Summary
Dengue is caused by four serotype-distinct dengue viruses (DENVs), and developing a multivalent vaccine against dengue has not been straightforward since partial immunity to DENV may predispose to more severe disease upon subsequent DENV infection. The vaccine that is furthest along in development is CYD™, a live attenuated tetravalent vaccine (LATV) produced by Sanofi Pasteur. Although the multi-dose vaccine demonstrated protection against severe dengue, its overall efficacy was limited by DENV serotype, serostatus at vaccination, region, and age. The National Institute of Allergy and Infectious Diseases has developed the LATV dengue vaccines TV003/TV005. A single dose of either TV003 or TV005 induced seroconversion to four DENV serotypes in 74%–92% (TV003) and 90% (TV005) of flavivirus seronegative adults and elicited near-sterilizing immunity to a second dose of vaccine administered 6–12 months later. The important differences in the structure, infectivity, and immune responses to TV003/TV005 are compared with CYD™.
Keywords: Dengue, live attenuated dengue vaccine, vaccine efficacy, vaccine immunogenicity, vaccine infectivity, vaccine attenuation strategies
Background
Dengue vaccine development has been ongoing for more than 70 years following Dr. Albert Sabin's inoculation of volunteers with mouse-brain passaged DENV-1 to determine if the virus was attenuated and immunogenic [1]. As evidenced by this long history, the hurdles for the development of a safe and effective dengue vaccine have been numerous. First, there are four individual DENV serotypes all of which can infect humans and cause the same spectrum of illness. Second, infection with one DENV serotype likely provides life-long protection against symptomatic illness with that particular DENV serotype (homotypic protection), but provides only short-term (1- 2 years) protection against symptomatic infection with a different DENV serotype (heterotypic protection). Not only does the first DENV infection fail to protect against the other 3 DENV serotypes, it may predispose to more severe dengue disease following a second, heterotypic DENV infection. Epidemiologic studies have determined that pre-existing immunity to one DENV serotype is the greatest risk factor for more severe disease upon secondary DENV infection [2]. This is thought to be due to the phenomenon of antibody dependent enhancement (ADE) of infection in which the heterotypic, non-neutralizing antibodies raised by the first DENV infection bind to the second infecting DENV but do not neutralize the virus. Instead, the antibody-virus complexes bind to Fcγ receptors on monocytes and macrophages and subsequently infect the cell with greater efficiency [3,4]. It is thought that entry into the cell by this means bypasses some of the innate immune responses targeted at controlling the virus. Because of the risk of infection by ADE, it is desirable that a dengue vaccine protect against all four DENV serotypes. Third, the epidemiology of dengue is diverse, making it difficult in some cases to identify the most appropriate age group to vaccinate. In some countries the majority of symptomatic infections occur in adults, in other countries children bear the major burden of disease. Despite these many hurdles, there is still one dengue vaccine that has completed 3 efficacy trials (Sanofi Pasteur), two dengue vaccines in Phase 2 clinical trials and awaiting Phase 3 efficacy evaluation (Takeda and NIAID/Institute Butantan), and many other candidates in early clinical development (Phase 1 clinical trials). The lead candidate vaccines are all live attenuated vaccines which are intended to infect recipients in a controlled manner, with replication of the vaccine virus (detectable as viremia) providing a biologically-relevant amplification of the antigen in a immunological context similar to natural infection.
The Sanofi Pasteur Vaccine CYD™
The pioneering efficacy trials of the Sanofi Pasteur live attenuated tetravalent CYD™ vaccine for dengue have been closely watched and the results eagerly anticipated, since a vaccine to control dengue is currently not available and long overdue. The CYD™ has now completed a Phase 2b and two Phase 3 efficacy trials with mixed results [5-8]. The efficacy of CYD™ in the three different trials is summarized in Table 1. Efficacy of the vaccine varied by DENV serotype, by age of the vaccine recipients, by region, and by serostatus at time of vaccination. Efficacy against DENV-3 and DENV-4 was greatest in all three studies. Importantly, the efficacy against DENV-2 did not meet statistical significance in CYD23 (9.2%; 95% CI: −75 to 51.3) or CYD14 (35%; 95% CI: −9.2 to 61) in the per-protocol analysis. Efficacy for DENV-1 ranged from 50% (95% CI: 24.6-66.8) in CYD14 to 55.6% (95% CI: −21.6 to 84) in CYD23. However, efficacy against DENV-2 (42.3%) did achieve statistical significance in CYD15 (CI: 14-61.1). Analyses also revealed much lower efficacy in vaccine recipients who were DENV sero-negative at the time of vaccination (Table 1). The realization of partial efficacy during the clinical evaluation of a vaccine is unfortunately not without precedent (malaria and human immunodeficiency virus), however, the question of whether the vaccine may still have an overall impact on control of disease is certainly worth consideration. Results from the first year of the long-term safety assessment for these trials (Year 3 of the trial) were recently published [8] and the efficacy of CYD™ against hospitalization was favorable, yet highly dependent on DENV serostatus and age at time of vaccination. The relative risk of hospitalization in vaccine recipients 2-5 years of age at vaccination compared to placebo recipients in CYD14 was 7.45 (95% CI 1.15-313.8) and in those less than 9 years of age was 1.58 (95% CI 0.61-4.83). In CYD15, the relative risk was only 0.53 (95% CI 0.25-1.16) however, the risk of hospitalization in both studies increased from that of the previous year (Table 1).
Table 1.
| Trial | Region | Vaccine recipients enrolled | Age | Overall Efficacy1 (95% CI) | Efficacy sero-positive at baseline (95% CI)1 | Efficacy sero-negative at baseline (95% CI)1 | Relative Risk of hospitalization Year 21 (compared to control) | Relative Risk of hospitalization Year 3 (compared to control) |
|---|---|---|---|---|---|---|---|---|
| CYD23 | Thailand | 2,669 | 4 – 11 | 30.22 (−13.4-56.6) | Not done | Not done | 0.52 (0.28-0.97) | 1.01 (0.47-2.3) |
| CYD14 | Southeast Asia | 10,275 | 2 – 14 | 56.53 (43.8-66.4) | 74.3 (53.2-86.3) | 35.5 (−26.8-66.7) | 0.29 (0.16-0.51) | 1.04 (0.52-2.19) |
| CYD15 | Latin America | 13,920 | 9 – 16 | 60.8 (52.0-68.0) | 83.7 (62.2-93.7) | 43.2 (−61.5-80) | 0.21 (0.10 to 0.43) | 0.53 (0.25 – 1.16) |
Period of primary efficacy evaluation was >28 days after the third dose to month 25 (12 month efficacy period). This period represents year 2 of the study.
Not significant. Efficacy against serotype 2 was 9.2% (95% CI −75 – 51.3)
Efficacy against DENV-2 was not significant (35%, 95% CI −9.2 to 61.0)
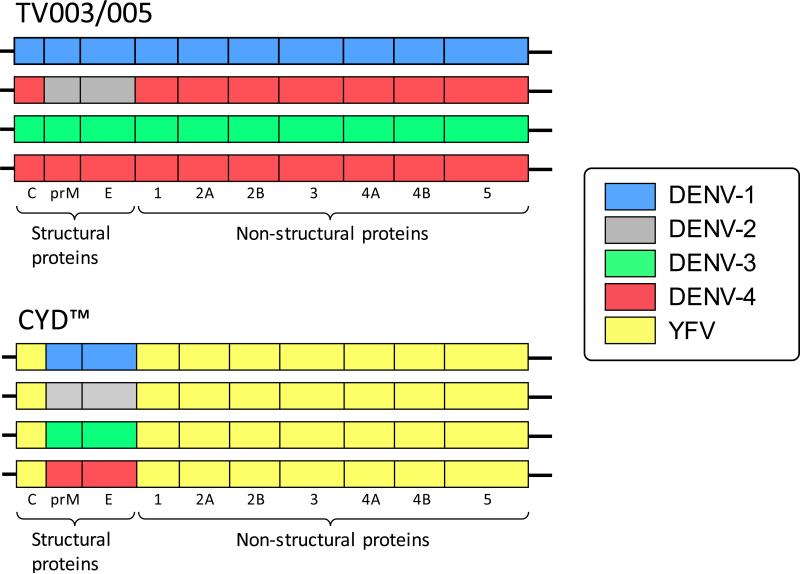
Why the efficacy of CYD™ is reduced in subjects who are sero-negative at the time of vaccination and why it appears to increase the risk of hospitalization over time in children less than 9 years of age remains unclear and is the topic of intense research. The Sanofi Pasteur CYD™ vaccine is comprised of 4 chimeric viruses in which the prM and E proteins of the yellow fever 17D vaccine have been replaced by those of each DENV serotype. The monovalent components of the vaccine each contain the non-structural proteins of the yellow fever 17D virus; they do not contain DENV non-structural proteins (Figure 1). Since the vast majority of the CD8 epitopes for DENV are located in the non-structural proteins, the CYD™ vaccine probably does not induce a significant CD8-mediated immune response against dengue [9,10].
Figure 1.
Graphical representation of the structural and non-structural proteins making up each of the four serotypes that comprise the live attenuated tetravalent dengue vaccines TV003/TV005 and CYD™. The individual viruses are delineated by color (blue = DENV-1, gray = DENV-2, green = DENV-3, red = DENV-4, and yellow = YFV).
The infectivity and resulting immunogenicity induced by the individual components of CYD™ may not be balanced, although these parameters were not studied separately for each individual vaccine component. However, this imbalance is suggested by the need for 3 doses of the vaccine to be given over a 12-month period of time. The majority of live viral vaccines that are administered parenterally (including flavivirus vaccines for YFV and JEV, and multivalent vaccines such as MMR) are suitable for delivery as a single primary dose, although revaccination at a later time may be used to seroconvert those who do not respond to the first dose. The principal reason for use of only a single dose is that the primary dose introduces replicating vaccine virus that elicits a neutralizing antibody that is capable of blocking infection and replication of a second live virus dose. In fact, if a second dose elicits a significant boost in antibody titer, it is likely due to a primary response that is sufficiently weak and unable to neutralize vaccine virus and allows replication following the second dose. In other words, a weak primary dose is incapable of “protecting” against infection with the second dose of live vaccine virus. In the case of CYD™, boosting was apparent after both the second and third dose for most of the DENV serotypes, with the noted exception of DENV-4 [11,12]. Several studies of CYD™ in subjects who were DENV seronegative at the time of vaccination have demonstrated a dominance or greater apparent infectivity of the DENV-4 component of the vaccine as measured by vaccine viremia and the neutralizing antibody response after the first dose [12-14]. In 101 dengue-virus-naïve adult subjects who received CYD™, the geometric mean titer (GMT) of neutralizing antibody to DENV-1 following the first dose was only 9.97 (95% CI: 8.11-12.3) and to DENV-2 was only 16.7 (95% CI: 12.3-22.6) [12]. This contrasts with a GMT to DENV-3 of 45.5 (95% CI: 34-60.9) and to DENV-4 of 438 (95% CI: 273 -704). Frequencies of seroconversion to DENV-1 and DENV-2 were 33% and 48%, respectively compared with those to DENV-3 of 80% and to DENV-4 of 85%. A subset of subjects in this study was selected to determine if vaccine virus could be detected following vaccination. Viremia was detected at day 7 in 57.7% of vaccine recipients, 86.7% of which was due to CYD-4 (the DENV-4 component of CYD™). Only 1/25 subjects had the DENV-2 component recovered following dose 1; and the DENV-1 component was not recovered from any subject. These data indicate that the DENV-4 component of CYD™ is fully infectious and the DENV-1 and DENV-2 components may be less infectious. It is not known if the antibody to the individual vaccine components is homotypic or if it is cross-reactive antibody induced by the highly immunogenic DENV-4 component. This imbalance in the infectivity of the components of CYD™ may be contributing to the lower efficacy of the vaccine to DENV-1 and DENV-2 as well as to the increased risk of hospitalization demonstrated in year 3 of follow-up in those subjects younger than 9 years and who were assumed to be seronegative at the time of vaccination [8]. Fortunately, the relative risk for hospitalization in this age group peaked in year 3 and was seen to decrease during year 4 of follow-up in Thailand. Whether this risk is principally due to a lower quality immune response or less-developed vascular physiology in this age group is the topic of ongoing investigations. Nevertheless, in order to compensate for this imbalance and boost seroconversion to all four DENV serotypes, the vaccine must be administered three times over the course of one year (0, 6, 12 month schedule).
The U.S. National Institute of Allergy and Infectious Diseases Vaccine TV003/TV005
The Laboratory of Infectious Diseases (LID) at the National Institute of Allergy and Infectious Diseases (NIAID) has been engaged in the clinical development of a dengue live attenuated tetravalent vaccine (LATV) for more than fifteen years. Knowing that partial immunity to dengue can increase the risk of more severe disease with subsequent infection, numerous monovalent and tetravalent DENV candidate vaccines were evaluated to identify those candidates with the most favorable safety, infectivity, and immunogenicity profiles. The goal of this program was to develop a live attenuated tetravalent dengue vaccine that could induce protection against all four DENV serotypes with a single dose. Using recombinant DNA technology, two primary attenuation strategies were utilized: deletions in the 3′ untranslated region and structural gene chimerization. The prototype monovalent candidate vaccine was rDEN4Δ30 and contains all the structural and non-structural proteins of a wild type DENV-4, but is attenuated by a 30-nucleotide deletion in the 3′ UTR. Similar removal of the analogous 30 nucleotides from the other DENV serotypes was used to create additional vaccine candidates. In addition, chimeric vaccine candidates were also created by substituting the prM and E genes from each serotype into the rDEN4Δ30 vaccine candidate. Nine of these recombinant monovalent DENV candidate vaccines have been evaluated in healthy, flavivirus-naïve adult volunteers [15]. A total of 5 different tetravalent admixtures or formulations (TV001 – TV005) were evaluated in clinical studies described below (Table 2) [16,17]. The early monovalent trials were extremely informative for development of the final LATV vaccine and provided invaluable data about the clinical, virological, and immunological phenotypes of these viruses when administered singly. Such studies also enabled comparison of the monovalent phenotypes with those observed in the eventual tetravalent formulations. A very important early observation was that chimerization of the viruses is highly attenuating, even over-attenuating in some cases. The first DENV-3 vaccine candidate that was evaluated was a chimeric virus constructed between DENV-3 and the rDEN4Δ30 background. This strategy is similar to that of CYD™ except that the prM and E of DENV-3 were substituted into another DENV virus rather than the YF 17D virus. The resulting virus, rDEN3/4Δ30, was evaluated in humans and found to be very poorly infectious as it induced seroconversion to DENV-3 in only 25% of subjects who were administered a dose of 105 PFU, 100-fold higher than the typical dose of the other monovalent dengue vaccines that were evaluated [15].
Table 2.
Admixtures of four serotypes in the live attenuated tetravalent dengue vaccine formulations
| Admixture | Dose of each component (log10PFU) | Monovalent vaccine component for indicated serotype |
|||
|---|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | ||
| TV001 | 3, 3, 3, 3 | rDEN1Δ30 | rDEN2/4Δ30 | rDEN3-3'D4Δ30 | rDEN4Δ30 |
| TV002 | 3, 3, 3, 3 | rDEN1Δ30 | rDEN2/4Δ30 | rDEN3-3'D4Δ30 | rDEN4Δ30-200,201 |
| TV003 | 3, 3, 3, 3 | rDEN1Δ30 | rDEN2/4Δ30 | rDEN3Δ30/31 | rDEN4Δ30 |
| TV004 | 3, 3, 3, 3 | rDEN1Δ30 | rDEN2/4Δ30 | rDEN3Δ30/31 | rDEN4Δ30-200,201 |
| TV005 | 3, 4, 3, 3 | rDEN1Δ30 | rDEN2/4Δ30 | rDEN3Δ30/31 | rDEN4Δ30 |
Following the failure of rDEN3/4Δ30, two other DENV-3 candidate vaccines were evaluated: rDEN3Δ30/31 and rDEN3-3′D4Δ30. rDEN3Δ30/31 includes a 31 nucleotide deletion in the 3′ UTR in addition to the 30-nucleotide deletion homologous to that in rDEN1Δ30 and rDEN4Δ30. rDEN3-3′D4Δ30 is a chimeric virus with the entire 3′ UTR of rDEN4Δ30 replacing that of DENV-3. Both viruses induced seroconversion to DENV-3 in more than 80% of subjects at a dose of 103 PFU when evaluated as monovalent viruses but rDEN3-3′D4Δ30 appeared to be less infectious than rDEN3Δ30/31 when administered as part of a tetravalent formulation (Table 3) [16]. For this reason, rDEN3Δ30/31 was ultimately chosen as the DENV-3 component for the tetravalent vaccine.
Table 3.
Seroconversion to individual DENV serotypes induced by a single dose of different tetravalent vaccine formulations
| Admixture | N | Days of sero analysis | % seroconversion (PRNT60 ≥ 10) |
|||
|---|---|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | |||
| TV001 | 20 | 421 | 80 | 65 | 60 | 95 |
| TV002 | 20 | 421 | 80 | 60 | 75 | 90 |
| TV003 | 20 | 42 1 | 100 | 50 | 85 | 100 |
| TV003 | 38 | 90 2 | 95 | 763 | 97 | 100 |
| TV004 | 20 | 421 | 75 | 50 | 85 | 85 |
| TV005 | 20 | 42 1 | 80 | 60 | 90 | 100 |
| TV005 | 39 | 90 2 | 92 | 973 | 97 | 97 |
Seroconversion was measured only through study day 42 post-vaccination
Seroconversion was measured through study 90 post-vaccination
p = 0.0068, 2-sided Fisher's Exact
Greater attenuation and reduced infectivity was also observed for another chimeric virus that was evaluated, rDEN2/4Δ30 [18]. Although this virus infected 100% of subjects at a dose of 103 PFU when tested as a monovalent virus, it was the least infectious virus when combined into tetravalent formulations (Table 3) [15,17,19]. Only about 5% of subjects who received any tetravalent formulation other than TV005 developed detectable DENV-2 viremia. rDEN1Δ30, rDEN3Δ30/31, and rDEN4Δ30 were each recovered from ≥ 20% of subjects who received a tetravalent formulation in which they were included. In addition, rDEN2/4Δ30 induced a somewhat lower frequency of seroconversion compared to the other components of the tetravalent formulations (Table 3). Dose ranging studies to determine the 50% infectious dose in humans (HID50) of the monovalent vaccine candidate viruses demonstrated the HID50 for both of the two chimeric viruses, rDEN2/4Δ30 and rDEN3/4Δ30, was ≥ 10 PFU, while the HID50 for rDEN1Δ30, rDEN3Δ30/31, and rDEN4Δ30 was well below 10 PFU (10 PFU infected ≥ 90% of subjects) [15,20].
Balanced infectivity for all four components of the LATV is essential to ensure the induction of homotypic antibody to each of the four DENV serotypes. To overcome the greater attenuation and reduced infectivity of the rDEN2/4Δ30 component of the LATV that was observed in TV001 – TV004, two strategies were developed. The first strategy was to increase the dose of rDEN2/4Δ30 by 10-fold in the tetravalent formulation. TV003 and TV005 share the same four monovalent components, however, in TV003 each component is given at the same dose (103 PFU), while in TV005 rDEN2/4Δ30 is given at a dose of 104 PFU (Table 2). The second strategy was to increase the period for serological evaluation from 42 to 90 days post-vaccination. Increasing the dose of rDEN2/4Δ30 by 10-fold was effective in overcoming the higher HID50 of the vaccine component as a significantly higher percentage of TV005 recipients had detectable rDEN2/4Δ30 virus in the blood following vaccination than did those who received TV003 (Table 4) [17,21]. In addition, the frequency of seroconversion to DENV-2 also improved significantly from 76% in those who received TV003 to 97% in those who received TV005 (p = 0.006806) when seroconversion was evaluated through 90 days post vaccination (Table 3) [17]. The overall frequency of tetravalent antibody response following a single dose of vaccine was also improved, increasing from 74% with TV003 to an unprecedented 90% with TV005 (Table 5) [17].
Table 4.
Frequency of detectable viremia for individual components of LATV in flavivirus-naive subjects
Table 5.
Percent and cumulative neutralizing antibody responses following a single dose of TV003 or TV005 in study CIR 268 and CIR 279
| % vaccinees with multivalent response (cumulative) |
||||||
|---|---|---|---|---|---|---|
| Admixture (Study) | N | Tetra | Tri | Bi | Mono | None |
| TV003 (CIR 268)1 | 20 | 45 (45) | 45 (90) | 10 (100) | 0 (100) | 0 |
| TV005 (CIR 268)1 | 20 | 60 (60) | 30 (90) | 0 (90) | 10 (100) | 0 |
| TV003 (CIR 279)2 | 38 | 74 (74) | 18 (92) | 8 (100) | 0 | 0 |
| TV005 (CIR 279)2 | 39 | 90 (90) | 8 (98) | 0 (98) | 2 (100) | 0 |
Based on PRNT60. Seropositive = PRNT60 of ≥1:10, peak titer through study day 42
Based on PRNT50. Seropositive = PRNT50 of ≥1:10, peak titer through study day 90
In the context of antiviral vaccination, sterilizing immunity can defined as inhibition or neutralization of subsequent infection by the virus against which one was vaccinated, with infection being evident by either detectable viremia or a significant boost (> 4-fold) in antibody titer. Although induction of sterilizing immunity is not always necessary to achieve the goal of reduced disease and morbidity, it is a quantifiable clinical outcome and an indicator of vaccine “take”. As described above, CYD™ requires 3 doses to be given over a 12-month period of time. Viremia, particularly of the DENV-4 component, was observed after the first dose (as expected for a live vaccine) and to a lesser extent, after the second and third doses [12-14]. The second and third doses also resulted in a boost in antibody titer to all DENV serotypes except DENV-4. These data indicate CYD™, when given to dengue-naïve individuals, is not able to induce sterilizing immunity against later infection with itself, a live attenuated virus. It is therefore not surprising that the efficacy of CYD™ was somewhat lower in dengue-naïve vaccine recipients. To determine if breakthrough infection would occur as was observed with CYD™, the effect of a second dose of TV003 or TV005 given 6 months after the first dose was evaluated [17]. Following the first dose of TV003, 75% of vaccine recipients had detectable vaccine virus in the blood and 62% developed a characteristic vaccine-associated rash. Seventy-seven percent of TV005 recipients developed detectable viremia and 62% developed a vaccine-associated rash following the first dose. Following the second dose of TV003 or TV005 administered 6 months later, neither rash nor viremia was observed in TV003 recipients and only a single TV005 recipient had detectable virus for one day (0.5 log10 PFU of rDEN3Δ30/31). Following the second dose of vaccine, there was a ≤ 2-fold increase in mean antibody titer to each of the four DENV serotypes compared with the nadir titer at Study Day 180, just prior to second vaccination (Table 6). Similar results were observed for a second dose of TV003 administered 12 months after the first (manuscript submitted). These results indicated that the vaccine was able to induce nearly complete sterilizing immunity in all vaccinated subjects for at least twelve months.
Table 6.
Neutralizing antibody titers following the first and second dose of TV003 or TV005 given 180 days apart
| Admixture | N | Dose | Day | Mean peak neutralizing antibody titer (Median)1 |
|||
|---|---|---|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | ||||
| TV003 | 38 | 1 | 28 – 90 | 63(58) | 39 (50) | 83 (72) | 144 (152) |
| 34 | 1 | 180 | 13(12) | 11 (9) | 37 (33) | 38 (41) | |
| 34 | 2 | 208 – 270 | 18 (15) | 23 (31) | 38 (53) | 78 (76) | |
| TV005 | 39 | 1 | 28 – 90 | 35 (32) | 91 (99) | 100 (99) | 205 (218) |
| 33 | 1 | 180 | 10 (9) | 35 (40) | 25 (28) | 40 (40) | |
| 33 | 2 | 208 – 270 | 15 (15) | 55 (65) | 36 (34) | 75 (74) | |
PRNT50 expressed as reciprocal geometric mean titer.
Although the neutralizing antibody response (and T-cell response) to TV003 and TV005 was measured 180 days after vaccination and shown to be sufficient to block boosting following subsequent vaccine administration, it is not known if these observed antibody levels will be protective against natural DENV infection. To more stringently assess the protective efficacy induced by a single dose of TV003 or TV005, a DENV-2 challenge model in humans was developed. Challenge strain rDEN2Δ30 was first evaluated in 10 healthy flavivirus-naïve subjects (Clinicaltrials.gov NCT01931176). Virus was recovered from 100% of inoculated subjects with a mean peak titer of 2.5 log10 PFU/mL serum (range 1.5 – 3.3 log10 PFU/mL). Although no subject developed dengue fever, 80% of subjects developed a diffuse macular-papular rash that was graded as moderate in 50% of those affected and 40% of subjects developed neutropenia that was also graded as moderate in 50% of those affected (manuscript submitted). To assess the protective efficacy of TV003 against DENV-2 infection, subjects were challenged with rDEN2Δ30 six months after receipt of TV003 (clinicaltrials.gov NCT02021968). TV003 provided complete protection against viremia, rash, and neutropenia induced by rDEN2Δ30 (manuscript submitted). A second study evaluating the protective efficacy of TV005 against DENV-2 challenge is ongoing (clinicaltrials.gov NCT02317900). A DENV-3 challenge model is also currently under development.
Conclusion
DENV is unique in that partial immunity to one or more serotypes may not only fail to confer protection, it may actually predispose individuals to more severe disease upon subsequent infection with a second, heterotypic DENV. This characteristic makes development of a safe and effective dengue vaccine challenging. The goal of a dengue vaccine is to simultaneously induce homotypic immunity to each of the four DENV serotypes. It is believed that cross-reactive, heterotypic antibody is poorly neutralizing (at least in the long term) and may contribute to enhanced DENV infection leading to more severe disease upon secondary infection [3,22,23]. For this reason, the ideal dengue vaccine should induce solid homotypic immunity to each of the four DENV serotypes. When subjects are inoculated with a tetravalent vaccine, it is difficult to assess how much of the measured neutralizing antibody is homotypic and how much antibody is heterotypic. In the absence of complicated processes, such as antibody depletions, the current assays also do not effectively measure neutralizing and enhancing antibody in a single assay, making it difficult to assess the overall neutralization capacity of serum from individual vaccine recipients. For these reasons, it was essential to carefully assess the infectivity and immunogenicity of each component of the LATV dengue vaccines to ensure these responses were relatively balanced. Of the five LATV formulations that were evaluated, TV003 and TV005 induced the most balanced neutralizing antibody response. Because the rDEN2/4Δ30 component appeared to be less infectious than the other components of TV003, based on viremia and the frequency of seroconversion, the dose of rDEN2/4Δ30 was increased 10-fold in TV005. This increase in dose improved the replication and subsequent immunogenicity of rDEN2/4Δ30 and resulted in a more balanced response. Although not definitive, the infectivity and immunogenicity data support the conclusion that a homotypic antibody response is being generated by each of the components of the tetravalent formulation. This hypothesis is supported by the inability of a second dose of TV003 or TV005 to induce viremia, rash, or a boost in antibody titer when given 6 or 12 months after the first dose.
The ability of a single dose of TV003 to TV005 to prevent infection by a second dose of vaccine when administered 6 or 12 months after the first dose is clearly different from what is observed with CYD™. Not only are the second and third doses of CYD™ still able to infect, they are required to induce seroconversion to all four DENV serotypes in >95% flavivirus-naïve subjects [12]. Vaccination with a live attenuated vaccine against DENV not only elicits protective neutralizing antibodies, but it also elicits an antigen-specific T cell response with activated CD8 cells exhibiting antiviral effector phenotypes [24]. Both TV003 and TV005 contain the wild type nonstructural proteins of three of the four DENV serotypes. This is also a key difference between those candidate vaccines and CYD™, which does not contain any DENV non-structural proteins. Following a primary DENV infection, the CD8+ T cell response is induced against serotype-specific epitopes residing primarily in the non-structural proteins. However, following a secondary DENV infection, there is a shift in the CD8+ T cell response to epitopes conserved across serotypes [9]. A comprehensive analysis of the CD8+ responses from samples collected in the hyper-endemic region of Sri Lanka demonstrated that low T-cell responses correlated with disease susceptibility and that a strong, multifunctional CD8+ T cell response was associated with protection from severe disease [9]. Although each of the components of TV003/TV005 induces a predominantly serotype-specific CD8+ T cell response when administered as monovalent vaccines, the tetravalent vaccine induces a multifunctional T cell response that is directed against highly conserved CD8+ epitopes [25]. Although the risk for more severe disease is associated with a second, heterotypic DENV infection, there is no such association with a third or fourth DENV infection. In fact, it appears that the risk for severe disease is much lower for a third or fourth DENV infection [26,27].The CD8+ T cell response elicited by the LATV dengue TV003 is very similar to that observed after multiple natural DENV infections suggesting that it would induce a T cell response protective against severe disease.
Although it is unknown if the tetravalent antibody response induced by both TV003 and TV005 is comprised of homotypic antibody to each of the four serotypes, the balanced infectivity of both vaccines would suggest this is the case. In addition, the cellular immune response induced by TV003 appears to be directed to CD8+ epitopes that are highly conserved across the four DENV serotypes [25]. The highly infectious phenotype of each of the monovalent components and the presence of DENV wild type non-structural proteins in TV003 and TV005 are critical to their ability to induce a balanced humoral and cellular immune response and differentiates these vaccines from CYD™. TV003/TV005 are currently being evaluated in endemic populations in Thailand and Brazil. The vaccine has been licensed to in-country manufacturers in Brazil (Instituto Butantan), Vietnam (Vabiotech), and India (Panacea Biotec and Serum Institute of India). The Instituto Butantan is completing a Phase 2 trial in both dengue sero-negative and sero-positive adults and is planning to begin a Phase 3 trial in Q4 2015. The phase 3 clinical trial will evaluate the efficacy of a single dose of TV003 in adult subjects down to children 1 year of age. In addition, Merck & Co. has licensed the vaccine for worldwide distribution and is beginning clinical development, and GSK has licensed the vaccine strains for use in their purified inactivated vaccine product.
Expert Commentary
This is a very exciting time for dengue vaccine development. The first dengue candidate vaccine, CYD™, will likely be licensed in 2015/2016. Although the vaccine demonstrated very good efficacy against severe dengue and hospitalized dengue in the first year, it demonstrated variable overall efficacy against symptomatic dengue of any severity depending upon the region is which it was evaluated (Asia or Latin America), the age of those vaccinated (poorer efficacy in children < 9 years), and by sero-status at vaccination (much lower efficacy in those who are dengue-naïve at first vaccination). In addition, there was an increased risk of hospitalization in the first year of the long-term safety follow-up period in subjects who were < 9 years of age at vaccination. The etiology of these observations is not known but is currently an active area of research. It is not known if these are unique to the CYD™ vaccine because of its mechanism of attenuation, or if similar observations will be seen with other dengue vaccines currently in clinical evaluation. Several candidate dengue vaccines are in Phase 1 and Phase 2 clinical trials with two LATV dengue vaccine candidates scheduled to begin Phase 3 efficacy trials very soon. Second-generation dengue vaccines will likely demonstrate improvements over the current lead candidate such as improved efficacy (overall and in younger children), reduced dosing schedule, and inclusion of younger children, perhaps as young as 1 year, in their target population. Long-term safety follow-up is essential for all dengue candidate vaccines, regardless of the type of vaccine (live attenuated, sub-unit protein, purified inactivate) to assess for waning immunity and increased risk of dengue over time in vaccinated subjects. In addition, it would be helpful to include overall efficacy determinations for more than the 12-month efficacy period in CYD23, CYD14, and CYD15 to better assess for waning protection against dengue. No correlate of protection or correlate of risk has been identified from these trials. Adequate sample collection should also be included in the study design so that samples are available to investigate the role of post-vaccination immune responses in both protection and association with long-term safety issues that may be identified. .
Five-year view
One or more dengue vaccines will be licensed within the next five years. Through Phase 3 efficacy studies and perhaps, dengue human challenge models, we will have a better understanding of dengue correlates of protection and risk and this will better inform the long-term strategy for dengue vaccine introduction and dengue control.
Key Issues.
Given the moderate overall efficacy and higher efficacy against hospitalization of the CYD™ vaccine, it is hoped that the vaccine can still have an overall impact on control of disease despite safety concerns in young vaccinees.
Long-term follow-up in the CYD™ vaccine studies was instrumental in identifying safety concerns that are currently being investigated.
In terms of overall infectivity for dengue naïve subjects, it appears that the NIAID dengue vaccine can be administered as a single dose without the requirement for immunological priming from prior DENV exposure.
The NIAID dengue vaccine elicits a robust, multifunctional T cell response that is directed against highly conserved CD8+ epitopes located primarily in the non-structural proteins of DENV and is likely to augment the humoral immune response.
The pioneering work on the Sanofi Pasteur CYD™ vaccine has paved the way for future vaccine efficacy studies to move forward safely with specific attention to details related age, pre-existing DENV immunity, and circulating DENV serotypes.
Acknowledgments
The NIAID research described in this review was supported by the Intramural Research Program of the NIH. SS Whitehead is a co-inventor of intellectual property owned by the US Department of Health and Human Services relating to TV005 and other formulations of the NIAID tetravalent dengue vaccine. The Laboratory of Infectious Diseases at NIAID has entered into a collaborative research agreement with Merck for co-development of the TV005 vaccine.
Footnotes
Financial and competing interests disclosure
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
REFERENCES
* Of interest
** Of considerable interest
- 1.Sabin AB, Schlesinger RW. Production of immunity to dengue with virus modified by propagation in mice. Science. 1945;101:640–642. doi: 10.1126/science.101.2634.640. [DOI] [PubMed] [Google Scholar]
- 2.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. The American journal of tropical medicine and hygiene. 1988;38(1):172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 3.Kliks S. Antibody-enhanced infection of monocytes as the pathogenetic mechanism for severe dengue illness. AIDS Res Hum Retroviruses. 1990;6(8):993–998. doi: 10.1089/aid.1990.6.993. [DOI] [PubMed] [Google Scholar]
- 4.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–467. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 5*.Sabchareon A, Wallace D, Sirivichayakul C, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380(9853):1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [Efficacy data for CYD™ vaccine in Thailand.] [DOI] [PubMed] [Google Scholar]
- 6*.Villar L, Dayan GH, Arredondo-Garcia JL, et al. Efficacy of a Tetravalent Dengue Vaccine in Children in Latin America. The New England journal of medicine. 2014;372(2):113–123. doi: 10.1056/NEJMoa1411037. [Phase III efficacy data for CYD™ in the Americas.] [DOI] [PubMed] [Google Scholar]
- 7*.Capeding MR, Tran NH, Hadinegoro SRS, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. The Lancet. 2014;384(9951):1358–1365. doi: 10.1016/S0140-6736(14)61060-6. [Phase III efficacy data for CYD™ vaccine in Asia.] [DOI] [PubMed] [Google Scholar]
- 8**.Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. The New England journal of medicine. 2015;373(13):1195–1206. doi: 10.1056/NEJMoa1506223. [Presents data for long-term safety evaluation of CYD™ vaccine.] [DOI] [PubMed] [Google Scholar]
- 9.Weiskopf D, Angelo MA, de Azeredo EL, et al. Comprehensive analysis of dengue virus- specific responses supports an HLA-linked protective role for CD8+ T cells. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1305227110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiskopf D, Sette A. T cell immunity to infection with dengue virus in humans. Frontiers in immunology. 2014;5 doi: 10.3389/fimmu.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villar LA, Rivera-Medina DM, Arredondo-Garcia JL, et al. Safety and Immunogenicity of a Recombinant Tetravalent Dengue Vaccine in 9-16 Year Olds: A Randomized, Controlled, Phase II Trial in Latin America. Pediatr Infect Dis J. 2013;32(10):1102–1109. doi: 10.1097/INF.0b013e31829b8022. [DOI] [PubMed] [Google Scholar]
- 12.Dayan GH, Thakur M, Boaz M, Johnson C. Safety and immunogenicity of three tetravalent dengue vaccine formulations in healthy adults in the USA. Vaccine. 2013;31(44):5047–5054. doi: 10.1016/j.vaccine.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 13.Poo J, Galan F, Forrat R, Zambrano B, Lang J, Dayan GH. Live-attenuated Tetravalent Dengue Vaccine in Dengue-naive Children, Adolescents, and Adults in Mexico City: Randomized Controlled Phase 1 Trial of Safety and Immunogenicity. Pediatr Infect Dis J. 2011;30(1):e9–e17. doi: 10.1097/INF.0b013e3181fe05af. [DOI] [PubMed] [Google Scholar]
- 14.Morrison D, Legg TJ, Billings CW, Forrat R, Yoksan S, Lang J. A novel tetravalent dengue vaccine is well tolerated and immunogenic against all 4 serotypes in flavivirus-naive adults. J Infect Dis. 2010;201(3):370–377. doi: 10.1086/649916. [DOI] [PubMed] [Google Scholar]
- 15*.Durbin AP, Kirkpatrick BD, Pierce KK, Schmidt AC, Whitehead SS. Development and clinical evaluation of multiple investigational monovalent DENV vaccines to identify components for inclusion in a live attenuated tetravalent DENV vaccine. Vaccine. 2011;29(42):7242–7250. doi: 10.1016/j.vaccine.2011.07.023. [Provides background material related to the development of the NIAID vaccine candidates.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durbin AP, Kirkpatrick BD, Pierce KK, et al. A Single Dose of Any of Four Different Live Attenuated Tetravalent Dengue Vaccines Is Safe and Immunogenic in Flavivirus-naive Adults: A Randomized, Double-blind Clinical Trial. J Infect Dis. 2013;207(6):957–965. doi: 10.1093/infdis/jis936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Kirkpatrick BD, Durbin AP, Pierce KK, et al. Robust and Balanced Immune Responses to All 4 Dengue Virus Serotypes Following Administration of a Single Dose of a Live Attenuated Tetravalent Dengue Vaccine to Healthy, Flavivirus-Naive Adults. J Infect Dis. 2015;212(5):702–710. doi: 10.1093/infdis/jiv082. [Comparison of NIAID vaccines TV003 and TV005 and its suitability as a single dose vaccine.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitehead SS, Hanley KA, Blaney JE, Jr., Gilmore LE, Elkins WR, Murphy BR. Substitution of the structural genes of dengue virus type 4 with those of type 2 results in chimeric vaccine candidates which are attenuated for mosquitoes, mice, and rhesus monkeys. Vaccine. 2003;21(27-30):4307–4316. doi: 10.1016/s0264-410x(03)00488-2. [DOI] [PubMed] [Google Scholar]
- 19.Durbin AP, McArthur JH, Marron JA, et al. rDEN2/4Delta30(ME), A Live Attenuated Chimeric Dengue Serotype 2 Vaccine Is Safe and Highly Immunogenic in Healthy Dengue-Naive Adults. Hum Vaccin. 2006;2(6):255–260. doi: 10.4161/hv.2.6.3494. [DOI] [PubMed] [Google Scholar]
- 20.Durbin AP, Whitehead SS, McArthur J, et al. rDEN4 Delta 30, a Live Attenuated Dengue Virus Type 4 Vaccine Candidate, Is Safe, Immunogenic, and Highly Infectious in Healthy Adult Volunteers. J Infect Dis. 2005;191(5):710–718. doi: 10.1086/427780. [DOI] [PubMed] [Google Scholar]
- 21.Durbin AP, Kirkpatrick BD, Pierce KK, et al. American Society of Tropical Medicine and Hygiene 59th Annual Meeting. American Society of Tropical Medicine and Hygiene; Atlanta, GA: 2010. Evaluation of the safety and immunogenicity of Tetravax-DV, a live attenuated tetravalent dengue vaccine. Abstract 248. [Google Scholar]
- 22.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. The American journal of tropical medicine and hygiene. 1988;38(2):411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 23.Halstead SB, Porterfield JS, O'Rourke EJ. Enhancement of dengue virus infection in monocytes by flavivirus antisera. The American journal of tropical medicine and hygiene. 1980;29(4):638–642. doi: 10.4269/ajtmh.1980.29.638. [DOI] [PubMed] [Google Scholar]
- 24.Rivino L, Kumaran EA, Thein TL, et al. Virus-specific T lymphocytes home to the skin during natural dengue infection. Science translational medicine. 2015;7(278):278ra235. doi: 10.1126/scitranslmed.aaa0526. [DOI] [PubMed] [Google Scholar]
- 25.Weiskopf D, Angelo MA, Bangs DJ, et al. The human CD8+ T cell responses induced by a live attenuated tetravalent dengue vaccine are directed against highly conserved epitopes. J Virol. 2015;89(1):120–128. doi: 10.1128/JVI.02129-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibbons RV, Kalanarooj S, Jarman RG, et al. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. The American journal of tropical medicine and hygiene. 2007;77(5):910–913. [PubMed] [Google Scholar]
- 27.Olkowski S, Forshey BM, Morrison AC, et al. Reduced risk of disease during postsecondary dengue virus infections. J Infect Dis. 2013;208(6):1026–1033. doi: 10.1093/infdis/jit273. [DOI] [PMC free article] [PubMed] [Google Scholar]