Abstract
BACKGROUND
The treatment of dolichoectatic basilar trunk aneurysms has been ineffectual or morbid due to non-saccular morphology, deep location, and involvement of brainstem perforators. Treatment with bypass surgery has been advocated to eliminate malignant hemodynamics and stabilize aneurysm growth.
OBJECTIVE
To validate that flow alteration with bypass and parent artery occlusion favorably impacts aneurysm progression.
METHODS
Surgical management evolved in 3 phases, each with different hemodynamic alterations.
RESULTS
During a 17-year period, 37 patients with dolichoectatic basilar trunk aneurysms were retrospectively identified, of whom 21 patients were observed, 12 treated immediately, and 4 selected for treatment after clinical progression. In Phase 1, flow reversal was overly thrombogenic, despite heparin (N=5, final mortality, 100%). In Phase 2, flow reduction with IC-IC bypass was safer than flow reversal but did not prevent progressive aneurysm enlargement (N=3, final mortality 67%). In Phase 3, distal clip occlusion of the basilar trunk aneurysm preserved anterograde flow in the aneurysm without rupture, but reduced flow threatened perforator patency, despite treatment with Clopidogrel (Plavix) (N=8, final mortality 62%).
CONCLUSION
Shifting treatment strategy for dolichoectatic basilar trunk aneurysms improved surgical (80% to 50%) and final mortalities (100% to 62%), with stabilization of aneurysms in the Phase 3 survivors. Good outcomes are determined by perforator preservation and mitigating aneurysm thrombosis. Occlusion techniques with increased distal run-off appear to benefit perforators. The treatment of dolichoectatic basilar trunk aneurysms can advance through concentrated management in dedicated centers, concerted efforts to study morphology/hemodynamics with computational methods, and widespread collection of registry data.
Keywords: Atherosclerosis, basilar artery aneurysm, basilar trunk, bypass, dolichoectasia, giant aneurysm, fusiform aneurysm, revascularization
“Diseases desperate grown must by desperate appliance be relieved, or not at all.”
- Shakespeare, from Hamlet, Act IV, Scene 3
There is no aneurysm more daunting than the dolichoectatic basilar trunk aneurysm. The pathogenesis of this aneurysm is not clear, but it appears to originate from circumferential atherosclerotic degeneration of the basilar artery, resulting in dilatation, elongation, and dolichoectasia. Progressive structural and morphological changes in the arterial walls are accompanied by hemodynamic changes, generating regions of abnormal flow velocity, wall shear stress, vorticity, turbulence, and stagnation, which in turn might exacerbate arterial degeneration through degradation, thrombus formation, and inflammation.1, 2 In addition to these structural, morphological, and hemodynamic changes, this disease may be fueled by anatomical variations, like the absence of posterior communicating arteries (PCoA) and a competent circle of Willis, or by other diseases, like arterial dissection or dysplasia. This pathogenesis can initiate a vicious cycle of clinical deterioration that results in aneurysm enlargement, brainstem and cranial nerve compression, thinning of the basilar arterial walls with aneurysmal rupture, and/or intraluminal thrombosis with perforator infarctions and embolic strokes. Patients may experience progressive neurological deficits from a growing mass or hydrocephalus, the sudden ictus of subarachnoid hemorrhage, or a stepwise decline from multiple strokes. Dolichoectatic basilar trunk aneurysms have a dismal natural history, with some estimates of 2-year survival rates as low as 20%.3
The treatment of these lesions has been either ineffectual or staggeringly morbid. Unlike saccular aneurysms with clippable necks, dolichoectatic aneurysms are unclippable and their location makes them poorly accessible surgically. Reconstruction with clips is possible with dolichoectatic aneurysms that are eccentric or involve only a portion of the arterial wall, but such lesions are rare and it is difficult to exclude all abnormal tissue while preserving perforators. These lesions are often accessible endovascularly, but they are equally uncoilable and adjunctive stents or balloon remodeling techniques fail to improve their coilability. Hunterian ligation of one or both vertebral arteries (VA) or the proximal basilar artery was practiced by Charles Drake,4 but this treatment depended on a sizable PCoA (> 2mm diameter) and its long-term efficacy was poorly established. Some have performed arterial transposition to decompress the brainstem or cranial nerves,5 but dolichoectatic basilar arteries are often immobile and, at best, this intervention offers only symptomatic relief temporarily. Advancements in endovascular devices have led to recent attempts to treat these aneurysms with flow diverters, but early results have been poor and have not matched the excellent results observed at other locations lacking perforators.3, 6–15 Failed efforts to treat this disease have led many to conclude that this problem is unsolvable, and that these patients should be managed medically with aspirin, blood pressure control, and cholesterol-lowering agents, and possibly with ventriculoperitoneal shunts when hydrocephalus becomes symptomatic.
Treatment of dolichoectatic basilar trunk aneurysms with bypass surgery has been advocated as means of eliminating malignant hemodynamics, redirecting flow away from areas of aneurysmal degeneration, and promoting intraluminal thrombosis, thereby stabilizing aneurysm growth and preventing hemorrhage.16–18 The strategy consists of an extracranial-to-intracranial bypass (EC-IC), usually a superficial temporal artery (STA) bypass to either the superior cerebellar artery (SCA) or posterior cerebral artery (PCA), followed by Hunterian ligation of the VA or basilar trunk proximally. Published experiences with these treatments are limited to case reports and small series, each with small variations in strategy or technique.16, 17, 19–32 These experiences are associated with significant morbidity and no clear solution has been demonstrated. In this report, we add our experience with dolichoectatic basilar trunk aneurysms to the literature. We embraced the concept that flow alteration by means of bypass and parent artery occlusion would favorably impact disease progression, and we sought to validate others’ experiences with this bypass/Hunterian ligation strategy or improve upon it in an effort to find an answer to this disease.
METHODS
Patients
This study was approved by the Institutional Review Board and conducted in compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations. Patients with basilar artery aneurysms were identified from a prospectively maintained database of the Vascular Neurosurgery Service, and those with dolichoectatic basilar trunk aneurysms were included. Dolichoectatic aneurysms were defined as fusiform, circumferential dilatations of the basilar trunk. Patients with aneurysms involving the basilar bifurcation, PCA, and SCA were excluded, as were those with aneurysms involving the vertebral arteries. Patients with saccular aneurysms on the basilar trunk, such as those originating from the anterior inferior cerebellar artery (AICA), vertebrobasilar junction, or basilar perforators, were also excluded. Excluded patients consisted of: 9 patients with saccular basilar trunk aneurysms that were clipped microsurgically (mean diameter, 10 mm); 14 with AICA aneurysms (3 proximal AICA aneurysms and 11 distal aneurysms) that were clipped (n=7) or trapped (n=7); and 2 patients with basilar perforating artery aneurysms that were trapped. Four other surgical patients with basilar trunk dolichoectatic aneurysms were excluded: 3 patients who underwent partial thrombectomy and clip reconstruction, and 1 patient who underwent combined endonasal-transclival/retrosigmoid mobilization of the aneurysm for brainstem decompression.
Medical records, including preoperative and postoperative radiographic imaging, operative reports, intraoperative photographs, videos, hospital course, and outpatient visits, were reviewed retrospectively. Clinicians not directly involved in the patients’ care (AAA and WCR) performed all outcome assessments using the modified Rankin Scale (mRS). Patient outcomes were reported using standard descriptive statistics.
Management Algorithm
The diagnosis of dolichoectatic basilar trunk aneurysm was established with CT scans, magnetic resonance (MR) imaging, and digital subtraction angiography (DSA). Patients with mild or stable symptoms were managed conservatively, whereas those with severe or progressive symptoms were managed surgically (Figure 1). High-resolution contrast-enhanced MR angiography (CE-MRA) generated volumetric models to measure aneurysm growth during observation. In addition, time-resolved phase-contrast MRI (4D PC-MRI) measured aneurysm hemodynamics. Observed patients were imaged annually, and those with aneurysm growth, symptom progression, or rupture were considered for treatment. In contrast, patients presenting with severe or progressive symptoms were immediately considered for treatment. 4D PC-MRI was again used to measure aneurysm hemodynamics and to provide flow boundary conditions for numerical simulations of the altered hemodynamics associated with bypass options.1, 2 Simulations were used to guide the selection of bypasses.
Figure 1.
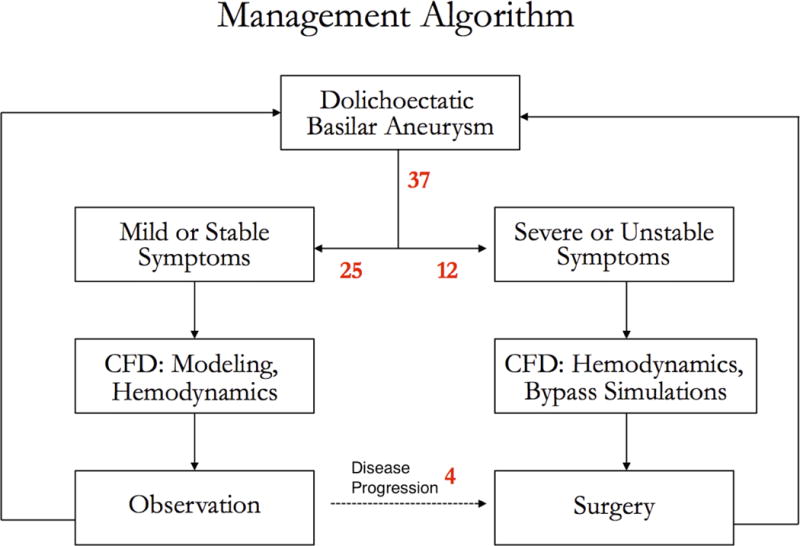
Management algorithm for 37 patients with dolichoectatic basilar trunk aneurysms. CFD = computational fluid dynamics.
Surgical management evolved in 3 distinct phases, each with different exposures, bypasses, methods of aneurysm occlusion, hemodynamic alterations, and postoperative managements (Figure 2 and Table 1). Surgical bypasses consisted of EC-IC bypasses (STA-SCA and STA-PCA bypasses in Phase 1) or intracranial-to-intracranial (IC-IC) bypasses using an interposition graft (VA-SCA in Phase 2 and MCA-PCA bypasses in Phase 3). When the STA was inadequate, the external carotid artery or other cervical donor arteries were used with a saphenous vein graft (SVG). For shorter IC-IC grafts, the radial artery was used preferentially in patients with a competent palmar arch on preoperative Allen testing. Bypasses were performed through orbitozygomatic craniotomies with either subtemporal (Phase 1) or pretemporal/transsylvian approaches (Phase 3), or through a combined far lateral/subtemporal craniotomy for the VA-SCA bypass (Phase 2). The indication for bypass in all patients was the planned occlusion of the aneurysm’s parent artery in the setting of diminutive or absent posterior communicating arteries. Aneurysm occlusion was performed in several ways: endovascularly in a second interventional stage, usually proximally in the vertebral arteries bilaterally (Phase 1); surgically at the time of bypass, in the dominant vertebral artery (Phase 2); or surgically at the time of bypass, at the distal aneurysm outflow, below the basilar apex and proximal to the SCAs (Phase 3).
Figure 2.
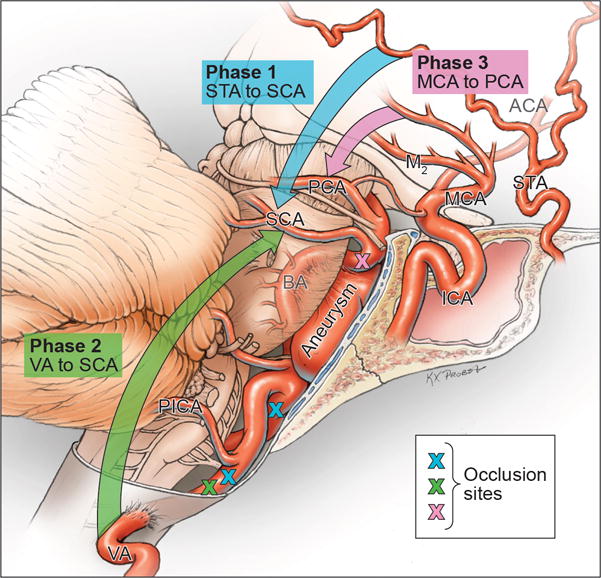
Summary of bypasses used to treat dolichoectatic basilar trunk aneurysms and the evolution in surgical management from extracranial-intracranial bypasses (superficial temporal artery (STA) to superior cerebellar artery (SCA) or posterior cerebral artery (PCA), Phase 1) to intracranial-intracranial bypasses with interposition grafts (V3 segment of vertebral artery (VA) to SCA, Phase 2, and middle cerebral artery (MCA) to PCA, Phase 3).
Table 1.
Summary of phases of management of dolichoectatic basilar trunk aneurysms.
| Phase 1 | Phase 2 | Phase 3 | |
|---|---|---|---|
| Exposure | OZ/Subtemporal | Far Lateral/Subtemporal | OZ/Pretemporal |
| Bypass | EC-IC: STA-SCA, STA-PCA | IC-IC: V3 VA-SCA | IC-IC: MCA-PCA |
| Aneurysm Occlusion | Endovascular (proximal, staged) | Surgical (proximal, dominant VA) | Surgical (distal, basilar artery) |
| Basilar Flow | Retrograde | Anterograde | Anterograde |
| Hemodynamic Alteration | Reversal | Reduction (mild – moderate) | Reduction (severe) |
| Postoperative Management | ASA, Heparin | ASA | ASA, Plavix |
During the anastomosis, heparin solution was irrigated locally. Systemic heparin was not used. Bypasses were checked for patency with indocyanine green (ICG) videoangiography and Doppler ultrasound. During clamp time, patients were maintained in barbiturate- or propofol-induced EEG burst suppression with mild elevation of the systolic arterial blood pressure. Postoperative angiography evaluated patency of the bypass and obliteration of the aneurysm and patients were maintained on ASA 325 mg indefinitely. Endovascular Hunterian occlusions were typically performed 3 days after bypass surgery, and patients were routinely heparinized during and for 3 days after their endovascular procedure (Phase 1). Clopidogrel (Plavix) was used later in the series as described below (Phase 3).
Computational Fluid Dynamics
Patient-specific computational fluid dynamic (CFD) modeling was used to assess postoperative flows that would result from alternative surgical options. High-resolution CE-MRA produced patient-specific vascular geometries of the aneurysm and its proximal and distal vessels. The branches of the basilar trunk, including the AICAs and pontine perforators, could not be imaged because of the limitations in imaging resolution, and idealized geometries of the AICAs and perforators were added, according to modeling procedures described previously.33 CE-MRA data were segmented using in-house software, and three-dimensional surfaces corresponding to the luminal boundaries were obtained for each patient. The idealized geometries of the basilar trunk branches were then added using Geomagic Design software (3D Systems).
Patient-specific inlet flow rates required for the flow computations were measured with through-plane PC-MRI technique, providing flow waveforms prescribing the inlet velocities through the cardiac cycle for each vertebral artery. Our previous studies1 demonstrated that the flow rates ratio of the jets entering from the VA has a major effect on the flow field in the basilar trunk and, subsequently, in the aneurysm. In most of the patients, preoperative flow was also measured with 4D PC-MRI, providing time-resolved velocity field in three dimensions. Numerical flow simulations were conducted in the preoperative vertebrobasilar geometries and compared to in vivo PC-MRI measurements to ensure the accuracy of the computations. Postoperative flow simulations were then carried out for the alternative surgical options. Contrast agent transport was simulated by solving the advection diffusion equation along with the Navier-Stokes equations describing the flow. This numerical technique provided flow residence times and determined intra-aneurysmal regions of flow separation and stagnation, which were found to correlate with thrombus deposition.2
RESULTS
Patients (participants) and Baseline Characteristics
During a 17-year period from September 1997 to September 2014, 37 patients with dolichoectatic basilar trunk aneurysms were identified, of whom 25 had mild or stable symptoms and were observed (Figure 1). The average patient was 63 years old (range, 34 – 80) and there was a male predominance (16 men and 9 women). Patients presented with strokes, brainstem compression, or incidentally during workup for memory loss or headache. The mean mRS at presentation was 1.5 (range, 0 – 4). The mean aneurysm diameter was 1.7 cm (range, 0.4 – 5.0), including 7 giant aneurysms and 9 thrombotic aneurysms.
These patients were observed for a mean duration of 3.7 years (range, 3 months – 11.7 years). During that time, 7 patients (28%) remained neurologically stable, and 11 patients (44%) deteriorated with new disabilities. 5 patients (20%) died of the following causes: myocardial infarction (n=1), complications from surgery (n=1), and unknown (n=3). Two patients (8%) were lost to follow-up. The mean mRS in the observation cohort at last follow-up was 2.8. Changes in mRS scores are shown (Figure 3). Four patients (16%) with deteriorating condition opted for surgical intervention.
Figure 3.
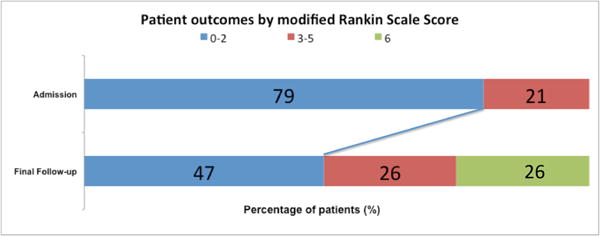
Patient outcomes by modified Rankin Scale scores in the 25 patients with dolichoectatic basilar trunk aneurysms that were managed conservatively initially. The mRS scores at final follow-up refer to those at the end of the observation period, including the preoperative mRS scores of the 4 patients who later went on to surgery. The postoperative mRS scores in these 4 patients are described later in the surgical results. The mRS scores of the 2 patients lost to follow-up were excluded.
Thirteen of the 25 observed patients were studied with CE-MRA. Initial mean luminal aneurysm volume was 851 mm3 (range, 155 – 2231 mm3). Volumetric growth curves demonstrated stable aneurysms in 10 patients and enlargement in 3 patients (Figure 4), who were later selected for surgery.
Figure 4.
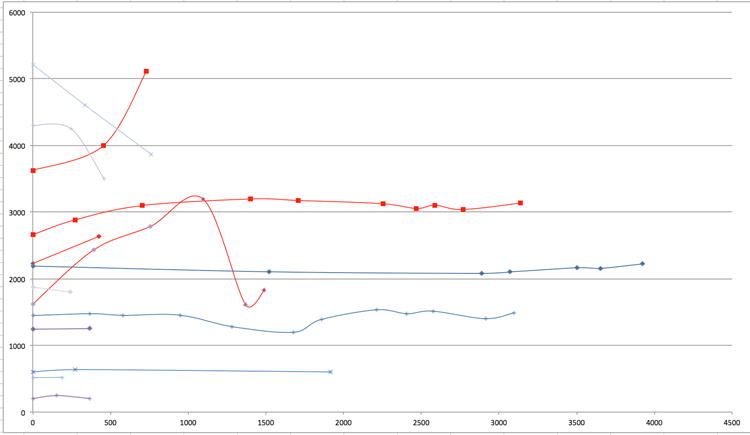
Aneurysm growth curves for 13 patients with observed dolichoectatic basilar trunk aneurysms. X-axis is observation time measured in days and Y-axis is aneurysm volume measured in cubic mm. Decreases in volume correlated with new intra-aneurysmal thrombosis, with or without clinical stroke. Curves in red highlight patients with aneurysm enlargement, of whom one stabilized and 3 went on to surgical intervention.
Sixteen surgical patients had a mean age of 59 years (range, 44 – 76 years) and a male predominance (12 men and 4 women). Presentations included subarachnoid hemorrhage in 3 patients (19%), brainstem compression in 10 patients (63%), cranial neuropathy in 5 patients (31%), stroke in 5 patients (31%), and hydrocephalus in 7 patients (44%). The mean mRS at presentation was 2.6 (range, 1 – 5). The mean aneurysm diameter was 2.7 cm (range, 1.5 – 5.0 cm), including 9 giant aneurysms and 9 thrombotic aneurysms.
Main Results: Surgical Results, Phase 1
In the first management phase, patients were bypassed with an EC-IC bypass consisting of either STA-SCA (n=3) or STA-PCA (n=1) bypass (Table 1, Figure 5). One patient with an inadequate STA required an ECA-SCA bypass with saphenous vein graft. Bypasses were performed through an orbitozygomatic craniotomy with subtemporal exposure of the recipient artery. Surgery was limited to bypass only, with the intent to endovascularly coil occlude the vertebral arteries bilaterally, one proximal to the PICA origin and the other distal to the PICA origin, in order to eliminate aneurysmal inflow, reverse blood flow in the basilar trunk, and use one PICA to draw or sump flow in this retrograde direction. All bypasses were performed successfully and were patent (Table 2). One patient who presented with SAH suffered a fatal re-rupture the night after his surgery. The remaining 4 patients were coiled on the 3rd postoperative day and heparinized to facilitate flow reversal. One patient experienced a fatal SAH 2 days after tolerating her endovascular occlusion (Figure 6). Another developed a sizable subdural hematoma requiring evacuation, and although he recovered from deficits related to the SDH, his family later withdrew support. Two patients developed brainstem and cerebellar infarctions after their coiling procedures from basilar artery thrombosis and expired.
Figure 5.
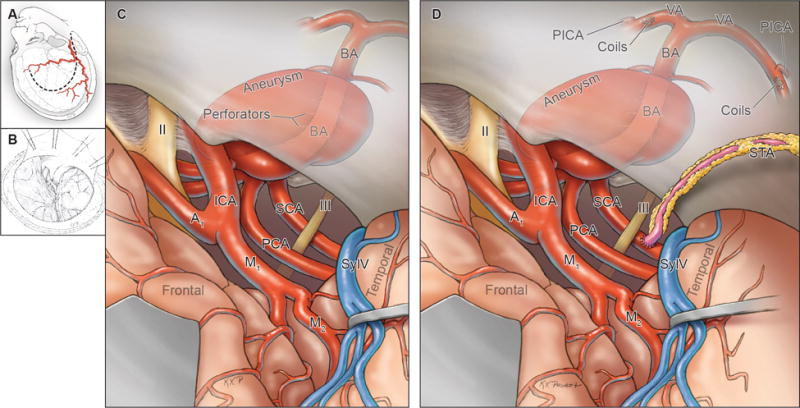
Summary of Phase 1 surgical management (STA to SCA or PCA bypass), with (A) harvest of donor STA, (B) exposure through an orbitozygomatic-pterional craniotomy, (C) exposure and access to recipient SCA or PCA subtemporally or pretemporally, (D) completion of the EC-IC anastomosis (STA to SCA bypass shown), and endovascular occlusion of the vertebral arteries bilaterally in a separate procedural stage. Abbreviations: ICA = internal carotid artery; A1 = anterior cerebral artery, A1 segment; M1 = middle cerebral artery, M1 segment; BA = basilar artery; VA = vertebral artery; PICA = posterior inferior cerebellar artery; STA = superficial temporal artery; SylV = Sylvian vein; II = optic nerve; III = oculomotor nerve.
Table 2.
Summary of results in phases of management of dolichoectatic basilar trunk aneurysms.
| Phase I | Phase II | Phase III | Total | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Treatment | Bypass/Flow Reversal | Bypass/Flow Reduction | Bypass/Distal Occlusion | |||||
| Patients | 5 | 3 | 8 | 16 | ||||
| Bypass Patency | 5 | 100% | 2 | 67% | 8 | 100% | 15 | 94% |
| Surgical Mortality | 4 | 80% | 0 | 0% | 4 | 50% | 8 | 50% |
| Final Mortality | 5 | 100% | 2 | 67% | 5 | 62% | 12 | 75% |
| Survivors | 0 | 0% | 1 | 33% | 3 | 38% | 4 | 25% |
| Final Mean mRS | 6 | 1.0 | 3.3 | 2.8 | ||||
| F/U Durations (yrs) | NA | 8 | 4.5 | 5.4 | ||||
| Aneurysm Stabilization | Unknown | No | Yes | |||||
Figure 6.
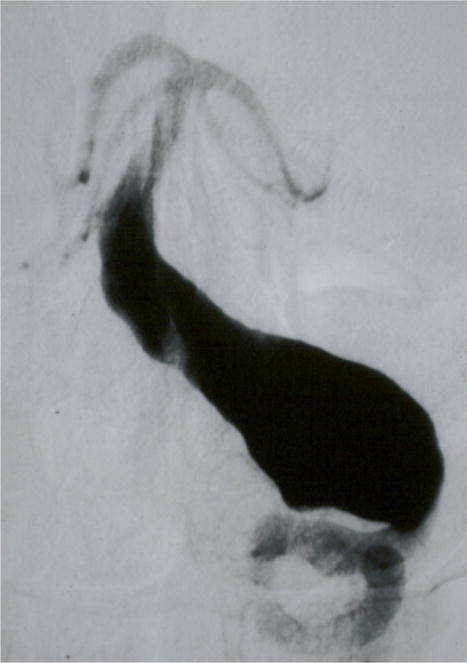
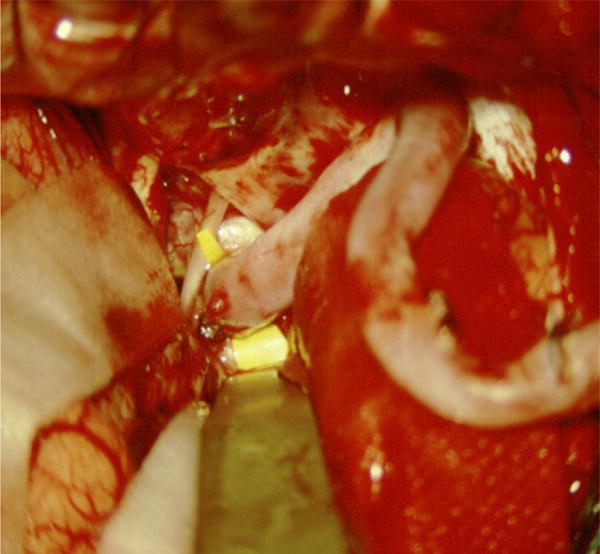
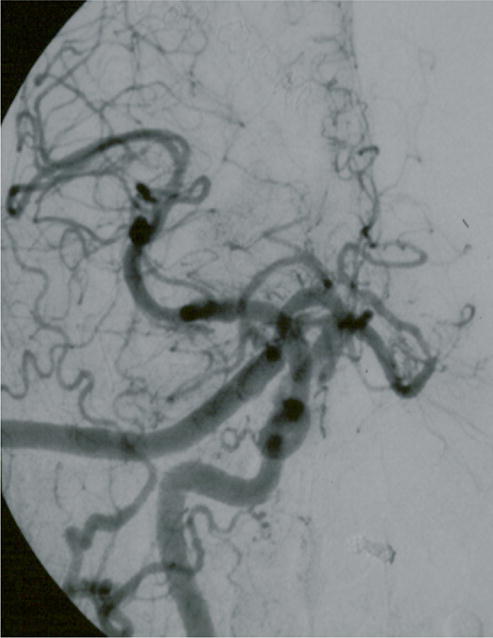
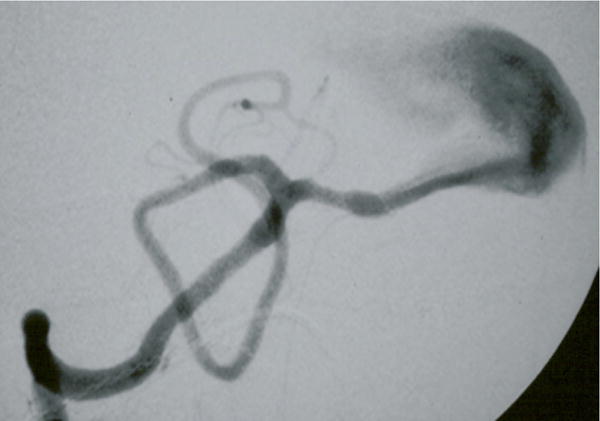
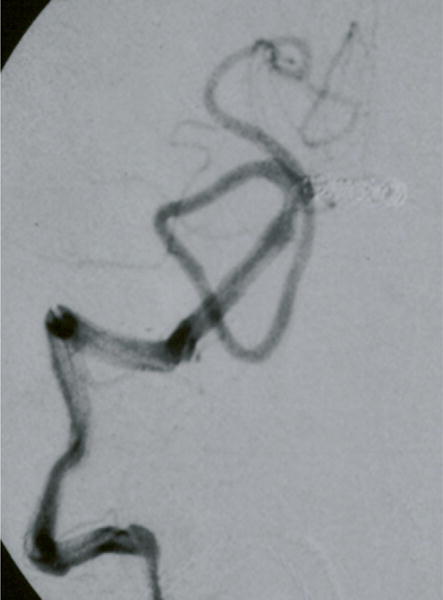
Case example of Phase I surgical management. (A) This 60-year-old woman had progressive brainstem compressive symptoms from this dolichoectatic basilar trunk aneurysm (left VA digital subtraction angiogram, anteroposterior view). (B) A bypass from the right external carotid artery to the SCA with saphenous vein graft was performed, (C) revascularizing the basilar apex (right common carotid angiogram, anteroposterior view) and (D, E) enabling the vertebral arteries to be occluded endovascularly 3 days later while heparinized (right VA angiogram, anteroposterior view, (D) before and (E) after coil occlusion distal to PICA). The patient tolerated her flow reversal for two days, and then experienced a fatal subarachnoid hemorrhage while on heparin.
In summary, although flow reversal with EC-IC bypass and staged endovascular occlusion of proximal inflow has been reported as a treatment option for dolichoectatic basilar trunk aneurysms, this hemodynamic alteration may be too thrombogenic, heparin may be too dangerous in the acute perioperative setting, and the temporal artery may be insufficient to fully revascularize the posterior circulation.
Surgical Results, Phase 2
In the second management phase, patients were bypassed with an IC-IC bypass consisting of a VA-SCA bypass with an interposition graft (n=2) (Figure 7). The dominant VA provided a donor site (proximal V3 segment) and was then occluded distally (distal V3 segment or proximal V4 segment) to reduce inflow to the basilar trunk aneurysm. One patient already had an occluded VA and the subclavian artery was used as the donor artery instead. This strategy was intended to maintain anterograde flow in the basilar trunk but reduce it with the combination of unilateral, dominant VA occlusion and increased distal flow to the basilar apex. The modified park bench position is used and the “hockey stick” incision of the far lateral approach is extended anterosuperiorly in front of the ear to incorporate the subtemporal exposure.
Figure 7.
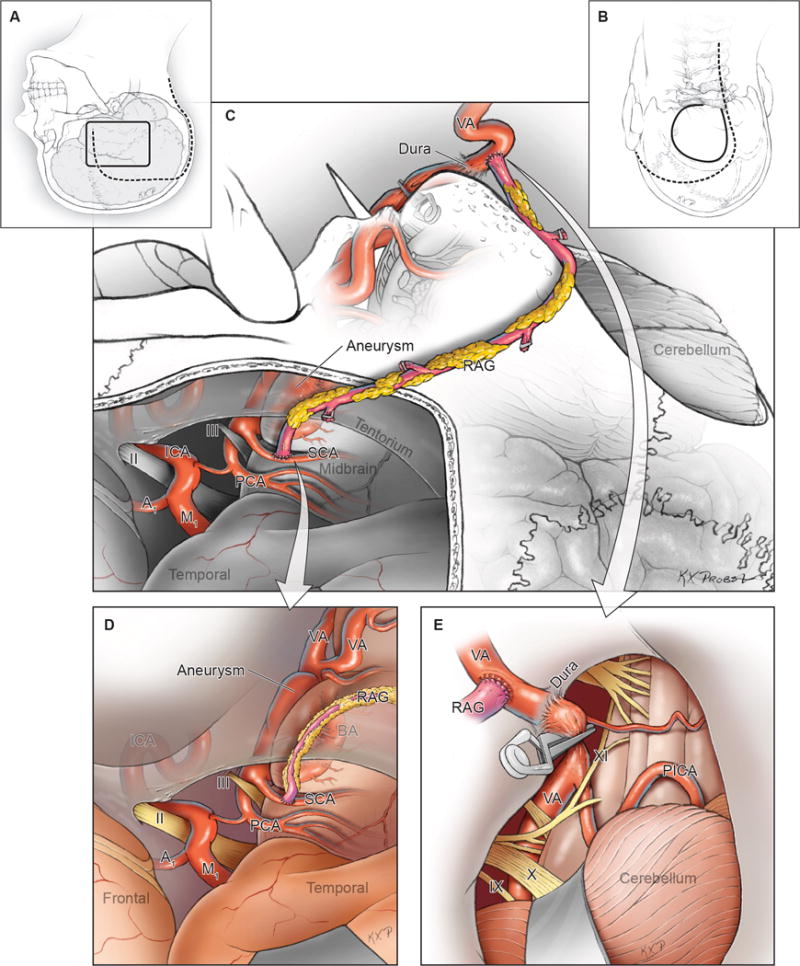
Summary of Phase 2 surgical management (VA to SCA bypass), with combined (A) temporal and (B) far lateral craniotomies through a single incision, (C) exposure of the donor V3 segment of VA and recipient SCA, (D) completion of the graft to SCA anastomosis, and (E) graft to VA anastomosis with clip occlusion of the dominant VA either extradurally (V3) or intradurally (V4). Suboccipital craniectomy and duroplasty creates space to accommodate cerebellar swelling. Abbreviations: ICA = internal carotid artery; A1 = anterior cerebral artery, A1 segment; M1 = middle cerebral artery, M1 segment; BA = basilar artery; VA = vertebral artery; PICA = posterior inferior cerebellar artery; SCA = superior cerebellar artery; PCA = posterior cerebral artery; RAG = radial artery graft; IX = glossopharyngeal nerve; X = vagus nerve; XI = accessory nerve.
The first patient had a reduction in aneurysm luminal volume after the procedure with no neurological complications (Figure 8), but her aneurysm continued to grow with symptom progression. One year postoperatively, follow-up angiography showed the bypass filling only the recipient SCA territory distally. Her contralateral VA was sacrificed endovascularly, resulting in a fatal basilar artery thrombosis. The bypass in the second patient occluded postoperatively due to technical complications: an inability to access his radial artery in the park bench position; the use of an allograft saphenous vein graft instead; and a small, duplicated SCA used as the recipient artery. His bypass occlusion prevented any additional treatment and his aneurysm grew. He died from complications related to an aspiration pneumonia one year postoperatively, and experienced progressive deficits during that year. The third patient presented with a ruptured aneurysm involving the upper basilar trunk that was clip reconstructed after performing her bypass – the only clip reconstruction in the series. She recovered completely and is free of aneurysm recurrence 8 years after surgery. None of these patients was treated with heparin.
Figure 8.
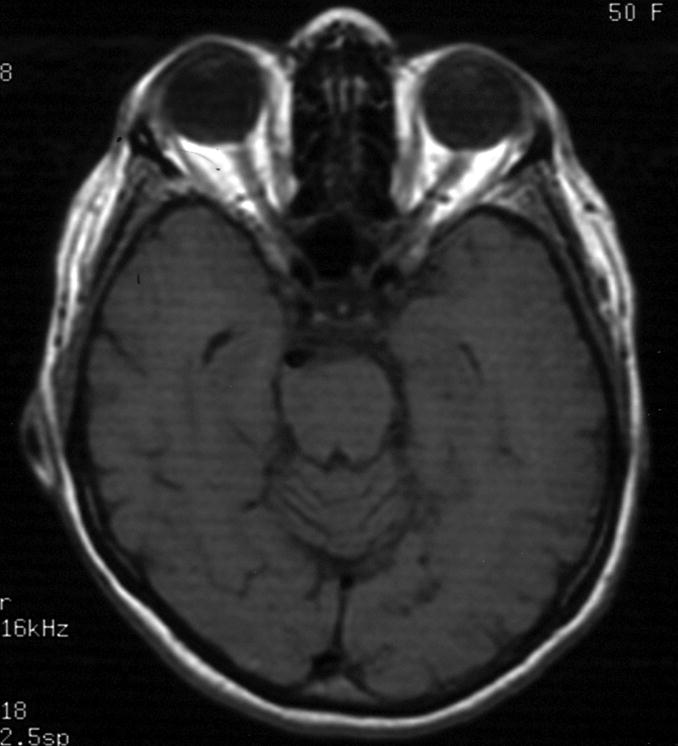
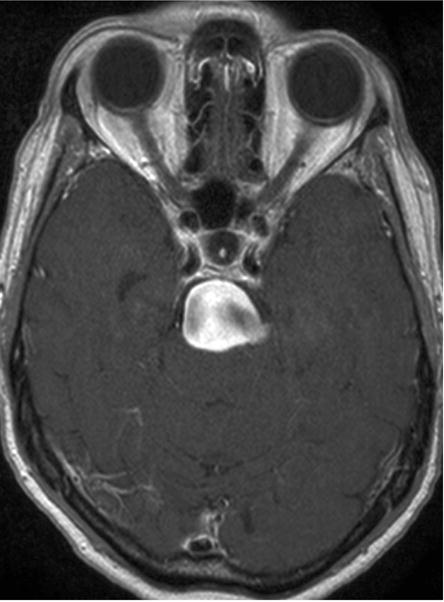
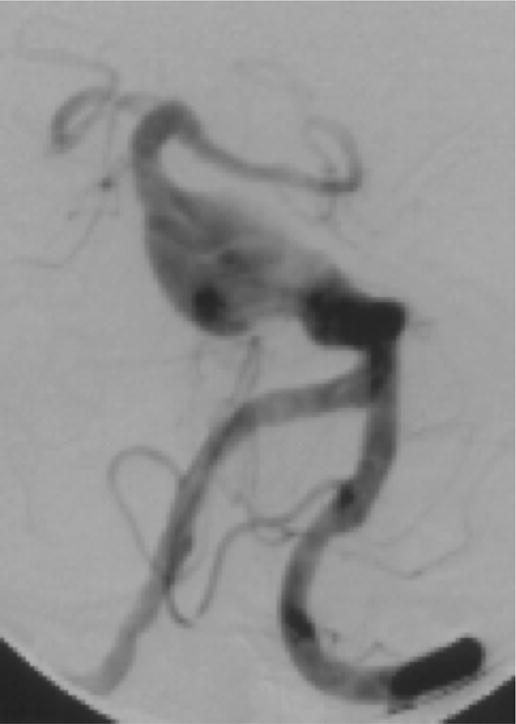
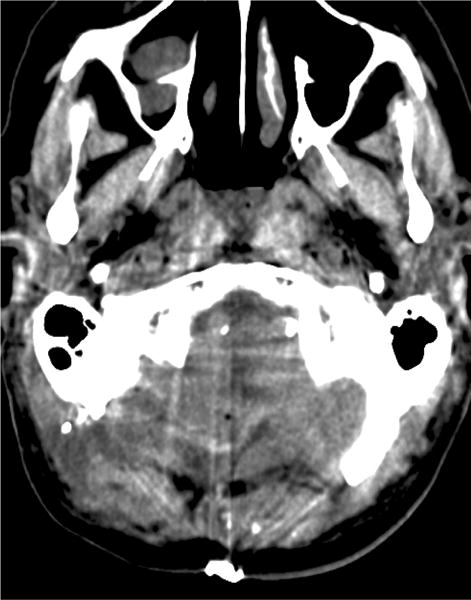
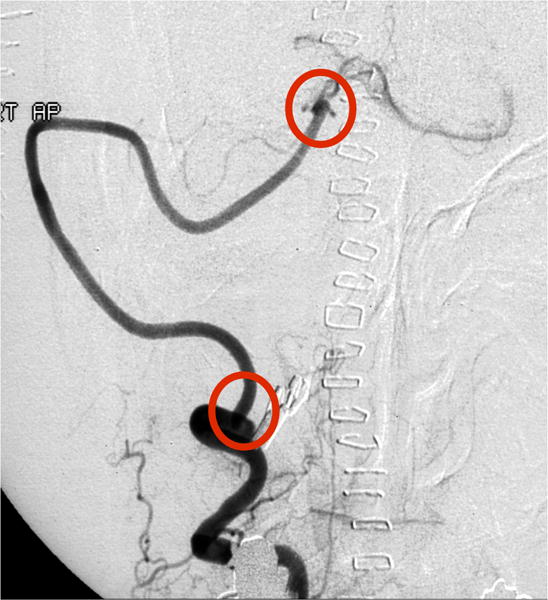
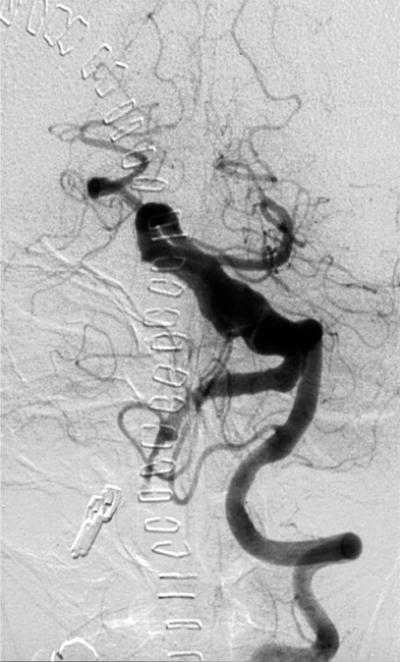
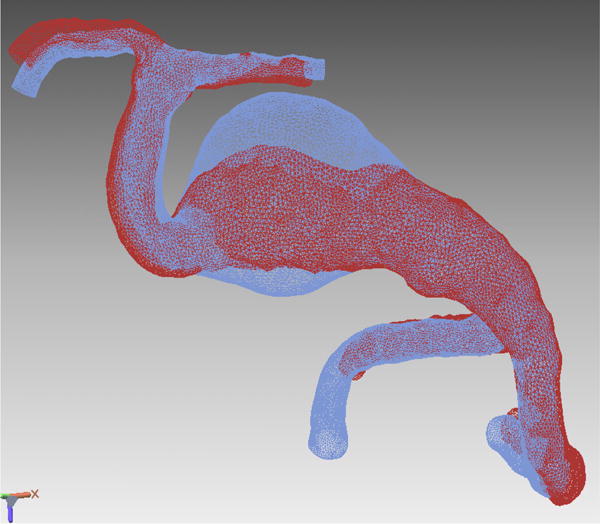
Case example of Phase 2 surgical management. (A) This 51-year-old woman initially presented with mild symptoms from mild basilar dilatation and tortuosity (axial magnetic resonance imaging, T1-weighted image), and was observed for 3 years. (B) During those years, her aneurysm grew and her symptoms became severe (axial magnetic resonance imaging, T1-weighted image with gadolinium; and (C) left vertebral artery digital subtraction angiogram, anteroposterior view). (D) The combined subtemporal-far lateral approach enabled a wide suboccipital craniectomy and duroplasty for posterior fossa relaxation, as seen on axial postoperative CT scan. (E) The postoperative angiogram (right VA angiogram, anteroposterior view) demonstrated patency of the right V3VA-SCA bypass, occlusion of the distal right VA, and filling of the basilar quadrification. (F) The left VA angiogram (anteroposterior view) demonstrated significant thrombosis of aneurysm lumen, (G) shown with superimposed luminal aneurysm volumes pre- (blue) and post-operatively (red) generated with CE-MRA techniques. Although endovascular occlusion of the left VA was intended postoperatively, it was deferred due to her excellent clinical and angiographic outcomes. Her symptoms progressed and the aneurysm enlarged 6 months later, prompting left VA coiling that caused a fatal thrombosis of the basilar trunk.
In summary, flow reduction appeared to be safer than flow reversal, but except for the clip reconstructed aneurysm, the other aneurysms still enlarged progressively and low demand on the bypass reduced its impact on distal basilar flow. The far lateral craniotomy accessed the posterior fossa for decompressive craniectomy and enabled simultaneous surgical occlusion of the dominant VA, which was not possible in Phase 1 with the orbitozygomatic approach. The distal anastomosis to the SCA and the harvest of the bypass graft can be awkward in the park bench position, making this strategy imperfect.
Surgical Results, Phase 3
In the third management phase, patients were bypassed with an IC-IC bypass consisting of an MCA-PCA bypass in all patients (n=8), with radial artery used as the graft in 6 patients and saphenous vein in 2 patients. All bypasses were performed through an orbitozygomatic-transsylvian exposure of the recipient P2 segment of PCA (Figure 9). The deep anastomosis was performed first and the M2 MCA segment was used as the donor site for the second anastomosis. All bypasses were confirmed to be patent, after which aneurysm outflow was occluded distally with clips on the basilar trunk. Interestingly, ICG demonstrated no bypass flow in 3 patients until demand was created by clip occlusion of the basilar artery. This strategy of MCA-PCA bypass and distal aneurysm occlusion maintained anterograde flow in the proximal basilar artery and aneurysm, albeit reduced; it split the posterior circulation into upper and lower halves, with the basilar quadrifurcation supplied by a more robust bypass than the temporal artery bypasses; and it required a standard approach rather than an awkward combination approach.
Figure 9.
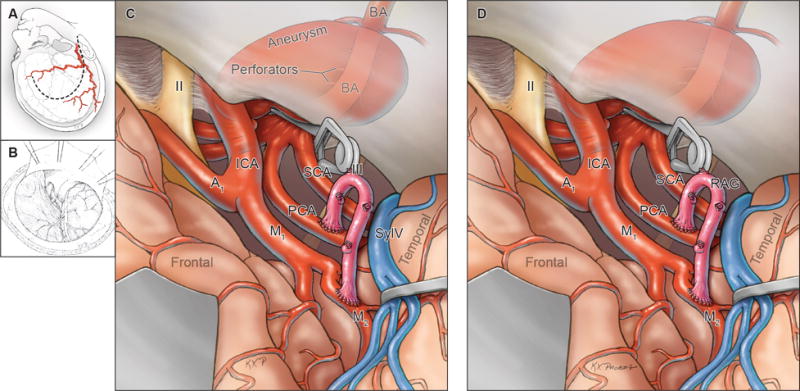
Summary of Phase 3 surgical management (MCA to PCA bypass), with (A, B) exposure through an orbitozygomatic-pterional craniotomy, (C) exposure via transsylvian access to the recipient PCA pretemporally, (D) completion of 2 anastomoses to the PCA and MCA, and distal clip occlusion of the aneurysm below the basilar quadrifurcation. Abbreviations: ICA = internal carotid artery; A1 = anterior cerebral artery, A1 segment; M1 = middle cerebral artery, M1 segment; BA = basilar artery; VA = vertebral artery; PICA = posterior inferior cerebellar artery; STA = superficial temporal artery; SylV = Sylvian vein; II = optic nerve; III = oculomotor nerve.
Despite good technical execution in all of the first 4 patients, all experienced brainstem perforator infarcts postoperatively that were minor in 2 patients (Figure 10), resulting in a drop in mRS scores from 2 to 3 in both, and fatal in 2 patients. These results indicated that the MCA-PCA bypass adequately revascularized the distal basilar circulation, but the anterograde basilar flow was still not sufficient to preserve perforator patency. Therefore, clopidogrel (Plavix) was added to the postoperative management regimen, with 75 mg administered immediately postoperatively and another 75 mg administered after confirming no hemorrhaging complications on CT scan. Clopidogrel (Plavix) was then maintained at 75 mg QD.
Figure 10.
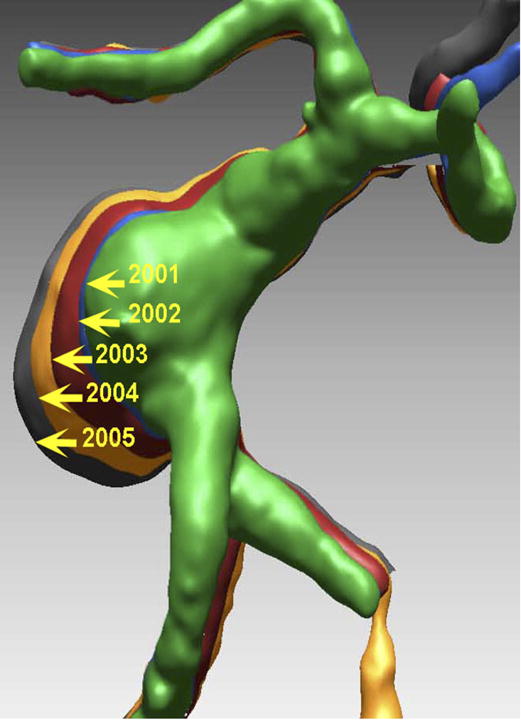
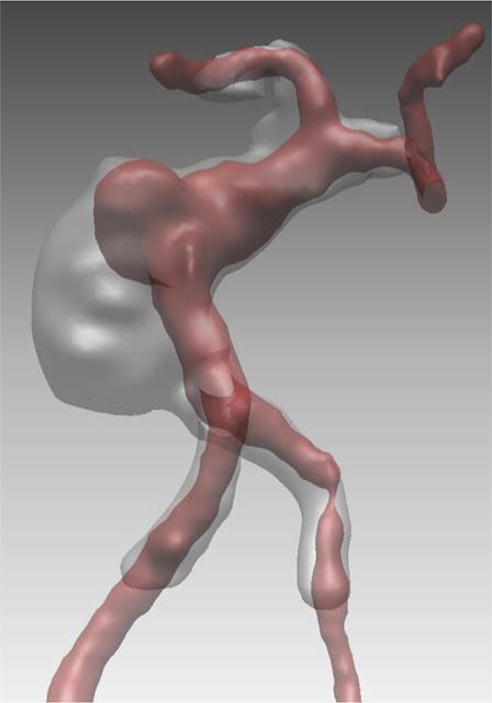
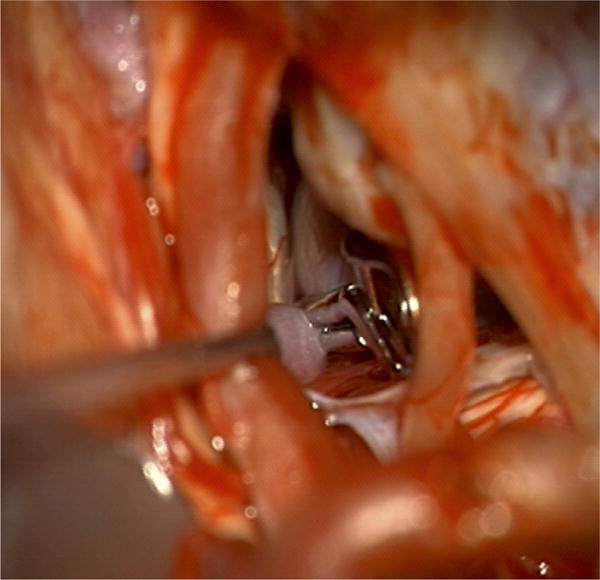
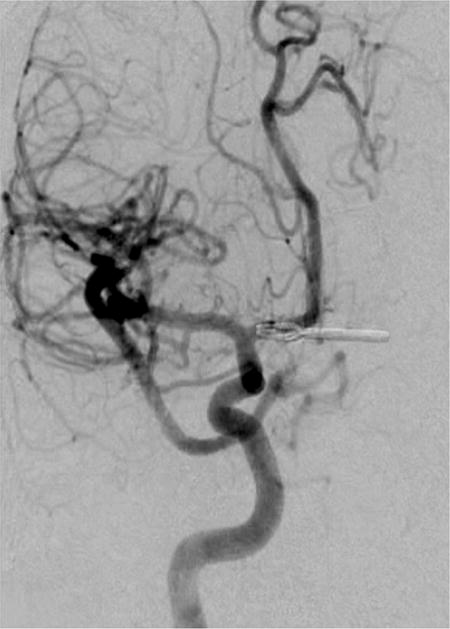
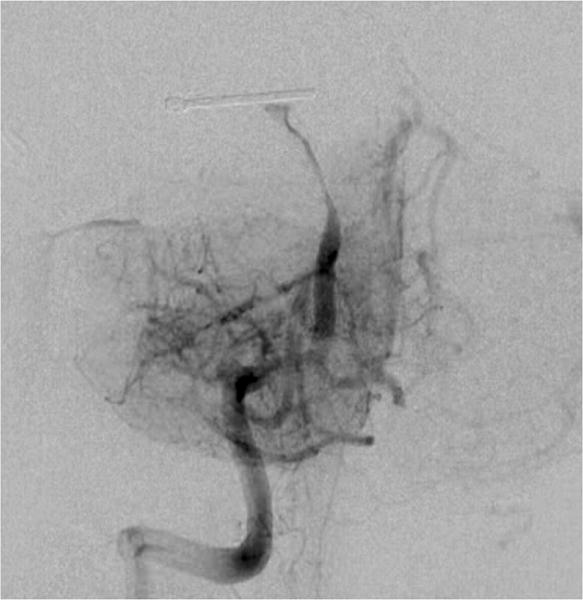
Case example of Phase 3 surgical management. (A) The 44-year-old man was followed with CE-MRA for 4 years with progressive aneurysm enlargement. (B) He then suffered a pontine stroke from acute thrombosis of the inferior portion of the aneurysm lumen, as shown on superimposed pre- (gray) and post-stroke luminal aneurysm volumes. (C) He underwent MCA-PCA bypass and distal clip occlusion of the aneurysm. (D) Postoperative angiography showed good perfusion of the basilar quadrifurcation through the bypass (right ICA angiogram, anteroposterior view) and (E) thrombosis of the remaining aneurysm lumen with anterograde flow up to the clip (right vertebral artery angiogram, anteroposterior view). The patient experienced a minor pontine perforator infarct resulting in a decrease in mRS score from 2 to 3, but he has required no further treatment in the subsequent 7 years and lives with some minor assistance.
With this clopidogrel (Plavix) regimen in the next 4 patients, 1 patient had no perforator infarcts and tolerated his surgery without complication; 1 patient experienced a minor brainstem perforator infarct resulting in a decrease in mRS score from 2 to 3; and 1 patient suffered fatal brainstem perforator infarcts. The final patient experienced an SCA territory infarction resulting from an attempt to clip occlude the distal basilar artery with the clip blades below one SCA to include it in the territory of the bypass and above the other to include it in the territory of the basilar artery. This “SCA cross-clipping” technique was intended to sump flow through the aneurysm to the SCA and thereby increase basilar artery blood flow to maintain perforator patency (Figure 11). ICG videoangiography demonstrated SCA patency intraoperatively but it occluded later and the patient died.
Figure 11.
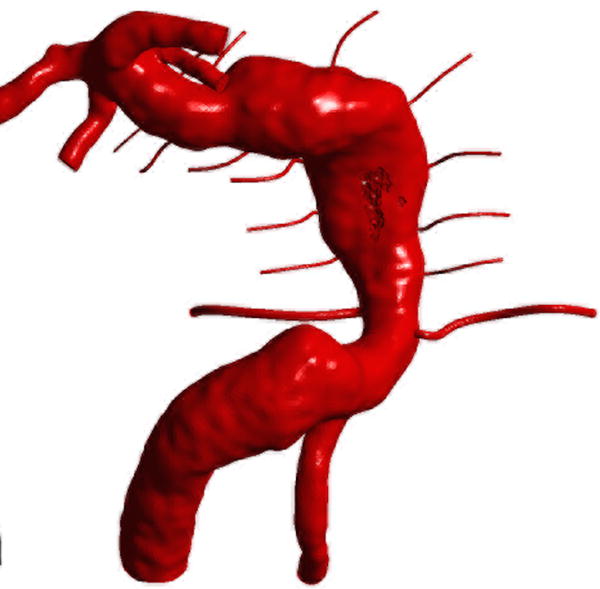
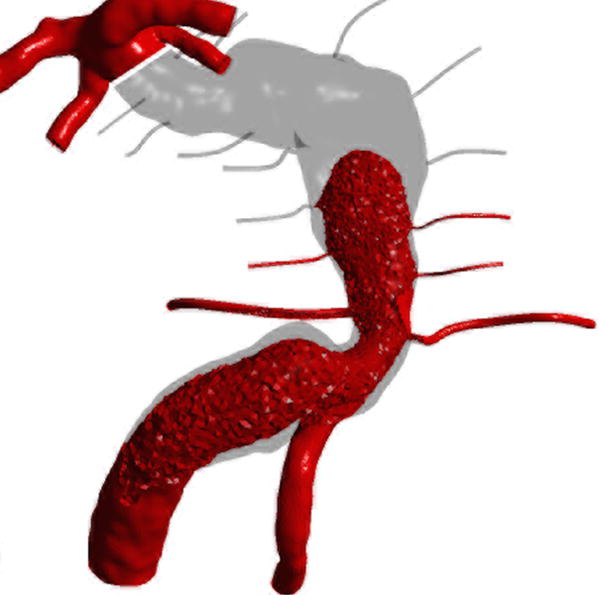
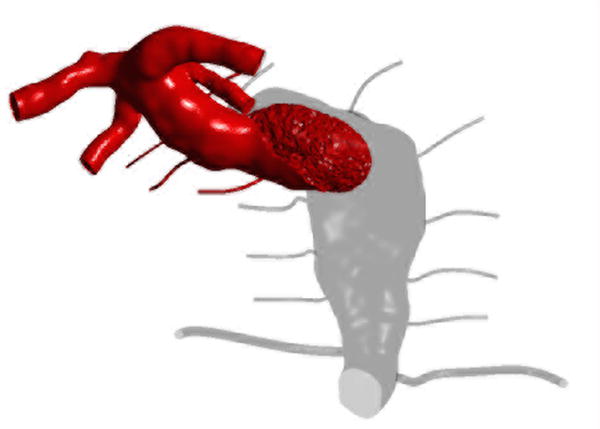
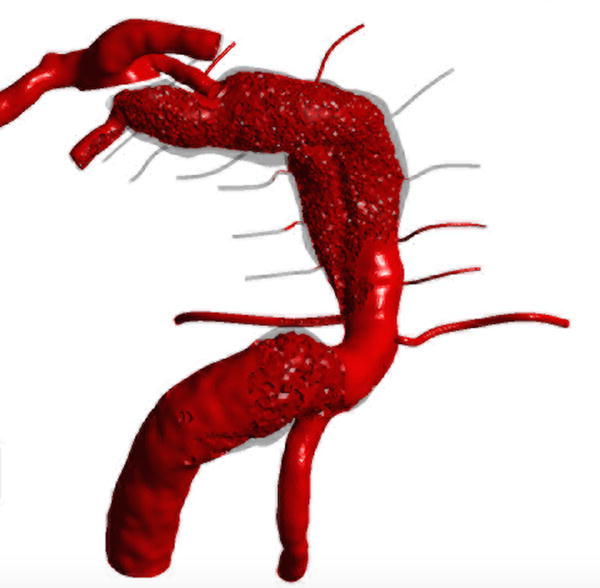
Computational fluid dynamic simulations of treatment options for a dolichoectatic basilar trunk aneurysm. (A) Virtual contrast transport is shown in red, demonstrating the branch and perforator anatomy of this aneurysm preoperatively. After alternative treatments, contrast transport is shown in red 8 cycles after the start of virtual injection, whereas increased flow residence time is shown in gray. (B) Right MCA-PCA bypass with distal aneurysm occlusion below the quadrifurcation, with a clip proximal to SCAs (Option 1). (C) Right MCA-PCA bypass with proximal aneurysm occlusion, with a clip on the basilar artery proximal to the AICAs (Option 2). (D) Right MCA-PCA bypass with SCA cross-clipping, with a clip on the distal basilar artery above one SCA to sump flow through the basilar artery and below the other SCA that is supplied by the bypass (Option 3). CFD simulations and computed velocities demonstrated slowed anterograde flow through the basilar trunk with distal occlusion and slowed retrograde flow through the basilar trunk with proximal occlusion, both with stagnation near the clip site, suggesting high risk of perforator occlusion. In contrast, SCA cross-clipping created an outlet for anterograde flow (run-off) that increased basilar trunk flow relative to option 1 and appeared more likely to maintain perfusion to pontine perforators.
In summary, MCA-PCA bypass created a “surgical posterior communicating artery” that robustly supplied the basilar quadrifurcation, but only after clip occlusion of the basilar artery. This bypass did not significantly diminish trans-aneurysmal flow by itself, based on ICG videography. Distal clip occlusion of the basilar trunk aneurysm preserved anterograde flow in the aneurysm and did not precipitate its rupture, but the resulting flow reduction threatened perforator patency. Clopidogrel (Plavix) was tolerated postoperatively and an increased loading dose may be indicated. Surviving patients tended to have smaller aneurysms with smaller luminal volumes, suggesting that earlier intervention may impact outcome favorably. Surviving patients also tended to have aneurysms located at the upper basilar trunk (above AICA and the midpoint of clivus), suggesting that perforator complications from aneurysm thrombosis involving the midbrain are less devastating than those involving the pons. Cross-clipping the SCA was technically difficult to accomplish. The shifting treatment strategy for dolichoectatic basilar trunk aneurysms (Table 1) resulted in small improvements in surgical and final mortalities (Table 2), with stabilization of aneurysms in the Phase 3 survivors.
DISCUSSION
This report describes a large experience managing patients with dolichoectatic basilar trunk aneurysms – one of, if not the most, difficult aneurysms encountered in practice. Although we focused on the evaluation of bypass as part of an evolving treatment paradigm, our conservative management posture should be emphasized. Less than a third of all patients (12 of 37) were initially selected for treatment, favoring a period of clinical and radiographic surveillance instead. The natural history of our observed patients was more benign than expected, with only 44% experiencing neurological deterioration and 20% dying, not always from their aneurysms. Furthermore, only one quarter of patients followed with CE-MRA demonstrated significant enlargement. This natural history in our series was more favorable than predicted by other reports on giant aneurysms and dolichoectatic basilar trunk aneurysms.3, 13, 14
Reports in the literature suggest that the treatment of these aneurysms with EC-IC bypass surgery and aneurysm occlusion can be performed with high but perhaps acceptable morbidity (approximately 20%) and mortality (approximately 18%), as well as with reasonable efficacy, as determined by low rates of subsequent aneurysm growth.16, 17, 19–32, 34–38 Phase I of our experience can be viewed as a validation study, and our findings were not confirmatory. Our poor results cannot be attributed to technical complications with bypass (100% patency), leading us to conclude that: reversal of flow in basilar trunk aneurysms is dangerously thrombogenic (Figure 12); the use of heparin postoperatively increases morbidity; and this strategy should be discouraged. Phase II demonstrated that flow reduction rather than reversal prevents catastrophic basilar thrombosis but fails to stabilize these aneurysms. Phase II also showed that a bypass to the posterior circulation distal to the aneurysm without the high demand created by an occlusive intervention fails to alter basilar hemodynamics and can lead to involution of the bypass. In Phase III, we expected the anterograde flow maintained by distal aneurysm occlusion to preserve blood flow in basilar perforators and were disappointed to find that patients still suffered postoperative brainstem infarcts (Figure 12). Overall, the results demonstrated that not just the right bypass determines good outcomes, but also perforator preservation and inhibition of aneurysm thrombosis after the flow-reducing intervention. The MCA-PCA bypass, with its intracranial location, robust flow, short length, and easier execution than other bypasses, is ideal and all were flawless in this series. However, the interplay between flow reduction and aneurysm thrombosis creates a dangerous therapeutic situation ranging from ineffective to catastrophic. The addition of antiplatelet agents appears to be an important addition to the management regimen and was tolerated better than expected, but perforator occlusion persisted and calls for further innovation, perhaps with a more aggressive loading dose.
Figure 12.
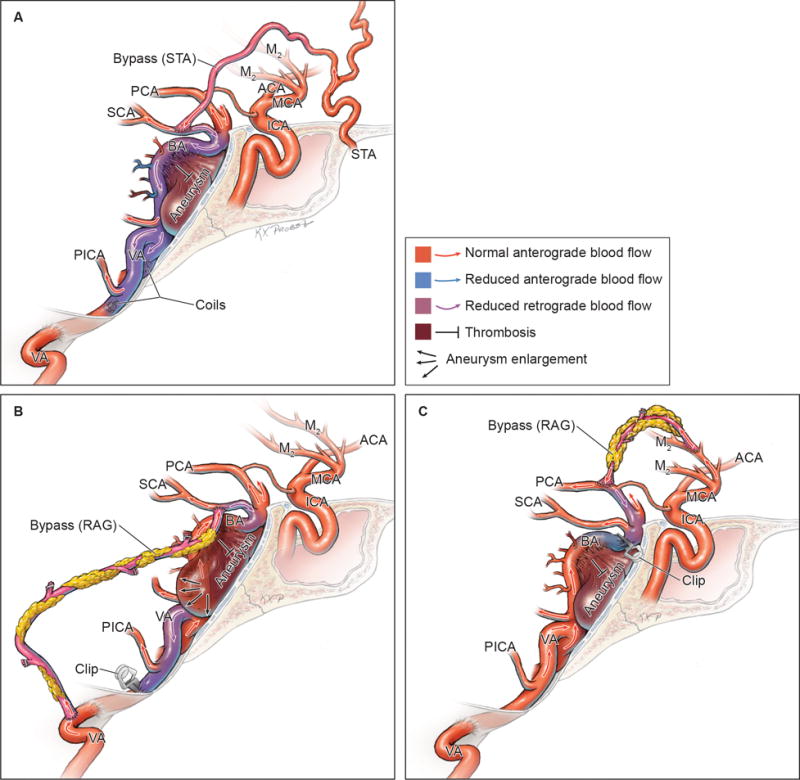
Outcomes after bypass and flow alteration. (A) STA-SCA bypass and flow reversal with bilateral vertebral artery coil occlusion resulted in aneurysm thrombosis and perforator occlusion (Phase 1). (B) VA-SCA bypass and flow reduction with unilateral vertebral artery clip occlusion preserved anterograde flow and precipitated some aneurysmal thrombosis but failed to stabilize the aneurysm (Phase 2). (C) MCA-PCA bypass and distal aneurysm clip occlusion also preserved anterograde flow but threatened distal basilar perforators in some patients (Phase 3).
The Next Phase
The reduction in surgical mortality from 80% in Phase I to 50% in Phase III represents some real progress, but our surgical efforts demonstrate that flow direction may not be the determinative factor. Flow above some threshold level, regardless of its direction, is needed to stave off complete thrombosis of the basilar lumen and occlusion of the perforators. Clip occlusion of the basilar artery, whether distal or proximal, eliminates basilar flow except what its branches can pull or sump either from the proximal basilar artery in the case of distal aneurysm occlusion or from the bypass in the case of proximal aneurysm occlusion. Small perforating branches are unable to sump enough flow for their preservation and are overwhelmed by stagnation of blood within a vast intra-aneurysmal space, leading to thrombosis. We have studied this process in the natural course of untreated giant aneurysms, observing spontaneous thrombosis in regions of low flow velocity, low wall shear stress, and long residence times. Extrapolating, therapeutic reduction of flow in the dolichoectatic basilar trunk aneurysm represents an extreme provocation of thrombosis that even the aggressive use of antiplatelets or anticoagulants may not be able to overcome.
One solution may be the creation of a distal run-off, or occluding the basilar artery but maintaining a major branch artery that can sump flow through the aneurysm and nourish the perforators. Cross-coiling the PICAs in Phase I is conceptually similar to distal run-off because one PICA sumps flow from the bypass. The SCA cross-clip is an example of this solution, but it is difficult to do in practice, as demonstrated by our last case in which the cross-clip occluded one of the SCA branches. An alternative might be cross-clipping the AICAs through a combined approach that exposes the basilar apex for the MCA-PCA bypass as well as the basilar trunk for proximal clip occlusion. Preliminary work in cadavers has demonstrated that a orbitozygomatic-extended retrosigmoid approach offers this exposure, but CFD simulations suggest that AICA has less sump effect than SCA due to its smaller caliber and the fact that flow is proportional to an artery’s radius to the fourth power.
The pathology and hemodynamics of dolichoectatic basilar trunk aneurysms leave little margin for error in their surgical treatment. The confluence of two arteries into one and the dolichoectatic morphology of the aneurysm generate complex flows with high-velocity jets, variable shear stresses, stagnation, inflammation, and thrombosis. In this state, perforators frequently occlude spontaneously and patent perforators may already be struggling to survive. In this context, it is remarkable that any intervention upsetting the delicate balance between flow and thrombosis would work, but results in our survivors and in others’ reports are dramatic.16, 26 The presence of intra-aneurysmal thrombus preoperatively is a feature shared by survivors, indicating that propagating thrombosis therapeutically may be safer than initiating it in an aneurysm that had none previously. Pre-existing thrombus may be a nidus for additional thrombus that avoids branch origins, or it may have already occluded perforators that would have caused surgical morbidity. Aneurysms with lower initial luminal volumes tended to fare better as well, perhaps because the dead space within the aneurysm where flow stagnates after occlusion is smaller, reducing the load of new thrombus. These observations are anecdotal and, despite our efforts to quantitate the morphometrics and hemodynamics of these aneurysms, there are no measures to guide management. Even with sophisticated CFD, threshold levels for flow velocity, shear stress, and residence time are unknown, as are their effects on thrombus propagation.
Endovascular Options
Stents or flow diverters were expected to solve the dolichoectatic basilar trunk aneurysm. Conceptually, an intra-aneurysmal conduit would eliminate malignant hemodynamics, preserve flow in branch arteries, and promote thrombosis outside the lumen, much like stent grafts used with aortic aneurysms. However, patients treated with the flow diverters have had complications related to perforator occlusion, just like surgical patients (Table 3).3, 6–15 Flow diverters did not actually divert flow unless multiple devices were deployed, resulting in occlusive coverage of the perforators and branch arteries originating from the trunk.14 Flow diverters were initially too short to treat the entire lesion and necessitated overlapping devices.14 In addition to perforator complications, the Pipeline device caused peri-procedural ruptures, even in aneurysms that had never ruptured before,14 thought to be due to a “ball-valve” mechanism where blood forcibly escaped through the pores of the flow diverter during systole and was trapped extraluminally where it slowly expanded the aneurysm. This complication resulted in devastating ruptures (while patients were anti-coagulated or taking anti-platelet agents, or both).
Table 3.
Published results for the endovascular treatment of dolichoectatic basilar trunk aneurysms.
| First author | Last author | Year, journal | Total # of fusiform BA/VBJ aneurysms |
# of
Basilar Trunk aneurysms |
Vertebro-basilar junction aneurysms |
Devices used | Deaths | Ischemic Complications |
Hemorrhagic or Mass Effect Complications |
Complications not
otherwise specified |
Angiographic Cures |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Byrne, J | Kamran, M | 2010, PlosOne | 10 | 3 | 7 | SILK | 2 | 0 | 1 (mass effect from aneurysm thrombosis – led to death) | NA | |
| Lubicz, B | Leclerc, X | 2010, Stroke | 3 | 3 | 0 | SILK | 0 | 1 (branch occlusion) | 0 | 1 | |
| Raphaeli G | Lubicz B | 2011, Neurosurgery | 10 [16 purely vertebral artery aneurysms not included] |
8 | 2 | SILK ENTERPRISE LEO SOLITAIRE COILS |
3 | NA | NA | 1 treatment failure (died before
treatment) 1 with transient hemiparesis at 2 weeks |
1 |
| Ertl L | Fesl G | 2014, AJNR | 6 | 2 | 4 | PIPELINE SILK, LEO |
4 (two addt’l with mRS 5) | 3 | 1 – brainstem compression (following pneumonia) | 0 | 1 |
| Monteith S | Jabbour P | 2014, JNS | 7 | 4 | 3 | PIPELINE | 1 | 1 | 0 | 0 | 2 |
| Meckel S | Klisch J | 2013, Neurosurgery | 6 | 0 | 6 | PIPELINE (n=9) SILK (n=1) |
4 | 3 ischemic strokes | 1 mass effect 1 hemorrhage |
1 flow diverter construct thrombosis | 2 |
| Siddiqui A | Levy E | 2012, JNS | 7 | 7 | 0 | SILK (n=1) PIPELINE (n=6) |
4 | 5 | 1 (SAH) | NA | 2 |
| Van Oel L | Peluso J | 2013, AJNR | 13 | NA | NA | Mix: Neuroform, Enterprise, LEO, SILK, and coiling | 3 | 3 | 0 | 1 – MI during anesthesia | 9 |
| Fischer, S | Henkes, H | 2014, Neurosurgery | 37 | 17 | 20 | PIPELINE | NA | NA | NA | NA | 16 of 25 with DSA |
| Toth, G | Hui F | 2014, JNIS | 2 | 2 | 0 | PIPELINE | 1 | 2 strokes (1 died, 1 mRS of 5) |
0 | NA | 1 (secondary to construct thrombosis and occlusion) |
| Munich S | Lopes D | 2014, JNS | 12 | NA | NA | PIPELINE | 1 | 2 strokes | 0 | 2 patients with new hemiparesis 1 patient with new nystagmus/dizziness (ambulatory at 2 mos.) |
9 |
| Total | BA trunk aneurysms | Deaths (in all aneurysms treated) | Ischemic Complications (in all aneurysms treated) | Hemorrhagic or Mass Effect Complications (in all aneurysms treated) | Complications not otherwise specified | ||||||
| 46 | 23 | 20 | 5 | 7 | 38 of 123 total aneurysms cured |
FD = flow diversion
SILK = SILK FLOW DIVERSION DEVICE
PIPELINE = PIPELINE EMBOLIZATION DEVICE (FLOW DIVERTER)
With the Pipeline device now offered in longer sizes, some have begun treating dolichoectatic basilar trunk aneurysms with a single device to minimize perforator coverage and with intra-aneurysmal coiling around the flow diverter to obliterate the surrounding space.39 Results with these technique modifications have not been reported. Endovascular technology is ever-evolving and new designs might offer a solution. We are intrigued by the possibility of parent artery occlusive devices that can be deployed endovascularly – an objective not typically pursued by industry but applicable in this setting. Occlusive devices might offer stand-alone therapies that reduce or redistribute flow to modify risk of hemorrhage or aneurysm growth, or they might be used in combination with a bypass procedure to obviate the need for clip occlusion. We are intrigued by the possibility of remodeling the basilar trunk with a flow diverter, allowing it time to endothelialize, and then debulking the aneurysm later to relieve mass effect symptoms. Many of these lesions present with brainstem compressive symptoms, and endovascular devices might enable safe decompressive thrombectomy, which is not currently part of existing interventions.
Personal Perspective and Limitations
Although it is humbling to report a surgical series where half the patient died, we submit this work to stimulate discourse about the problem of the dolichoectatic basilar trunk aneurysm. These are rare cases, accounting for a fraction of one percent of a large microsurgical experience with nearly 4,000 aneurysms, but they are painful and disturbing. Patients confront an incurable disease with a dismal prognosis, often with severe disabilities and high hopes for treatment. Neurosurgeons invest tremendous, and sometimes flawless, effort in the procedure, only to have an abrupt fatality that is difficult to explain, hard for families to accept, and visible to colleagues in the hospital. These defeats call into question the advisability and ethics of further efforts. While many clinicians have already given up on this disease and abandoned surgical options, our failures, perhaps more than our successes, have forced us to study this pathology in autopsy specimens and in the cadaver lab, stimulating insights, technical developments, and improvements. In our opinion, the treatment of dolichoectatic basilar trunk aneurysms will advance only through concentrated management in select few centers and concerted effort between neurosurgeons, neurologists, and interventional neuroradiologists to study the morphology and hemodynamics with sophisticated computational methods. Multicenter registry data must be collected on these patients. In the vascular neurosurgical subspecialty, this disease is akin to glioblastoma multiforme in the tumor neurosurgical subspecialty, in that the underlying biology is complex, cures are elusive, and the fight will involve unconventional teams of scientists. Therapeutic advancement with the dolichoectatic basilar aneurysm is hampered not only by its rarity but also by a reluctance to intervene.
CONCLUSION
In his first report on Bleeding Aneurysms of the Basilar Artery in 1960 in which he described results in 4 patients (3 basilar bifurcation aneurysm and 1 SCA aneurysm with 2 deaths),40 Drake summarized: “the surgical treatment of these aneurysms will undoubtedly develop in some degree and it would appear that in certain instances in which expectancy of life is poor … a direct attack is justifiable.” In 1968, he updated: “It is nice to be able to alter the gloomy statements of 2 years ago and encourage others with experience in the surgical treatment of aneurysms to operate on these lesions, with reason and caution, for they may not be as formidable as has been thought in the past.”41 The situation may be similar down the basilar trunk. There is a will amongst some neurosurgeons to fight this disease, and they need referrals, brave patients, supportive colleagues, and strong constitutions to persevere. It is difficult to say where this “work in progress” will lead, but it is, in our opinion, worth pursuing. There will certainly be more casualties along the way, but we hope to redeem ourselves and honor the patients who were harmed by applying the lessons we have learned and discovering a better solution for the dolichoectatic basilar trunk aneurysm, whatever it may be.
Acknowledgments
Dr Saloner and his research work in this field are partly supported by NIH grant NS059944. Dr Rayz and his research work in this field are partly supported by NIH grant R01 HL115267.
Footnotes
Disclosure: The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Rayz VL, Boussel L, Lawton MT, et al. Numerical modeling of the flow in intracranial aneurysms: prediction of regions prone to thrombus formation. Annals of biomedical engineering. 2008 Nov;36(11):1793–1804. doi: 10.1007/s10439-008-9561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rayz VL, Boussel L, Ge L, et al. Flow residence time and regions of intraluminal thrombus deposition in intracranial aneurysms. Annals of biomedical engineering. 2010 Oct;38(10):3058–3069. doi: 10.1007/s10439-010-0065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Oel LI, van Rooij WJ, Sluzewski M, Beute GN, Lohle PN, Peluso JP. Reconstructive endovascular treatment of fusiform and dissecting basilar trunk aneurysms with flow diverters, stents, and coils. AJNR. American journal of neuroradiology. 2013 Mar;34(3):589–595. doi: 10.3174/ajnr.A3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drake CG. Ligation of the vertebral (unilateral or bilateral) or basilar artery in the treatment of large intracranial aneurysms. Journal of neurosurgery. 1975 Sep;43(3):255–274. doi: 10.3171/jns.1975.43.3.0255. [DOI] [PubMed] [Google Scholar]
- 5.Anson JA, Lawton MT, Spetzler RF. Characteristics and surgical treatment of dolichoectatic and fusiform aneurysms. Journal of neurosurgery. 1996 Feb;84(2):185–193. doi: 10.3171/jns.1996.84.2.0185. [DOI] [PubMed] [Google Scholar]
- 6.Byrne JV, Beltechi R, Yarnold JA, Birks J, Kamran M. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: a multicentre prospective study. PloS one. 2010;5(9) doi: 10.1371/journal.pone.0012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ertl L, Holtmannspotter M, Patzig M, Bruckmann H, Fesl G. Use of flow-diverting devices in fusiform vertebrobasilar giant aneurysms: a report on periprocedural course and long-term follow-up. AJNR. American journal of neuroradiology. 2014 Jul;35(7):1346–01352. doi: 10.3174/ajnr.A3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer S, Perez MA, Kurre W, Albes G, Bazner H, Henkes H. Pipeline embolization device for the treatment of intra- and extracranial fusiform and dissecting aneurysms: initial experience and long-term follow-up. Neurosurgery. 2014 Oct;75(4):364–374. doi: 10.1227/NEU.0000000000000431. discussion 374. [DOI] [PubMed] [Google Scholar]
- 9.Lubicz B, Collignon L, Raphaeli G, et al. Flow-diverter stent for the endovascular treatment of intracranial aneurysms: a prospective study in 29 patients with 34 aneurysms. Stroke; a journal of cerebral circulation. 2010 Oct;41(10):2247–2253. doi: 10.1161/STROKEAHA.110.589911. [DOI] [PubMed] [Google Scholar]
- 10.Meckel S, McAuliffe W, Fiorella D, et al. Endovascular treatment of complex aneurysms at the vertebrobasilar junction with flow-diverting stents: initial experience. Neurosurgery. 2013 Sep;73(3):386–394. doi: 10.1227/01.neu.0000431472.71913.07. [DOI] [PubMed] [Google Scholar]
- 11.Monteith SJ, Tsimpas A, Dumont AS, et al. Endovascular treatment of fusiform cerebral aneurysms with the Pipeline Embolization Device. Journal of neurosurgery. 2014 Apr;120(4):945–954. doi: 10.3171/2013.12.JNS13945. [DOI] [PubMed] [Google Scholar]
- 12.Munich SA, Tan LA, Keigher KM, Chen M, Moftakhar R, Lopes DK. The Pipeline Embolization Device for the treatment of posterior circulation fusiform aneurysms: lessons learned at a single institution. Journal of neurosurgery. 2014 Nov;121(5):1077–1084. doi: 10.3171/2014.7.JNS132595. [DOI] [PubMed] [Google Scholar]
- 13.Raphaeli G, Collignon L, De Witte O, Lubicz B. Endovascular treatment of posterior circulation fusiform aneurysms: single-center experience in 31 patients. Neurosurgery. 2011 Aug;69(2):274–283. doi: 10.1227/NEU.0b013e31821723f2. [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui AH, Abla AA, Kan P, et al. Panacea or problem: flow diverters in the treatment of symptomatic large or giant fusiform vertebrobasilar aneurysms. Journal of neurosurgery. 2012 Jun;116(6):1258–1266. doi: 10.3171/2012.2.JNS111942. [DOI] [PubMed] [Google Scholar]
- 15.Toth G, Bain M, Hussain MS, et al. Posterior circulation flow diversion: a single-center experience and literature review. Journal of neurointerventional surgery. 2014 Jul 1; doi: 10.1136/neurintsurg-2014-011281. [DOI] [PubMed] [Google Scholar]
- 16.Kalani MY, Zabramski JM, Nakaji P, Spetzler RF. Bypass and flow reduction for complex basilar and vertebrobasilar junction aneurysms. Neurosurgery. 2013 May;72(5):763–775. doi: 10.1227/NEU.0b013e3182870703. discussion 775–766. [DOI] [PubMed] [Google Scholar]
- 17.Russell SM, Post N, Jafar JJ. Revascularizing the upper basilar circulation with saphenous vein grafts: operative technique and lessons learned. Surgical neurology. 2006 Sep;66(3):285–297. doi: 10.1016/j.surneu.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 18.Mai JC, Tariq F, Kim LJ, Sekhar LN. Flow diversion radial artery bypass graft coupled with terminal basilar artery occlusion for the treatment of complex basilar apex aneurysms: operative nuances. Neurosurgery. 2013 Jun;72(2 Suppl Operative):ons116–126. doi: 10.1227/NEU.0b013e31827bf2d8. discussion ons126. [DOI] [PubMed] [Google Scholar]
- 19.Evans JJ, Sekhar LN, Rak R, Stimac D. Bypass grafting and revascularization in the management of posterior circulation aneurysms. Neurosurgery. 2004 Nov;55(5):1036–1049. doi: 10.1227/01.neu.0000140822.64362.c6. [DOI] [PubMed] [Google Scholar]
- 20.Ewald CH, Kuhne D, Hassler WE. Bypass-surgery and coil-embolisation in the treatment of cerebral giant aneurysms. Acta neurochirurgica. 2000;142(7):731–737. doi: 10.1007/s007010070087. discussion 737–738. [DOI] [PubMed] [Google Scholar]
- 21.Gobble RM, Hoang H, Jafar J, Adelman M. Extracranial-intracranial bypass: resurrection of a nearly extinct operation. Journal of vascular surgery. 2012 Nov;56(5):1303–1307. doi: 10.1016/j.jvs.2012.03.281. [DOI] [PubMed] [Google Scholar]
- 22.Horie N, Kitagawa N, Morikawa M, et al. Giant thrombosed fusiform aneurysm at the basilar trunk successfully treated with endovascular coil occlusion following bypass surgery: a case report and review of the literature. Neurological research. 2007 Dec;29(8):842–846. doi: 10.1179/016164107X217392. [DOI] [PubMed] [Google Scholar]
- 23.Jafar JJ, Russell SM, Woo HH. Treatment of giant intracranial aneurysms with saphenous vein extracranial-to-intracranial bypass grafting: indications, operative technique, and results in 29 patients. Neurosurgery. 2002 Jul;51(1):138–144. doi: 10.1097/00006123-200207000-00021. discussion 144–136. [DOI] [PubMed] [Google Scholar]
- 24.Kaku Y, Funatsu N, Tsujimoto M, Yamashita K, Kokuzawa J. STA-MCA/STA-PCA Bypass Using Short Interposition Vein Graft. Acta neurochirurgica. Supplement. 2014;119:79–82. doi: 10.1007/978-3-319-02411-0_14. [DOI] [PubMed] [Google Scholar]
- 25.Kalani MY, Elhadi AM, Ramey W, et al. Revascularization and pediatric aneurysm surgery. Journal of neurosurgery. Pediatrics. 2014 Jun;13(6):641–646. doi: 10.3171/2014.3.PEDS13444. [DOI] [PubMed] [Google Scholar]
- 26.Kalani MY, Zabramski JM, Nakaji P, Spetzler RF. Twenty-year follow-up of flow reversal and revascularization for a giant serpentine basilar artery aneurysm. Neurosurgery. 2014 Sep;10(Suppl 3):E493–497. doi: 10.1227/NEU.0000000000000438. discussion E497. [DOI] [PubMed] [Google Scholar]
- 27.Kang HS, Oh CW, Han MH, Byun HS, Han DH. Treatment of a sequential giant fusiform aneurysm of the basilar trunk. Korean journal of radiology. 2005 Apr-Jun;6(2):125–129. doi: 10.3348/kjr.2005.6.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim LJ, Tariq F, Sekhar LN. Pediatric bypasses for aneurysms and skull base tumors: short- and long-term outcomes. Journal of neurosurgery. Pediatrics. 2013 May;11(5):533–542. doi: 10.3171/2013.1.PEDS12444. [DOI] [PubMed] [Google Scholar]
- 29.Shi X, Qian H, K CK, Zhang Y, Zhou Z, Sun Y. Bypass of the maxillary to proximal middle cerebral artery or proximal posterior cerebral artery with radial artery graft. Acta neurochirurgica. 2011 Aug;153(8):1649–1655. doi: 10.1007/s00701-011-1070-x. discussion 1655. [DOI] [PubMed] [Google Scholar]
- 30.Shi X, Qian H, Singh KC, et al. Surgical management of vertebral and basilar artery aneurysms: a single center experience in 41 patients. Acta neurochirurgica. 2013 Jun;155(6):1087–1093. doi: 10.1007/s00701-013-1656-6. [DOI] [PubMed] [Google Scholar]
- 31.Terasaka S, Itamoto K, Houkin K. Basilar trunk aneurysm surgically treated with anterior petrosectomy and external carotid artery-to-posterior cerebral artery bypass: technical note. Neurosurgery. 2002 Oct;51(4):1083–1087. doi: 10.1097/00006123-200210000-00044. discussion 1087–1088. [DOI] [PubMed] [Google Scholar]
- 32.Tulleken CA, van der Zwan A, van Rooij WJ, Ramos LM. High-flow bypass using nonocclusive excimer laser-assisted end-to-side anastomosis of the external carotid artery to the P1 segment of the posterior cerebral artery via the sylvian route. Technical note. Journal of neurosurgery. 1998 May;88(5):925–927. doi: 10.3171/jns.1998.88.5.0925. [DOI] [PubMed] [Google Scholar]
- 33.Rayz VL, Abla A, Boussel L, et al. Computational modeling of flow-altering surgeries in basilar aneurysms. Annals of biomedical engineering. 2015 May;43(5):1210–1222. doi: 10.1007/s10439-014-1170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chwajol M, Munson TA, Alaraj A, Charbel FT, Aletich VA, Amin-Hanjani S. Extracranial carotid-vertebral bypass for endovascular access to complex posterior circulation aneurysms: a novel management approach. Neurosurgery. 2012 May;70(5):1296–1303. doi: 10.1227/NEU.0b013e318241374b. discussion 12303–12304. [DOI] [PubMed] [Google Scholar]
- 35.Jiang H, Ni W, Lei Y, Li Y, Gu Y. Combined extracranial-intracranial bypass surgery with stent-assisted coil embolization for moyamoya disease with a ruptured wide-necked basilar trunk aneurysm: a case report. Turkish neurosurgery. 2015;25(1):180–185. doi: 10.5137/1019-5149.JTN.10043-13.0. [DOI] [PubMed] [Google Scholar]
- 36.Kakarla UK, Beres EJ, Ponce FA, et al. Microsurgical treatment of pediatric intracranial aneurysms: long-term angiographic and clinical outcomes. Neurosurgery. 2010 Aug;67(2):237–249. doi: 10.1227/01.NEU.0000371727.71991.64. discussion 250. [DOI] [PubMed] [Google Scholar]
- 37.Ponce FA, Albuquerque FC, McDougall CG, Han PP, Zabramski JM, Spetzler RF. Combined endovascular and microsurgical management of giant and complex unruptured aneurysms. Neurosurgical focus. 2004 Nov 15;17(5):E11. doi: 10.3171/foc.2004.17.5.11. [DOI] [PubMed] [Google Scholar]
- 38.Streefkerk HJ, Wolfs JF, Sorteberg W, Sorteberg AG, Tulleken CA. The ELANA technique: constructing a high flow bypass using a non-occlusive anastomosis on the ICA and a conventional anastomosis on the SCA in the treatment of a fusiform giant basilar trunk aneurysm. Acta neurochirurgica. 2004 Sep;146(9):1009–1019. doi: 10.1007/s00701-004-0296-2. discussion 1019. [DOI] [PubMed] [Google Scholar]
- 39.Lin N, Brouillard AM, Krishna C, et al. Use of coils in conjunction with the pipeline embolization device for treatment of intracranial aneurysms. Neurosurgery. 2015 Feb;76(2):142–149. doi: 10.1227/NEU.0000000000000579. [DOI] [PubMed] [Google Scholar]
- 40.Drake CG. Bleeding aneurysms of the basilar artery. Direct surgical management in four cases. Journal of neurosurgery. 1961 Mar;18:230–238. doi: 10.3171/jns.1961.18.2.0230. [DOI] [PubMed] [Google Scholar]
- 41.Drake CG. Further experience with surgical treatment of aneurysm of the basilar artery. Journal of neurosurgery. 1968 Oct;29(4):372–392. doi: 10.3171/jns.1968.29.4.0372. [DOI] [PubMed] [Google Scholar]


