Abstract
We describe the 2-year follow-up of an open-label trial (CT-AMT-011-01) of AAV1-LPLS447X gene therapy for lipoprotein lipase deficiency (LPLD), an orphan disease associated with chylomicronemia, severe hypertriglyceridemia, metabolic complications and potentially life-threatening pancreatitis. The LPL S447X gene variant, in an adeno-associated viral vector of serotype 1 (alipogene tiparvovec), was administered to 14 adult LPLD patients with a prior history of pancreatitis. Primary objectives were to assess the long-term safety of alipogene tiparvovec and achieve a ≥40% reduction in fasting median plasma triglyceride (TG) at 3–12 weeks compared with baseline. Cohorts 1 (n=2) and 2 (n=4) received 3 × 1011gc/kg, and cohort 3 (n=8) received 1 × 1012gc/kg. Cohorts 2 and 3 also received immunosuppressants from the time of alipogene tiparvovec administration and continued for 12 weeks. Alipogene tiparvovec was well tolerated, without emerging safety concerns for 2 years. Half of the patients demonstrated a ≥40% reduction in fasting TG between 3–12 weeks. TG subsequently returned to baseline, although sustained LPL S447X expression and long-term changes in TG-rich lipoprotein characteristics were noted independently of the effect on fasting plasma TG.
Keywords: LPL deficiency, familial chylomicronemia, alipogene tiparvovec, gene therapy, AAV1-LPLS447X
INTRODUCTION
Lipoprotein lipase (LPL) deficiency (LPLD) is a rare autosomal recessive disease with an estimated prevalence of 1–2 in 1,000,000. It is characterized by severe hypertriglyceridemia, chylomicronemia, and risk of recurrent and potentially fatal pancreatitis.1 Signs and symptoms include hepatosplenomegaly, eruptive xanthomas, lipemia retinalis, severe abdominal pain, peripheral neuropathy and an increased risk of cardiometabolic complications1–6. Patients with recurrent episodes of acute pancreatitis may also develop chronic pancreatitis and signs of exocrine or endocrine pancreatic insufficiency, including diabetes mellitus.1,3,7
LPL is a central enzyme in the catabolism of triglyceride (TG)-rich lipoproteins, namely chylomicrons (CMs) and very-low-density lipoproteins (VLDL)8,9. The adipose and skeletal muscle tissue are important sites of LPL production. Once produced, LPL is secreted and translocated to the luminal surface of endothelial cells to ensure direct contact with circulating TG-rich lipoproteins. When LPL is deficient, CM levels and pancreatitis risk increase dramatically.10 The pathophysiology underlying CM-related pancreatitis has not been completely elucidated. One hypothesis is that large CMs lodged in pancreatic capillaries expose them to pancreatic lipase, with the subsequent release of free fatty acids (FFAs) through the hydrolysis of CM-associated triglycerides. High concentrations of FFAs are thought to damage pancreatic cells leading to pancreatitis 11–15.
Fasting plasma TG concentrations exceed normal values 10–100 fold in LPLD5,16. Chylomicronemia is observed when fasting plasma TG concentration is > 10.0 mmol/L, and the risk of pancreatitis importantly increases with values above 20 mmol/L (approximately 2000 mg/dL)1,7,16. Traditional disease management in LPLD aims to decrease fasting TGs to below or near 10 mmol/L by restricting the fat intake to 10–15% of the total daily calorie intake, requiring the use of medium-chain triglyceride (MCT) oil and excluding alcohol from the diet throughout life1. This restrictive diet limits social freedom and adds to the burden of disease; it is not always sufficient to eliminate the risk of complications, even when patients are compliant. Currently available TG-lowering agents are not effective in controlling chylomicronemia in LPLD patients16–18. Additional disease management approaches are therefore required. Enzyme supplementation therapy is not feasible due to the very short half-life of LPL in the circulation. Gene therapy, which aims to induce the expression of functional LPL in muscle, offers an attractive therapeutic approach.
Pre-clinical gene therapy studies have shown that biologically active LPL can be produced in muscle by non-pathogenic, non-integrating19,20, adeno-associated virus (AAV) type 1-mediated gene transfer 21–23 without tissue-specific regulation of the transgene. AAV1-LPLS447X encodes a naturally-occurring gain-of-function LPL variant associated with lower plasma TG and a lower rate of cardiovascular disease24 than wild-type LPL. Intramuscular (IM) administration of AAV1-LPLS447X has been shown to result in the resolution of chylomicronemia and a life-long reduction in plasma TG concentrations in LPL-deficient mice23. In humans, the first interventional clinical study was carried out in eight LPLD patients using AAV1-LPLS447X produced using plasmid-based production in human embryonic kidney (HEK293) cells. Results of this study demonstrated statistically significant TG reductions in all patients up to 12 weeks25. Follow-up after 18–31 months showed a return of TG to baseline levels, which at the time was hypothesized to be related to an immune response to AAV1-capsid proteins25,26. AAV1-LPLS447X produced in HEK293 cells was not amenable to large-scale production therefore a switch was made to using baculovirus-based production in insect cells; AAV1-LPLS447X produced in this manner was termed alipogene tiparvovec27. We describe herein results from the > 2 years follow-up of the first clinical study conducted with alipogene tiparvovec (clinical study CT-AMT-011-01; ClinicalTrials.gov number: NCT01109498).
STUDY DESIGN
This open-label, dose-escalation clinical trial assessing the safety and efficacy of alipogene tiparvovec28 was conducted at the ECOGENE-21 Clinical Research Center, Chicoutimi, Quebec, Canada. Twenty-two adult LPLD patients with a history of pancreatitis participated in a prospective observational study (PREP-02) of > 4 months (range: 18–78 weeks) duration to determine baseline disease manifestations and the maximum effect of a controlled low-fat diet on chylomicronemia and fasting plasma TG levels (S.1). LPLD diagnosis was ascertained by genotyping and post-heparin measure of LPL activity. Among the participants to PREP-02, 14 subjects meeting eligibility criteria received alipogene tiparvovec while continuing the same low-fat diet in the interventional study (S.2). All participants kept a diary of food and beverage intake for the three days before each study visit and were thus instructed to carefully adhere to a low-fat diet where fat intake represented no more that 20–25% of caloric intake (< 55 g fat per day, assuming a 2,000 calorie diet) during both the observational and interventional studies to minimize diet-induced fluctuation in plasma TG levels. These subjects were assigned to 3 cohorts. Cohorts 1 (n=2) and 2 (n=4) received 3 × 1011gc/kg, and cohort 3 (n=8) received 1 × 1012gc/kg (Table 1). Cohorts 2 and 3 also received immunosuppressants from the time of alipogene tiparvovec administration and continued for 12 weeks. The immune suppression regimen consisted of cyclosporine A (CSA; 3 mg/kg/day) and mycophenolate mofetil (MMF; 2 gram per day) initiated at the time of alipogene tiparvovec administration and maintained for 12 weeks thereafter. A period of 12 weeks was considered sufficient for the prevention of potential capsid-related immunogenicity, based on observations in Rhesus macaques.29
Table 1.
Characteristics and Treatment Regimen of the 14 Subjects who Received Alipogene Tiparvovec
| Subject ID | Genotype | Gender | Age | Cohort | Dose alipogene tiparvovec [gc/kg] | Immuno-suppressant regimen |
|---|---|---|---|---|---|---|
| 01 | P207L/P207L | F | 60 | 2 | 3 × 1011 | CsA + MMF |
| 04 | P207L/P207L | M | 50 | 1 | 3 × 1011 | none |
| 06 | P207L/P207L | M | 51 | 1 | 3 × 1011 | none |
| 07 | P207L/P207L | F | 56 | 2 | 3 × 1011 | CsA + MMF |
| 08 | P207L/P207L | M | 28 | 3 | 1 × 1012 | CsA + MMF |
| 09 | P207L/G188E | F | 62 | 3 | 1 × 1012 | CsA + MMF |
| 10 | P207L/D9N | M | 42 | 2 | 3 × 1011 | CsA + MMF |
| 11 | P207L/P207L | F | 48 | 3 | 1 × 1012 | CsA + MMF |
| 13 | P207L/G188E | F | 40 | 3 | 1 × 1012 | CsA + MMF |
| 14 | P207L/P207L | F | 51 | 2 | 3 × 1011 | CsA + MMF |
| 15 | P207L/P207L | F | 50 | 3 | 1 × 1012 | CsA + MMF |
| 18 | P207L/P207L | M | 37 | 3 | 1 × 1012 | CsA + MMF |
| 19 | P207L/P207L | F | 28 | 3 | 1 × 1012 | CsA + MMF |
| 20 | P207L/P207L | F | 36 | 3 | 1 × 1012 | CsA + MMF |
CsA: cyclosporine A (3mg/kg/day)
MMF: mycophenolate mofetil (2g/day)
Primary objectives were to assess the long-term safety profile of alipogene tiparvovec and achieve a reduction in fasting median plasma TG of at least 40%, 3–12 weeks after therapy compared to baseline. Secondary objectives were to achieve a reduction in fasting TG to ≤10.0 mmol/L within 12 weeks, to measure the biological activity and expression of LPLS447X in the muscle after 26 weeks, to evaluate potential immune responses against LPLS447X and AAV1 capsid proteins, and to assess biodistribution and shedding of AAV1-LPLS447X vector DNA.
The study, conducted in accordance with Good Clinical Practices (CPMP/ICH/135/95) and the Declaration of Helsinki, was approved by Health Canada, the Board of the Chicoutimi Hospital and its Ethics Committee. All subjects provided written informed consent. The Ethics Committee of the Ministry of Health (FRSQ), Quebec Province, provided long-term study supervision. Monitoring of safety data was carried out by the Academic Medical Center, Amsterdam, The Netherlands. The ECOGENE-21 clinical site was audited by the European Medicines Agency (EMA).
RESULTS
Adverse events
One serious adverse event (SAE) (acute pancreatitis) occurred during the observational (PREP-02) study, and another subject experienced acute pancreatitis between the PREP-02 and interventional study, resulting in an overall pancreatitis incidence of 0.20 event/subject/year during prospective observation (S.3). Following injection of alipogene tiparvovec, all subjects (N=14) reported one or multiple mild to moderate adverse events (AEs). These did not cluster to one organ system and showed no dose relationship. Twelve subjects reported injection site events, such as local and transient bruising, edema, sensitivity and/or pain lasting a few days/weeks. One subject (cohort 3) developed a serious AE, considered probably related to drug administration, with fever (39.9°C) 10 hours after injection. This resolved spontaneously within 12 hours. No clinically relevant changes in clinical laboratory assessments, vital signs, chest X-rays and physical examination occurred during the study. No treatment-related changes in creatine phosphokinase (CPK), high sensitivity C-reactive protein or lactate dehydrogenase were observed. Immunosuppression did not result in any untoward side effects and did not have an impact on biochemical/inflammatory markers. Twelve subjects experienced non-severe infections (mostly nasopharyngitis [59%]), but incidence was within the expected seasonal range. Six SAEs were reported during the long-term follow-up (LTFU) including one severely affected diabetic patient, with history of frequent recurrent pancreatitis and chronic renal failure before treatment, who had a cardiac arrest leading to death 2 years into the LTFU period. None of the SAEs were related to alipogene tiparvovec administration.
Viral biodistribution and shedding
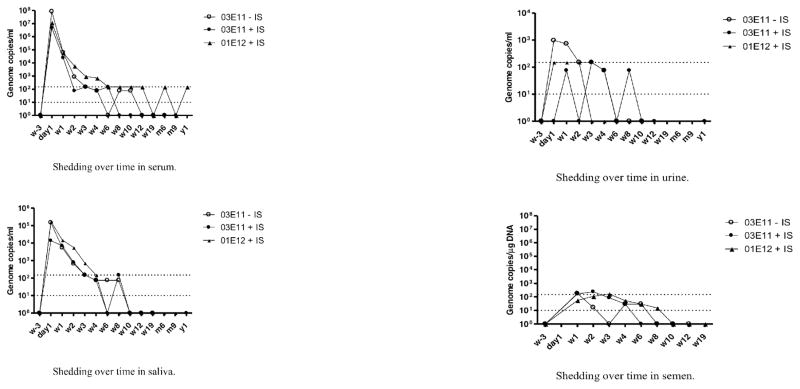
Peak levels of alipogene tiparvovec-derived vector DNA were detected 24 hours after administration in serum (max. 1.7 × 108 gc/mL), saliva (max. 8.0 × 105 gc/mL) and urine (max. 2.0 × 103 copies/mL). Levels in semen were first measured at Week 1 (four of five male subjects (max. 3.6 × 102 gc/μg DNA)). Vector DNA in most samples dropped to around/below the limit of detection (1.0 × 101 gc/μg DNA) within 4–6 weeks except in one subject (Figure 1). Vector DNA clearance from semen occurred after 6–10 weeks, although low levels, barely above the limit of detection, were later found once in two of the five male subjects.
Figure 1.
Presence of AMT-011 Vector DNA in body fluids: Results are depicted as median values per treatment group, per time point. Dashed lines indicate limit of detection (bottom line) and limit of quantification (top line) of the assay
(A: shedding in serum; B; shedding in saliva; C: shedding in urine; D: shedding in semen).
Host immune response
Anti-LPLS447X antibodies were not observed in any subject. Anti-AAV1 antibodies were detected in approximately half (n=8) of the subjects before alipogene tiparvovec administration, and all subjects, whether exhibiting pre-existing antibodies or not, showed a treatment-emergent increase in anti-AAV1 antibodies after administration, persisting at high titer through the post-treatment period (Table 2). Treatment-emergent anti-AAV antibody responses were not affected by immune suppression or the termination of the immunosuppressive regimen. Based on the data obtained from PBMCs of adequate quality, a moderate and non-persistent T-cell response was observed directed against the AAV1 capsid (and not against LPLS447X) in 9 out of the 14 subjects.
Table 2.
Humoral immune response to AAV1
| Subject | Dose | Antibodies against AAV1 | |
|---|---|---|---|
|
| |||
| (gc/kg) | Pre-administration | Post-administration | |
| 01 | 3×1011 +ISR | ++ | ++ |
| 04 | 3×1011 | ND | ++ |
| 06 | 3×1011 | − | ++ |
| 07 | 3×1011 +ISR | + | ++ |
| 08 | 1×1012 +ISR | − | ++ |
| 09 | 1×1012 +ISR | − | ++ |
| 10 | 3×1011 +ISR | − | ++ |
| 11 | 1×1012 +ISR | + | ++ |
| 13 | 1×1012 +ISR | + | ++ |
| 14 | 3×1011 +ISR | ++ | ++ |
| 15 | 1×1012 +ISR | + | ++ |
| 18 | 1×1012 +ISR | + | ++ |
| 19 | 1×1012 +ISR | − | ++ |
| 20 | 1×1012 +ISR | + | ++ |
ISR: immunosuppressants
The test results of the samples were scored by comparison with those of a negative control, (a serum sample from a healthy human control). To this end, algorithms were developed to convey the optical density results into a semi-quantitative scoring system. Based on the algorithms, samples were said to be strongly positive (++), weakly positive (+) or negative (−) for AAV1 antibodies.
Local injection site response
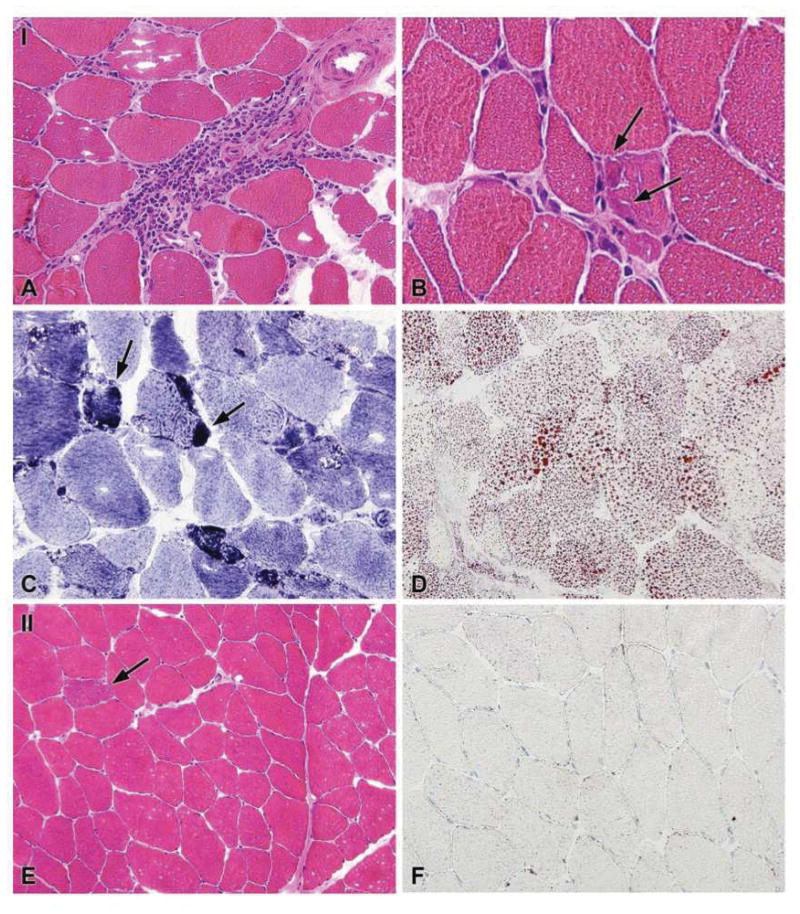
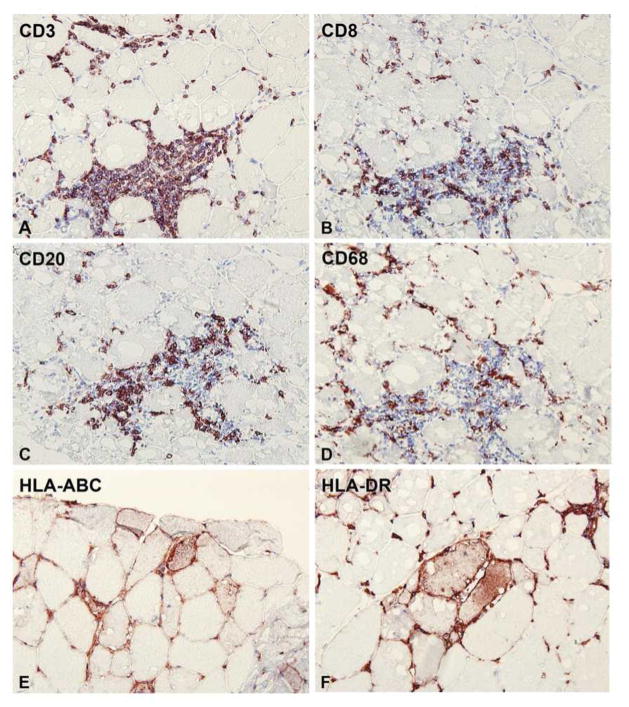
Variable local responses were observed in injected muscle tissue compared with non-injected muscle. These responses ranged from none or minor (subjects 01 and 13), to slight (06), moderate (04 and 10) and a more pronounced local response (09 and 11). A more pronounced local response was characterized by non-specific muscle fiber degeneration and regeneration, neutral lipid accumulation within fibers, and perivascular to endomysial infiltration by CD8+ T-cells, CD20+ B-cells and CD68+ macrophages (Figures 2 and 3B). Subsarcolemmal accumulations (positive in NADH-oxidoreductase, PAS, and Gomori-Trichrome stains; negative in SDH stain) were observed and identified by EM as tubular aggregates (Figure 3A). Positive staining for SERCA-1 and -2 suggests that these accumulations represent a proliferation of the sarcoplasmic reticulum. The expression of MHC class I/II surface receptors was up-regulated in the sarcolemma of a few fibers close to cellular infiltrates. In its more moderate form (04 and 10), fiber de- and regeneration was noted but tubular aggregates were not always observed (04 only), and there was less lipid accumulation. Fewer perivascular and endomysial infiltrates consisted mainly of CD8+ T-cells and CD68+ macrophages, with only a minor CD20+ component; there was no expression of MHC class I/II on fibers. Progressively less degenerative muscle fiber responses, less lipid accumulation and less tissue infiltration were observed for biopsy samples from subjects 06, 13, and 01 (Table 3). There was a trend towards a more pronounced local response in samples showing more LPL expression (Tables 3 and 5). Apoptosis, excess fibrosis or widespread necrosis were not observed in injected muscle tissue, and there were no gross abnormalities; overall muscle structure and function was preserved.
Figure 2.

Paraffin-embedded cross-section of the muscle biopsy (injected muscle) from subject 09, stained with H&E; showing a large focal infiltrate and diffuse infiltration throughout the biopsy
Figure 3.
Figure 3A: General histology as noted in cryosections of the injected muscle from subject 11 who was one of two patients (09 and 11) to show a more pronounced local response. (A–D) injected muscle (I): (A & B) H&E staining showing (large) perivascular and endomysial infiltration of the injected tissue. Some freezing artifacts are observed. Arrows in (B) indicate small, irregularly shaped degenerating fibers with subsarcolemmal accumulations with neighboring endomysial infiltrates; (C) uneven NADH stain, arrows pointing to increased staining of the subsarcolemmal accumulations noted in (B); (D) accumulation of neutral lipid, visualized by Oil Red O staining. (E&F) non-injected muscle; (E) H&E staining showing largely normal histology except for a single ragged red fiber (indicated by arrow); (F) normal neutral lipid content as visualized using Oil Red O.
Figure 3B: Immunohistochemical staining of cryosections of injected muscle from subject 09 who was one of two patients (09 and 11) to show a more pronounced local response. (A) CD3+ T-lymphocytes found within the injected tissue as a large perivascular infiltrate, and more diffuse endomysial infiltration; (B) staining for CD8+ T-lymphocytes in the same area; (C) CD20+ B-lymphocytes in the same area; (D) CD68+ macrophages within the same area; (E and F) staining for MHC class I and –II surface receptors more distal to large infiltrates, showing positivity on the surface of muscle fibers (in addition to positivity of infiltrates).
Table 3.
Summary of immunohistochemical staining of cryosections of injected muscle biopsies isolated from 7 LPLD subjects following IM administration of alipogene tiparvovec
| Patient | Dose (gc/kg) | ISR | CD3 | CD4 | CD8 | CD68 | CD20 | HLA-ABC (fibers/infl.cells) | HLA-DR fibers/infl.cells) |
|---|---|---|---|---|---|---|---|---|---|
| 01 | 3×1011 | ISR | - | - | - | - | - | - | - |
| 04 | 3×1011 | - | 2+ | 1+ | 2+ | 2+ | 1+ | −/+ | −/+ |
| 06 | 3×1011 | - | 1+ | 1+ | 1+ | 1+ | - | −/+ | −/+ |
| 09 | 1×1012 | ISR | 3+ | 2+ | 3+ | 3+ | 3+ | +/+ | +/+ |
| 10 | 3×1011 | ISR | 2+ | 2+ | 2+ | 2+ | 1+ | +*/+ | +*/+ |
| 11 | 1×1012 | ISR | 3+ | 2+ | 3+ | 3+ | 3+ | +/+ | +/+ |
| 13 | 1×1012 | ISR | - | - | - | - | - | - | - |
ISR: immunosuppressants
Scoring reflects relative levels of infiltration: - none; 1+ rare; 2+ moderate; 3+ high number;
single fiber affected.
Scores provide a semi-quantitative and relative means of discriminating between patients. A score of 3+ represents the highest level of infiltration observed in this study.
Table 5.
Alipogene tiparvovec derived DNA sequence and LPL expression in muscle of 7 LPLD patients following intramuscular administration of alipogene tiparvovec
| Subject | Dose (gc/kg) | QPCR (gc/μg gDNA) | LPL mass (ng/ml) | LPL activity (nmol/min/mg) | LPL IHC | Oil Red O | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| I | C | I | C | I | C | I | C | I | C | ||
| 01 | 3 × 1011 + ISR | 900 | 0 | 0 | 0 | 0 | 0 | - | - | - | - |
| 04 | 3 × 1011 | 170000 | 110 | 24.06 | 0 | 5.91 | 0 | ++ | - | ++ | - |
| 06 | 3 × 1011 | 22000 | 30 | 0 | 0 | 0 | 0 | - | - | + | - |
| 09 | 1 × 1012 + ISR | 77000 | 0 | 85.75 | 0 | 23.29 | 0 | ++ | - | +++ | - |
| 10 | 3 × 1011 + ISR | 110000 | 30 | 0 | 0 | 0 | 0 | + | - | ++ | - |
| 11 | 1 × 1012 + ISR | 630000 | 0 | 182.76 | 0 | 77.52 | 0 | +++ | - | +++ | - |
| 13 | 1 × 1012 + ISR | 13000 | 30 | 0 | 0 | 0 | 0 | - | - | - | - |
QPCR: alipogene tiparvovec derived DNA sequence (encoding LPLS447X) gene copy numbers as measured in biopsy tissue homogenate.
LPL mass and LPL activity as measured in muscle tissue homogenates.
I: injected muscle; C: non-injected muscle; ISR: immunosuppressants regimen;
LPL IHC: detection of LPL by immunohistochemistry on tissue sections; ORO: (intracellular) neural lipids as detected in tissue sections stained by Oil Red O.
Stained tissue sections were examined by two trained observers, with respect to the presence or absence of various histopathological parameters and specific immunoreactivity for the different markers.
Two representative frozen sections per individual & biopsy specimen were stained for CD3, CD4, CD8, CD20, CD68, HLA-DR and HLA-ABC and assessed by the two observers independently; a consensus score was obtained.
The degree of staining was rated on a semi-quantitative three-point scale: 0, no positive cells; 1+: rare positive cells; 2+: moderate number of positive cells; 3+: high number of positive cells.
A similar three-point scale was used to score the amount of lipid detected in the Oil-Red-O stained sections. It should be noted that this scoring was intended to discriminate between subjects, relating only to the relative amount of infiltration or lipid accumulation noted in the biopsy specimens, and does not represent an overall severity score.
AAV1 vector DNA sequence and LPLS447X expression in injected muscle
All seven biopsies showed AAV1 vector DNA, 26 weeks after therapy (Table 5). Levels in corresponding non-injected samples were at or below the level of quantification irrespective of dose. Muscle tissue homogenates tested positive for LPL protein and activity in three of seven injected muscle samples. Expression of LPL protein was confirmed in four of seven subjects by staining serial cross-sections of injected muscle using the antibody 5D2. Sections for five of seven subjects also showed an increase in intracellular lipids (Oil Red O stain for neutral lipid) consistent with increased local LPL activity (Figure 3A, Table 5). These findings agree with other studies that show persistent transgene expression in man following AAV-mediated gene transfer to muscle30–32
Effect on fasting TG, TG-rich lipoproteins characteristics and clinical outcomes
As expected, fasting TG levels during the PREP-02 study remained >10.0 mmol/L and comparable to historical values in all subjects. Three to twelve weeks after alipogene tiparvovec administration, all but two subjects demonstrated reduced median TG compared to baseline (average reduction: 39.53%); 50% achieved the primary objective of a ≥40 % reduction in TG. Four subjects achieved the secondary endpoint of TG ≤10.0 mmol/L. Two subjects showed no response on fasting TG levels. TG reductions during week 3–12 were statistically significant for the total group (p = 0.0009) and cohort 3 (p = 0.0023) (Table 4). TG returned to baseline values in all subjects by week 16–26. The distribution of TG, lipids and apolipoprotein B (apoB) concentration was also studied in the different lipoprotein fractions until week 52, through ultracentrifugation and isolation of the lipoproteins according to floating (Sf) and density criteria. The analyses of the Sf >400 fraction (corresponding to the density of chylomicrons) and less buoyant Sf 20–400 fraction (corresponding to the density of VLDL particles) showed that the total apoB concentration in the Sf 20–400 fraction, a surrogate of the number of circulating (TG-rich) lipoproteins within this fraction, increased at 12 and 52 weeks compared to baseline (cohort 3; p = 0.009) (Figure 4A). This coincided with significant increases in cholesterol (p = 0.04) and TG (p= 0.04) in this less buoyant (Sf 20–400) fraction and with a trend towards decreased cholesterol and TG in the more buoyant fraction (Sf >400) (Figure 4B). These modifications in TG-rich lipoprotein characteristics were independent of the effect of alipogene tiparvovec on total TG33 and correlated with signs of clinical improvement.
Table 4.
Primary efficacy outcome in individual subjects
Fasting plasma triglycerides (TG, mmol/L)* before and 3–12 weeks after administration of alipogene tiparvovec
| Subject | Median pre-therapy TG ** | Median post-therapy TG (W3–W12) | % Reduction W3–W12 vs pre-therapy |
|---|---|---|---|
| 1 | 22.55 | 21.39 | 5.15 |
| 4 | 49.10 | 30.41 | 38.07 |
| 6 | 15.88 | 4.37 | 72.50 |
| 7 | 23.30 | 6.73 | 71.11 |
| 8 | 22.90 | 18.72 | 18.25 |
| 9 | 23.81 | 5.38 | 77.42 |
| 10 | 21.87 | 29.01 | −32.68 |
| 11 | 28.38 | 24.44 | 13.89 |
| 13 | 34.21 | 10.17 | 70.29 |
| 14 | 22.38 | 14.76 | 34.07 |
| 15 | 65.48 | 25.17 | 61.56 |
| 18 | 16.52 | 9.56 | 42.16 |
| 19 | 13.02 | 13.95 | −7.15 |
| 20 | 21.39 | 10.99 | 48.62 |
Normal fasting whole plasma TG levels in unaffected individuals range between 1 and 2.3 mmol/L
Median of last five values from PREP-02 and of Week -3 baseline visit from CT-AMT-011-01
TG values generally had reverted back to around baseline values by the Week 26 visit.
None of the participants had historical plasma TG values < 10 mmol/L
Figure 4.
Figure 4A: Distribution of plasma TG and cholesterol in the Sf >400 and Sf 20–400 fractions before therapy and 52 weeks after alipogene tiparvovec administration.
Figure 4B: Total APOB in the Sf 20–400 fraction before therapy and 12 and 52 weeks after alipogene tiparvovec administration.
The assessment of clinical outcomes was not a primary objective of the CT-AMT 011–01 study. However, more than two years after alipogene tiparvovec injection, most (N=9) patients self-reported signs of clinical benefits such as a capacity to eat more or to eat food they were unable to before, increased energy levels or improved abdominal comfort. Although based on a small number of patients and episodes, overall pancreatitis incidence up to 2 years post-alipogene tivarvovec injection decreased by 5-fold, 0.04 event/year compared with 0.20 event/year from enrolment into PREP-02 to the day of administration of alipogene tiparvovec. Two patients had acute pancreatitis during LTFU. One had a severe pancreatitis event during week 1. The other events occurred in a subject non-compliant to diet for several days. This patient reported a decrease of the intensity, duration and peak of the abdominal pain during the pancreatitis crisis compared to pre-treatment.
DISCUSSION
Alipogene tiparvovec was generally well tolerated for up to two years and was associated with signs of clinical benefits and persistent LPL expression in muscle, independently of the effect on plasma TG. Signs of long-term transgene expression are consistent with results of previous studies having shown multi-year expression following a single IM administration of AAV30–31. AAV is a non-pathogenic, non-integrating viral19,20, and AAV genomes persist as extrachromosomal monomers or concatamers which support long-term expression.
Alipogene tiparvovec-derived vector DNA was present transiently in serum, urine, saliva and, at extremely low levels, in semen. Other clinical studies have indicated the absence of the vector sequence in semen following intramuscular AAV2 or AAV1 administration.30,31 However, the sensitivity of the PCR-based assay used in our study was 5 to 10-fold higher than those used in previous studies. Overall, data indicate minimal risk, if any, of germ-line transmission. The risk to the environment or other individuals associated with shedding such low amounts of AAV is limited, since further spread of replication-deficient AAV vectors is highly unlikely.
All subjects showed a robust antibody response, and more than half (9 out of 14) demonstrated a moderate and non-persistent T-cell response to AAV1 capsid proteins. Muscle biopsy assessments suggest a relationship between the extent of the local injection site response and the level of local LPL activity (e.g., LPL protein and activity, accumulation of intracellular lipids) rather than with observed anti-AAV1 immune responses.17 The safety evaluation demonstrated that, with the exception of one SAE, drug-related AEs were mild or moderate, did not cluster to one organ system and showed no dose relationship. One subject suffered an episode of pancreatitis following a high-fat meal within one week of alipogene tiparvovec administration, when transgene expression is not expected to be optimal. Preclinical observations have shown that LPL is unlikely to be expressed before 3 weeks after alipogene tiparvovec administration leading to the CT-AMT-011-01 protocol criteria that measure changes in TGs from 3 weeks onwards.
TG values during the observational PREP-02 study confirmed previous findings that the prescription of a severe dietary fat restriction does not reduce fasting TG to a level at which the risk of pancreatitis and other chylomicronemia symptoms may be eliminated.1,7,12,13 In LPLD, the extent of chylomicronemia, and hence plasma TG levels, are affected by diet. Alipogene tiparvovec in addition to a low-fat diet was effective in lowering fasting plasma TG levels 3–12 weeks after administration in all but two patients. The fact that TG reverted to baseline after 19–26 weeks was initially interpreted as a sign of transient efficacy25. However, several signs of clinical efficacy independent of plasma TG were noticed up to 2 years after LPL gene transfection and raised the possibility that TG-rich lipoprotein characteristics, particularly the size, lipid content and kinetics of CMs, rather than plasma TG concentration per se, are the best surrogate markers of pancreatitis risk in LPLD. Signs of efficacy beyond week 12 included: (a) sustained modification in TG-rich lipoprotein characteristics independent of the effect on total TG; (b) persistent vector DNA, transgene expression, and biological activity of LPLS447X in injected muscle after 26 weeks; (c) self-reported signs suggestive of improvement of the quality of life; (d) reduction in overall pancreatitis incidence and/or intensity of the crisis up to 2 years post-alipogene tivarvovec injection. These results and observations have led to the conception and execution of two additional studies specifically designed to evaluate the effect of alipogene tiparvovec on chylomicron metabolism, kinetics and clearance (CT-AMT-011-02) and on pancreatitis and abdominal pain (CT-AMT-011-03). The later is designed as a case review study to retrospectively and prospectively assess the prevalence, severity and incidence of abdominal pain crises and acute pancreatitis in LPLD patients, including those having received alipogene tiparvovec or participated in the PREP-study.
CMs are responsible for the transportation and delivery of fat following a meal. LPLD is thus, by its very nature, a postprandial disease. It would appear that there is no simple correlation between fasting whole plasma TG and pancreatitis risk after therapy. The pathogenesis of pancreatitis associated with chylomicronemia is not completely understood, and factors other than total fasting plasma TG may be involved.13 Lipoprotein fractionation studies showed an apparent sustained shift of TG and cholesterol from the buoyant CM fraction (Sf >400) to a less buoyant fraction corresponding to the VLDL density (Sf 20–400) without changes in the number and composition of LDL and HDL particles. The conditions for ultracentrifugation used here preclude a complete separation of CM from VLDL and other lipoproteins. The fixed spin time is likely to allow separation of only the more buoyant CM. After therapy, on average more TG, cholesterol and apoB were recovered from the Sf 20–400 fraction suggesting that TG-rich particles became less buoyant. We hypothesize that such modifications in CM characteristics contribute to decreased pancreatitis and clinical benefits. This is supported by recent data from the CT-AMT-011-02 study suggesting that chylomicron clearance and TG-rich lipoprotein characteristics, rather than fasting TG, might be a more appropriate indicator of alipogene tiparvovec efficacy33,34. The clinical significance of CM composition and its possible utility as a surrogate marker of alipogene tiparvovec efficacy is the subject of ongoing studies. Although involving a small number of patients and events, the five-fold reduction in the 2-year incidence of pancreatitis is compatible with a long-term expression of LPLS447X.
CONCLUSION
A single (one-time) intramuscular administration of alipogene tiparvovec, in addition to a low-fat diet, was well tolerated. Alipogene tiparvovec was associated with a transient effect on fasting TG, persistent gene expression, sustained alterations in TG-rich lipoprotein distribution profiles and signs of clinical improvement including a clinically meaningful decrease in pancreatitis incidence and characteristics. This is the first demonstration of a single gene therapy intervention leading to persistent transgene expression and sustained clinical benefit in a systemic metabolic disorder.
METHODS
Study drug
Alipogene tiparvovec is a recombinant AAV vector of serotype 1. The vector contains the coding sequence for the human gene variant LPLS447X. Transcription is driven by the CMV promoter and terminated by a bovine growth hormone polyadenylation sequence. Alipogene tiparvovec was produced using insect cells and baculoviruses by Amsterdam Molecular Therapeutics (AMT) B.V., the Netherlands, in accordance with Good Manufacturing Practice guidelines (S.5).
Drug administration
Subjects, under spinal anesthesia, received alipogene tiparvovec by multiple intramuscular injections divided equally between the musculus vastus lateralis and musculus vastus medialis of both the left and right musculus femoralis. The calf muscles were also injected if the number of injections exceeded 40 injection sites (S6). Follow-up evaluations took place at 1 day and 1, 2, 3, 4, 6, 8, 10 and 12 weeks (main study), and at 19, 26, 39, 52 weeks and 2 years after alipogene tiparvovec administration (LTFU) (S.6).
Adverse events
The coding of AEs was performed using MedDRA version 9.1. Routine assessments included physical examination, vital signs and weight, chest X-ray ECG, blood pressure, urine analysis, serum biochemistry, hematology assessments and serology (S.7 and S.8). In addition, systemic humoral and cell-based immune responses to AAV-1 and LPLS447X were assessed, and biodistribution and shedding of AAV1-LPLS447X monitored. Studies to examine local tolerance to alipogene tiparvovec and the injection procedure were carried out in muscle biopsy samples from injected and non-injected muscles taken 26 weeks after administration.
Any untoward clinical signs and symptoms, including injection site reactions and pancreatitis, were recorded and graded. Pancreatitis events during observation, the main 12-week study phase and LTFU were determined by the attending physician according to the 2004 guideline of the Society for Surgery of the Alimentary Tract on the treatment of acute pancreatitis.35 The frequency of pancreatitis events was calculated by dividing the total number of events observed in all patients by the total number of years of follow-up.
Humoral immune responses to AAV-1 and LPLS447X were determined by measuring serum antibodies against the AAV1 capsid proteins and LPLS447X protein by ELISA (general antibody titre, non-discriminatory for immunoglobulin subclasses) (Xendo Drug Development B.V., Groningen, the Netherlands, now part of QPS Holding LLC). Serum samples were collected at baseline, at weeks 1, 2, 3, 4, 6, 8, 10 and 12, and at all time points during LTFU. Peripheral blood mononuclear cells (PBMC), employed to measure cell-mediated immune responses, were used in an enzyme-linked immunospot (ELISpot) assay for the detection of IFN-γ secretion in response to antigen stimulation; PBMCs were incubated with whole AAV1 capsid particles, with pools of peptides derived from the AAV1 capsid (VP1 protein sequence) or with recombinant LPLS447X protein, for 20–24 hours, and cytokine secretion was detected with an anti-human IFN-γ antibody. Positive and negative controls were also included in the assay; assays were performed by Mingozzi and co-workers (CHOP, Philadelphia PA, USA).26 PBMC were collected from whole blood collected at baseline, at weeks 2, 4, 6, 8 and 12, and at all time points during LTFU.
Biodistribution and shedding of AAV1-LPLS447X to evaluate the potential risk of transmission to third parties or the environment was assessed by detection of vector DNA in serum, saliva, urine and semen using quantitative-PCR (Q-PCR). Primers and probe (Taqman) used in the assay were specific for the boundary between the LPLS447X sequence and WPRE element. The assays were performed by JSW Life Sciences GmbH (Grambach, Austria). Samples were collected at baseline, at day 1, at all time points and until three consecutive samples were negative. During the LTFU, additional semen samples were collected at least 75 days after administration of alipogene tiparvovec, until three consecutive samples were negative. DNA isolation methods (also used for vector DNA and LPL expression studies) were optimized and spiking experiments were performed to determine the sensitivity of the method. Ten copies of the vector DNA sequence could be detected.
Muscle biopsies
Biopsies used in both safety (to assess local tolerance to the study drug) and efficacy assessments were obtained from injected and non-injected muscle of 7 of 14 subjects enrolled in the trial (two from cohort 1, two from cohort 2 and three from cohort 3; the remaining subjects did not consent to the biopsy procedure). Biopsies were taken ~26 weeks after vector administration.
Each muscle biopsy was divided into several parts; these separate parts were used for: Q-PCR (to measure vector DNA levels in muscle homogenate), transgene expression (LPL protein mass and activity in muscle homogenate) and histology (basic histology including immunohistochemical staining to characterize muscle fibers and inflammatory cells). A separate section was fixed in Karnovsky fixative for electron microscopy (EM). Muscle histology was performed by Dr. Eleonora Aronica of the Department of Neuro-Pathology of the Academic Medical Centre (AMC), Amsterdam, The Netherlands. Paraffin sections of formalin-fixed material were prepared for general histology. Six μm-thick cryosections were routinely stained for ATPase pre-incubated at pH4.3, NADH–Oxidoreductase, Succinate Dehydrogenase (SDH), Cytochrome Oxidase (Cox)/SDH, Periodic Acid-Shiff (PAS), Oil Red-O (ORO), Hematoxylin and Eosin (H&E), Acid Phosphatase, Congo Red, Non-specific Esterase (NE) and Gomori Trichrome. Immunohistochemistry (IHC) was performed for CD3, CD4, CD8, CD20, CD68, and human leukocyte antigens HLA-DR and HLA-ABC.36–39 Additional immunohistochemistry was performed for sarco(endo)plasmic reticulum Ca2+ATPases (SERCA1 and SERCA2), myosin heavy chain (MHC) and membrane attack complex (MAC or C5b-9).
Stained tissue sections were examined by two independent examiners for the presence of histopathological parameters and specific immunoreactivity to determine a consensus score. The degree of staining was rated on a semi-quantitative three-point scale: 0, no positive cells; 1+: rare positive cells; 2+: moderate number of positive cells; 3+: high number of positive cells. This scoring allowed discrimination between subjects based on the relative degree of infiltration or lipid accumulation in specimens, and does not represent an overall severity score.
Efficacy assessments
Efficacy assessment included determination of plasma TG levels, and presence of vector DNA and LPL protein and LPL activity in muscle biopsies. Plasma TG levels were measured taking free glycerol into account, using standard colorimetric methods, by PPD Global Central Labs, Kentucky, USA. Extra to the protocol, ultracentrifugation studies were performed to assess TG, cholesterol and apoB levels in plasma lipoprotein fractions, and plasma phospholipid levels were determined.
Statistical analysis
Because of intra-subject variability in TG levels, multiple data points were used to derive pre- and post-therapy values. The median of the six most recent measurements before the day of alipogene tiparvovec administration was used for pre-therapy values. All TG data from week 3 until and including week 12 were used for the main study post-administration TG response assessment. A linear mixed model was used to estimate the average reduction in individual TG after alipogene tiparvovec administration and whether there was a statistically significant reduction in TG calculated using median and mean values. Individual pre-therapy and post-therapy TG values until week 12, and 26, were compared using the non-parametric Wilcoxon test. A score of 0 or 1 was assigned to subject’s TG levels to indicate success or failure (TG ≤10.00 mmol/L or > 10.00 mmol/L, respectively). Using a Chi-squared statistic, a Mixed Model Repeated Measures and Wilcoxon Signed Rank test, it was determined whether alipogene tiparvovec, or a specific dose, lowers TG significantly. All hypotheses were tested with an overall two-sided significance level of 0.05.
Supplementary Material
Acknowledgments
Authors would like to thank: all participants in the clinical study; staff from the Academic Medical Center (AMC), Amsterdam, the Children’s Hospital of Philadelphia; IATEC, TCTC, ECOGENE-21 Clinical Research Center, the Chicoutimi Hospital and Amsterdam Molecular Therapeutics (AMT) B.V. Medical writing support was provided by Pam Pickering, Conscience Creative LLP, Leatherhead, Surrey, UK. K.T. is a Université de Montréal post-doctoral and CCRP fellow and receives support from the Canadian Heart and Stroke Foundation. J.M was a Université de Montréal post-doctoral fellow and received support from the Canadian Institutes for Health Research (CIHR) during the study, and D.G. was holder of the Canada Research Chair in preventive genetics and community genomics (www.chairs.gc.ca), which is also supported by a CIHR team grant (# CTP-82941).
Sponsor: AMT, the Netherlands.
Footnotes
ClinicalTrials.gov number: NCT01109498.
Supplementary information (S.1–S.8) is available at Gene Therapy’s website
Conflict of Interest
The funding body (AMT) was involved in all aspects of the study (study design, data collection and analysis, and data interpretation in collaboration with the CRO and principal investigator. Five authors (J.dW., J.T., S.vD., N.vdB. and V.S-F) are employees of AMT. The remaining authors declare no conflict of interest. The principal investigator of the study (DG) has no financial interest in AMT and made all final editorial decisions regarding the manuscript.
References
- 1.Brunzell JD, Deeb SS. Familial lipoprotein lipase deficiency, Apo C-II deficiency and hepatic lipase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic Basis of Inherited Disease. 8. New York, NY: McGraw-Hill; 2001. pp. 2789–2816. [Google Scholar]
- 2.Black DM, Sprecher DL. Dietary treatment and growth of hyperchylomicronemic children severely restricted in dietary fat. Am J Dis Child. 1993;147:60–62. doi: 10.1001/archpedi.1993.02160250062018. [DOI] [PubMed] [Google Scholar]
- 3.Chait A, Robertson HT, Brunzell JD. Chylomicronemia syndrome in diabetes mellitus. Diabetes Care. 1981;4:343–348. doi: 10.2337/diacare.4.3.343. [DOI] [PubMed] [Google Scholar]
- 4.Chait A, Brunzell JD. Chylomicronemia syndrome. Adv Int Med. 1992;37:249–273. [PubMed] [Google Scholar]
- 5.Santamarina-Fojo S. The familial chylomicronemia syndrome. Endocrinol Metab Clin North Am. 1998;27:551–567. doi: 10.1016/s0889-8529(05)70025-6. [DOI] [PubMed] [Google Scholar]
- 6.Benlian P, De Gennes JL, Foubert L, Zhang H, Gagné SE, Hayden M. Premature atherosclerosis in patients with familial chylomicronaemia caused by mutations in the lipoprotein lipase gene. N Engl J Med. 1996;335:848–854. doi: 10.1056/NEJM199609193351203. [DOI] [PubMed] [Google Scholar]
- 7.Fortson MR, Freedman SN, Webster PD., III Clinical assessment of hyperlipidemic pancreatitis. Am J Gastroenterol. 1995;90:2134–2139. [PubMed] [Google Scholar]
- 8.Goldberg IJ. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res. 1996;37:693–707. [PubMed] [Google Scholar]
- 9.Mahley RW, Ji ZS. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein. E J Lipid Res. 1999;40:1–16. [PubMed] [Google Scholar]
- 10.Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med. 2002;80:753–769. doi: 10.1007/s00109-002-0384-9. [DOI] [PubMed] [Google Scholar]
- 11.Mössner J, Bödeker H, Kimura W, Meyer F, Böhm S, Fischbach W. Isolated rat pancreatic acini as a model to study the potential role of lipase in the pathogenesis of acinar cell destruction. Int J Pancreatol. 1992;12:285–296. doi: 10.1007/BF02924368. [DOI] [PubMed] [Google Scholar]
- 12.Kimura W, Meyer F, Hess D, Kirchner T, Fischbach W, Mössner J. Comparison of different treatment modalities in experimental pancreatitis in rats. Gastroenterology. 1992;103:1916–1924. doi: 10.1016/0016-5085(92)91452-a. [DOI] [PubMed] [Google Scholar]
- 13.Kimura W, Mössner J. Role of hypertriglyceridemia in the pathogenesis of experimental acute pancreatitis in rats. Int J Pancreatol. 1996;20:177–184. doi: 10.1007/BF02803766. [DOI] [PubMed] [Google Scholar]
- 14.Saharia P, Margolis S, Zuidema GD, Cameron JL. Acute pancreatitis with hyperlipemia: studies with an isolated perfused canine pancreas. Surgery. 1977;82:60–67. [PubMed] [Google Scholar]
- 15.Wang Y, Sternfeld L, Yang F, Rodriguez JA, Ross C, Hayden MR, et al. Enhanced susceptibility to pancreatitis in severe hypertriglyceridaemic lipoprotein lipase-deficient mice and agonist-like function of pancreatic lipase in pancreatic cells. Gut. 1992;58:422–430. doi: 10.1136/gut.2007.146258. [DOI] [PubMed] [Google Scholar]
- 16.Tremblay K, Méthot J, Brisson D, Gaudet D. Etiology and risk of lactescent plasma and severe hypertriglyceridemia. J Clin Lipidol. 2011;5:37–44. doi: 10.1016/j.jacl.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Gaudet D, de Wal J, Tremblay K, Déry S, van Deventer S, Freidig A, et al. Review of the clinical development of alipogene tiparvovec gene therapy for lipoprotein lipase deficiency. Atheroscler Suppl. 2010;11:55–60. doi: 10.1016/j.atherosclerosissup.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Brisson D, Méthot J, Tremblay K, Tremblay M, Perron P, Gaudet D. Comparison of the efficacy of fibrates on hypertriglyceridemic phenotypes with different genetic and clinical characteristics. Pharmacogenet Genomics. 2010;20:742–747. doi: 10.1097/FPC.0b013e328340095e. [DOI] [PubMed] [Google Scholar]
- 19.Schnepp BC, Jensen RL, Clark KR, Johnson PR, et al. Infectious molecular clones of Adeno-associated virus isolated directly from human tissues. J Virol. 2009;83:1456–1464. doi: 10.1128/JVI.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rip J, Nierman MC, Sierts JA, Petersen W, Van den Oever K, Van Raalte D, et al. Gene therapy for lipoprotein lipase deficiency: working toward clinical application. Hum Gene Ther. 2005;16:1276–1286. doi: 10.1089/hum.2005.16.1276. [DOI] [PubMed] [Google Scholar]
- 22.Ross CJ, Twisk J, Bakker AC, Miao F, Verbart D, Rip J, et al. Correction of feline lipoprotein lipase deficiency with adeno-associated virus serotype 1-mediated gene transfer of the lipoprotein lipase S447X beneficial mutation. Hum Gene Ther. 2006;17:487–499. doi: 10.1089/hum.2006.17.487. [DOI] [PubMed] [Google Scholar]
- 23.Ross CJ, Twisk J, Meulenberg JM, Liu G, van den Oever K, Moraal E, et al. Long-term correction of murine lipoprotein lipase deficiency with AAV1-mediated gene transfer of the naturally occurring LPL(S447X) beneficial mutation. Hum Gene Ther. 2004;15:906–919. doi: 10.1089/hum.2004.15.906. [DOI] [PubMed] [Google Scholar]
- 24.Rip J, Nierman MC, Ross CJ, Jukema JW, Hayden MR, Kastelein JJ, et al. Lipoprotein lipase S447X: a naturally occurring gain-of-function mutation. Arterioscler Thromb Vasc Biol. 2006;26:1236–1245. doi: 10.1161/01.ATV.0000219283.10832.43. [DOI] [PubMed] [Google Scholar]
- 25.Stroes ES, Nierman MC, Meulenberg JJ, Franssen R, Twisk J, Henny CP, et al. Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients. Arterioscler Thromb Vasc Biol. 2008;28:2303–2304. doi: 10.1161/ATVBAHA.108.175620. [DOI] [PubMed] [Google Scholar]
- 26.Mingozzi F, Meulenberg JJ, Hui DJ, Basner-Tschakarjan E, Hasbrouck NC, Edmonson SA, et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114:2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burnett JR, Hooper AJ. Alipogene tiparvovec, an adeno-associated virus encoding the Ser(447)X variant of the human lipoprotein lipase gene for the treatment of patients with lipoprotein lipase deficiency. Curr Opin Mol Ther. 2009;11:681–691. [PubMed] [Google Scholar]
- 28.Clinical Trials.gov. Safety and Efficacy in LPL-Deficient Subjects of AMT-011, an Adeno-Associated Viral Vector Expressing Human Lipoprotein Lipase [S447X] [Last accessed on September 27 2010];Protocol summary. at http://clinicaltrials.gov/ct2/show/NCT01109498?term=LPLD&rank=1.
- 29.Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang H, Pierce GF, Ozelo MC, de Paula EV, Vargas JA, Smith P, et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther. 2006;14:452–455. doi: 10.1016/j.ymthe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Flotte TR, Trapnell BC, Humphries M, Carey B, Calcedo R, Rouhani F, et al. Phase 2 Clinical Trial of a Recombinant Adeno-associated Virus Vector Expressing Alpha 1 Antitrypsin: Interim Results. Hum Gene Ther. 2011;22:1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpentier A, Frisch F, Labbe SM, Methot J, Gagné C, Déry S, et al. Gene Therapy With Alipogene Tiparvovec Results In Enhanced Post-prandial Clearance of Chylomicrons In LPLD Patients. J Clin Endocrinol Metab. 2012 (in press) [Google Scholar]
- 34.Gaudet D, Methot J, Gagné C, Déry S, Tremblay K, De Wal J, et al. Modifications in Triglyceride-Rich Lipoprotein Metabolism Induced by Alipogene Tiparvovec (AAV1-LPLS447X Gene Therapy) Correlate With Clinical Benefit in Patients with Lipoprotein Lipase Deficiency (LPLD). Circulation; Proceedings of the American Heart Association; November 2010; Abstract 21355. [Google Scholar]
- 35.The Society for Surgery of the Alimentary Tract. [last accessed 12 July 2011];Guideline of on the treatment of acute pancreatitis. 2004 ( http://www.ssat.com/cgi-bin/acupanc6.cgi)
- 36.Iverius PH, Brunzell JD. Human adipose tissue lipoprotein lipase: changes with feeding and relation to postheparin plasma enzyme. Am J Physiol. 1985;249:E107–114. doi: 10.1152/ajpendo.1985.249.1.E107. [DOI] [PubMed] [Google Scholar]
- 37.Aronica E, van Kempen AA, van der Heide M, Poll-The BT, van Slooten HJ, Troost D, et al. Congenital disorder of glycosylation type Ia: a clinicopathological report of a newborn infant with cerebellar pathology. Acta Neuropathol. 2005;109:433–442. doi: 10.1007/s00401-004-0975-3. [DOI] [PubMed] [Google Scholar]
- 38.Miller FW, Love LA, Barbieri SA, Balow JE, Plotz PH. Lymphocyte activation markers in idiopathic myositis: changes with disease activity and differences among clinical and autoantibody subgroups. Clin Exp Immunol. 1990;81:373–379. doi: 10.1111/j.1365-2249.1990.tb05341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Troost D, Das PK, van den Oord JJ, Louwerse ES. Immunohistological alterations in muscle of patients with amyotrophic lateral sclerosis: mononuclear cell phenotypes and expression of MHC products. 1992;11:115–120. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.