human embryonic stem cell-derived cardiomyocytes through activation of pro-proliferative PI3K/Akt signaling, as well as promotes expansion of early mesoderm during cardiomyocyte differentiation in mouse embryonic stem cells24, 25. Periostin, a ligand for the alpha-V/beta-3 and alpha-V/beta-5 integrins, was reported by Kuhn et al to induce cell cycle reentry of differentiated myocytes in vitro and promote mononucleated myocytes to enter mitosis via activation of PI3K signaling. Remarkably, this study showed that periostin treatment results in enhanced myocardial function post-infarction in rat hearts26. Bersell et al demonstrated that Neuregulin 1 stimulates cell cycle reentry, karyokinesis, and cytokinesis of rat ventricular cardiomyocytes by activating tyrosine kinase signaling downstream of the ErbB4 receptor27. However, the validity of these results is actively debated28. Nevertheless, neuregulin treatment was reported to improved cardiac function post-infarction27. Alternatively, thyroid hormone treatment has been reported to induce cardiomyocyte proliferation29. A thyroid hormone surge during neonatal development was reported to be able to induce a proliferative burst in predominantly binuclear cardiomyocytes via activation of the IGF signaling pathway. Low levels of the cardiac natriuretic peptides ANP and BMP were also shown to enhance CM proliferation via activation of Npr3 and cAMP pathways in vitro in neonatal rat ventricular myocytes and also in the zebrafish heart in vivo30. In rat myocytes, stromal cell-derived factor 1 alpha enhanced the proliferation ability of cardiomyocytes via the PI3K/Akt and ERK signaling pathway31. More recently in human iPSC-CMs and primate ESC-CMs, granulocyte colony stimulating factor (GCSF) was shown to promote proliferation of developing CMs by activating the JAK/STAT signaling pathway32. Additionally, intrauterine GCSF injection promoted cardiomyocyte hyperplasia in developing mouse embryos32. Cytokine interleukin 13 stimulated cell cycle reentry of neonatal rat ventricular cardiomyocytes and preferentially induced either proliferation or survival of CMs compared with other cell types33. TGF-beta superfamily growth factor BMP10 induced dose-dependent CM proliferation and cell cycle reentry of differentiated rat ventricular cardiomyocytes in vitro, and stimulated CM cell-cycle reentry and mitosis post-infarction in vivo by enhancing expression of the cardiac transcription factor Tbx2034. Similarly, paracrine factor C3orf58, a hypoxia and Akt induced stem cell factor, was shown to enhance cardiac repair in vivo and a 60% increase in DNA synthesis in neonatal rat ventricular myocytes35. Finally, FGF ligands were able to enhance regional CM proliferation in the fetal murine heart via the FOXO3/p27 signaling pathway, and their overexpression in adult mice promotes myocyte cell cycle reentry36, 37. These studies demonstrate that extracellular ligands, secreted hormones, and growth factor treatments are able to exert a proliferation-enhancing effect in mammalian cardiomyocytes both in vitro and in vivo.
For translational purposes, small molecules may be preferable as agents for stimulating cardiomyocyte proliferation as they are relatively cheap in comparison to growth factors and, as synthetic molecules, can be produced under xeno-free conditions in a GMP compliant fashion. For example, Engel et al demonstrated that the small molecule p38 inhibitor SB203580 induced DNA synthesis, karyokinesis, cytokinesis of adult rat ventricular CMs through dedifferentiation of sarcomeric structures38. Likewise, BIO (6-bromoindirubin-30-oxime), a GSK3beta inhibitor, promoted cell cycle reentry and progression and increased mitosis through activation of the canonical Wnt pathway in multiple cardiomyocyte types39, 40. Uosaki et al also demonstrated that small molecules SU1498 and KN93 increased PSC-CM proliferation through activation of the Raf-MEK-ERK signal cascade and inhibition of CAMKII, respectively40. These studies demonstrate that small molecules are effective and stable regulators of myocyte cell cycle activity and some may even improve the cardiomyocyte yield in chemically-defined cardiomyocyte differentiation protocols14.
MicroRNAs have recently garnered much interest for their abilities to control gene expression via RNA silencing. Multiple miRNA families have been studied in the context of cardiac development, and some are noted for their abilities to promote myocyte replication in vitro and in vivo. Knockout of miR-133a was able to enhance CM proliferation through elevation of cyclin D2 in the mouse heart41. A functional screen identified that miR-199a-3p and miR-590-3p were able to induce cell cycle reentry and cytokinesis of mouse adult and neonatal cardiomyocytes in vitro and in vivo following MI42. Knockdown of the miR-15 family increased mitotic activation of cardiomyocytes via de-repression of Chek143. Overexpression of miR-17-92 was sufficient to promote CM proliferation in vitro and in vivo in rat and mouse CMs by repressing the tumor suppressor PTEN44. MiR-499 was able to enhance neonatal rat ventricular CM proliferation in vitro through an effect on Sox6 and cyclin D145. The reactivation of cell cycle activity in postnatal mouse CMs and reduction of scar formation was achieved by increasing the expression of miR302-367 in vivo. Interestingly, this activity of miR302-37 required the presence of multiple members of the Hippo signaling pathway46. Treatment with anti-miR-34 was able to enhance post-MI remodeling in the adult murine heart by inhibiting the function of miR-34 during apoptosis through its target genes Bcl2, Cyclin D1, and Sirt147. Finally, mir-99/100 and Let-7a/c and their downstream targets smarca5 and FNTB were identified as regulators of heart regeneration in zebrafish and overexpression of these target proteins enhanced the proliferative response in murine models48. These studies illustrate that miRNAs, a relatively new topic in cardiac biology, play a significant role in regulating cardiomyocyte proliferation.
In addition to exogenous introduction of pro-proliferative proteins and other factors, a number of studies have utilized genetic manipulation of critical cell cycle regulating pathways to hyperactivate cardiomyocyte proliferation. While genetic overexpression of pathways that enhance cardiomyocyte proliferation may not be directly translatable to human therapy, it can provide important mechanistic insights for studying the signaling network regulating cardiomyocyte proliferation. Overexpression of a number of cyclin proteins and cyclin dependent kinases has proven effective in enhancing cardiomyocyte proliferation in mouse models as well as in in vitro systems. Bicknell et al demonstrated that overexpression of CCNB1 induced multinucleation, cell cycle reentry, as well as DNA synthesis in adult rat cardiomyocytes49. Likewise, overexpression of Cyclins A2, D1, D2, and others induced postnatal mitosis of adult myocytes and functional benefit after myocardial infarction in mouse models50–53. Deletion of the FOXO1 transcription factor was able to downregulate cyclin kinase inhibitor expression, thereby increased myocyte proliferation54. Similarly, knockout of the Jumonji protein was able to increase myocyte mitosis in primary mouse cardiomyocytes by upregulating cyclins D1 and D255. Taken together, these studies illustrate that cardiomyocytes are susceptible to cell cycle reentry following stimulation with a number of exogenous factors.
Conclusions
Effective induction of proliferation in adult human cardiomyocytes represents a significant goal for the cardiovascular regenerative medicine community. A number of lower vertebrates, adult zebrafish3, and neonatal mouse hearts5 have all been shown to exhibit significant cardiac regenerative potential following experimental induction of myocardial damage, raising the possibility that with the right mechanistic insight a similar process may be recapitulated in humans. Other studies have demonstrated the effects of cell cycle activating compounds, identified through in vitro myocyte screening, to enhance cardiomyocyte division in animal studies in vivo. Alternatively, direct reprogramming of existing cardiac fibroblast population in the mammalian heart both in vitro56 and in vivo towards the cardiomyocyte lineage has been reported to work in murine models, although at an extremely low efficiency. Because of recent advances in cardiomyocyte differentiation protocols from pluripotent stem cells, these cells can now be mass-produced for the screening of novel regulators of cell cycle activity. We believe that human stem cell-derived cardiomyocytes, because of their similar gene expression pattern and cell cycle regulation to primary human cardiomyocytes, represent the best current surrogate cell type for understanding human cardiomyocyte biology and cell cycle induction. Still, much work remains to be done with regards to PSC-CM cell production and maturation. Advancements must still be made in scale of production as well as reproducibility of cardiomyocyte differentiation from pluripotent stem cells, as cardiomyocyte differentiation yield remains variable from stem cell line to stem cell line. Additionally, developing directed differentiation protocols for production of atrial, ventricular, and nodal-specific cardiomyocyte subtypes would be valuable, as different cardiomyocyte populations may respond differently to treatment with proliferation-inducing compounds. Finally, because current cardiomyocyte differentiation protocols produce PSC-CMs that are fetal-like in their gene expression patterns and structure, induced maturation of these cells will be necessary in order to accurately model cardiovascular disease phenotypes and cardiotoxic drug responses. Likely, these issues will be addressed, as cardiomyocyte differentiation protocols and developments in iPSC reprogramming have been advancing at breakneck speed. In spite of their present limitations, we ultimately believe that stem cell derived cardiomyocytes represent an excellent platform for screening for novel regulators of cardiomyocyte proliferation, which could eventually be utilized for translational therapies.
Opinion Statement.
Adult human cardiomyocytes are terminally-differentiated and have limited capacity for cell division. Hence, they are not naturally replaced following ischemic injury to the heart. As such, cardiac function is often permanently compromised after an event such as myocardial infarction. In recent years, investigators have focused intensively on ways to reactivate cardiomyocyte mitotic activity in both in vitro cell culture systems and in vivo animal models. In parallel, advances in stem cell biology have allowed for the mass production of patient-specific human cardiomyocytes from human induced pluripotent stem cells. These cells can be produced via chemically-defined differentiation of human pluripotent stem cells in a matter of weeks and could theoretically be utilized directly for therapeutic purposes to replace damaged myocardium. However, stem cell-derived cardiomyocytes, like their adult counterparts, are post-mitotic and incapable of large-scale expansion after reaching a certain stage of in vitro differentiation. Due to this shared characteristic, these stem cell-derived cardiomyocytes may provide a platform for studying genes, pathways, and small molecules that induce cell cycle reentry and proliferation of human cardiomyocytes. Ultimately, the discovery of novel mechanisms or pathways to induce human cardiomyocyte proliferation should improve our ability to regenerate adult cardiomyocytes and help restore cardiac function following injury.
Acknowledgments
This work is supported by a National Science Foundation Graduate Research Fellowship (to A. S.), and an NIH Director’s Pioneer Award, NIH/NHLBI U01 HL099067, American Heart Association Grant-in-Aid (to S.M.W.).
Footnotes
Conflict of Interest
None of the authors declare a conflict related to this paper.
Contributor Information
Arun Sharma, Email: arun.sharma@stanford.edu.
Yuan Zhang, Email: yzhang17@stanford.edu.
Sean M. Wu, Email: smwu@stanford.edu.
References
- 1.Pagidipati NJ, Gaziano TA. Estimating deaths from cardiovascular disease: A review of global methodologies of mortality measurement. Circulation. 2013;127:749–756. doi: 10.1161/CIRCULATIONAHA.112.128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. J. Exp. Zool. 1974;187:249–253. doi: 10.1002/jez.1401870208. [DOI] [PubMed] [Google Scholar]
- 3.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 4.Li F, Wang X, Capasso JM, Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J. Mol. Cell. Cardiol. 1996;28:1737–1746. doi: 10.1006/jmcc.1996.0163. [DOI] [PubMed] [Google Scholar]
- 5.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturzu AC, Rajarajan K, Passer D, Plonowska K, Riley A, Tan TC, Sharma A, Xu AF, Engels MC, Feistritzer R, Li G, Selig MK, Geissler R, Robertson KD, Scherrer-Crosbie M, Domian IJ, Wu SM. Fetal mammalian heart generates a robust compensatory response to cell loss. Circulation. 2015;132:109–121. doi: 10.1161/CIRCULATIONAHA.114.011490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 8.Kimura W, Xiao F, Canseco DC, Muralidhar S, Thet S, Zhang HM, Abderrahman Y, Chen R, Garcia JA, Shelton JM, Richardson JA, Ashour AM, Asaithamby A, Liang H, Xing C, Lu Z, Zhang CC, Sadek HA. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature. 2015;523:226–230. doi: 10.1038/nature14582. [DOI] [PubMed] [Google Scholar]
- 9.Ryan TJ, Anderson JL, Antman EM, Braniff BA, Brooks NH, Califf RM, Hillis LD, Hiratzka LF, Rapaport E, Riegel BJ, Russell RO, Smith EE, 3rd, Weaver WD. Acc/aha guidelines for the management of patients with acute myocardial infarction: Executive summary. A report of the american college of cardiology/american heart association task force on practice guidelines (committee on management of acute myocardial infarction) Circulation. 1996;94:2341–2350. doi: 10.1161/01.cir.94.9.2341. [DOI] [PubMed] [Google Scholar]
- 10.Abouna GM. Organ shortage crisis: Problems and possible solutions. Transplant. Proc. 2008;40:34–38. doi: 10.1016/j.transproceed.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 11.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and bmp signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical wnt signaling. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC. Chemically defined generation of human cardiomyocytes. Nat. Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niebruegge S, Nehring A, Bar H, Schroeder M, Zweigerdt R, Lehmann J. Cardiomyocyte production in mass suspension culture: Embryonic stem cells as a source for great amounts of functional cardiomyocytes. Tissue Eng. Part A. 2008;14:1591–1601. doi: 10.1089/ten.tea.2007.0247. [DOI] [PubMed] [Google Scholar]
- 16.Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22:1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, Feldman O, Gepstein A, Arbel G, Hammerman H, Boulos M, Gepstein L. Modelling the long qt syndrome with induced pluripotent stem cells. Nature. 2011;471:225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 18.Sharma A, Wu JC, Wu SM. Induced pluripotent stem cell-derived cardiomyocytes for cardiovascular disease modeling and drug screening. Stem cell research & therapy. 2013;4:150. doi: 10.1186/scrt380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma A, Marceau C, Hamaguchi R, Burridge PW, Rajarajan K, Churko JM, Wu H, Sallam KI, Matsa E, Sturzu AC, Che Y, Ebert A, Diecke S, Liang P, Red-Horse K, Carette JE, Wu SM, Wu JC. Human induced pluripotent stem cell-derived cardiomyocytes as an in vitro model for coxsackievirus b3-induced myocarditis and antiviral drug screening platform. Circ. Res. 2014;115:556–566. doi: 10.1161/CIRCRESAHA.115.303810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mordwinkin NM, Burridge PW, Wu JC. A review of human pluripotent stem cell-derived cardiomyocytes for high-throughput drug discovery, cardiotoxicity screening, and publication standards. J Cardiovasc. Transl. Res. 2013;6:22–30. doi: 10.1007/s12265-012-9423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human esc-derived cardiomyocytes. Biomaterials. 2013;34:5813–5820. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens KR, Pabon L, Muskheli V, Murry CE. Scaffold-free human cardiac tissue patch created from embryonic stem cells. Tissue Eng. Part A. 2009;15:1211–1222. doi: 10.1089/ten.tea.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDevitt TC, Laflamme MA, Murry CE. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the igf/pi 3-kinase/akt signaling pathway. J. Mol. Cell. Cardiol. 2005;39:865–873. doi: 10.1016/j.yjmcc.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engels MC, Rajarajan K, Feistritzer R, Sharma A, Nielsen UB, Schalij MJ, de Vries AA, Pijnappels DA, Wu SM. Insulin-like growth factor promotes cardiac lineage induction in vitro by selective expansion of early mesoderm. Stem Cells. 2014;32:1493–1502. doi: 10.1002/stem.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat. Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 27.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/erbb4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 28.Reuter S, Soonpaa MH, Firulli AB, Chang AN, Field LJ. Recombinant neuregulin 1 does not activate cardiomyocyte DNA synthesis in normal or infarcted adult mice. PLoS ONE. 2014;9:e115871. doi: 10.1371/journal.pone.0115871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naqvi N, Li M, Calvert JW, Tejada T, Lambert JP, Wu J, Kesteven SH, Holman SR, Matsuda T, Lovelock JD, Howard WW, Iismaa SE, Chan AY, Crawford BH, Wagner MB, Martin DI, Lefer DJ, Graham RM, Husain A. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell. 2014;157:795–807. doi: 10.1016/j.cell.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker JR, Chatterjee S, Robinson TY, Bennett JS, Panakova D, Galindo CL, Zhong L, Shin JT, Coy SM, Kelly AE, Roden DM, Lim CC, MacRae CA. Differential activation of natriuretic peptide receptors modulates cardiomyocyte proliferation during development. Development. 2014;141:335–345. doi: 10.1242/dev.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou CJ, Qi YM, Zhang DZ, Wang QG, Cui CS, Kuang L, Chen B. The proliferative and migratory effects of physical injury and stromal cell-derived factor-1alpha on rat cardiomyocytes and fibroblasts. Eur. Rev. Med. Pharmacol. Sci. 2015;19:1252–1257. [PubMed] [Google Scholar]
- 32.Shimoji K, Yuasa S, Onizuka T, Hattori F, Tanaka T, Hara M, Ohno Y, Chen H, Egasgira T, Seki T, Yae K, Koshimizu U, Ogawa S, Fukuda K. G-csf promotes the proliferation of developing cardiomyocytes in vivo and in derivation from escs and ipscs. Cell Stem Cell. 2010;6:227–237. doi: 10.1016/j.stem.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 33.O'Meara CC, Wamstad JA, Gladstone RA, Fomovsky GM, Butty VL, Shrikumar A, Gannon JB, Boyer LA, Lee RT. Transcriptional reversion of cardiac myocyte fate during mammalian cardiac regeneration. Circ. Res. 2015;116:804–815. doi: 10.1161/CIRCRESAHA.116.304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun L, Yu J, Qi S, Hao Y, Liu Y, Li Z. Bone morphogenetic protein-10 induces cardiomyocyte proliferation and improves cardiac function after myocardial infarction. J. Cell. Biochem. 2014;115:1868–1876. doi: 10.1002/jcb.24856. [DOI] [PubMed] [Google Scholar]
- 35.Beigi F, Schmeckpeper J, Pow-Anpongkul P, Payne JA, Zhang L, Zhang Z, Huang J, Mirotsou M, Dzau VJ. C3orf58, a novel paracrine protein, stimulates cardiomyocyte cell-cycle progression through the pi3k-akt-cdk7 pathway. Circ. Res. 2013;113:372–380. doi: 10.1161/CIRCRESAHA.113.301075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novoyatleva T, Sajjad A, Pogoryelov D, Patra C, Schermuly RT, Engel FB. Fgf1-mediated cardiomyocyte cell cycle reentry depends on the interaction of fgfr-1 and fn14. FASEB J. 2014;28:2492–2503. doi: 10.1096/fj.13-243576. [DOI] [PubMed] [Google Scholar]
- 37.Rochais F, Sturny R, Chao CM, Mesbah K, Bennett M, Mohun TJ, Bellusci S, Kelly RG. Fgf10 promotes regional foetal cardiomyocyte proliferation and adult cardiomyocyte cell-cycle re-entry. Cardiovasc. Res. 2014;104:432–442. doi: 10.1093/cvr/cvu232. [DOI] [PubMed] [Google Scholar]
- 38.Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. P38 map kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tseng AS, Engel FB, Keating MT. The gsk-3 inhibitor bio promotes proliferation in mammalian cardiomyocytes. Chem. Biol. 2006;13:957–963. doi: 10.1016/j.chembiol.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Uosaki H, Magadum A, Seo K, Fukushima H, Takeuchi A, Nakagawa Y, Moyes KW, Narazaki G, Kuwahara K, Laflamme M, Matsuoka S, Nakatsuji N, Nakao K, Kwon C, Kass DA, Engel FB, Yamashita JK. Identification of chemicals inducing cardiomyocyte proliferation in developmental stage-specific manner with pluripotent stem cells. Circ. Cardiovasc. Genet. 2013;6:624–633. doi: 10.1161/CIRCGENETICS.113.000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu N, Bezprozvannaya S, Williams AH, Qi X, Richardson JA, Bassel-Duby R, Olson EN. Microrna-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22:3242–3254. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies mirnas inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 43.Porrello ER, Johnson BA, Aurora AB, Simpson E, Nam YJ, Matkovich SJ, Dorn GW, 2nd, van Rooij E, Olson EN. Mir-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ. Res. 2011;109:670–679. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J, Huang ZP, Seok HY, Ding J, Kataoka M, Zhang Z, Hu X, Wang G, Lin Z, Wang S, Pu WT, Liao R, Wang DZ. Mir-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ. Res. 2013;112:1557–1566. doi: 10.1161/CIRCRESAHA.112.300658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Wang J, Jia Z, Cui Q, Zhang C, Wang W, Chen P, Ma K, Zhou C. Mir-499 regulates cell proliferation and apoptosis during late-stage cardiac differentiation via sox6 and cyclin d1. PLoS ONE. 2013;8:e74504. doi: 10.1371/journal.pone.0074504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian Y, Liu Y, Wang T, Zhou N, Kong J, Chen L, Snitow M, Morley M, Li D, Petrenko N, Zhou S, Lu M, Gao E, Koch WJ, Stewart KM, Morrisey EE. A microrna-hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci. Transl. Med. 2015;7:279ra238. doi: 10.1126/scitranslmed.3010841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Y, Cheng H, Qiu Y, Dupee DK, Noonan M, Lin YD, Fisch S, Unno K, Sereti KI, Liao R. Microrna-34a plays a key role in cardiac repair and regeneration following myocardial infarction. Circ. Res. 2015 doi: 10.1161/CIRCRESAHA.117.305962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aguirre A, Montserrat N, Zacchigna S, Nivet E, Hishida T, Krause MN, Kurian L, Ocampo A, Vazquez-Ferrer E, Rodriguez-Esteban C, Kumar S, Moresco JJ, Yates JR, 3rd, Campistol JM, Sancho-Martinez I, Giacca M, Izpisua Belmonte JC. In vivo activation of a conserved microrna program induces mammalian heart regeneration. Cell Stem Cell. 2014;15:589–604. doi: 10.1016/j.stem.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bicknell KA, Coxon CH, Brooks G. Forced expression of the cyclin b1-cdc2 complex induces proliferation in adult rat cardiomyocytes. Biochem. J. 2004;382:411–416. doi: 10.1042/BJ20031481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaudhry HW, Dashoush NH, Tang H, Zhang L, Wang X, Wu EX, Wolgemuth DJ. Cyclin a2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J. Biol. Chem. 2004;279:35858–35866. doi: 10.1074/jbc.M404975200. [DOI] [PubMed] [Google Scholar]
- 51.Woo YJ, Panlilio CM, Cheng RK, Liao GP, Atluri P, Hsu VM, Cohen JE, Chaudhry HW. Therapeutic delivery of cyclin a2 induces myocardial regeneration and enhances cardiac function in ischemic heart failure. Circulation. 2006;114:I206–I213. doi: 10.1161/CIRCULATIONAHA.105.000455. [DOI] [PubMed] [Google Scholar]
- 52.Tamamori-Adachi M, Ito H, Sumrejkanchanakij P, Adachi S, Hiroe M, Shimizu M, Kawauchi J, Sunamori M, Marumo F, Kitajima S, Ikeda MA. Critical role of cyclin d1 nuclear import in cardiomyocyte proliferation. Circ. Res. 2003;92:e12–e19. doi: 10.1161/01.res.0000049105.15329.1c. [DOI] [PubMed] [Google Scholar]
- 53.Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ. Targeted expression of cyclin d2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ. Res. 2005;96:110–118. doi: 10.1161/01.RES.0000152326.91223.4F. [DOI] [PubMed] [Google Scholar]
- 54.Evans-Anderson HJ, Alfieri CM, Yutzey KE. Regulation of cardiomyocyte proliferation and myocardial growth during development by foxo transcription factors. Circ. Res. 2008;102:686–694. doi: 10.1161/CIRCRESAHA.107.163428. [DOI] [PubMed] [Google Scholar]
- 55.Jung J, Kim TG, Lyons GE, Kim HR, Lee Y. Jumonji regulates cardiomyocyte proliferation via interaction with retinoblastoma protein. J. Biol. Chem. 2005;280:30916–30923. doi: 10.1074/jbc.M414482200. [DOI] [PubMed] [Google Scholar]
- 56.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


