Abstract
Intracerebral hemorrhage (ICH) is a major health concern, with high rates of mortality and morbidity and no highly effective clinical interventions. Basic research in animal models of ICH has provided insight into its complex pathology, in particular revealing the role of inflammation in driving neuronal death and neurologic deficits after hemorrhage. The response to ICH occurs in four distinct phases: (1) initial tissue damage and local activation of inflammatory factors, (2) inflammation-driven breakdown of the blood–brain barrier, (3) recruitment of circulating inflammatory cells and subsequent secondary immunopathology, and (4) engagement of tissue repair responses that promote tissue repair and restoration of neurologic function. The development of CNS inflammation occurs over many days after initial hemorrhage and thus may represent an ideal target for treatment of the disease, but further research is required to identify the mechanisms that promote engagement of inflammatory versus anti-inflammatory pathways. In this review, the authors examine how experimental models of ICH have uncovered critical mediators of pathology in each of the four stages of the inflammatory response, and focus on the role of the immune system in these processes.
Keywords: intracerebral hemorrhage, stroke, neuroinflammation, neuroimmunology
Stroke is a major international health concern and the cause of death for an estimated 11% of deaths worldwide.1 Intracerebral hemorrhage (ICH), while accounting for just 10 to 25% of total strokes, is responsible for greater than half of stroke-related deaths, and less than half of ICH patients survive for 1 year.2 Survivors of ICH often suffer severe morbidity, with just 20% living independently at 6 months.3 Despite efforts to improve therapy and develop new treatments for the disease, the overall mortality rate for ICH has not declined in the past 10 years, and many recent clinical trials for novel surgical or pharmaceutical interventions have failed to show substantial benefit.2 Therefore, there is a critical need for improvement in our understanding of the basic mechanisms governing the severe pathology and resulting morbidity associated with ICH to appropriately target future therapies.
Experimental models of ICH have to this point provided important insight into the systemic and local processes that occur after hemorrhage. In particular, these studies have clarified that though direct mechanical, chemical, and oxidative insults resulting from the hemorrhage are largely responsible for the immediate loss of tissue at the site of the hematoma, inflammatory processes engaged by this initial injury drive continued neuronal death in the perihematomal region.4 The secondary tissue damage driven by recruitment and activation of the immune system occurs predominantly between 12 hours and 72 hours after initial hemorrhage, and the region of neuronal loss associated with ICH expands substantially during this period.5–7 Thus, preclinical data suggest that there is an extended time window in which inhibition of central nervous system (CNS) inflammation may provide substantial benefit to ICH patients.
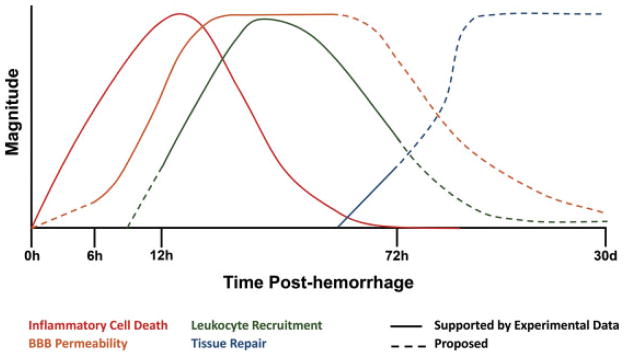
The inflammatory response to ICH can be divided into four distinct stages (Fig. 1):
Fig. 1.
Time course of inflammatory and tissue repair responses after intracerebral hemorrhage (ICH). The onset of ICH causes inflammatory cell death, resulting in the release of proinflammatory factors. Neuronal death occurs rapidly after hemorrhage and continues for the first 3 days posthemorrhage. Expression of factors that regulate the blood–brain barrier (BBB), including matrix metalloproteinases, is first detected at 6 hours post-ICH, and increased BBB permeability is observed for an extended period after hemorrhage. The recruitment of peripheral leukocytes results in significant accumulation of blood-derived macrophages and neutrophils detected at 12 hours post-ICH and peaking at ~24 hours after hemorrhage. At 3 days after hemorrhage, the initiation of pathways involved in tissue repair and hematoma resolution can be detected; however, the duration of these processes remains unclear.
Initial tissue damage and activation of local inflammatory factors
Immune activation in the CNS and remodeling of the blood–brain barrier (BBB)
Recruitment of circulating leukocytes and subsequent secondary immunopathology
Engagement of anti-inflammatory responses that promote tissue repair and restoration of neurologic function
In this review, we will first briefly examine the experimental animal models of ICH that have been developed and the relative strengths and weaknesses of each model. After this, we will explore how these models have informed our knowledge of key biological processes engaged by ICH at each stage, including various forms of cell death, inflammatory and inhibitory cytokines, matrix metalloproteinases, oxidative stress and damage resulting from free radicals, and components of the clotting cascade. Although great progress has been made thus far in understanding these interconnected pathways during the course of ICH and its resolution, there remain substantial gaps in our understanding of this disease; in this review, we will attempt to identify and comment upon these opportunities for future research.
Experimental Models of Intracerebral Hemorrhage
Due to their small size, high reproductive rate, and ease of manipulation, rats and mice have been the predominant mammalian experimental models of human disease for nearly 100 years, and this is likewise true of basic research on ICH. It is important to note, however, that rodent brains differ in many important ways from humans, including the proportion of white matter as well as physiological and physical factors associated with size.8 Studies on surgical approaches to ICH treatment, as well as those focused on loss of white matter and higher cognition deficits, have often utilized larger animal models, including dogs and pigs.8–10 Nonetheless, the ease of use and increasing genetic tractability of murine models has resulted in the majority of current work being performed in these species, particularly the mouse. There are two common approaches to induction of ICH in experimental models: injection of bacterial collagenase (either type IV or type VII) into the striatum and intrastriatal injection of autologous blood.
The collagenase injection model, first developed in rats,11 and then adapted for use in a wide range of species,8 acts by dissolving the collagen-based periendothelial extracellular matrix resulting in rapid spontaneous hemorrhage of small blood vessels near the site of injection. Sham injections or injections of saline are common controls. This approach mimics some key elements of clinical ICH, including endothelial damage and continued bleeding/rebleeding. Additionally, it is highly reproducible and hematoma volume can be controlled by the dose of collagenase used.8,11 However, this approach typically generates higher levels of inflammation than similar-sized hematomas in other models,12–14 suggesting that collagenase may initiate inflammatory pathways independent of its effect on hemorrhage. This may be the result of trace bacterial contaminants in the collagenase capable of potently activating bacterial pattern recognition receptors, or simply the continued dissolution of basement membrane.
Injection of autologous blood in the rat striatum was first developed as a model of ICH in 1985,10 and like collagenase injection, has been adapted for a wide range of species including mice.8,15 In this model, a controlled volume of whole blood is slowly infused directly into the striatum of immobilized mice to mimic the bleeding resulting from hemorrhage. This approach allows more precise control of hemorrhage size than the collagenase model, and avoids the complications of introducing an active bacterial enzyme into the site of injury, but it does not effectively model the rupture of blood vessels or sustained bleeding associated with ICH in the clinical setting. Soluble factors associated with endothelial damage, including activators of the complement cascade and thrombin, are believed to be important early signals in the pathology of ICH,16 and the absence of endothelial breakdown is a drawback of the autologous blood model.
In addition to the collagenase and autologous blood models, injection of blood components, such as thrombin, hemoglobin, and iron, have been used to model ICH in rodents.17,18 However, as these systems are likely less physiologically relevant than the prevailing models, they are best used when assessing the particular contributions of these components to the pathology of ICH. More recently, genetically manipulated mice have been utilized to develop models of spontaneous ICH without the need for injection, thus avoiding any inflammation driven by the surgeries necessary for intracerebral injection of collagenase or autologous blood.19,20 Although the drawback to these approaches has been the inability to control the onset of hemorrhage and therefore closely examine the early effects of the disease, a recent study has described an inducible endothelial-specific genetic strategy in which hemorrhage occurs only after administration of high-affinity synthetic estrogen,20 and may represent a promising means by which to study ICH in a controlled manner without surgical intervention. As each model of ICH possesses distinct strengths and weaknesses, the combined use of multiple approaches is recommended for preclinical and translational studies.
Stage I (0–6 Hours): Cell Death and Local Inflammation
Neuronal death at the site of the hematoma occurs rapidly upon the onset of ICH, and apoptotic as well as necrotic neurons have been observed in surgical evacuates.21,22 Although cell death at the immediate site of hemorrhage is believed to result from mechanical disruption of the tissue and the resulting mass effect,23 preclinical studies have identified distinct mechanisms of secondary neuronal injury resulting from blood-derived factors released during hemorrhage.3,16 Direct exposure of neurons to components of peripheral blood, including hemoglobin, can induce oxidative stress and engage apoptotic pathways on neurons.16,24–26 Simultaneously, endothelial damage during hemorrhage can activate proteases associated with initiation of the clotting cascade, such as complement serine proteases and thrombin, which activate CNS-resident microglia.16,27–30 Furthermore, these signals can act together to drive a recently discovered form of inflammatory cell death, necroptosis, which results in the release by dying neurons of potent inflammatory factors that propagate neuronal degeneration while simultaneously potentiating immune activation.31–36 This initial cascade of cell death and subsequent localized immune activation that occurs in the CNS during the first 6 hours posthemorrhage results in the remodeling of the BBB observed at later time-points after ICH.37
Oxidative Stress
Upon hemorrhage, iron, in the form of the cofactor heme, is released from the peripheral blood into the brain parenchyma.26 Heme is broken down into free iron and bilirubin by heme oxygenases (HO) expressed in neurons, astrocytes, and microglia.24,38,39 The subsequent release of free iron acts as a potent oxidative insult,17 and markers of oxidative stress neurons have been observed in many studies of ICH.17,24,25,40 The exact role of HO enzymes in ICH pathology remains controversial, with some studies finding that HO-1-or HO-2-deficient mice have decreased neurologic deficits and edema,25,39 whereas others have reported that HO-1 and HO-2 protect against neuronal death by sequestering free iron within microglia and astrocytes.38,41 The deleterious effect of free iron is better supported in experimental models, as treatment with the iron chelator deferoxamine decreased white matter loss and ameliorated the neurologic deficits in experimental models of ICH.9,42,43 Notably, the protective effect of deferoxamine was most potent when administered within 2 to 4 hours after hemorrhage, and treatment > 24 hours post-ICH had no significant effect on subsequent inflammation and behavioral outcomes,44,45 supporting the role of blood-derived free iron in the earliest stages of the inflammatory response to ICH. Thus, early intervention to prevent oxidative stress induced by blood components is a promising target for therapeutic intervention; a clinical trial to assess the efficacy and safety of deferoxamine treatment for ICH is ongoing.46
Thrombin and Complement Activation
Thrombin is a critical serine protease in the coagulation cascade produced on the plasma membrane of platelets and leukocytes and released into the brain parenchyma early during ICH.16,18,26,47 Beyond its role in driving the clotting of blood in the CNS after ICH, thrombin can bind directly to a family of protease-activated receptors (PARs), most notably PAR-1.48 PAR-1 expression is increased in the brains of rats after ICH,48 and thrombin contributes to the pathology of ICH by acting directly on PAR-1 expressing neurons and microglia.16 Indeed, intracerebral injection of rat striata activated resident microglia and increased vascular permeability of the BBB.18,30 PAR-1 signaling on neurons potentiates N-methyl-D-aspartate (NMDA) receptor activation of ion channels and can drive glutamate-mediated hypertoxicity and apoptosis.29,30,49,50 Indeed, PAR-1-deficient mice have decreased neuronal death and smaller lesion size after ICH, an effect reversed by administration of an NMDA antagonist.49,51 Thrombin also acts through PAR-1 to activate the transcription factor NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) in microglia, resulting in production of the inflammatory cytokines tumor necrosis factor α (TNF-α) and interleukin 1 β (IL-1β).52,53
Thrombin, as well as other components of the clotting cascade such as plasmin and factor X, can initiate activation of the complement cascade by catalyzing the cleavage of C3 and C5.54 Complement activation has been observed after experimental ICH,27,55 and may perpetuate inflammation and tissue damage via formation of the membrane attack complex and subsequent lysis of damaged erythrocytes as well as by recruitment and activation of leukocytes via the action of the C3a and C5a anaphylatoxins.28 Supporting the role of complement-mediated damage and inflammation in ICH, C3-deficient mice displayed improved behavioral outcomes and decreased HO-1 activity after hemorrhage,56 and pharmacological complement inhibition decreased leukocyte invasion and improved behavioral responses in both mice and rats.55,57,58 In contrast, a study of C5-deficient mice found small increases in edema after ICH compared with wild-type (WT) controls, suggesting that the various components of the complement cascade may play distinct roles after ICH.15
Production of Inflammatory Factors
Neuronal death after ICH has long been noted to include both apoptotic and necrotic cell death pathways.21,22,59 However, the recent discovery of a novel inflammatory cellular death pathway, necroptosis, has led to a series of studies investigating the role of this unique form of inflammatory cell death in ICH.31,32,34,60–63 Necroptosis is engaged by engagement of innate pattern recognition receptors that respond to both foreign pathogens as well as endogenous “danger signals” of tissue injury. Activation of these receptors results in formation of an intracellular “inflammasome” complex that activates caspase 1 as well as caspase 3, which promotes the release of inflammatory factors such as IL-1β and high-mobility group box 1 (HMGB1), as well as initiation of a controlled cell death resembling, but distinct from, apoptosis.60 Several recent studies have noted the activation of necroptosis in the affected brain as early as 3 hours post-ICH.32,34,62
The importance of necroptosis in the propagation of inflammation following ICH has been supported by recent studies inhibiting this process by administration of necrostatin, which prevents activation of the RIP kinases necessary for initiation of necroptosis. Necrostatin administration to rats at the time of experimental ICH resulted in dramatic improvement in behavioral outcomes at 24 and 72 hours post-ICH, as well as observed decreases in hematoma volume, neuronal death, astroglial and microglia/macrophage activation, and production of TNF-α and IL-1β.31,34,61 Activation of NLR family pyrin domain containing 3 (NLRP3) occurs in response to oxidative stress and is a potent driver of necroptosis.64 NLRP3 inhibition also improved behavioral outcomes and decreased microglia/macrophage activation and cytokine production after ICH,62,63 supporting the role of oxidative damage caused by free iron in the activation of innate inflammatory pathways immediately following ICH. Thus, the presence of blood components in the brain parenchyma acts as a danger signal, activating neuron necroptosis and resulting in the release of inflammatory factors in the perihematomal region.
Stage II (~6 Hours): Glial Activation and Breakdown of the Blood–Brain Barrier
Microglia are the resident innate phagocytes of the CNS and constitutively survey the CNS for the presence of infectious microbes and for endogenous danger signals of tissue damage and inflammation.65 After ICH, inflammatory factors generated by blood components and neuronal cell death bind receptors on microglia, engaging signaling cascades that result in activation of NF-κB and subsequent microglial activation. Indeed, decreased NF-κB expression and microglial activation after ICH has been observed upon inhibition of signaling by TNF-α,66 IL-1β,67 thrombin,30,52 heme,66,67 and HMGB1,68 demonstrating the ability of these remarkable cells to respond to a wide range of inflammatory stimuli in this setting. Microglial activation results in the production of matrix metalloproteinases (MMPs), a family of potent proteinases involved in remodeling of extracellular matrix and regulation of the BBB.69 Secretion of MMPs has been observed in the perihematomal region in experimental models of ICH,70–74 as well as in patients after hemorrhage.72,75 These studies have found that MMP9 is particularly important for disruption of endothelial tight junctions, BBB remodeling, and increased vascular permeability after ICH, and high serum levels of MMP9 in ICH patients are associated with hematoma growth and poor clinical outcome.76–78
The primary cellular sources of MMPs after ICH remain unclear. Several studies have reported MMP expression in perihematomal macrophages,70,73,75 but the methodologies used in these studies do not allow clear distinction between activated microglia and recruited blood-derived macrophages.65 Furthermore, MMP production by astrocytes and neutrophils after ICH has been reported69,72,75 and endothelial cells are also capable of MMP production.79 Matrix metalloproteinase activity has been observed as early as 6 hours post-ICH, with higher levels detected at 12 to 24 hours post-ICH.69,70 These findings support a model in which early MMP production by CNS-resident microglia and astrocytes promotes degradation of the BBB and initial recruitment of inflammatory leukocytes, which in turn participate in MMP production and contribute to progressively increased permeability of cerebral vasculature. Regulation of the BBB during inflammation is a complex process, and increased vesicular transport of proteins by endothelium as well the activity of complement and von Willebrand factor have been shown to mediate endothelial permeability after ICH.28,37,80,81 Recruited inflammatory leukocytes are a major source of the secondary neuronal injury after ICH, and the BBB thus represents an ideal target for therapy to inhibit continued inflammation after hemorrhage. However, there is substantial need for improvement in our understanding of the pathways and factors regulating vascular permeability after CNS injury to develop targeted therapies.
Stage III (12 Hours–7 Days): CNS Inflltration of Circulating Leukocytes
Monocytes and Neutrophil Recruitment
The presence of leukocytes in the CNS after ICH has been observed for more than 40 years.82 The importance of these cells to ICH pathology was first demonstrated when whole-body irradiation resulting in severe depletion of circulating leukocytes dramatically reduced edema and inflammation after ICH.83 The role of recruited polymorphonuclear and mononuclear leukocytes in the pathology of ICH has remained an area of great interest. Although the recruitment of neutrophils to the perihematomal region between 1 and 3 days posthemorrhage has been widely reported, the contribution of blood-derived macrophage populations in the CNS has only recently begun to be appreciated.6,7,41,57,84–87 The majority of studies examining macrophage activation after ICH have identified these cells by immunohistochemistry or immunofluorescence using expression of OX-42 or Iba-1.6,30,65,84,88–90 However, these markers are expressed both by activated microglia as well as monocyte-derived macrophages recruited from the blood65; thus the cellular identity of perihematomal phagocytes remained unclear. More recently, flow cytometry has been used to distinguish between myeloid populations in the CNS after ICH, with neutrophils identified as CD11b+ Ly6G+ Ly6Cmid CD45mid-hi, blood-derived macrophage populations as CD11b+ Ly6G− Ly6Chi CD45hi, and microglia as CD11b+ Ly6G− CD45low.85,91–95 Using this approach, we and others have found that inflammatory macrophages and neutrophils in-filtrate the CNS after ICH, with blood-derived macrophages comprising the dominant phagocyte population in the ipsilateral hemisphere from 12 hours to 7 days posthemorrhage.7,85,87,96 Notably, few leukocytes from the blood injection used to induce ICH were observed in the CNS,7 indicating that leukocyte recruitment after ICH is a distinct process and not an artifact of leukocytes from within the initial modeling of ICH.
Chemotaxis and extravasation of leukocytes from the circulation into the CNS after ICH are carefully controlled, and the signals governing these processes continue to be investigated in experimental models. CCR2 and CCR7, the two chemokines most responsible for monocyte recruitment to inflamed tissues,97 are increased in brain tissue after experimental ICH and peak at 1 day posthemorrhage.7,87 We found that CCL2-CCR2 signaling on blood-derived Ly6Chi macrophages, but not microglia, is critical for CNS invasion of these cells, and CCR2-deficient macrophages failed to accumulate in the ipsilateral hemisphere after ICH.7 This study provides context to the earlier finding that CCL2 or CCR2 deficiency resulted in decreased macrophage activation in the perihematomal region after ICH.89 Underscoring the clinical relevance of this signaling pathway, serum levels of CCL2 in patients 24 hours post-ICH correlated with modified Rankin Scale scores after 7 days.7
The contribution of cell adhesion molecules to leukocyte invasion after ICH has also been investigated. Mice deficient in β2 integrin (CD18), an integrin important in myeloid cell-to-cell contact and extravasation, displayed decreased neutrophil recruitment after ICH.98 Similarly, inhibition of vascular adhesion protein 1 (VAP-1) resulted in an approximate 50% decrease in neutrophil infiltration after ICH, despite no observed change in hematoma volume.99 Recently, we have reported increased expression of α4 integrin by blood-derived macrophages, neutrophils, and T cells in the brain after ICH.96 Antibody blockade of α4 prior to induction of ICH resulted in decreased recruitment of T cells and macrophages 2 days posthemorrhage,96 indicating that α4 integrin is important for the early recruitment of certain leukocyte populations after ICH.
Monocytes and Neutrophils Contribute to ICH Pathology
Blood-derived macrophages and neutrophils become highly activated upon recruitment to the perihematomal region after ICH in response to cytokines such as TNF-α and IL-1β, as well as the inflammation-associated factors thrombin, heme, and HMGB1. These inflammatory signals bind cell surface receptors on myeloid cells, resulting in NF-κB activation and production of inflammatory cytokines, reactive oxygen species, and nitric oxide that contribute to tissue damage.4,100 Direct evidence of inflammatory cytokine production by recruited leukocytes during ICH is limited, although we have recently demonstrated TNF-α production by recruited Ly6Chi macrophages in the CNS.7 However, recruitment of infiltrating leukocytes peaks between 1 and 3 days post-ICH,6,7,13,84 coinciding with observation of the highest levels of IL-1β and IL-6,87,92,101 and expansion of the region of neuronal death.5,40 Systemic depletion of neutrophils or monocytes or blockade of their entry into the CNS has consistently been found to decrease inflammation and improve functional outcomes, further supporting the pathological role of these recruited leukocytes early after ICH.7,80,81,86,89,96,98,99,102 Thus, the prevailing model is that early local inflammation after ICH precipitates recruitment of neutrophils and inflammatory monocytes/macrophages that subsequently contribute to an increasing inflammatory response in a feed-forward loop for approximately the first 3 days after hemorrhage.
Tumor necrosis factor α and IL-1β are believed to play an important role in the activation of recruited myeloid cells after ICH, and indeed inhibition of signaling by these cytokines has been shown to reduce macrophage and neutrophil activation and improve behavioral outcomes.103–105 However, these studies were unable to differentiate between the roles of these cytokines in early microglial activation versus the pathology induced by recruited cells; further study in exactly the role these factors play at various stages of ICH is required.
As described earlier in this review, the early stages of ICH result in release of heme, thrombin, and HMGB1 into the brain parenchyma. Each of these signals bind pattern-recognition receptors expressed on blood-derived macrophages and neutrophils and contribute to their activation. In particular, all three of these factors are believed to bind Toll-like receptor 4 (TLR4).16,92 Toll-like receptor 4-deficient mice demonstrate decreased levels of IL-1β, IL-6, and TNF-α in the brain as well as improved behavioral outcomes after ICH.66,92,106 Intriguingly, experiments conducted with wild type and TLR4-deficient blood injected into the striatum revealed that TLR4 signaling in the hemorrhage appears to contribute importantly to the inflammation subsequent to ICH in addition to its activity in recruited monocytes and neutrophils.92 High-mobility group box 1 can also bind toll-like receptor 2 (TLR2) and the receptor for advanced glycation end-products (RAGE), and likely contributes to inflammation after ICH via its action on these receptors.68,94 Indeed, inhibition of HMGB1 signaling resulted in decreased hematoma volume, neuronal death, and leukocyte recruitment, as well as improved behavioral outcomes after ICH.35,91,107 Accordingly, high serum levels of HMGB1 upon admission correlated with worse patient outcomes after 3 months.36
Stage IV: Engagement of Anti-Inflammatory and Wound Healing Responses (~72 Hours–?)
Anti-Inflammatory Pathways Engaged After Intracerebral Hemorrhage
Hematoma size, neuron death, and inflammation peak between 1 and 3 days after ICH.6,7,13,101,108 Notably, while at 72 hours post-ICH levels of IL-1β and IL-6 remain elevated, at this time point the presence of factors associated with inhibition of inflammation in the CNS, such as TGF-β and CXCL1, have increased.92,101 By 7 days posthemorrhage, the hematoma has largely been cleared and inflammation has receded.6,7,101 Additionally, expression of CD36, a scavenger receptor important for phagocytosis of apoptotic and necrotic cells, is increased on blood-derived macrophages and microglia at 3 and 7 days post-ICH.7,92,95 CD36-deficient mice displayed increased hematoma volume 5 days post-ICH and had worse behavioral outcomes than wild-type controls,95 indicating that CD36 may play an important role in hematoma clearance and recovery of neurologic function after ICH.
Recovery from ICH may also involve activation of pathways that regulate oxidative damage from free iron, ROS, and NO.4 Mice deficient for nuclear factor erythroid 2-related factor 2 (Nrf2), an important regulator of antioxidative responses during inflammation, demonstrated higher levels of neuronal death and worse functional outcomes after ICH.109,110 Experimental studies in which drugs that activate Nrf2 were administered 30 minutes to 2 hours after induction of ICH have found decreased inflammation and improved behavioral outcomes after 5 days.110,111 Similarly, therapeutic activation of peroxisome proliferator-activated receptor γ(PPARγ), which has also been shown to regulate antioxidative enzymes such as catalase and superoxide dismutase, decreased neuronal death and leukocyte recruitment, resulting in improved behavioral outcomes after ICH.90,112 Notably, both Nrf2 and PPARγ have been shown to inhibit NF-κB activation,109,112 and PPARγ activation also increased CD36 expression in the brain,90 suggesting that these factors may inhibit inflammation after ICH via multiple pathways.
Recent studies of statin use by ICH patients found that statin users had increased survival and decreased morbidity 30 days after ICH, and that discontinuation of statin use was associated with worse outcome.113,114 Experimental models of ICH have supported a protective role for statins in ICH. Statin treatment following induction of ICH in murine models resulted in decreased hematoma volume and neuron death, as well as improved behavioral outcomes.115–117 In addition to their effects on blood pressure, statins are believed to have anti-inflammatory properties.118 Indeed, statin administration after ICH decreased microglial activation and improved BBB integrity.116,117,119 These changes were associated with decreased expression of TNF-α as well as increased expression of the anti-inflammatory cytokine interleukin 10 and increased neurogenesis during the recovery from ICH.115,117
Stem Cell Therapy after Intracerebral Hemorrhage
Administration of pluripotent stem cells to stimulate neural regeneration after injury is currently being investigated in a wide range of conditions, including ICH. Bone marrow stromal cells administered by intraperitoneal injection at the time of induction of ICH homed to the damaged striatum, proliferated, and acquired expression of neuron and astrocyte markers.120 More promising for translation to the clinic, intracerebral transplantation of neural stem cells at 3 days post-ICH resulted in differentiation of transplanted cells into neurons and astrocytes and dramatically improved behavioral outcomes 1 month after ICH.121 These early studies have demonstrated incredible promise for stem cell therapy in treating ICH, but greater understanding of the mechanisms of neuronal regeneration in these settings is required.
Discussion
In this review we summarize what we have learned to date about the pathology of ICH from experimental models of the disease. Several potential immunomodulatory therapies have now been tested in clinical trials for ICH (Table 1). As of yet, no specific therapy has been identified, and it is apparent is how much we have yet to understand. In particular, certain aspects of the progression of ICH deserve greater attention moving forward. Intracerebral hemorrhage is characterized by an increased permeability of the BBB and the recruitment of circulating leukocytes. The clear role of recruited monocytes and neutrophils in driving tissue pathology and neuronal death during ICH suggests that therapies preventing BBB remodeling after ICH hold great promise, but we lack an understanding of the signals involved in this process. Moreover, most studies of ICH have not utilized approaches that allow a clear distinction between blood-derived macrophages and CNS-resident microglia; moving forward we must assess the role of each of these cells in ICH separately and more specifically, as initial evidence suggests they may play greatly differing roles in inflammation after hemorrhage and during recovery. To date, very few studies have investigated thoroughly the later stages of ICH and the pathways involved in clearance of the hematoma, resolution of inflammation, and other reparative processes. These natural pathways may be ideal targets to augment in clinical settings to promote neurologic repair. Finally, murine brain anatomy and immunological processes differ greatly from those observed in human patients, and when possible, data derived from clinical settings of patients with ICH may help to inform future basic and translational research.
Table 1.
Targeted anti-inflammatory therapies tested in early-phase clinical trials for the treatment of intracerebral hemorrhage
| Medication | Target(s) | Proposed mechanism | Trial results |
|---|---|---|---|
| Dexamethasone | Glucocorticoid receptor agonist | Inhibition of NF-κB, proapoptotic effect on lymphocytes | Mixed results in safety of patients due to hyperglycemia122,123 |
| Anakinra | IL-1 receptor antagonist | Inhibition of activation of microglia, neutrophils, and macrophages | No specific safety concerns in patients with ICH (n = 5)124 |
| Celecoxib | Cyclo-oxygenase-2 (COX-2) inhibitor | Inhibition of prostaglandin synthesis | Reduced perihematomal edema and ICH expansion125 |
| Fingolimod (FTY720) | Sphingosine 1-phosphate receptor blockade | Reduction in circulating lymphocytes | Reduced edema at day 7 and 14 in treated patients; improved neurologic outcomes at days 7, 14, and 30126 |
| Pioglitazone | PPAR γ agonist | Activation of antioxidative pathways | Trial evaluating safety and tolerability completed; results pending127 |
| Deferoxamine | Iron chelator | Prevention of iron induced oxidative injury | Trial evaluating safety and tolerability in progress128 |
Abbreviations: ICH, intracerebral hemorrhage; IL-1, interleukin 1; NF-κB, nuclear factor kappa-light-chain enhancer of activated B cells; PPARγ, peroxisome proliferator-activated receptor gamma.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Aiyagari V. The clinical management of acute intracerebral hemorrhage. Expert Rev Neurother. 2015;15(12):1421–1432. doi: 10.1586/14737175.2015.1113876. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373(9675):1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11(8):720–731. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felberg RA, Grotta JC, Shirzadi AL, et al. Cell death in experimental intracerebral hemorrhage: the “black hole” model of hemorrhagic damage. Ann Neurol. 2002;51(4):517–524. doi: 10.1002/ana.10160. [DOI] [PubMed] [Google Scholar]
- 6.Xue M, Del Bigio MR. Intracerebral injection of autologous whole blood in rats: time course of inflammation and cell death. Neurosci Lett. 2000;283(3):230–232. doi: 10.1016/s0304-3940(00)00971-x. [DOI] [PubMed] [Google Scholar]
- 7.Hammond MD, Taylor RA, Mullen MT, et al. CCR2+ Ly6C(hi) inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J Neurosci. 2014;34(11):3901–3909. doi: 10.1523/JNEUROSCI.4070-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James ML, Warner DS, Laskowitz DT. Preclinical models of intracerebral hemorrhage: a translational perspective. Neurocrit Care. 2008;9(1):139–152. doi: 10.1007/s12028-007-9030-2. [DOI] [PubMed] [Google Scholar]
- 9.Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40(6):2241–2243. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takasugi S, Ueda S, Matsumoto K. Chronological changes in spontaneous intracerebral hematoma—an experimental and clinical study. Stroke. 1985;16(4):651–658. doi: 10.1161/01.str.16.4.651. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21(5):801–807. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- 12.MacLellan CL, Silasi G, Auriat AM, Colbourne F. Rodent models of intracerebral hemorrhage. Stroke. 2010;41(10, Suppl):S95–S98. doi: 10.1161/STROKEAHA.110.594457. [DOI] [PubMed] [Google Scholar]
- 13.Xue M, Del Bigio MR. Comparison of brain cell death and inflammatory reaction in three models of intracerebral hemorrhage in adult rats. J Stroke Cerebrovasc Dis. 2003;12(3):152–159. doi: 10.1016/S1052-3057(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 14.Barratt HE, Lanman TA, Carmichael ST. Mouse intracerebral hemorrhage models produce different degrees of initial and delayed damage, axonal sprouting, and recovery. J Cereb Blood Flow Metab. 2014;34(9):1463–1471. doi: 10.1038/jcbfm.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura T, Xi G, Hua Y, Schallert T, Hoff JT, Keep RF. Intracerebral hemorrhage in mice: model characterization and application for genetically modified mice. J Cereb Blood Flow Metab. 2004;24(5):487–494. doi: 10.1097/00004647-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Babu R, Bagley JH, Di C, Friedman AH, Adamson C. Thrombin and hemin as central factors in the mechanisms of intracerebral hemorrhage-induced secondary brain injury and as potential targets for intervention. Neurosurg Focus. 2012;32(4):E8. doi: 10.3171/2012.1.FOCUS11366. [DOI] [PubMed] [Google Scholar]
- 17.Huang F-P, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002;96(2):287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- 18.Lee KR, Kawai N, Kim S, Sagher O, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of thrombin on cerebral blood flow, blood-brain barrier permeability, and cell survival in a rat model. J Neurosurg. 1997;86(2):272–278. doi: 10.3171/jns.1997.86.2.0272. [DOI] [PubMed] [Google Scholar]
- 19.Iida S, Baumbach GL, Lavoie JL, Faraci FM, Sigmund CD, Heistad DD. Spontaneous stroke in a genetic model of hypertension in mice. Stroke. 2005;36(6):1253–1258. doi: 10.1161/01.str.0000167694.58419.a2. [DOI] [PubMed] [Google Scholar]
- 20.Weinl C, Castaneda Vega S, Riehle H, et al. Endothelial depletion of murine SRF/MRTF provokes intracerebral hemorrhagic stroke. Proc Natl Acad Sci U S A. 2015;112(32):9914–9919. doi: 10.1073/pnas.1509047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qureshi AI, Ling GS, Khan J, et al. Quantitative analysis of injured, necrotic, and apoptotic cells in a new experimental model of intracerebral hemorrhage. Crit Care Med. 2001;29(1):152–157. doi: 10.1097/00003246-200101000-00030. [DOI] [PubMed] [Google Scholar]
- 22.Qureshi AI, Suri MFK, Ostrow PT, et al. Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery. 2003;52(5):1041–1047. discussion 1047–1048. [PubMed] [Google Scholar]
- 23.Suzuki J, Ebina T. Sequential changes in tissue surrounding ICH. In: Pia HW, Langmaid C, Zierski J, editors. Spontaneous Intracerebral Haematomas: Advances in Diagnosis and Therapy. Berlin, Heidelberg: Springer; 2012. [Google Scholar]
- 24.Matz PG, Weinstein PR, Sharp FR. Heme oxygenase-1 and heat shock protein 70 induction in glia and neurons throughout rat brain after experimental intracerebral hemorrhage. Neurosurgery. 1997;40(1):152–160. doi: 10.1097/00006123-199701000-00034. discussion 160–162. [DOI] [PubMed] [Google Scholar]
- 25.Qu Y, Chen-Roetling J, Benvenisti-Zarom L, Regan RF. Attenuation of oxidative injury after induction of experimental intracerebral hemorrhage in heme oxygenase-2 knockout mice. J Neurosurg. 2007;106(3):428–435. doi: 10.3171/jns.2007.106.3.428. [DOI] [PubMed] [Google Scholar]
- 26.Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke. 2007;38(2, Suppl):759–762. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- 27.Hua Y, Xi G, Keep RF, Hoff JT. Complement activation in the brain after experimental intracerebral hemorrhage. J Neurosurg. 2000;92(6):1016–1022. doi: 10.3171/jns.2000.92.6.1016. [DOI] [PubMed] [Google Scholar]
- 28.Ducruet AF, Zacharia BE, Hickman ZL, et al. The complement cascade as a therapeutic target in intracerebral hemorrhage. Exp Neurol. 2009;219(2):398–403. doi: 10.1016/j.expneurol.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donovan FM, Pike CJ, Cotman CW, Cunningham DD. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and RhoA activities. J Neurosci. 1997;17(14):5316–5326. doi: 10.1523/JNEUROSCI.17-14-05316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujimoto S, Katsuki H, Ohnishi M, Takagi M, Kume T, Akaike A. Thrombin induces striatal neurotoxicity depending on mitogen-activated protein kinase pathways in vivo. Neuroscience. 2007;144(2):694–701. doi: 10.1016/j.neuroscience.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 31.Chang P, Dong W, Zhang M, et al. Anti-necroptosis chemical necrostatin-1 can also suppress apoptotic and autophagic pathway to exert neuroprotective effect in mice intracerebral hemorrhage model. J Mol Neurosci. 2014;52(2):242–249. doi: 10.1007/s12031-013-0132-3. [DOI] [PubMed] [Google Scholar]
- 32.Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol. 2014;75(2):209–219. doi: 10.1002/ana.24070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11(10):700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 34.Su X, Wang H, Kang D, et al. Necrostatin-1 ameliorates intracerebral hemorrhage-induced brain injury in mice through inhibiting RIP1/RIP3 pathway. Neurochem Res. 2015;40(4):643–650. doi: 10.1007/s11064-014-1510-0. [DOI] [PubMed] [Google Scholar]
- 35.Lei C, Lin S, Zhang C, et al. High-mobility group box 1 protein promotes neuroinflammation after intracerebral hemorrhage in rats. Neuroscience. 2013;228:190–199. doi: 10.1016/j.neuroscience.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Xiong K-L, Lin S, et al. Elevation of high-mobility group protein box-1 in serum correlates with severityof acute intracerebral hemorrhage. Mediators Inflamm. 2010:2010. doi: 10.1155/2010/142458. Article ID 142458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knowland D, Arac A, Sekiguchi KJ, et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82(3):603–617. doi: 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schallner N, Pandit R, LeBlanc R, III, et al. Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase-1. J Clin Invest. 2015;125(7):2609–2625. doi: 10.1172/JCI78443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Doré S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130(Pt 6):1643–1652. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura T, Keep RF, Hua Y, Hoff JT, Xi G. Oxidative DNA injury after experimental intracerebral hemorrhage. Brain Res. 2005;1039(1–2):30–36. doi: 10.1016/j.brainres.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Doré S. Heme oxygenase 2 deficiency increases brain swelling and inflammation after intracerebral hemorrhage. Neuroscience. 2008;155(4):1133–1141. doi: 10.1016/j.neuroscience.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100(4):672–678. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- 43.Ni W, Okauchi M, Hatakeyama T, et al. Deferoxamine reduces intracerebral hemorrhage-induced white matter damage in aged rats. Exp Neurol. 2015;272:128–134. doi: 10.1016/j.expneurol.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okauchi M, Hua Y, Keep RF, Morgenstern LB, Schallert T, Xi G. Deferoxamine treatment for intracerebral hemorrhage in aged rats: therapeutic time window and optimal duration. Stroke. 2010;41(2):375–382. doi: 10.1161/STROKEAHA.109.569830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui H-J, He H-Y, Yang A-L, et al. Efficacy of deferoxamine in animal models of intracerebral hemorrhage: a systematic review and stratified meta-analysis. PLoS ONE. 2015;10(5):e0127256. doi: 10.1371/journal.pone.0127256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selim M. Deferoxamine mesylate: a new hope for intracerebral hemorrhage: from bench to clinical trials. Stroke. 2009;40(3, Suppl):S90–S91. doi: 10.1161/STROKEAHA.108.533125. [DOI] [PubMed] [Google Scholar]
- 47.Lee KR, Colon GP, Betz AL, Keep RF, Kim S, Hoff JT. Edema from intracerebral hemorrhage: the role of thrombin. J Neurosurg. 1996;84(1):91–96. doi: 10.3171/jns.1996.84.1.0091. [DOI] [PubMed] [Google Scholar]
- 48.Zheng G-Q, Wang X-T, Wang X-M, et al. Long-time course of protease-activated receptor-1 expression after intracerebral hemorrhage in rats. Neurosci Lett. 2009;459(2):62–65. doi: 10.1016/j.neulet.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Hamill CE, Mannaioni G, Lyuboslavsky P, Sastre AA, Traynelis SF. Protease-activated receptor 1-dependent neuronal damage involves NMDA receptor function. Exp Neurol. 2009;217(1):136–146. doi: 10.1016/j.expneurol.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee S-T, Chu K, Jung K-H, et al. Memantine reduces hematoma expansion in experimental intracerebral hemorrhage, resulting in functional improvement. J Cereb Blood Flow Metab. 2006;26(4):536–544. doi: 10.1038/sj.jcbfm.9600213. [DOI] [PubMed] [Google Scholar]
- 51.Xue M, Hollenberg MD, Demchuk A, Yong VW. Relative importance of proteinase-activated receptor-1 versus matrix metalloproteinases in intracerebral hemorrhage-mediated neurotoxicity in mice. Stroke. 2009;40(6):2199–2204. doi: 10.1161/STROKEAHA.108.540393. [DOI] [PubMed] [Google Scholar]
- 52.Wu J, Yang S, Xi G, Fu G, Keep RF, Hua Y. Minocycline reduces intracerebral hemorrhage-induced brain injury. Neurol Res. 2009;31(2):183–188. doi: 10.1179/174313209X385680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryu J, Pyo H, Jou I, Joe E. Thrombin induces NO release from cultured rat microglia via protein kinase C, mitogen-activated protein kinase, and NF-kappa B. J Biol Chem. 2000;275(39):29955–29959. doi: 10.1074/jbc.M001220200. [DOI] [PubMed] [Google Scholar]
- 54.Amara U, Flierl MA, Rittirsch D, et al. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185(9):5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xi G, Hua Y, Keep RF, Younger JG, Hoff JT. Systemic complement depletion diminishes perihematomal brain edema in rats. Stroke. 2001;32(1):162–167. doi: 10.1161/01.str.32.1.162. [DOI] [PubMed] [Google Scholar]
- 56.Yang S, Nakamura T, Hua Y, et al. The role of complement C3 in intracerebral hemorrhage-induced brain injury. J Cereb Blood Flow Metab. 2006;26(12):1490–1495. doi: 10.1038/sj.jcbfm.9600305. [DOI] [PubMed] [Google Scholar]
- 57.Garrett MC, Otten ML, Starke RM, et al. Synergistic neuroprotective effects of C3a and C5a receptor blockade following intracerebral hemorrhage. Brain Res. 2009;1298:171–177. doi: 10.1016/j.brainres.2009.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rynkowski MA, Kim GH, Garrett MC, et al. C3a receptor antagonist attenuates brain injury after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2009;29(1):98–107. doi: 10.1038/jcbfm.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delgado P, Cuadrado E, Rosell A, et al. Fas system activation in perihematomal areas after spontaneous intracerebral hemorrhage. Stroke. 2008;39(6):1730–1734. doi: 10.1161/STROKEAHA.107.500876. [DOI] [PubMed] [Google Scholar]
- 60.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517(7534):311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 61.King MD, Whitaker-Lea WA, Campbell JM, Alleyne CH, Jr, Dhandapani KM. Necrostatin-1 reduces neurovascular injury after intracerebral hemorrhage. Int J Cell Biol. 2014;2014:495817. doi: 10.1155/2014/495817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng L, Chen Y, Ding R, et al. P2×7R blockade prevents NLRP3 inflammasome activation and brain injury in a rat model of intracerebral hemorrhage: involvement of peroxynitrite. J Neuroinflammation. 2015;12(1):190. doi: 10.1186/s12974-015-0409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan B, Shen H, Lin L, Su T, Zhong S, Yang Z. Recombinant adenovirus encoding NLRP3 RNAi attenuate inflammation and brain injury after intracerebral hemorrhage. J Neuroimmunol. 2015;287:71–75. doi: 10.1016/j.jneuroim.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev. 2011;243(1):152–162. doi: 10.1111/j.1600-065X.2011.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol. 2013;2013:746068. doi: 10.1155/2013/746068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin S, Yin Q, Zhong Q, et al. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intra-cerebral hemorrhage. J Neuroinflammation. 2012;9(1):46. doi: 10.1186/1742-2094-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Z, Liu Y, Yuan F, et al. Sinomenine inhibits microglia activation and attenuates brain injury in intracerebral hemorrhage. Mol Immunol. 2014;60(2):109–114. doi: 10.1016/j.molimm.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Li D, Lei C, Zhang S, Zhang S, Liu M, Wu B. Blockade of high mobility group box-1 signaling via the receptor for advanced glycation end-products ameliorates inflammatory damage after acute intracerebral hemorrhage. Neurosci Lett. 2015;609:109–119. doi: 10.1016/j.neulet.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 69.Xue M, Yong VW. Matrix metalloproteinases in intracerebral hemorrhage. Neurol Res. 2008;30(8):775–782. doi: 10.1179/174313208X341102. [DOI] [PubMed] [Google Scholar]
- 70.Power C, Henry S, Del Bigio MR, et al. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol. 2003;53(6):731–742. doi: 10.1002/ana.10553. [DOI] [PubMed] [Google Scholar]
- 71.Wang J, Tsirka SE. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005;128(Pt 7):1622–1633. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- 72.Tejima E, Zhao B-Q, Tsuji K, et al. Astrocytic induction of matrix metalloproteinase-9 and edema in brain hemorrhage. J Cereb Blood Flow Metab. 2007;27(3):460–468. doi: 10.1038/sj.jcbfm.9600354. [DOI] [PubMed] [Google Scholar]
- 73.Lee J-M, Yin K-J, Hsin I, et al. Matrix metalloproteinase-9 and spontaneous hemorrhage in an animal model of cerebral amyloid angiopathy. Ann Neurol. 2003;54(3):379–382. doi: 10.1002/ana.10671. [DOI] [PubMed] [Google Scholar]
- 74.Zhao L, Arbel-Ornath M, Wang X, et al. Matrix metalloproteinase 9-mediated intracerebral hemorrhage induced by cerebral amyloid angiopathy. Neurobiol Aging. 2015;36(11):2963–2971. doi: 10.1016/j.neurobiolaging.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosell A, Ortega-Aznar A, Alvarez-Sabín J, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37(6):1399–1406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- 76.Abilleira S, Montaner J, Molina CA, Monasterio J, Castillo J, Alvarez-Sabín J. Matrix metalloproteinase-9 concentration after spontaneous intracerebral hemorrhage. J Neurosurg. 2003;99(1):65–70. doi: 10.3171/jns.2003.99.1.0065. [DOI] [PubMed] [Google Scholar]
- 77.Alvarez-Sabín J, Delgado P, Abilleira S, et al. Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: relationship to clinical and radiological outcome. Stroke. 2004;35(6):1316–1322. doi: 10.1161/01.STR.0000126827.69286.90. [DOI] [PubMed] [Google Scholar]
- 78.Silva Y, Leira R, Tejada J, Lainez JM, Castillo J, Dávalos A Stroke Project, Cerebrovascular Diseases Group of the Spanish Neurological Society. Molecular signatures of vascular injury are associated with early growth of intracerebral hemorrhage. Stroke. 2005;36(1):86–91. doi: 10.1161/01.STR.0000149615.51204.0b. [DOI] [PubMed] [Google Scholar]
- 79.Genersch E, Hayess K, Neuenfeld Y, Haller H. Sustained ERK phosphorylation is necessary but not sufficient for MMP-9 regulation in endothelial cells: involvement of Ras-dependent and -independent pathways. J Cell Sci. 2000;113(Pt 23):4319–4330. doi: 10.1242/jcs.113.23.4319. [DOI] [PubMed] [Google Scholar]
- 80.Chang C-F, Chen S-F, Lee T-S, Lee H-F, Chen S-F, Shyue S-K. Caveolin-1 deletion reduces early brain injury after experimental intracerebral hemorrhage. Am J Pathol. 2011;178(4):1749–1761. doi: 10.1016/j.ajpath.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cai P, Luo H, Xu H, et al. Recombinant ADAMTS 13 attenuates brain injury after intracerebral hemorrhage. Stroke. 2015;46(9):2647–2653. doi: 10.1161/STROKEAHA.115.009526. [DOI] [PubMed] [Google Scholar]
- 82.Lee MC, Heaney LM, Jacobson RL, Klassen AC. Cerebrospinal fluid in cerebral hemorrhage and infarction. Stroke. 1975;6(6):638–641. doi: 10.1161/01.str.6.6.638. [DOI] [PubMed] [Google Scholar]
- 83.Kane PJ, Modha P, Strachan RD, et al. The effect of immunosuppression on the development of cerebral oedema in an experimental model of intracerebral haemorrhage: whole body and regional irradiation. J Neurol Neurosurg Psychiatry. 1992;55(9):781–786. doi: 10.1136/jnnp.55.9.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gong C, Hoff JT, Keep RF. Acute inflammatory reaction following experimental intracerebral hemorrhage in rat. Brain Res. 2000;871(1):57–65. doi: 10.1016/s0006-8993(00)02427-6. [DOI] [PubMed] [Google Scholar]
- 85.Mracsko E, Javidi E, Na S-Y, Kahn A, Liesz A, Veltkamp R. Leukocyte invasion of the brain after experimental intracerebral hemorrhage in mice. Stroke. 2014;45(7):2107–2114. doi: 10.1161/STROKEAHA.114.005801. [DOI] [PubMed] [Google Scholar]
- 86.Sansing LH, Harris TH, Kasner SE, Hunter CA, Kariko K. Neutrophil depletion diminishes monocyte infiltration and improves functional outcome after experimental intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:173–178. doi: 10.1007/978-3-7091-0693-8_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hammond MD, Ai Y, Sansing LH. Gr1+ macrophages and dendritic cells dominate the inflammatory infiltrate 12 hours after experimental intracerebral hemorrhage. Transl Stroke Res. 2012;3(1):s125–s131. doi: 10.1007/s12975-012-0174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27(3):268–279. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- 89.Yao Y, Tsirka SE. The CCL2-CCR2 system affects the progression and clearance of intracerebral hemorrhage. Glia. 2012;60(6):908–918. doi: 10.1002/glia.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao X, Sun G, Zhang J, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007;61(4):352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- 91.Yang F, Wang Z, Zhang JH, et al. Receptor for advanced glycation end-product antagonist reduces blood-brain barrier damage after intracerebral hemorrhage. Stroke. 2015;46(5):1328–1336. doi: 10.1161/STROKEAHA.114.008336. [DOI] [PubMed] [Google Scholar]
- 92.Sansing LH, Harris TH, Welsh FA, Kasner SE, Hunter CA, Kariko K. Toll-like receptor 4 contributes to poor outcome after intracerebral hemorrhage. Ann Neurol. 2011;70(4):646–656. doi: 10.1002/ana.22528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FMV. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14(9):1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y-C, Zhou Y, Fang H, et al. Toll-like receptor 2/4 heterodimer mediates inflammatory injury in intracerebral hemorrhage. Ann Neurol. 2014;75(6):876–889. doi: 10.1002/ana.24159. [DOI] [PubMed] [Google Scholar]
- 95.Fang H, Chen J, Lin S, et al. CD36-mediated hematoma absorption following intracerebral hemorrhage: negative regulation by TLR4 signaling. J Immunol. 2014;192(12):5984–5992. doi: 10.4049/jimmunol.1400054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hammond MD, Ambler WG, Ai Y, Sansing LH. α4 integrin is a regulator of leukocyte recruitment after experimental intracerebral hemorrhage. Stroke. 2014;45(8):2485–2487. doi: 10.1161/STROKEAHA.114.005551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Titova E, Ostrowski RP, Kevil CG, et al. Reduced brain injury in CD18-deficient mice after experimental intracerebral hemorrhage. J Neurosci Res. 2008;86(14):3240–3245. doi: 10.1002/jnr.21762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma Q, Manaenko A, Khatibi NH, Chen W, Zhang JH, Tang J. Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. J Cereb Blood Flow Metab. 2011;31(3):881–893. doi: 10.1038/jcbfm.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mracsko E, Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Front Cell Neurosci. 2014;8:388. doi: 10.3389/fncel.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wasserman JK, Zhu X, Schlichter LC. Evolution of the inflammatory response in the brain following intracerebral hemorrhage and effects of delayed minocycline treatment. Brain Res. 2007;1180:140–154. doi: 10.1016/j.brainres.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 102.Moxon-Emre I, Schlichter LC. Neutrophil depletion reduces blood-brain barrier breakdown, axon injury, and inflammation after intracerebral hemorrhage. J Neuropathol Exp Neurol. 2011;70(3):218–235. doi: 10.1097/NEN.0b013e31820d94a5. [DOI] [PubMed] [Google Scholar]
- 103.Mayne M, Ni W, Yan HJ, et al. Antisense oligodeoxynucleotide inhibition of tumor necrosis factor-alpha expression is neuroprotective after intracerebral hemorrhage. Stroke. 2001;32(1):240–248. doi: 10.1161/01.str.32.1.240. [DOI] [PubMed] [Google Scholar]
- 104.Masada T, Hua Y, Xi G, Yang GY, Hoff JT, Keep RF. Attenuation of intracerebral hemorrhage and thrombin-induced brain edema by overexpression of interleukin-1 receptor antagonist. J Neurosurg. 2001;95(4):680–686. doi: 10.3171/jns.2001.95.4.0680. [DOI] [PubMed] [Google Scholar]
- 105.King MD, Alleyne CH, Jr, Dhandapani KM. TNF-alpha receptor antagonist, R-7050, improves neurological outcomes following intracerebral hemorrhage in mice. Neurosci Lett. 2013;542:92–96. doi: 10.1016/j.neulet.2013.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fang H, Wang P-F, Zhou Y, Wang Y-C, Yang Q-W. Toll-like receptor 4 signaling in intracerebral hemorrhage-induced inflammation and injury. J Neuroinflammation. 2013;10(1):27. doi: 10.1186/1742-2094-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ohnishi M, Katsuki H, Fukutomi C, et al. HMGB1 inhibitor glycyrrhizin attenuates intracerebral hemorrhage-induced injury in rats. Neuropharmacology. 2011;61(5–6):975–980. doi: 10.1016/j.neuropharm.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 108.Zhao X, Zhang Y, Strong R, Zhang J, Grotta JC, Aronowski J. Distinct patterns of intracerebral hemorrhage-induced alterations in NF-kappaB subunit, iNOS, and COX-2 expression. J Neurochem. 2007;101(3):652–663. doi: 10.1111/j.1471-4159.2006.04414.x. [DOI] [PubMed] [Google Scholar]
- 109.Wang J, Fields J, Zhao C, et al. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med. 2007;43(3):408–414. doi: 10.1016/j.freeradbiomed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao X, Sun G, Zhang J, et al. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007;38(12):3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- 111.Zhao X, Sun G, Zhang J, Ting S-M, Gonzales N, Aronowski J. Dimethyl fumarate protects brain from damage produced by intracerebral hemorrhage by mechanism involving Nrf2. Stroke. 2015;46(7):1923–1928. doi: 10.1161/STROKEAHA.115.009398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao X, Zhang Y, Strong R, Grotta JC, Aronowski J. 15d-Prostaglandin J2 activates peroxisome proliferator-activated receptor-gamma, promotes expression of catalase, and reduces inflammation, behavioral dysfunction, and neuronal loss after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2006;26(6):811–820. doi: 10.1038/sj.jcbfm.9600233. [DOI] [PubMed] [Google Scholar]
- 113.Flint AC, Conell C, Rao VA, et al. Effect of statin use during hospitalization for intracerebral hemorrhage on mortality and discharge disposition. JAMA Neurol. 2014;71(11):1364–1371. doi: 10.1001/jamaneurol.2014.2124. [DOI] [PubMed] [Google Scholar]
- 114.Tapia Pérez JH, Yildiz OC, Schneider T, Nimsky C. Meta-analysis of Statin Use for the Acute Therapy of Spontaneous Intracerebral Hemorrhage. J Stroke Cerebrovasc Dis. 2015;24(11):2521–2526. doi: 10.1016/j.jstrokecerebrovasdis.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 115.Karki K, Knight RA, Han Y, et al. Simvastatin and atorvastatin improve neurological outcome after experimental intracerebral hemorrhage. Stroke. 2009;40(10):3384–3389. doi: 10.1161/STROKEAHA.108.544395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Indraswari F, Wang H, Lei B, et al. Statins improve outcome in murine models of intracranial hemorrhage and traumatic brain injury: a translational approach. J Neurotrauma. 2012;29(7):1388–1400. doi: 10.1089/neu.2011.2117. [DOI] [PubMed] [Google Scholar]
- 117.Ewen T, Qiuting L, Chaogang T, et al. Neuroprotective effect of atorvastatin involves suppression of TNF-α and upregulation of IL-10 in a rat model of intracerebral hemorrhage. Cell Biochem Biophys. 2013;66(2):337–346. doi: 10.1007/s12013-012-9453-z. [DOI] [PubMed] [Google Scholar]
- 118.Antonopoulos AS, Margaritis M, Lee R, Channon K, Antoniades C. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Des. 2012;18(11):1519–1530. doi: 10.2174/138161212799504803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang D, Knight RA, Han Y, et al. Statins protect the blood brain barrier acutely after experimental intracerebral hemorrhage. J Behav Brain Sci. 2013;3(1):100–106. doi: 10.4236/jbbs.2013.31010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seyfried DM, Han Y, Yang D, et al. Localization of bone marrow stromal cells to the injury site after intracerebral hemorrhage in rats. J Neurosurg. 2010;112(2):329–335. doi: 10.3171/2009.2.JNS08907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Z, Cui C, Li Q, et al. Intracerebral transplantation of foetal neural stem cells improves brain dysfunction induced by intra-cerebral haemorrhage stroke in mice. J Cell Mol Med. 2011;15(12):2624–2633. doi: 10.1111/j.1582-4934.2011.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Desai P, Prasad K. Dexamethasone is not necessarily unsafe in primary supratentorial intracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 1998;65(5):799–800. doi: 10.1136/jnnp.65.5.799a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Poungvarin N, Bhoopat W, Viriyavejakul A, et al. Effects of dexamethasone in primary supratentorial intracerebral hemorrhage. N Engl J Med. 1987;316(20):1229–1233. doi: 10.1056/NEJM198705143162001. [DOI] [PubMed] [Google Scholar]
- 124.Emsley HCA, Smith CJ, Georgiou RF, et al. Acute Stroke Investigators. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76(10):1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chu K, Jeong S-W, Jung K-H, et al. Celecoxib induces functional recovery after intracerebral hemorrhage with reduction of brain edema and perihematomal cell death. J Cereb Blood Flow Metab. 2004;24(8):926–933. doi: 10.1097/01.WCB.0000130866.25040.7D. [DOI] [PubMed] [Google Scholar]
- 126.Fu Y, Hao J, Zhang N, et al. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol. 2014;71(9):1092–1101. doi: 10.1001/jamaneurol.2014.1065. [DOI] [PubMed] [Google Scholar]
- 127.Gonzales NR, Shah J, Sangha N, et al. Design of a prospective, dose-escalation study evaluating the Safety of Pioglitazone for Hematoma Resolution in Intracerebral Hemorrhage (SHRINC) Int J Stroke. 2013;8(5):388–396. doi: 10.1111/j.1747-4949.2011.00761.x. [DOI] [PubMed] [Google Scholar]
- 128.Yeatts SD, Palesch YY, Moy CS, Selim M. High dose deferoxamine in intracerebral hemorrhage (HI-DEF) trial: rationale, design, and methods. Neurocrit Care. 2013;19(2):257–266. doi: 10.1007/s12028-013-9861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]